Abstract
The feasibility of potato-based bioplastics as food or fresh produce packaging materials was investigated using glycerol and sorbitol plasticizers at three different concentrations (30, 50, and 70%). The effects of plasticizers on the tensile strength, water vapour transmission rate, water absorption, and contact angle of the plasticized bioplastic were compared to the pure potato starch-based bioplastic. At same concentration, glycerol-plasticized bioplastic films were more flexible and stickier than sorbitol-plasticized bioplastic films. At all concentrations, however, the tensile strength and elongation at break were consistently lower than the latter. The experimental results revealed that glycerol with a higher hydrophilicity produced bioplastic films with higher water vapour transmission (63.68–80.91%), water absorption (252.65–432.71%), and lower contact angle (36.669°–50.506°) in comparison with sorbitol-plasticized bioplastics. In Fourier transform infrared spectroscopy, the major absorption bands characteristics of all bioplastics, with or without the presence of plasticizers, were found to be identical. A weight loss test on strawberries stored at different temperatures was used to investigate the feasibility of plasticized potato starch-based bioplastics for use as food and fresh produce packaging materials. Increase in the concentration of both plasticizers from 30 to 70% reduced the weight loss of strawberries kept at 4 °C and room temperature, with no significant difference in weight loss observed at the same concentration of the plasticizers. It was also found that when strawberries were wrapped in plasticized bioplastics and kept at 4 °C, water loss was reduced by 4 to 6.8 fold compared to room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic is one of humanity's greatest inventions (North and Hladen 2013). It is lightweight, durable, soft, flexible, transparent, corrosion-resistant, and versatile even at extreme conditions (Sam et al. 2016; Sanyang et al. 2016; Siracusa et al. 2008). These characteristics make it suitable for a wide range of applications, including packaging, construction, and electronic component manufacturing, among others (Phil 2019). According to Geyer et al. (2017), the amount of plastic waste generated since 1950 has exceeded 8.3 billion tonnes, with the number expected to rise due to high demand in the market. The most common plastics on the market today are petroleum-based plastics, which are made up of synthetic organic polymers and derived from natural gas, crude oil, or coal (Suman et al. 2020). Although petroleum-based plastics can be disintegrated, they cannot decompose due to the addition of additives such as UV and thermal stabilizers, lubricants, photoinitiations, and antioxidants, which give them extreme stability. As a result of the massive amount of plastic waste that is released into the environment each year, its stability poses serious environmental and health risks (Ciriminna and Pagliaro 2019).
According to Plastic Oceans International (2021), approximately half of all plastics produced are designed for single-use purposes in the packaging industry and are discarded as trash after one use. Plastic bags, in particular, take nearly 10 to 20 years to degrade, which is a very long time (Chamas et al. 2020). Improper plastic waste management will result in the accumulation of plastic waste as well as plastic pollution, which will have a number of significant negative effects on the environment and endanger ecosystems (Prata et al. 2019). Walker et al. (2018) emphasized this, stating that if plastic waste is not properly managed and handled, it will contaminate water sources as it flows into the water stream or runoff, causing flooding or even leaching of toxic chemicals such as mercury, lead, and cadmium. As a result, aquatic food chains will be disrupted as aquatic lives consume the polluted water contaminated with toxic chemicals. Furthermore, greenhouse gases will be released from buried plastics, and bacteria that eat nylon, such as pseudomonas and flavobacteria, will release methane gas when they feed on plastic waste (nylon and polymer), contributing to global warming (Hossain et al. 2020).
When producing petroleum-based plastics, hazardous chemical substances such as bisphenol A (BPA), phthalates, antiminitroxide, brominated flame retardants, and polyfluorinated chemicals are used (Halden 2010). These chemicals may leach into the environment and harm humans. In the context of food packaging, harmful chemicals such as BPA and phthalates may leach out and diffuse into food. Plastic manufacturing also releases a variety of hazardous gaseous chemicals into the atmosphere, including hydrogen cyanide, particulate matter, and carbon monoxide, resulting in air pollution (Proshad et al. 2017). Plastic applications are common in food and fresh produce packaging and are commonly used to pack food and beverages in hawker stores. Despite the fact that its applications benefit human lives, environmentalists around the world have highlighted it as one of the most severe pollutions to the environment in recent years, as well as the threats that plastic wastes pose to the health of living organisms and the environment. To address all of the issues raised by the use and disposal of plastics, much attention has been paid to the research and development of bioplastics in the hopes of reducing reliance on petroleum-based plastics. Alternatives are being investigated, to replace petroleum-based plastics with other materials that have similar properties but are degradable, less toxic, and more affordable. Aside from that, the development and application of bioplastics not only helps to reduce environmental problems, but it also helps to alleviate petroleum oil shortages (Bangar et al. 2021).
Bioplastic, in general, is made from renewable raw materials such as starch and cellulose and can be composted and degraded after use, reducing the need for landfill. Bioplastic that is made from plants or starch is deemed as a feasible solution to this issue. Apart from enhanced properties compared to petroleum-based plastic, biodegradability is the main contributor to the increasing demand for bioplastic in various industries nowadays. One of the factors for bioplastic being popular in the food packaging industry is that it only takes up to three to six months to decompose through soil burial. Among all, starch-based bioplastic is extensively studied with starch being utilized as the main raw material because of its characteristics such as inexpensive, renewable, non-toxic, versatile, biodegradable, has good film-forming capabilities and can be found abundantly (Nouraddini et al. 2018; Jafarzadeh et al. 2018; López et al. 2015).
Starch is typically extracted from plant seeds, tubes, and roots. Many recent studies have been conducted to investigate the physiochemical properties of starch-based bioplastics derived from corn, cassava, yam, potato, and other sources (Ng et al. 2022; Lim et al. 2021). Native starch, on the other hand, has some drawbacks, such as a narrow viscosity range, poor mechanical properties, a high ageing temperature, and a high susceptibility to moisture, all of which limit its application in the packaging industry (Chen et al. 2017). Therefore, plasticizers like polyols are frequently used in the synthesis of starch-based bioplastics to improve mechanical and physicochemical properties like fragility, modulus of elasticity, and glass transition temperature (Ng et al. 2022; Vieira et al. 2011). This phenomenon may be attributed to the incorporation of plasticizer with starch, which is capable of decreasing the starch-starch intermolecular molecular and thus lowering the glass transition temperature, allowing polymer chain mobility (Ballesteros-Mártinez et al. 2020; Sanyang et al. 2016; Sanyang et al. 2015). As a result, the flexibility and stiffness of starch-based bioplastics improved, allowing them to be used in a wider range of applications.
To date, little research has been conducted to determine the effects of various plasticizers, such as glycerol and sorbitol, on bioplastic for use in food and fresh produce packaging. Glycerol is one of the most commonly used plasticizers in the production of bioplastics due to its high plasticizing capacity and ability to remain thermally stable at elevated temperatures (Bilck et al. 2015). According to Ooi et al. (2012), sorbitol plasticizer not only improved the overall water intake capacity of bioplastic film while also demonstrating improved rigidity, but its ability to biodegrade has also demonstrated it to be a suitable alternative to conventional petroleum-based plastics. Depending on the application, the type and concentration of plasticizer used influences the properties of starch-based films. Most previous research focused on the physical properties of the plasticized bioplastic, with little information available on the effect of different plasticizers and their concentrations on bioplastic application. Therefore, the present research also aims to reveal the potential of a potato starch-based bioplastic containing different plasticizers for use as a fruit packaging material.
In this study, potato starch was used as the raw material for bioplastic synthesis. Potatoes are a popular staple food around the world. They come in over a hundred species and thousands of varieties all over the world, with enormous potential that has yet to be fully realized. The use of potato starch as a bioplastic raw material is deemed interesting and worth exploring in the development of bioplastics due to the high availability of potato as a source of starch extraction. The effects of different types and concentrations of plasticizer on the mechanical and physicochemical properties of the synthesized bioplastics were investigated by adding glycerol and sorbitol to this particular starch at various concentrations. Tensile strength and elongation at break tests, water vapour transmission rate, water absorption test, water solubility test, Fourier transform infrared (FTIR) spectroscopy, contact angle measurement, and general appearance were all part of the characterization study for the comparison. Finally, the feasibility of using them as fruits packaging materials was investigated. The findings of this study should shed light on the mechanical and physicochemical properties of potato starch-based bioplastics, as well as their potential for use as food and fresh produce wrappers when different types and concentrations of plasticizer are used, which are not widely reported in the literature at the moment.
The experiments of this work were conducted at the Chemical Engineering Laboratory of UCSI University, Kuala Lumpur, Malaysia and Malaysia—Japan International Institute of Technology, Universiti Teknologi Malaysia from May to September 2020. The tensile tests and FTIR analysis were performed at GT Instruments Sdn Bhd Malaysia and Universiti Tunku Abdul Rahman, Perak, Malaysia, respectively.
Materials and methods
Preparation of bioplastics
Preparation of potato starch-based bioplastics
The preparation of potato starch bioplastics was performed according to the procedures described by Ng et al. (2022). First of all, a specific amount of potato starch and distilled water were mixed together. To obtain a homogeneous starch suspension, the mixture was heated in a water bath at 95 °C for 30 min with constant stirring. The mixture was then poured onto a baking tray and allowed to cool for about 10 min before being dried in an oven at 40 °C overnight. The dried bioplastic film was made entirely of potato starch and was designated as sample P-100.
Preparation of potato starch/glycerol bioplastics
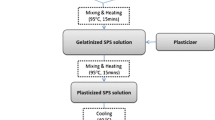
The synthesis of potato starch bioplastics with glycerol blends was carried out in accordance with the procedures described by Sanyang et al. (2016). Thirty grams of starch was mixed with 300 ml of distilled water and heated in a water bath at 95 °C for 15 min with constant stirring. This was done to allow the starch granules to disintegrate and provide a uniform dispersion. Following that, a specific amount of glycerol plasticizer was added to the mixture, and stirring was continued at 95 °C for another 15 min. The mixture was then poured onto a baking tray and allowed to cool for about 10 min before drying. The sample was dried in an oven at 40 °C overnight. Similar procedures were used to produce potato starch bioplastics with varying glycerol concentrations (30%, 50%, and 70%), as shown in Table 1.
Preparation of potato starch/sorbitol bioplastics
The preparation of potato starch bioplastic with sorbitol blends was similar to that of potato starch bioplastic with glycerol blends, except that the plasticizer used was sorbitol rather than glycerol. The composition of the potato starch/sorbitol blends prepared in this study is given in Table 2.
Characterization of bioplastics
General appearance
The general film appearance of synthesized potato starch bioplastics blended with different types and concentrations of plasticizer was observed and recorded. Typical physical appearances such as cracks, bubble formation, and opacity extent were observed with naked eyes.
Tensile strength/elongation at break
The tensile strength and elongation at break of all bioplastics were measured according to ASTM D882-97 method using Universal Testing Machine (TC200200147). Tensile strength is the maximum stress that a material can withstand while being stretched at an increasing rate or pull to fail. The unit measured for the ultimate tensile strength was expressed in MPa. For elongation, it is defined as the break point of a material. It represents the elongation or increase in length of a tensile sample as a result of pulling. The elongation unit was expressed as a percentage of the original length. In order to increase the accuracy of the results, average value from 5 measurements was recorded for each bioplastic composition.
Water vapour transmission rate
Seven impermeable plastic cups were filled with desiccant silica and completely sealed with bioplastic film on top. There were seven bioplastic samples with varying types and concentrations of plasticizers, and one plastic sample served as a control sample. All of the sealed impermeable plastic cups were placed in the desiccator, along with one additional plastic cup containing distilled water. For a week, the weight of each sealed impermeable plastic cup was measured on a daily basis. The water vapour transmission rate was calculated by using Eq. (1) (Lim et al. 2021).
where W is the weight change of samples (g), t is the duration in which weight change (W) occurred (day), \(\frac{W}{t}\) is the rate change of weight of sample (\(\frac{g}{{{\text{day}}}}\)), and A is the test area (m2).
The water vapour transmission rate measured for each plasticizer concentration was based on the average of 2 samples.
Water absorption test
All bioplastic samples were prepared in 2 cm × 2 cm sizes. After that, the samples were pre-dried in an oven at 50 °C for a day. After a day of pre-drying, they were cooled at room temperature in a desiccator to determine the initial dry weight (W0). The samples were then immersed in distilled water for a day. Following the immersion, the weight of the bioplastic samples was recorded. To obtain the average value, three measurements were taken for each sample. The water absorption percentage was then calculated based on Eq. (2) (Lim et al. 2021).
where Wa is the water absorption capacity (g), Wt is the nett weight after immersion (g), and Wo is the initial dry weight (g).
Fourier transform infrared (FTIR) spectroscopy
Fourier transform infrared spectroscopy was performed using PerkinElmer Spectrum Two FTIR machine to identify the functional group of the synthesized bioplastics. The spectra were obtained using an FTIR spectrometer in the range 4000–400 cm−1.
Contact angle measurement
The hydrophilic and hydrophobic properties of the bioplastics were tested using a tensiometer for the contact angle measurement. All of the bioplastics were cut to a 2 cm2 size and laid out horizontally on an acrylic sheet. The contact angle value was measured using a water droplet on the bioplastic samples. To better evaluate the surface properties, each bioplastic film sample was tested at least 2 times to obtain an average value for greater accuracy.
Food packaging application
Strawberries were used as fruit samples to investigate the feasibility of using plasticized potato starch bioplastics as fruit packaging materials. The initial weight of the synthesized bioplastic film sample and strawberry sample was first recorded (Wo). The strawberry was then wrapped in bioplastic and stored in the fridge (4 °C) for 7 days. For comparison, a similar set of strawberries was kept at room temperature. The weight of the strawberries was recorded on a daily basis in order to investigate moisture loss or changes in fruit quality as storage time was extended. The overall weight loss percentage was calculated using Eq. (3). In addition to this, the physical appearance of the strawberry was examined with the naked eye for the presence of mould or decolorization.
where Wf is the weight of sample on the last day of study (g), while Wo is the initial weight of sample on the first day of study (g).
The weight loss measured for each plasticizer concentration at both 4 °C and room temperature was based on the average of 2 samples.
Result and discussion
Characterization of bioplastics
General appearance
All bioplastic films were found to be clear and transparent in general. In comparison with plasticized bioplastic films, bioplastic film made with pure potato starch was found to be the most brittle, fragile with a hard surface, inflexible, and thus difficult to remove from the baking tray. Plasticized bioplastic films were less brittle, sticky, and had a smoother surface than pure potato starch bioplastic films, regardless of the types of plasticizers used. The flexibility of the resulting bioplastic films improved as the plasticizer concentration was increased, making them easier to peel off. According to Ng et al. (2022), this phenomenon could be attributed to the plasticizers' small molecular size allowing them to penetrate intermolecular vacancies on the chain of polymers. As a result of the weakening of the hydrogen bonding between starch molecules, molecule mobility increased, and bioplastics became more flexible and easier to peel off the casting surface. Furthermore, Lim et al. (2021) stated that plasticizers were used to reduce polymer matrix and make starch films more flexible by increasing intermolecular dispersion between polymer chains, regardless of the type or concentration of plasticizers used. Hazrati et al. (2021), Nasir and Othman (2021) and Sanyang et al. (2016) found similar results in terms of bioplastic appearance when Dioscorea hispida starch, corn starch and sugar palm starch were used as the raw materials for the preparation of bioplastic films.
When comparing the effects of different plasticizers, it was discovered that glycerol-plasticized bioplastic films were more flexible and stickier than sorbitol-plasticized bioplastic films at the same concentration. Furthermore, as the concentration of plasticizer increases, plasticized films become stickier. This phenomenon could be caused by phase separation and plasticizer diffusion to the film's surface (Talja et al. 2007). Surprisingly, the sorbitol-plasticized bioplastic films were consistently more transparent than glycerol at all concentrations tested (30%, 50%, and 70%). The summary of the visual appearance and characteristics of bioplastic films prepared in this study is presented in Table 3.
Tensile strength/elongation at break
Tensile tests were performed to determine the viability of potato-based bioplastic films plasticized with glycerol and sorbitol for future applications such as food and fresh produce packaging. All of the bioplastic films synthesized in this study were tested for tensile strength using the ASTM D882 tensile test. Figure 1 depicts the tensile strength results for the investigated films. It was clear that the pure potato starch bioplastic demonstrated the highest tensile strength at 34 MPa when compared to the other plasticized film samples, regardless of plasticizer type or concentration. This finding was supported by Sanyang et al. (2016) and Sanyang et al. (2015) who found that the degree of hydrogen bonding in starch-to-starch intermolecular interaction was greater than in starch-to-plasticizer attraction.
Besides, the concentration of plasticizer added had a significant impact on the tensile strength of bioplastics. Based on the results in Fig. 1, the higher the concentration of each glycerol and sorbitol plasticizer, the lower the tensile strength of the bioplastic films, indicating an inversely proportional relationship between them. According to Abdullah et al. (2018), the plasticizer composition increased, resulting in an increase in the tensile strength of bioplastics. This trend was supported by Ibrahim et al. (2019), Sofiah et al. (2019), Sanyang et al. (2016); Sanyang et al. (2015), and Muscat et al. (2012), who stated that as the plasticizer concentration increased, the strong intramolecular bonding between polymer chains was reduced, which reinforced the hydrogen bond formation between the starch and plasticizer molecules instead. As a result, the flexibility of bioplastics increased, and thus, the tensile strength decreased.
Another interesting observation from Fig. 1 is that bioplastic films plasticized with sorbitol had higher tensile strength than glycerol at the same plasticizer concentration. Several studies have reported a similar trend, indicating that glycerol films had lower tensile strength than sorbitol at the same concentration (Lusiana et al. 2019; Dawam Abdullah et al. 2018; Sanyang et al. 2016; Sanyang et al. 2015). Hazrati et al. (2021) recently demonstrated that sorbitol-plasticized films had higher tensile strength ranging from 11.28 to 4.4 MPa and glycerol-plasticized films had lower tensile strength ranging from 6.13 to 1.40 MPa at concentrations ranging from 30 to 60%. Luisana et al. (2019), Tapia-Blácido et al. (2013) and Aguirre et al. (2013) found that bioplastic plasticized with sorbitol was stiffer and stronger than bioplastic made with glycerol, which required more stress to achieve the same degree of deformation. According to Ng et al. (2022) and Hazrati et al. (2021), this could be attributed to difference in molecular weight between glycerol and sorbitol. Glycerol and sorbitol have molecular weights of 92.093 g/mol and 182.17 g/mol, respectively, with sorbitol having nearly twice the molecular weight of glycerol. Based on this difference, it is possible to conclude that glycerol has a better plasticizing effect than sorbitol due to its smaller size, which facilitates interaction with starch.
In addition, the amount of water in a bioplastic film affects its mechanical properties. This phenomenon, according to Luisana et al. (2019), is due to the hydrophilic nature of glycerol and sorbitol plasticizers. This is because when higher concentrations of plasticizers are used, the moisture content of bioplastics rises, disrupting starch integrity, reducing hydrogen bonding, and promoting mobility of amylose and amylopectin chains, overcoming the recrystallization effect. This claim was supported by Jafarzadeh et al. (2020), who found that hydroscopic films absorbed water more quickly at high moisture content, enhancing the plasticizing effect, lowering tensile strength, and improving film flexibility.
On the other hand, elongation at break is an important plastic characterization parameter because it indicates the maximum length that a bioplastic film can withstand before rupture (Sofiah et al. 2019). Figure 2 shows the results of the elongation at break for all the bioplastic samples prepared in this study. It was discovered that the results of elongation at break followed the opposite trend of the tensile results, and that it was directly proportional to plasticizer concentration. The pure potato starch film had the lowest elongation at break reading of 5.5%, with an increasing trend as the concentration of both glycerol and sorbitol was increased. Ng et al. (2022), Dawam Abdullah et al. (2018) and Luisana et al. (2013) reported similar findings regarding the increase in elongation at break of plasticized films.
Figure 2 shows that sorbitol demonstrated a higher viscoelastic response than glycerol, resulting in a higher elongation at break value for sorbitol-plasticized bioplastics at concentrations greater than 30%. When 30% plasticizer was added to starch-based bioplastics, the elongation at break value did not differ significantly between glycerol- and sorbitol-plasticized bioplastics. According to Zavareze et al. (2012), elongation at break value of a polymeric material was an indication of the molecular chain's flexibility. When compared to the pure potato starch-based bioplastic (P-100), the plasticizers improved polymer chain mobility by weakening intermolecular bonds between amylose and amylopectin in the starch matrix, which were replaced by hydrogen bonding between starch and plasticizer. This disruption caused starch molecular chains to rebuild, resulting in lower rigidity but higher flexibility of films due to increased chain mobility, eventually allowing the films to demonstrate higher elongation at break (Ballesteros-Mártinez et al. 2020; Lusiana et al. 2019; Laohakunjit and Noomhorm 2004). The results obtained in this study was also supported by Ballesteros-Mártinez et al. (2020) and Lusiana et al. (2019), who found that bioplastics with sorbitol as the plasticizer had a higher elongation at break value than those of using glycerol at the same plasticizer concentration.
Water vapour transmission rate
According to Ghasemlou et al. (2013), the water vapour transmission analysis on bioplastics for packaging material is critical because moisture transfer from the inner surface to the surrounding environment must be minimized. To maximize the shelf-life of food and fresh produce, water vapour transmission must be as low as possible (Dehghani et al. 2018). This is due to the fact that food and fresh produce necessitate a high water transmission permeability for respiration and transpiration in order to maintain their freshness (Zhou 2016). Figure 3 depicts the results of the water vapour transmission measurement in this study. Based on the results, it is possible to conclude that the higher the concentration of plasticizer added to the bioplastic blend, the greater the water vapour transmission. As the sorbitol concentration increased from 0 to 70% in sorbitol-plasticized bioplastics, the water vapour transmission rate increased from 36.23 to 51.67 g/day m2. Meanwhile, increasing the glycerol concentration from 0 to 70% increased the water vapour transmission from 63.68 to 80.91 g/day m2. These were significantly higher than the pure potato starch-based bioplastic at 35.62 g/day m2.
Geleta et al. (2020), Ballesteros-Mártinez et al. (2020) and Sanyang et al. (2016) all found a similar increasing trend in water vapour transmission rate as plasticizer concentration increased in bioplastics made from enset starch, sweet potato starch and sugar palm starch, respectively. This observation could be explained by the fact that as plasticizer concentration increased, starch polymer chains became more mobile and flexible, resulting in a looser structure and weaker starch-to-starch molecular interaction. According to Bilck et al. (2015), who investigated the amount of glycerol plasticizer used in cassava starch-based films, a higher amount of glycerol added to the film resulted in a higher water vapour transmission rate. The authors explained that glycerol altered polymer structure and increased polymer molecular mobility, promoting water diffusivity. Figure 3 also shows that plasticized bioplastics had better water vapour transmission than pure starch film in this study, which could be attributed to the hygroscopic properties of plasticizers. According to Isotton et al. (2015), the presence of hydroxyl groups in plasticizers enhanced the films solubility and hygroscopic properties, which were related to the hydrophilicity of the plasticized films. As a result, improved water transmission across bioplastic films was possible.
It was also discovered that bioplastic films plasticized with glycerol had a higher rate of water vapour transmission than bioplastic films plasticized with sorbitol. This could be due to glycerol's higher hydrophilic nature, which allowed it to absorb more water than sorbitol. Also, Jost et al. (2014) explained that glycerol was more effective at increasing water vapour transmission rate by interfering with molecular bonding and thus reducing molecular attraction of the polymer, resulting in more free volume in the matrix. Furthermore, different results among different plasticizers could be linked to the molecular structure of sorbitol, which was denser than glycerol and resulted in greater intermolecular interaction between starch and sorbitol polymer chains (Sanyang et al. 2016). Therefore, the rate of water transmission in sorbitol-plasticized bioplastics was lower than in glycerol-plasticized bioplastics.
Water absorption test
Water absorption analysis on bioplastics to be used as food and fresh produce packaging is critical because the results can be used to determine the stability of the bioplastics, as well as to investigate the effect of plasticizer concentrations on the hydrophilic characteristics of bioplastics in moist or humid environment. The weight of all bioplastic films increased after one day of immersion in distilled water, as shown in Fig. 4, indicating that all samples absorbed water. This could be due to the presence of unoccupied sites caused by the bioplastic films' abundant active hydroxyl groups. Water molecules were expected to diffuse into the starch polymer. As a result, the bioplastic films began to absorb water and expand. However, once the active sites of bioplastic films were completely filled, they could no longer absorb water molecules and equilibrium was attained. Sanyang et al. (2016) and Sanyang et al. (2015) explained that the net movement of water reached equilibrium when all active sites of the bioplastic matrix lost their ability to hold more water molecules. The weight of the expanded bioplastic film then remained constant. Nonetheless, it was obvious that the water absorption percentage decreased when plasticizer was added to the bioplastic blend (Mali et al. 2006). This was attributed by the substitution reaction from free hydroxyl groups in plasticized bioplastics. The introduction of functional groups onto the surface of starch molecules with free hydroxyl was likely to change some physiochemical properties of the bioplastics through starch esterification (Bartz et al. 2012).
The present study revealed that the concentration of plasticizer affected the bioplastic water absorption ability, with the water absorption percentage being inversely proportional to the concentration of plasticizer. The percentage of water absorbed decreased as the concentration of plasticizer increased. This trend was consistent with the findings of Hazrati et al. (2021), Maulida et al. (2018) and Sanyang et al. (2016), who found that increasing the concentration of plasticizer resulted in a decrease in water uptake. This phenomenon may be explained by the fact that bioplastics with lower plasticizer concentrations have more vacant active sites than bioplastics with higher plasticizer concentrations, allowing water to easily penetrate vacant spaces and resulting in high water absorption.
When the results of similar plasticizer concentrations were compared, it was found that the water absorption of bioplastic films plasticized with glycerol was higher than that of bioplastic films plasticized with sorbitol. Ng et al. (2022) and Lusiana et al. (2019) both reported a similar trend. This was also supported by Sanyang et al. (2016) and Sanyang et. al. (2015) who reported that bioplastic films plasticized with glycerol were found to be more hygroscopic and could absorb water more quickly than bioplastic films plasticized with sorbitol. According to the authors, sorbitol had the ability to restrict water molecules and the interaction of intermolecular hydrophilic functional groups in starch, resulting in a reduction in water absorption by plastics. This was consistent with the results obtained in this study (Fig. 4).
Fourier transform infrared (FTIR) spectroscopy
Figure 5a and b shows the FTIR spectra of all bioplastics synthesized in this study. The peaks obtained for bioplastic samples containing different plasticizers and pure potato starch-based film were found to be similar, indicating the presence of a similar functional group in all samples. However, the peak intensity of the pure potato starch-based bioplastic sample was significantly lower than that of the plasticized bioplastic samples.
Figure 5 shows that the absorption bands had a wide range. It was associated with the formation of hydrogen bonds between O–H groups at the end of starch and plasticizer chains in the 3290 to 3295 cm−1 range. The findings agreed with those of Hazrati et al. (2021), Dawam Abdullah et al. (2018), and Sanyang et al. (2016). According to Ooi et al. (2012), the higher the concentration of glycerol and sorbitol plasticizer, the higher the concentration of O–H functional group in the sample. This was reflected in the present findings, which showed that the spectra of pure potato starch-based bioplastic (without plasticizer) did not reveal any peak within this wavelength range, but that the peak was visible in the spectra of glycerol-plasticized sample when compared to the sorbitol counterpart. Furthermore, the inclusion of plasticizer could have disrupted the intramolecular and intermolecular hydrogen bonds between the hydroxyl groups in the starch chains. In place of these interactions, more stable hydrogen bonds were formed between the -OH groups of starch and plasticizer (Paluch et al. 2022).
The next peak in Fig. 5 was in the range of 2923 to 2928 cm−1, which corresponded to the aliphatic absorption peak for bond stretching of C-H in alkene group in matrix structure of starch (Nordin et al. 2020; Sanyang et al. 2016). The following peak was observed in the range of 1361 to 1415 cm−1, indicating the aliphatic absorption peak for O–H bond bending. The most intense peak for all samples was identified in the 990 to 1000 cm−1 range. According to Ng et al. (2022), Nordin et al. (2020) and Sanyang et al. (2016), the presence of C-O bond stretching on the C–O–C group in the anhydro-glucose starch rings was attributed to this.
The absorption bands characteristics of all samples were found to be identical based on the FTIR spectra. Therefore, the presence or absence of plasticizers in bioplastic films had no effect on the functional groups when compared to the pure potato starch-based bioplastic film.
Contact angle measurement
Contact angle measurement was taken to determine whether the synthesized bioplastics were hydrophilic or hydrophobic (Li et al. 2013). Theoretically, when the droplet shape is more spherical, the film demonstrates higher water barrier properties, implying lower water absorption and less hydrophilicity of the surface. Bioplastic films with high hydrophilicity, on the other hand, are unfavourable for use as food or fresh produce packaging. This is due to the fact that when the moisture content of the bioplastics is low, it reduces the possibility of mould growth, which ultimately improves the mechanical properties and appearance of the bioplastic films (Azmin et al. 2020). On the contrary, high moisture content increases the activity of microorganism metabolism, which is undesirable (Borah et al. 2019). Depending on the wettability, the contact angle will range from 0 to 180°. Low water contact angles of < 90° indicate that the bioplastic film is highly hydrophilic, whereas high contact angles of > 90° indicate that the bioplastic film is highly hydrophobic (Abdullah and Dong 2019).
In this study, pure potato starch-based bioplastic film had the highest contact angle reading, with a value of 67.228° indicating the lowest wettability and the highest hydrophobicity when compared to plasticized films. The results of the contact angle measurement of the bioplastic samples with plasticizer concentrations up to 50% in this study are illustrated in Fig. 6. According to Dawam et al. (2018), the higher the starch concentration, the greater the contact angle value, indicating an increase in the hydrophobicity of bioplastics. In contrast, the high concentration of plasticizer with hygroscopicity characteristics could be attributed to the low contact angle, higher wettability, and increased hydrophilicity of the bioplastic synthesized. Several studies in the literature found that increasing the glycerol content of starch-based plastic films resulted in increased hygroscopic properties, which were attributed to an increase in hydroxyl groups available to form hydrogen bonds with water (Bilck et al. 2015).
In the case of plasticized bioplastic films, the contact angle was found to be slightly higher in sorbitol-based bioplastic films than in glycerol-based bioplastic films. According to the findings of Isotton et al. (2015), etherified maize starch films synthesized with 20% sorbitol and 20% glycerol resulted in contact angles of 82° and 72°, respectively. The author attributed this to the fact that glycerol is more hydrophilic than sorbitol, resulting in a lower contact angle value and higher wettability when compared to sorbitol. Basiak et al. (2018) increased the amount of glycerol in the bioplastic synthesis from 33 to 50% and thereby observed a reduction of contact angle value from 103° to 43°. Similarly, Heydari et al. (2013) reported that when the glycerol concentration was increased to 35%, the contact angle of bioplastic films plasticized with 25% glycerol at 49° was reduced to 35°. This could be attributed to an increase in polyol concentration, which increased the polarity of bioplastics, facilitating adhesion and cohesiveness while increasing water spreading.
In short, the findings of this study showed that the addition of glycerol and sorbitol plasticizers aided in the promotion of water spreading on plastic surfaces and reduced the contact angle of the bioplastic film, which was consistent with the findings of Basiak et al. (2018). The current results in Fig. 6 also demonstrated that glycerol was more hydrophilic than sorbitol, which corroborated the earlier results of water vapour transmission rate and water absorption.
Food packaging application
Using strawberries as a fruit sample, the feasibility of using plasticized bioplastic films as food and fresh produce packaging materials was investigated. The strawberries were wrapped in all bioplastic films and stored at two different temperatures: 25 °C (room temperature) and 4 °C (fridge). After one week of storage, the overall appearance and weight loss of the strawberries were compared and analysed.
Based on visual observation, the general appearance of strawberries kept in the refrigerator did not differ significantly from the appearance on the first day of the experiment. Strawberries have exceptionally soft flesh and a thin fruit skin, according to a study conducted by Ikegaya et al. (2020). Strawberries typically have a shelf life of 7 days when stored in the fridge (Ayala-Zavala et al. 2004). However, according to Ikegaya et al. (2020), the quality of fruits stored in the refrigerator with packaging can be preserved better than those stored without packaging. The authors were successful in providing solid support for the findings that the general appearance of strawberries kept in the fridge did not change much and that there was no significant weight loss.
Ahimbisibwe et al. (2019) stated that high temperatures and humidity promote the degradation of bioplastics. In this study, after one week of being kept at room temperature, it was discovered that all strawberries began to decay, and mould growth could be seen. Based on the study conducted by Maraei et al. (2017), strawberries were highly perishable, with a shelf-life of 2 to 3 days if stored at room temperature and were relatively vulnerable to postharvest. Zhang et al. (2003) described that the rapid decay rate of strawberries kept at room temperature was caused by high respiration rates, pathogenic attacks, and environmental stresses. Food and fresh produce kept in the refrigerator with relatively low humidity and temperature are able to inhibit mould and fungus growth and restrict moisture transfer when compared to room temperature. Temperature, humidity, and biological activities are the main environmental factors that influence the decomposition rate of plastic material, and food or fresh produce.
From the weight loss results shown in Fig. 7, the overall weight loss of strawberries stored at 4 °C was significantly lower than the weight loss of strawberries stored at room temperature. The weight loss of strawberries was found to be reduced when the bioplastics contained plasticizer concentrations ranging from 30 to 70% were used as the packaging material, regardless of the types of plasticizers used or the temperature at which the strawberries were stored. This meant that as the concentration of plasticizer in the bioplastic film increased, the weight loss of strawberries decreased. It was clear from Fig. 7 that as the sorbitol concentration increased from 30 to 70%, the weight loss decreased from 7.25 to 5.75% at 4 °C, whereas the weight loss for strawberries wrapped using glycerol-plasticized bioplastics was 6.14 and 4.00%, respectively. The overall weight loss for strawberries kept at room temperature, on the other hand, was relatively higher. Similar to the strawberries kept at 4 °C, the weight loss of strawberries decreased as the concentration of plasticizers added increased. It was discovered that as the sorbitol plasticizer concentration increased from 30 to 70%, the weight loss percentage recorded was 44.33 and 17.90%, respectively, at room temperature. With the addition of glycerol plasticizer, the weight loss of strawberries decreased from 42.62 to 23.69%.
The physiological weight loss observed in fruits was due to water evaporation, degradation, and respiration. It was known that when fruits were stored in a high-temperature, low-humidity environment, the rate of weight loss was accelerated (Rahman et al. 2016). Moreover, Kumar et al. (2012) found that the longer the fruits were stored, the higher the rate of weight loss. The weight loss of strawberries in both fridge and room temperatures was consistent with the results of water vapour transmission, with the higher the water vapour transmission rate of bioplastic films (at higher plasticizer concentrations) achieved, the lower the weight loss of strawberries recorded. There was the presence of humidity in the wrapped region as a result of fruit respiration and evaporation. However, bioplastic films with low water permeability will restrict moisture transfer through the surface, resulting in a relatively optimum environment for microbial growth and fruit oxidation and deterioration if the films are used. Consequently, food and fresh produce packaging must have a high water vapour permeability in order to allow moisture transfer and maintain freshness.
The distinct observation from Fig. 7 was that a higher concentration of plasticizer promoted better strawberry preservation. Despite the fact that glycerol induced better water affinity than sorbitol due to its hydrophilic nature, as evidenced by the water vapour transmission test, water absorption analysis, and contact angle measurement in this study, the weight loss of strawberries wrapped at the same plasticizer concentrations was not significantly different, regardless of whether it was glycerol or sorbitol. This is most likely due to the wrapping method used in this study, in which the strawberries were not completely sealed in the plasticized bioplastic. If bioplastics are to be used as packaging materials, appropriate or better wrapping methods could be investigated further to better understand the role and effect of various plasticizers on the preservation of food or fresh produce.
Another interesting finding in this study is the inability of the plasticized bioplastics to preserve strawberries kept at room temperature. Despite the fact that increased plasticizer concentrations contributed to lower strawberry weight loss at room temperature, the overall weight loss recorded was still high, ranging from 20 to 40%. The result necessitates more research to reinforce the bioplastic with inorganic or organic fillers, as well as the incorporation of bioactive components with antimicrobial properties, consistent with the suggestions by Harnkarnsujarit et al (2021).
Conclusion
In this study, bioplastic films were successfully synthesized from potato starch and plasticized with different plasticizers namely glycerol and sorbitol. A number of characterization studies were conducted, including tensile strength, elongation at break, water vapour transmission rate, water absorption, FTIR spectroscopy, and contact angle measurement. The feasibility of plasticized bioplastics for use as food and fresh produce packaging materials was investigated using a weight loss test on strawberries stored at various temperatures. Even though glycerol was more hydrophilic than sorbitol in the characterization results, the weight loss of strawberries wrapped in bioplastics with the same plasticizer concentrations was not significantly different. The higher the concentration of plasticizer, however, the less weight loss of strawberries stored at both refrigerated and room temperatures. The findings of this study suggested that bioplastics made from potato starch and plasticized with either glycerol or sorbitol could be used as packaging materials for food and fresh produce, though more research is needed to assess the industrial limitations and economic feasibility of its application in food preservation.
Availability of data and material
All data generated or analysed during this study are included in this published article.
Code availability
Not applicable.
References
Abdullah ZW, Dong Y (2019) Biodegradable and water resistant poly(vinyl) alcohol (PVA)/Starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front Mater 6:58. https://doi.org/10.3389/fmats.2019.00058
Abdullah AHD, Pudjirahar S, Karina M, Dwi Putri O, Fauziyyah RH (2018) Fabrication and characterization of sweet potato starch-based bioplastics plasticized with glycerol. J Biol Sci 19(1):57–64. https://doi.org/10.3923/jbs.2019.57.64
Aguirre A, Borneo R, León AE (2013) Properties of triticale protein films and their relation to plasticizing-antiplasticizing effects of glycerol and sorbitol. Ind Crops Prod 50:297–303. https://doi.org/10.1016/j.indcrop.2013.07.043
Ahimbisibwe M, Banadda N, Seay J, Nabuuma B, Atwijukire E, Wembabazi E, Nuwamanya E (2019) Influence of weather and purity of plasticizer on degradation of cassava starch bioplastics in natural environmental conditions. J Agric Food Chem Environ 8(4):237–250. https://doi.org/10.4236/jacen.2019.84018
Ayala-Zavala J, Wang SY, Wang CY, González-Aguilar GA (2004) Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT Food Sci Technol 37(7):687–695. https://doi.org/10.1016/j.lwt.2004.03.002
Azmin SNHM, Hayat NABM, Nor MSM (2020) Development and characterization of food packaging bioplastic film from cocoa pod husk cellulose incorporated with sugarcane bagasse fibre. J Bioresour Bioprod 5(4):248–255. https://doi.org/10.1016/j.jobab.2020.10.003
Ballesteros-Mártinez L, Pérez-Cervera C, Andrade-Pizarro R (2020) Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J 20:1–9. https://doi.org/10.1016/j.nfs.2020.06.002
Bangar SP, Purewal SS, Trif M, Maqsood S, Kumar M, Manjunatha V, Rusu AV (2021) Functionality and applicability of starch-based films: an eco- friendly approach. Foods 10(9):2181. https://doi.org/10.3390/foods10092181
Bartz J, Madruga KM, Klein B, Pinto VZ, Dias LRG (2012) Pasting properties of native and acetylated rice starches. Braz J Food Technol. https://doi.org/10.1590/s1981-67232012005000040
Basiak E, Lenart A, Debeaufort F (2018) How glycerol and water contents affect the structural and functional properties of starch-based edible films. Polymers 10(4):412. https://doi.org/10.3390/polym10040412
Bilck AP, Müller CMO, Olivato JB, Mali S, Grossmann MVE, Yamashita F (2015) Using glycerol produced from biodiesel as a plasticiser in extruded biodegradable films. Polímeros 25(4):331–335. https://doi.org/10.1590/0104-1428.1803
Borah A, Balasubramanian S, Kaur A, Kaur J, Sukhija S, Balasubramanian S (2019) Thermal and microbial characteristics of barley pasta as affected by moisture content. Our Heritage 67(7):300–314
Chamas A, Moon H, Zheng J, Qiu Y, Tabassum T, Jang JH, Abu-Omar M, Scott SL, Suh S (2020) Degradation rates of plastics in the environment. ACS Sustainable Chem Eng 8(9):3494–3511. https://doi.org/10.1021/acssuschemeng.9b06635
Chen Y, Wei X, Chang G, Fu T, Cui L, Li J (2017) Study of bagasse/tapioca starch film preparation and characterization. IOP Conf Ser Earth Environ Sci 69:012053. https://doi.org/10.1088/1755-1315/69/1/012053
Ciriminna R, Pagliaro M (2019) Biodegradable and compostable plastics: a critical perspective on the dawn of their global adoption. ChemistryOpen 9(1):8–13. https://doi.org/10.1002/open.201900272
Dawam Abdullah AH, Pudjirahar S, Karina M, Dwi Putri O, Fauziyyah RH (2018) Fabrication and characterization of sweet potato starch-based bioplastics plasticized with glycerol. Sci J Biol Sci 19(1):57–64. https://doi.org/10.3923/jbs.2019.57.64
Dehghani S, Hosseini SV, Regenstein JM (2018) Edible films and coatings in seafood preservation: a review. Food Chem 240:505–513. https://doi.org/10.1016/j.foodchem.2017.07.034
Geleta TT, Habtegebreil SA, Tolesa GN (2020) Physical, mechanical, and optical properties of enset starch from bulla films influenced by different glycerol concentrations and temperatures. J Food Process Preserv 44(8):e14586. https://doi.org/10.1111/jfpp.14586
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3(7):e1700782. https://doi.org/10.1126/sciadv.1700782
Ghasemlou M, Aliheidari N, Fahmi R, Shojaee-Aliabadi S, Keshavarz B, Cran MJ, Khaksar R (2013) Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr Polym 98(1):1117–1126. https://doi.org/10.1016/j.carbpol.2013.07.026
Halden RU (2010) Plastics and health risks. Annu Rev Public Health 31(1):179–194. https://doi.org/10.1146/annurev.publhealth.012809.103714
Harnkarnsujarit N, Wongphan P, Chatkitanan T, Laorenza Y, Srisa A (2021) Chapter 7 - Bioplastic for Sustainable Food Packaging. In: Galanakis CM (ed) Sustainable Food Processing and Engineering Challenges. Elsevier, Amsterdam, pp 203–277
Hazrati KZ, Sapuan SM, Zuhri MYM, Jumaidin R (2021) Preparation and characterization of starch-based biocomposite films reinforced by Dioscorea hispida Fibers. J Mater Res Technol 15:1342–1355. https://doi.org/10.1016/j.jmrt.2021.09.003
Heydari A, Alemzadeh I, Vossoughi M (2013) Functional properties of biodegradable corn starch nanocomposites for food packaging applications. Mater Des 50:954–961. https://doi.org/10.1016/j.matdes.2013.03.084
Hossain S, Rahman MA, Chowdhury AM, Mohonta KS (2020) Plastic pollution in bangladesh: a review on current status emphasizing the impacts on environment and public health. Environ Eng Res 26(6):200535. https://doi.org/10.4491/eer.2020.535
Ibrahim MIJ, Sapuan SM, Zainudin ES, Zuhri MYM (2019) Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers. Int J Food Prop 22(1):925–941. https://doi.org/10.1080/10942912.2019.1618324
Ikegaya A, Ohba S, Nakajima T, Toyoizumi T, Ito S, Arai E (2020) Practical long-term storage of strawberries in refrigerated containers at ice temperature. Food Sci Nutr 8(9):5138–5148. https://doi.org/10.1002/fsn3.1817
Isotton F, Bernardo G, Baldasso C, Rosa L, Zeni M (2015) The plasticizer effect on preparation and properties of etherified corn starchs films. Ind Crops Prod 76:717–724. https://doi.org/10.1016/j.indcrop.2015.04.005
Jafarzadeh S, Alias AK, Ariffin F, Mahmud S (2018) Physico-mechanical and microstructural properties of semolina flour films as influenced by different sorbitol/glycerol concentrations. Int J Food Prop 21(1):983–995. https://doi.org/10.1080/10942912.2018.1474056
Jafarzadeh S, Jafari SM, Salehabadi A, Nafchi AM, Kumar USU, Abdul Khalil HPS (2020) Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci Technol 100:262–277. https://doi.org/10.1016/j.tifs.2020.04.017
Jost V, Kobsik K, Schmid M, Noller K (2014) Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr Polym 110:309–319. https://doi.org/10.1016/j.carbpol.2014.03.096
Kumar PS, Sagar VR (2012) Drying kinetics and physico-chemical characteristics of osmo-dehydrated mango, guava and aonla under different drying conditions. J Food Sci Technol 51(8):1540–1546. https://doi.org/10.1007/s13197-012-0658-3
Laohakunjit N, Noomhorm A (2004) Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch 56:348–356. https://doi.org/10.1002/star.200300249
Li Y, Ceylan M, Shrestha B, Wang H, Lu QR, Asmatulu R, Yao L (2013) Nanofibers support oligodendrocyte precursor cell growth and function as a neuron-free model for myelination study. Biomacromol 15(1):319–326. https://doi.org/10.1021/bm401558c
Lim R, Kiew PL, Lam MK, Yeoh WM, Ho MY (2021) Corn starch/PVA bioplastics—the properties and biodegradability study using Chlorella vulgaris cultivation. Asia-Pac J Chem Eng 16(3):e2622. https://doi.org/10.1002/apj.2622
López OV, Castillo LA, García MA, Villar MA, Barbosa SE (2015) Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll 43:18–24. https://doi.org/10.1016/j.foodhyd.2014.04.021
Lusiana SW, Putri D, Nurazizah IZ, Bahruddin, (2019) Bioplastic properties of sago-PVA starch with glycerol and sorbitol plasticizers. J Phys Conf Ser 1351:012102. https://doi.org/10.1088/1742-6596/1351/1/012102
Mali S, Grossmann MVE, García MA, Martino MN, Zaritzky NE (2006) Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. J Food Eng 75(4):453–460. https://doi.org/10.1016/j.jfoodeng.2005.04.031
Maraei RW, Elsawy KM (2017) Chemical quality and nutrient composition of strawberry fruits treated by γ-irradiation. J Radiat Res Appl 10(1):80–87. https://doi.org/10.1016/j.jrras.2016.12.004
Maulida KT, Harahap MB, Ginting MHS (2018) Utilization of mango seed starch in manufacture of bioplastic reinforced with microparticle clay using glycerol as plasticizer. IOP Conf Ser Mater Sci Eng 309:012068. https://doi.org/10.1088/1757-899X/309/1/012068
Muscat D, Adhikari B, Adhikari R, Chaudhary D (2012) Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J Food Eng 109(2):189–201. https://doi.org/10.1016/j.jfoodeng.2011.10.019
Nasir NN, Othman SA (2021) The physical and mechanical properties of corn-based bioplastic films with different starch and glycerol content. J Phys Sci 32(3):89–101
Ng JS, Kiew PL, Lam MK, Yeoh WM, Ho MY (2022) Preliminary evaluation of the properties and biodegradability of glycerol- and sorbitol-plasticized potato-based bioplastics. Int J Environ Sci Technol 19(3):1545–1554. https://doi.org/10.1007/s13762-021-03213-5
Nordin N, Othman SH, Rashid SA, Basha RK (2020) Effects of Glycerol and Thymol on Physical, Mechanical, and Thermal Properties of Corn Starch Films. Food Hydrocoll 106:105884. https://doi.org/10.1016/j.foodhyd.2020.105884
North EJ, Halden RU (2013) Plastics and environmental health: the road ahead. Rev Environ Health 28(1):1–8. https://doi.org/10.1515/reveh-2012-0030
Nouraddini M, Esmaiili M, Mohtarami F (2018) Development and characterization of edible films based on eggplant flour and corn starch. Int J Biol Macromol 120:1639–1645. https://doi.org/10.1016/j.ijbiomac.2018.09.126
Ooi ZX, Ismail H, Bakar AA, Aziz NAA (2012) The Comparison effect of sorbitol and glycerol as plasticizing agents on the properties of biodegradable polyvinyl alcohol/rambutan skin waste flour blends. Polym Plast Technol Eng 51(4):432–437. https://doi.org/10.1080/03602559.2011.639827
Paluch M, Ostrowska J, Tyński P, Sadurski W, Konkol M (2022) Structural and thermal properties of starch plasticized with glycerol/urea mixture. J Polym Environ 30:728–740. https://doi.org/10.1007/s10924-021-02235-x
Phil C R (2019) Plastics in the Environment. https://www.researchgate.net/publication/335338250_Plastics_in_the_Environment. Accessed 28 September 2021.
Plastic Oceans International (2021) Plastic Pollution Facts. https://plasticoceans.org/the-facts/. Accessed 1 March 1 2021.
Prata JC, Silva ALP, da Costa JP, Mouneyrac C, Walker TR, Duarte AC, Rocha-Santos T (2019) Solutions and integrated strategies for the control and mitigation of plastic and microplastic pollution. Int J Environ Res Public Health 16(13):2411. https://doi.org/10.3390/ijerph16132411
Proshad R, Kormoker T, Islam MS, Haque MA, Rahman MM, Mithu MMR (2017) Toxic effects of plastic on human health and environment: a consequences of health risk assessment in bangladesh. Int J Health 6(1):1–5. https://doi.org/10.14419/ijh.v6i1.8655
Rahman MM, Moniruzzaman M, Ahmad MR, Sarker B, Khurshid Alam M (2016) Maturity stages affect the postharvest quality and shelf-life of fruits of strawberry genotypes growing in subtropical regions. J Saudi Soc Agric Sci 15(1):28–37. https://doi.org/10.1016/j.jssas.2014.05.002
Sam ST, Nuradibah MA, Chin KM, Hani N (2016) Current Application and Challenges on Packaging Industry Based On Natural Polymer Blending. In: Olatunji O (ed) Natural Polymers-Industry Techniques and Applications. Springer, London, pp 163–184
Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2015) Effect of plasticizer type and concentration on dynamic mechanical properties of sugar palm starch–based films. Int J Polym Anal Charact 20(7):627–636. https://doi.org/10.1080/1023666X.2015.1054107
Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2016) Effect of plasticizer type and concentration on physical properties of biodegradable films based On Sugar Palm (Arenga pinnata) starch for food packaging. J Food Sci Technol 53(1):326–336. https://doi.org/10.1007/s13197-015-2009-7
Siracusa V, Rocculi P, Romani S, Rosa MD (2008) Biodegradable Polymers for Food Packaging: A Review. Trends Food Sci Technol 19(12):634–643. https://doi.org/10.1016/j.tifs.2008.07.003
Sofiah Y, Aznury M, Melianti, (2019) Mechanical Properties of Bioplastics Product from Musa Paradisica Formatypica Concentrate with Plasticizer Variables. J Phys Conf Ser 1167:012048. https://doi.org/10.1088/1742-6596/1167/1/012048
Suman TY, Li WG, Alif S, Faris VRP, Amarnath DJ, Ma JG, Pei DS (2020) Characterization of Petroleum-based Plastics and Their Absorbed Trace Metals from the Sediments of the Marina Beach in Chennai. Environ Sci Eur, India. https://doi.org/10.1186/s12302-020-00388-5
Talja RA, Helén H, Roos YH, Jouppila K (2007) Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr Polym 67(3):288–295. https://doi.org/10.1016/j.carbpol.2006.05.019
Tapia-Blácido DR, Sobral PJDA, Menegalli FC (2013) Effect of drying conditions and plasticizer type on some physical and mechanical properties of amaranth flour films. LWT Food Sci Technol 50:392–400. https://doi.org/10.1016/j.lwt.2012.09.008
Vieira MGA, da Silva MA, dos Santos LO, Beppu MM (2011) Natural-based plasticizers and biopolymer films: a review. Eur Polym J 47(3):254–263. https://doi.org/10.1016/j.eurpolymj.2010.12.01
Walker TR, Xanthos D (2018) A call for canada to move toward zero plastic waste by reducing and recycling single-use plastics. Resour Conserv Recycl 133:99–100. https://doi.org/10.1016/j.resconrec.2018.02.014
Zavareze EDR, Pinto VZ, Klein B, El Halal SLM, Elias MC, Prentice-Hernández C, Dias ARG (2012) Development of oxidised and heat-moisture treated potato starch film. Food Chem 132(1):344–350. https://doi.org/10.1016/j.foodchem.2011.10.090
Zhang M, Xiao G, Peng J, Salokhe V (2003) Effects of modified atmosphere package on preservation of strawberries. Int Agrophysics 17(3):143–148
Zhou H (2016) Physio-Chemical Properties of Bioplastics and Its Application for Fresh-Cut Fruits Packaging. Hokkaido University. http://hdl.handle.net/2115/61802. Accessed 18 September 2021.
Acknowledgements
This work is financially supported by the Universiti Teknologi Malaysia, UTM Encouragement Research (UTMER) with reference No. PY/2021/01231/Q.K130000.3843.20J90.
Funding
This work is financially supported by the Universiti Teknologi Malaysia, UTM Encouragement Research (UTMER) with reference No. PY/2021/01231/Q.K130000.3843.20J90.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: Lau Hou Yip and Kiew Peck Loo were involved in the paper conception and design; Tan Lian See and Lau Hou Yip contributed to the literature review and data collection; Lam Man Kee and Yeoh Wei Ming assisted in the characterization of bioplastic. Lau Hou Yip and Kiew Peck Loo prepared the draft manuscript. All authors reviewed the write-up and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Not applicable.
Ethics approval
Not applicable.
Consent to participate
This paper has not been published and is not being considered for publication elsewhere. All authors have been agreed to submit this paper to International Journal of Environmental Science and Technology.
Consent for publication
This paper has not been published and is not being considered for publication elsewhere. All authors have been agreed to submit this paper to International Journal of Environmental Science and Technology.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lau, H.Y., Kiew, P.L., Tan, L.S. et al. Deciphering the effects of plasticizers in potato-based bioplastic for food and fresh produce packaging. Int. J. Environ. Sci. Technol. 20, 13703–13716 (2023). https://doi.org/10.1007/s13762-023-04936-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04936-3