Abstract
Water security may be regarded as a first step toward achieving food and energy security. Efficient use of fresh water resources and recycling of wastewater after proper treatment are viewed as tools to achieve water sustainability. Sugar industry can have good potential to treat and reuse its effluents. This potential is not realized by prevailing effluent treatment technologies because of high capital and operation cost of treatment process. More upon, these technologies require substantial amount of energy (electricity) as well as chemicals and labors. We have therefore focused on the development of a technology that would help to overcome these limitations. The alga—Spirulina—was our choice to (1) treat the effluent and (2) use the sugar mill effluent as its growth medium. Experiments using Spirulina at secondary treatment stage showed 91 % reduction in chemical oxygen demand in 108-h treatment time. Further, biochemical analysis of Spirulina harvested from the sugar mill effluent treatment tanks revealed that the harvested biomass has high protein levels. Spirulina is well known for its usage as a protein supplement and therefore can be used as an additional source of revenue generation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water, food and energy securities are issues of global concern. Water security is the foundation for food and energy security and for overall long-term social and economic development. Water is directly or indirectly linked with health, nutrition, social well-being and economic progress, especially in developing countries (Bigas 2012). In case of India, with the present population growth rate (1.9 % per annum), the population is expected to cross the 1.5 billion mark by 2050. Therefore, there is an urgent need for well-organized water resource management through effective usage of available water and cost as well as energy-efficient technologies for wastewater treatment, its reuse/recycling. Such technology also needs to achieve zero liquid discharge norms set by the Central Pollution Control Board of India (CPCB 2003). Experiments described in this paper were focused on feasibility of such a technology.
Sugar production is a seasonal agro-based industry. In India, this is the second largest agro-based industry after textile. Maharashtra State is one of the leading Indian states manufacturing sugar from sugar cane. Effluent generated in sugar mills during various manufacturing processes has a very high potential for causing pollution of surface water, ground water and soil (Upadhyay et al. 2009). Effluents from sugar industry, if directly released in water for irrigation, affect soil fertility as well as plant growth and seed germination (Ramkrishan et al. 2001). The physicochemical characteristics of sugar mill effluents discloses its organic strength i.e., chemical oxygen demand (COD) value ranges from 1500 to 4000 mg/L and biochemical oxygen demand (BOD) values from 500 to 800 mg/L (Deshmane et al. 2015). Because the effluent was from agro-based industry, it contains nitrogen (N), phosphorous (P), and potassium (K) in substantial quantities (Rajagopal et al. 2013).
Presently, many sugar factories in Maharashtra are operating effluent treatment plants based on activated sludge process in combination with preliminary, primary, secondary and/or tertiary treatment units (personal observations of the authors). These plants require large amounts of energy for the operation of various electromechanical units (Mara 2006). Moreover, the sugar industry suffers from scarcity of skilled manpower and to a lesser extent supervisory staff (personal observations of the authors). These factors are becoming hurdle in reusing/recycling of treated sugar mill effluent (SME). The present research was therefore initiated to simplify sugar effluent treatment and make it environment friendly.
Literature review shows that microalgae/cyanobacteria are effective in removing various kinds of nutrients and inorganic compounds including metals from wastewater/effluents (de-Bashan and Bashan 2010). Their findings indicate that microalgae can grow in wastewaters, making them suitable for a low-cost and effective biological wastewater treatment. Further, 1 kg of BOD removed in activated sludge process requires 1 kWh of electricity for aeration. The electrical power used also produces 1 kg of carbon dioxide (Oswald 2003). By contrast, 1 kg of BOD removed by photosynthetic oxygenation requires no energy inputs, and it produces enough algal biomass for fuel generation (Oswald 2003). Pedroni et al. (2001) observed better results for microalgae in the wastewater treatment as compared to conventional processes such as activated sludge. Filamentous algae/cyanobacteria can be as effective as microalgae, and they may be harvested relatively easily by filtration. Moreover, some filamentous algae/cyanobacteria form aggregates and can be harvested by sedimentation or by flotation (Hori et al. 2002). Therefore, in the present research, Spirulina was used for SME treatment. Experiments were conducted with an aim of achieving reduction in COD/BOD of SME at lower energy (in the form of electrical, mechanical as well as labor). In short, the study was to explore feasibility of effectiveness of Spirulina for SME treatment as well as use of SME medium for the growth of Spirulina (Fig. 1).
Materials and method
Materials
SME is a wastewater from the sugar manufacturing processes. It was freshly collected from three sugar factories of Pune District of Maharashtra (See Acknowledgment). The effluent was brought to the laboratory and stored at 4 °C. It was used the next day for corresponding experimental batch. Cold-stored effluent was allowed to reach ambient temperature (diurnal range 20–31 °C) before commencing experimentation. Pure culture of Spirulina species was obtained from Krishi Vidnynan Kendra (Agriculture Science Centre), located at Babhaleshwar, District Ahmednagar, Maharashtra State, India. Rectangular tanks (open photo-bioreactor) made up of glass of dimension 60 cm (l) × 30 cm (b) × 45 cm (h) were used for the experiments. Aerators were used for continuous supply of air to the medium in the tanks, at the rate of 3 L/min. A rotator device was prepared by mounting a simple steel paddle on a motor, rotating six rotations per minute (RPM). This was used in two tanks for continuous stirring of the medium present in the tank. Lutron make light meter (model LX 101A, made in Taiwan) was used to measure the light (in lux). Other instruments such as pH meter and spectrophotometer (Hach make, model DR 2000) were used.
Method
Sugar effluent treatment and its analysis
Preparatory treatment
Initial pH of the effluent ranged from 3.6 to 4.1. Because algal cultures are maintained between pH 7 to 9 (Lavens and Sorgeloos 1996), lime [Ca(OH)2—0.1 g/L of effluent] was used to adjust the pH of the effluent, to a level of 7.2–7.3. In addition, alum (potassium alum) dosing was also carried out (~0.29 g/L of effluent), to settle suspended solids. This was mainly to reduce the possible interference of suspended solids from effluent in photosynthetic activity of Spirulina. The settled solids were removed by simple filtration using filter paper. The initial and final (after completion of effluent treatment) characteristics of effluent were determined in accordance with standard methods prescribed by APHA (Eaton et al. 2005).
Experimental setup
Five glass tanks labeled A, B, C, D and E were used for the experiment. In each tank, 10 L of above-described SME was poured. In tanks A and B, 1.5 L of Spirulina was added to the effluent. This volume was determined through earlier experiments conducted by the authors (data not given). For tanks C and D, Spirulina was added to the effluent in a quantity of 0.250 L every day. This was to maintain exponential nature of growth pattern of Spirulina; some of the culture need to be recycled. Therefore, through this experiment, i.e., addition of 0.250 L/day of fresh culture (which is in log phase), it was tried to mimic the commercial process of harvesting of algae. Tank E was used as a control, i.e., without Spirulina—water was added in lieu of Spirulina. Air was supplied using aerators to tanks B and D. The supply of air was to support aerobic bacteria presumed to be present with Spirulina culture (Ciferri 1983) and in the effluent or introduced in the medium through air. In case of tanks A and C, the effluent with added Spirulina was continuously mixed by pre-fabricated rotation device. Cell density of Spirulina equivalent to 0.590 ± 0.010 O.D (at 660 nm) was maintained uniform for tanks A, B, C and D using a spectrophotometer.
All tanks were kept under natural environmental conditions outside the laboratory. Ambient temperature recorded during the experiment was 24 °C (±4 °C) to 34 °C (±4 °C). Spirulina grow well in the range of 30–38 °C (Vonshak and Tomaselli 2003; Ogbonda et al. 2007). Sun was the source of light, and the tanks were 90 % covered by a shade net only at the top. Shed net permitted only 25 % of light to pass. This was to avoid photo-inhibition due to excessive light, nuisance of birds feeding on algae, avoid green house effect inside the tank and minimize evaporation losses. Light energy was measured at the surface of the medium using a light meter; details of the experimental setup are provided in Table 1.
Treatment time
Bio-chemical oxygen demand and chemical oxygen demand are important qualitative indicators of wastewater. According to effluent discharge norms of Ministry of Environment and Forests, Government of India (General Effluent Standards Schedule—VI, dated May 19, 1993), wastewater having BOD equal to or less than 100 mg/L can be utilized for irrigation purpose. It was not possible to measure BOD on a daily basis. Therefore, COD was used as a key test parameter, as it could be measured every day within few hours. The end point of experiment was COD (of filtrate of SME) reduction of >80 % of initial value or COD value of <250 mg/L, whichever occurred earlier. Filtered effluent samples from all the tanks were collected at 12-h intervals for testing of COD and BOD, nitrogen (N), phosphorous (P), and potassium (K) were measured only at the initial and final stages of the experiment.
Study of protein content of Spirulina used in the SME treatment
Spirulina is well known for its high protein content (Avila-Leon et al. 2012). Therefore, this parameter was examined for Spirulina used in SME treatment. The biomass collected after SME filtration was allowed to dry to a constant weight at ambient temperature (maximum 34 ± 4 °C) and was used for protein estimation by the method of Lowry et al. (1951).
Results and discussion
Discussion: COD reduction of sugar mill effluent
As mentioned earlier, the experiment was replicated three times and observations for COD discussed below were of individual cycle/batch of the same. A pre-defined end point (mentioned earlier at “Treatment time”) for the experiment (SME treatment) was COD reduction of more than 80 % of its initial value or COD value of less than 250 mg/L. In this experiment, 53 and 50 % of COD reduction of SME was achieved in just 24-h time, in tanks A and B, respectively. For tank A, overall COD reduction of 91 % (final COD was 195 mg/L ± 12) was achieved in 108-h treatment time. In the same treatment time, COD reduction of SME in tank B was of 89 % (final COD was 242 mg/L ± 9).
Most striking observation of this experimental set was COD reduction of SME by 81 % for tank C (provided with external rotation device) and 77 % for tank D in 108 h (provided with aerator). In these two tanks, instead of adding a bulk amount of Spirulina at start up, the same was provided freshly in a small amount i.e., 250 ml every day, in the SME.
Reduction in BOD of SME was highest in tank A that was 91.3 %, followed by 89.4 % for tank B, 84.9 % for tank C and 81.2 % for tank D (Fig. 2).
The results of change in COD during the experiment were analyzed statistically by one-way ANOVA method using GraphPad Prism software (version 5.01). Change in COD of tanks A, B, C and D was compared with E. The significance value p was observed <0.001, whereas when the statistical significance was tested by comparing the results of COD reduction of tanks A versus B, tanks A versus C, tanks B versus D and tanks C versus D, it was observed that the results were not significant. In other words, the COD reduction observed in these tanks was quite comparable to each other.
On the basis of the above observations as well as work done earlier by other workers, the reasoning of COD/BOD reduction using algae has been tried to explore here. It has been found that all species of Spirulina so far isolated even from the most alkaline lakes are always contaminated by bacteria. The bacterial flora associated with the cultures of Spirulina is varied but with a preponderance of gram-negative rods (Ogawa and Terui 1970). It is not known whether any mutualistic relations exist between these bacteria and the Spirulina. It is possible to distinguish two main groups of bacterial contaminants that of the organisms present mostly in the culture medium and loosely adhering to the trichomes and those, called epiphytic contaminants, bound or tightly adhering to the thin sheath enclosing the trichomes (Ciferri 1983). Based on these observations, a logical reason for COD/BOD reduction emerges as the symbiotic action of Spirulina and associated microorganisms. Spirulina produce oxygen within the water column where it was readily available to aerobic bacteria (in this case bacteria associated with Spirulina) that could have oxidized complex organic material of SME into its constituent plant nutrients (as reported by Green et al. 1995). As a result, there was significant reduction in the BOD/COD (observed in tank A, B, C and D as compared to tank E). In addition, Spirulina has shown excellent uptake of nutrients such as nitrogen (N), phosphorous (P), and potassium (K) (de-Bashan and Bashan 2010). Therefore, these two factors have resulted into reduction in COD to the extent of 80–91 % (Fig. 3).
Another reason is the observation reported by Ciferri (1983) that if media with pH close to neutrality are used, very few bacterial colonies are found. In the present experiments, the pH of SME was near neutral (7.1–7.3). Costa et al. (2003) investigated the feasibility of using fresh water from Mangueira Lagoon (Rio Grande do Sul, Brazil) for biomass production in open raceway ponds (0.7 m long, 0.18 m wide, 0.075 m deep). In this study, they collected water sample in aseptic conditions and observed the microbiological growth. The results of microbiological analysis showed that the levels of bacterial contamination in all experiments were <1.6 × 104 CFU/ml lesser than that previously described for dried Spirulina. These counts were taken before drying of the Spirulina culture. Further, the drying process would probably decrease the level of contamination.
Thus, the investigations were continued further to find out the other reasons, if any. The observations reported by Ogawa and Terui (1972) were found to be interesting. These workers verified utilization of glucose by supplying it to cultures of S. platensis with 14C-glucose. Within less than 4 days of culture, all labeled glucose disappeared from the medium and almost 50 % of the label was recovered with the cells of Spirulina, the rest being released either as CO2 (34 %) or as organic by-products excreted into the medium (19 %). The growth of S. platensis with or without addition of glucose was quantitatively analyzed by Marquez et al. (1993) under light and dark conditions. It was shown that S. platensis is capable to grow on glucose heterotrophically under aerobic-dark conditions and that the photosynthetic activity and oxidative assimilation of glucose can independently operate mixotrophically under light conditions.
In case of SME, the organic elements are due to contamination of cane juice (mono and disaccharides) at various stages of the process, fine particles of bagasse, etc. The observations reported by Ogawa and Terui (1972) and Marquez et al. (1993) suggest that the organic matter partially consumed by the Spirulina itself. The bacteria associated with Spirulina were more active when pH of the SME reached near eight and thus helped in further reduction of COD/BOD. Removal of nutrients from SME during the treatment by Spirulina could have minor-to-moderate contribution in reduction in COD.
Nitrogen removal and protein content of Spirulina
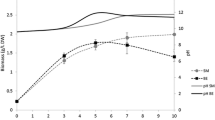
Nitrogen is an important nutrient for the production of any microalgal biomass. Nitrogen can be utilized by algae as nitrate, nitrite, or ammonia and elemental nitrogen. Some cyanobacteria, such as Spirulina, are diazotrophic, which means they are capable of utilizing elemental nitrogen as their sole nitrogen source by the reduction of N2 to NH4 + (Benemann 1979). Spirulina has a high protein concentration of 65–70 % of its dry weight (Phang et al. 2000). Uslu et al. (2011) observed that protein content of Spirulina related to nitrogen available in the medium. In the present case, total available nitrogen of SME was 120.07 mg/L (± 21.4). Protein content of Spirulina was estimated for tanks A and B. Protein content of Spirulina was observed to be 661.3 ± 38.0 mg/g (Fig. 4), in all three cycles. Highest protein contents of 699.3 and 686.3 mg/g (on the dry weight basis of biomass) were observed for Spirulina collected from tanks A and B, respectively, after 72-h interval. Sassano et al. (2010) reported the increase in the protein content of cyanobacterial biomass to some extent with the increasing nitrogen content. Wu and Pond (1981) observed a protein content of 60.1 and 71.8 % for Spirulina maxima grown on fermented cattle and poultry manure, respectively. Therefore, sugar mill effluent rich in nitrogen may be considered a suitable cultivation medium for protein production, using Spirulina (Fig. 4).
Conclusion
Our study demonstrates the viability of experimented technology for the treatment and recycling of SME. This simple, less energy-consuming technology option treats the effluent by reducing its COD up to 91 % in 108 h. Reduction of COD over 50 % was achieved in 24 h. In addition, nitrogen, phosphorous, and potassium of effluent acted as nutrient for Spirulina growth. Due to this, these inorganic elements were significantly reduced during the treatment. High level of protein content of Spirulina biomass signifies the role of SME in protein production. Protein production is immensely important to encourage the industry for generating revenue and recover the cost of effluent treatment. Therefore, the industry may seriously invest in ETP and operate it sensibly. Reduction of COD/BOD of SME and growth of Spirulina are interdependent and therefore beneficial to the sugar industry.
References
Avila-Leon I, Chuei Matsudo M, Sato S, de Carvalho JCM (2012) Arthrospira platensis biomass with high protein content cultivated in continuous process using urea as nitrogen source. J Appl Microbiol 112:1086–1094
Benemann JR (1979) Production of nitrogen fertilizer with nitrogen-fixing blue-green algae. Enzyme Microb Technol 1:83–90
Bigas H (ed) (2012) The global water crisis: addressing an urgent security issue. Papers for the InterAction Council, 2011–2012. Hamilton, Canada: The United Nations University—Institute for Water, Environment and Health (UNU-INWEH)
Central Pollution Control Board (2003) Charter on corporate responsibility for environmental protection
Ciferri O (1983) Spirulina, the edible microorganism. Microbiol Rev 47(4):551–578
Costa JAV, Colla LM, Filho PD (2003) Spirulina platensis growth in open raceway ponds using fresh water supplemented with carbon, nitrogen and metal ions. Z Naturforsch 58c:76–80
De-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bio-resour Technol 101:1611–1627
Deshmane A, Nimbalkar D, Nikam TD, Ghole VS (2015) Exploring alternative treatment method for sugar industry effluent using ‘Spirulina platensis’. Sugar Tech. doi:10.1007/s12355-015-0366-1
Eaton AD, Clesceri LS, Rice EW, Greenberg AE (eds) (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF), Washington, DC
Green FB, Lundquist TJ, Oswald WJ (1995) Energetics of advanced integrated wastewater pond system. Water Sci Technol 31(12):9–20
Hori K, Ishii S, Ikeda G, Okamoto J, Tanji Y, Weeraphasphong C (2002) Behavior of filamentous cyanobacterium Anabaena spp. in water column and its cellular characteristics. Biochem Eng J 10:217–225
Lavens P, Sorgeloos P (eds) (1996) Manual on the production and use of live food for aquaculture. Food and Agriculture Organization (FAO) fisheries technical paper 361. FAO, Rome
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mara DD (2006) Natural wastewater treatment; good practice in water and environmental management. In: Nigel Horan (ed) Aqua Enviro Technology Transfer, Wakefield
Marquez Facundo J, Sasaki Ken, Kakizono Toshihide, Nishio Naomichi, Nagap Shiro (1993) Growth characteristics of Spirulina platensis and in mixotrophic and heterotrophic conditions. J Ferment Bioeng 76(5):408–410
Ogawa T, Terui G (1970) Studies on the growth of Spirulina platensis. (I) On the pure culture of Spirulina platensis. J Ferment Technol 48:361–367
Ogawa T, Terui G (1972) Growth kinetics of Spirulina platensis in autotrophic and mixotrophic cultures. In: Terui G (ed) Fermentation technology today. Society of Fermentation Technology, Tokyo, pp 543–549
Ogbonda KH, Aminigo RE, Abu GO (2007) Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bio-resource Technol 98:2207–2211
Oswald WJ (2003) My sixty years in applied algology. J Appl Phycol 15:99–106
Pedroni P, Davison J, Beckert H, Bergman P, Benemann J (2001) A proposal to establish an international network on biofixation of CO2 and greenhouse gas abatement with microalgae. J Energy Environ Res 1:136–150
Phang SM, Miah MS, Yeoh BG, Hashim MA (2000) Spirulina cultivation in digested sago starch factory wastewater. J Appl Phycol 12:395–400
Rajagopal R, Noori M, Saady Cata, Torrijos M, Thanikal JV, Hung YT (2013) Sustainable agro-food industrial wastewater treatment using high rate anaerobic process. Water 5:292–311
Ramkrishan K, Srirama CM, Thamizhin IP, Sundar MP (2001) Effect of sugar mill effluent polluted soil on VAM fungi. J Ecobiol B(3):187–192
Sassano CEN, Gioielli LA, Ferreira LS, Rodrigues MS, Sato S, Converti A (2010) Evaluation of the composition of continuously-cultivated Arthrospira (Spirulina) platensis using ammonium chloride as nitrogen source. Biomass Bioenergy 34:1732–1738
Upadhyay RN, Pandey VC, Tewari DD, Verma SC, Pandey K (2009) Study of the groundwater quality contaminated with sugar mill effluent. Nat Environ Pollut Technol 7:249–252
Uslu L, Isik O, Koç K, Göksan T (2011) The effects of nitrogen deficiencies on the lipid and protein contents of Spirulina platensis. Afr J Biotechnol 10(3):386–389
Vonshak A, Tomaselli L (2003) Arthrospira (Spirulina): systematics and ecophysiology biochemistry. In: Vonshak A (ed) Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. Taylor & Francis, London, pp 505–522
Wu JF, Pond WG (1981) Amino acid composition and microbial contamination of Spirulina maxima, a blue-green alga, grown on the effluent of different fermented animal wastes. Bull Environ Contam Toxicol 27:151–159
Acknowledgments
We thank Vasantdada Sugar Institute for funding the present research, Dr Latey for his guidance to improve the language of the manuscript and the following industries for providing effluent used in this study: Yashwant Sahakari Sakhar Karkhana Limited, Theur, District Pune, Maharashtra; Sant Tukaram Sahakari Sakhar Karkhana Limited, Kasarsai-Darumbre, District Pune, Maharashtra; Shri Nath Mhaskoba Sahakari Sakhar Karkhana Limited, Rahu, Daund District Pune, Maharashtra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deshmane, A.B., Darandale, V.S., Nimbalkar, D.S. et al. Sugar mill effluent treatment using Spirulina for recycling of water, saving energy and producing protein. Int. J. Environ. Sci. Technol. 13, 749–754 (2016). https://doi.org/10.1007/s13762-015-0891-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-015-0891-1