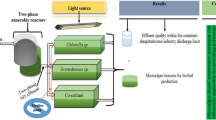
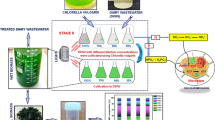
Abstract
Using effluents as a growth medium for microalgae contributes to the effluent treatment and reduces the costs associated with biomass production. Dairy effluent (DE) is among the most voluminous food industry effluents generated. This study aimed to produce biomass and agricultural biostimulants by cultivating Chlorella fusca LEB 111 and Spirulina sp. LEB 18 in dairy effluent. The microalgae were grown in pure and diluted DE at 25%, 50%, and 75% in water and, BG 11 and Zarrouk culture media. The treated DE (culture supernatant) was utilized as a biostimulant in the germination of red pear tomato (Lycopersicon esculentum) seeds. After the cultivation process, treated effluent was obtained with BOD removal efficiency of 98.5% and 99.1%, COD 96.5% and 97.7%, total phosphorus 98.8% and 85.3%, and ammonia nitrogen 98% and 99% for C. fusca LEB 111 and Spirulina sp. LEB 18, respectively. Under the condition in which 100% DE was used, the maximum biomass concentrations of 1.03 g L−1 and 1.98 g L−1 and maximum biomass productivity of 159.70 mg L−1 day−1 and 187.70 mg L−1 day−1 were achieved for Chlorella and Spirulina, respectively. The C. fusca LEB 111 supernatant grown with 100% DE increased seed germination, resulting in 100% germinated seeds. Therefore, this study presents an alternative for microalgae cultivation utilizing DE as an alternative medium, resulting in biomass and treated effluent with high potential for application in agriculture.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global consequences of population growth, industrial activities, climate change, and modern lifestyles have led to the excessive use of fossil fuels, agrochemicals and the high generation of wastewater (Peng et al. 2023). Urban effluents and effluents from food industries, such as dairy products, are among the wastewater generated in larger volumes (Ma et al. 2023). Nitrogen chemical fertilizers, which are heavily used in agriculture, directly contribute to climate change, as the nitrous oxide emitted has a high global warming potential compared to other greenhouse gases produced in agricultural practices (Ammar et al. 2022).
The dairy industry is one of the largest water consumers and producers of effluent among food industries, mainly due to milk treatment, sterilization processes, and equipment cleaning (Kumar et al. 2020). A high organic content with significant organic matter, nitrogen, phosphorus, and micronutrients characterizes dairy effluent (DE). The organic residues in DE are considered environmentally harmful due to their high chemical (COD) and biochemical oxygen demand (BOD) and the associated problems of rapid degradation. If not adequately managed, DE can threaten the ecological balance (Gogoi et al. 2021). By specific legislation in each location, effluents require treatment before being released into water bodies. After conventional wastewater treatment, the generated waste must be sent off-site for further treatment and disposal, increasing operating costs (Ma et al. 2023). However, DE can be an excellent source of nutrients for microalgae cultivation, presenting a potential biological treatment option for this waste (Costa et al. 2021).
Industrial-scale microalgae cultivation demands high nutrient and water concentrations (Kumar et al. 2019). Therefore, microalgae can consume high levels of nitrogen and phosphorus found in wastewater, utilizing only solar energy and producing biomass (Acién Fernández et al. 2018). Using effluents as a microalgae cultivation medium has become an alternative to reduce process costs and obtain microalgae biomass with diverse applications (Costa et al. 2019). The microalgal genera Chlorella and Spirulina (Arthrospira) have gained attention in using effluents as an alternative culture medium due to their resistance to cellular stress conditions (Bezerra et al. 2022).
Chlorella and Spirulina are known to produce biomass rich in protein, with an average content of 50% of their dry weight, along with carbohydrates, lipids, vitamins, minerals, and chlorophyll (Becker 2007; Morais et al. 2015). Consequently, a microalgae biorefinery approach can be employed, where microalgae are cultivated in DE, generating biomass that can be utilized to produce biofuels, animal feed, and in agriculture. Additionally, this process generates treated effluent that can be used as biostimulants for seed germination in agriculture (Costa et al. 2021). Biostimulants are natural substances that, when applied in small quantities, can stimulate plant growth and development, whether under optimal growth conditions or under conditions that cause cellular stress (Kapoore et al. 2021). Microalgae biostimulants pose a low risk of toxicity to the environment and living beings. By utilizing microalgae biostimulants, farmers can reduce their dependence on synthetic chemicals, such as fertilizers and growth regulators, thus mitigating the negative environmental impact associated with the overuse of these products (Braun and Colla 2022).
Therefore, this study aimed to produce biomass and agricultural biostimulants by cultivating Chlorella fusca LEB 111 and Spirulina sp. LEB 18 in dairy effluent, thus providing products with broad potential for application in agriculture and contributing to the reduction of pollution caused by the disposal of effluents and agrochemicals. This research is aligned with the goal of sustainable agriculture based on microalgae. It contributes to achieving the 17 Sustainable Development Goals (SDGs), particularly SDG-2 "Zero Hunger and Sustainable Agriculture", SDG-6 “Clean Water and Sanitation”, SDG-12 "Responsible Consumption and Production", and SDG-13 "Climate Action".
Material and methods
Microalgae and cultivation conditions
The microalgae Spirulina sp. LEB 18 and Chlorella fusca LEB 111 were obtained from the Biochemical Engineering Laboratory at the Federal University of Rio Grande (FURG) in Rio Grande do Sul, Brazil. The effluent used was obtained from a dairy industry in Timbó, Santa Catarina, Brazil. It was filtered to remove suspended solid particles before being stored at -20 °C until use (Schulze et al. 2017). Zarrouk medium (Zarrouk 1966) and BG 11 medium (Rippka et al. 1979) were used as control media for Spirulina sp. LEB 18 and Chlorella fusca LEB 111, respectively.
Six experiments were conducted in duplicate for each alga to test the growth of the alga. Various concentrations of dairy effluent diluted in distilled water and BG 11 culture media (for C. fusca LEB 111) and Zarrouk (for Spirulina sp. LEB 18) were used: 0% (control), 25%, 50%, and 100%. The experiments were carried out in autotrophic growth conditions, with a 12 h light/dark photoperiod, for 20 days in 0.5 L Erlenmeyer flasks with a useful volume of 0.4 L and an initial biomass concentration of 0.2 g L−1. The cultures were kept in a thermostated chamber at 30 °C with 70 μmolphotons m−2 s−1 of light and agitation was achieved by injecting sterile air.
Biomass recovery
At the end of the cultivations the biomass was recovered by centrifugation at 9000 ×g for 20 min, resuspending the pellet in distilled water and centrifuging again for 20 min under the same conditions for removing salts from the culture medium. The centrifuged biomass was frozen at – 80 °C for 48 h and then lyophilized. Lyophilized samples were stored at – 20 °C for further characterization. The supernatant of the cultures was stored at – 20 ºC for further characterization and use in seed germination tests.
Microalgal growth
Biomass concentration was determined daily by measuring the culture optical density (OD) in a spectrophotometer at 670 nm and plotting a standard curve of the OD in relation to dry biomass. The maximum biomass productivity (Pmax, mg L−1 day−1) was determined according to the equation Pmax = (Xt − X0) / (t − t0), where Xt is the biomass concentration (g L−1) at time t (day) and X0 is the biomass concentration (g L−1) at time t0 (day) (Bailey and Ollis 1986). The pH of the cultures was also determined daily using direct digital pH meter readings (Mettler Toledo FiveGo, Switzerland).
Effluent characterization and removal efficiency
The dairy effluent used was characterized both before and after the cultivation of C. fusca LEB 111 and Spirulina sp. LEB 18 with 100% dairy effluent. The following parameters were determined: pH, sedimentable solids, electrolytic conductivity, hardness, turbidity, nitrate, ammonia, chemical and biochemical oxygen demand (COD and BOD), organic phosphorus, phosphate, total phosphorus, and oils and greases (Baird et al. 2017). The removal efficiency was calculated as the difference between the parameters evaluated before and after microalgae cultivation in the raw effluent (Ji et al. 2012).
Characterization of microalgal biomass
Protein and carbohydrate analyses were performed at the end of the experiments using biomass extracts prepared with 5 mg lyophilized biomass and 10 mL distilled water using an ultrasonic probe (COLE PARMER CPX 130, USA) to extract the intracellular compounds for 10 min in cycles of 59 s. The total carbohydrate concentration in the biomass was determined using the phenol–sulfuric acid method with glucose as the standard (Dubois et al. 1956). The protein content in the biomass was determined by the colorimetric method of Lowry et al. (1951), using thermal and alkaline pretreatment with sodium hydroxide to solubilize insoluble proteins and quantification with a standard curve of bovine serum albumin. The lipid concentration in the biomass was determined in 10 mg lyophilized biomass using the colorimetric method of Marsh and Weinsteins (1966). This method is based on the extraction of lipids using a chloroform and methanol mix (1:2 v/v) reaching polar and non-polar lipids and quantification with a standard tripalmitin curve. The moisture content was determined by the methodology described by Official Methods of Analysis—AOAC (2000).
Seed germination test using supernatants
Supernatants from pure dairy effluent (100%) and dairy effluent diluted in Zarrouk and BG 11 media containing Spirulina sp. LEB 18 and C. fusca LEB 111 were selected to evaluate their potential as biostimulants in the germination of red pear tomato seeds (Lycopersicon esculentum). The seeds were sterilized with a 5% sodium hypochlorite solution for 8 min and then rinsed with distilled water. Subsequently, the seeds were treated with culture supernatants and the control condition (distilled water) for 70 min. The germination test was conducted with 50 seeds per treatment, distributed in plastic boxes (240 × 166 × 101 mm) containing 1 kg of vermiculite moistened with 500 mL of distilled water, with four repetitions per treatment. All experiments were carried out in quadruplicate, in a thermostated chamber, at 30 °C and 100 μmolphotons m−2 s−1, with a photoperiod of 12 h light/dark. The boxes were kept closed and moistened daily with distilled water.
The evaluation of germination was conducted at 5, 7, 9, and 11 days after sowing by counting the number of germinated seedlings. The first count was performed on the 5th day after the initiation of the germination test. The germination speed index was calculated by dividing the number of germinated seeds by the number of days elapsed between sowing and germination. After the germination test, the length of the seedlings and roots were determined. The fresh and dry weight of the seedlings was also determined (Brasil 2009).
Statistical analysis
All analyses were performed in triplicate and evaluated using analysis of variance, followed by Tukey’s post-hoc test, to compare the means with a 95% confidence level.
Results
Growth of microalgae in dairy effluent
Based on the biomass concentration curves of microalgae C. fusca LEB 111 and Spirulina sp. LEB 18 (Fig. 1), it is evident that dairy effluent was conducive to microalgal growth, even when used as the sole culture medium (100% dairy effluent). Furthermore, the microalgae did not display any adaptation phase during the experiments in all tested conditions. In the study by Kumar et al. (2018), raw dairy effluent was also used to cultivate Ascochloris sp. ADW007, replacing BG 11 and TAP media, and a significantly higher maximum biomass concentration (2.23 g L−1) was obtained compared to the other conditions, indicating the potential of this residue as a microalgae culture medium. However, the microalgae used in their study were already adapted to the effluent as it was isolated from the same. Therefore, this study is noteworthy as the microalgae Chlorella and Spirulina exhibited Xmax 1.03 g L−1 and 1.98 g L−1 and Pmax 159.70 mg L−1 day−1 and 187.70 mg L−1 day−1, respectively, with 100% effluent, without any adaptation phase, even without adaptation to the effluent (Table 1). Moreover, the condition where 25% of effluent and 75% of BG 11 or Zarrouk medium were used proved more favorable for the growth and biomass production of C. fusca LEB 111 and Spirulina sp. LEB 18, with Xmax 1.60 g L−1 and 3.11 g L−1, respectively.
Biomass concentration (g L−1) during the cultivation of Chlorella fusca LEB 111 (a) and Spirulina sp. LEB 18 (b) grown in dairy effluent under the conditions: Control (100% Medium) (♦), 100% Dairy effluent (▲), 50% Dairy effluent/ 50% Medium (■), 25% Dairy effluent / 75% Medium (●), 50% Dairy Effluent/50% Water (□), 25% Dairy Effluent/75% Water (○)
During the cultivation of Spirulina sp. LEB 18 in dairy effluent, the pH remained within the range of 10.0 to 11.0, with variations occurring due to microalgal growth. The different concentrations of effluent, culture media, and distilled water did not show a significant change in this parameter. The Zarrouk medium contains sodium bicarbonate as a carbon source, which microalgae use in their carbon assimilation mechanism (Ota et al. 2009). This leads to a pH of around 10 to 10.5. As time progresses, two bicarbonate ions are consumed by the cell, with one internalized in the form of carbon dioxide and the other released in the form of the carbonate, causing the pH of the medium to increase to values greater than 10.5 (Shiraiwa et al. 1993).
The pH of the C. fusca LEB 111 cultivation in dairy effluent remained in the range of 8.0 to 11.0, with lower pH values recorded in the first few days and increasing according to the growth of the microalgae, as expected for this species (Duarte et al. 2017). Despite the raw dairy effluent having an alkaline pH of 11.77, it did not affect microalgae growth in any tested conditions. Therefore, adjusting the pH was unnecessary as the effluent’s pH did not differ significantly from the media used. Additionally, the experiments were examined daily under an optical microscope, and no contamination was detected until the last day, even though the effluent had not been sterilized before the cultivations.
Concentration of macromolecules in Chlorella fusca LEB 111 and Spirulina sp. LEB 18 grown in dairy effluent
The concentrations of carbohydrates, proteins, and lipids were determined in the biomass of C. fusca LEB 111 and Spirulina sp. LEB 18 cultured in dairy effluent to verify the variation of these macromolecules when using this alternative culture medium. Carbohydrates (Table 2) were the predominant biomass fraction in C. fusca LEB 111 grown with 100% dairy effluent, an increase of 111.5% about the control. In the case of Spirulina sp. LEB 18, the condition that most favored the biosynthesis of carbohydrates was with 50% of effluent diluted in 50% of water, resulting in an increase of 455.8% of the control.
Regarding protein concentration, C. fusca LEB 111 showed the highest content when cultured with 25% and 50% of effluent diluted in BG 11 medium, with results statistically similar to the control (as shown in Table 2). In the case of Spirulina sp. LEB 18, using 25% of effluent and 75% of Zarrouk medium, led to biomass with over 70% protein content, a promising result for agricultural and animal feed applications. Notably, this condition also resulted in a lipid concentration of more than 18%, further supporting the potential use of this biomass as animal feed. In summary, these findings suggest that dairy effluent, when used in appropriate dilutions and media combinations, can promote the biosynthesis of key macromolecules in microalgae, opening up opportunities for sustainable and cost-effective bioproduction.
Effluent characterization and removal efficiency
The chemical oxygen demand (COD) measurement is used to quantify the amount of organic compounds in wastewater indirectly. Regarding the characterization of the raw effluent used in this study (Table 3), it is evident that this residue has a high organic matter content (BOD 1652.00 mg L−1, COD 3840 mg L−1), high turbidity (> 1000 NTU), and other characteristics that require treatment to meet the conditions for wastewater release.
Seed germination test with supernatants of Spirulina sp. LEB 18 and Chlorella fusca LEB 111 grown in dairy effluent
The germination test conducted on red pear tomato (L. esculentum) seeds demonstrated the positive effects of seed treatment with supernatants of C. fusca LEB 111 and Spirulina sp. LEB 18 grown in dairy effluent. The culture supernatant of C. fusca LEB 111 produced with 100% dairy effluent increased seed germination, resulting in 100% germination (Fig. 2, Table 4). Moreover, there was an increase in the count of first germinated seeds, total seed germination, germination speed index, seedling length, root length, fresh and dry matter mass, seedling and root height, and number of leaves in all conditions tested, as compared to the control (water), indicating the potential of these conditions.
Discussion
All kinetic parameters evaluated in C. fusca LEB 111 and Spirulina sp. LEB 18 cultivated in dairy effluent under different conditions were higher or statistically equal to those of the controls (Table 1). These findings demonstrate that dairy effluent is a suitable culture medium for the growth of these microalgae, as it provides the necessary nutrients for their growth. The microalga's ability to efficiently absorb nutrients from dairy effluent and produce value-added products makes it a potential and cost-effective method for biomass production (Costa et al. 2021).
Regarding biomass composition, when microalgae are cultured in a medium supplemented with wastewater that has a higher nitrogen load, their biomass tends to have higher nitrogen and protein contents. This is because nitrogen is essential for synthesizing amino acids and proteins (Ferreira et al. 2019; Braun and Colla 2022). Using higher proportions of wastewater with alternative nutrient sources can increase the protein percentage in microalgae biomass. Additionally, the increase in nitrogen content may be related to the higher nitrate levels in the medium and the rate of nitrate removal by the microalgae. On the other hand, effluents with higher phosphorus percentages can lead to biomass with higher lipid contents (Baldisserotto et al. 2020). These findings suggest that the nutrient composition of the wastewater used as a culture medium can significantly influence the composition of the microalgae biomass. Therefore, wastewater management and treatment strategies can be crucial in optimizing the production of specific target molecules in microalgae, such as proteins and lipids.
The results regarding the potential use of microalgae biomass in agriculture are once again promising, given that higher protein concentrations in the biomass are necessary for plant growth, as nitrogen is a key component in the process. However, chemical fertilizers can negatively impact the environment due to excess nitrogen lost through denitrification, leaching, or volatilization (Ahmed et al. 2017). In contrast, microalgae-based organic fertilizers can offer several advantages, including the gradual release of nitrogen, phosphorus, and potassium, as well as the presence of plant growth-promoting substances such as phytohormones, vitamins, carotenoids, amino acids, and antifungal compounds (Coppens et al. 2016).
One of the main limitations preventing the incorporation of microalgae into animal feed is the high raw material cost and its limited availability. However, as demonstrated in this study, dairy effluent can provide nutrients for the production of biomass, which can then serve as a raw material for the production of animal feed that is rich in proteins, fatty acids, antioxidants, antimicrobial compounds, and other essential compounds that can prevent diseases and prolong the animals' lifespan. Furthermore, this biomass can be used for extracting compounds such as pigments and antioxidants, which can be purified and utilized in the food and pharmaceutical industries (Dineshbabu et al. 2019; Debeni Devi et al. 2023).
As previously mentioned, there is a need to reduce the production costs of microalgae biomass to enable their biotechnological application, including the Chlorella species (Andrade and Andrade 2017). Therefore, the use of C. fusca LEB 111 and Spirulina sp. LEB 18 could be implemented by dairy industries located near effluent treatment plants. By doing so, in addition to generating additional income, the company would also reap environmental benefits related to water quality improvement and carbon dioxide assimilation.
After cultivating microalgae using raw dairy effluent, the culture supernatant was characterized to determine the removal efficiency of pollutants. Iliopoulou et al. (2022) reported that contaminants in dairy effluent decrease over time during microalgae cultivation, indicating that pollutant consumption is proportional to species growth. This phenomenon was also observed in our study (Table 3), as all pollutants present in the raw effluent were reduced after 20 days of cultivation, confirming the potential of this medium as an alternative for the cultivation of C. fusca LEB 111 and Spirulina sp. LEB 18.
The cultivation of C. fusca LEB 111 and Spirulina sp. LEB 18 proved to be an excellent biological treatment for this effluent, exhibiting a removal efficiency greater than 95% for COD and BOD. The reduction in COD by more than 95% demonstrated that the microalgal cells effectively utilized the organic carbon as a substrate for their growth and energy source, producing biomass rich in macromolecules and facilitating the treatment of the raw effluent. The BOD reduction achieved in this study complies with the resolution of the effluent discharge conditions of the 2011 National Environment Council—CONAMA, which mandates a minimum removal of 60% BOD from the effluent.
Microalgae can remove all forms of nitrogen present in wastewater. In microalgae, the assimilation of nitrate involves two transport and two reduction steps: first, nitrate (NO3−) is transported into the cell, then the cytosolic nitrate reductase enzyme catalyzes the reduction of nitrate to nitrite, which is subsequently transported to the chloroplast, where the nitrite reductase enzyme catalyzes its reduction to ammonium (Lachmann et al. 2019). Finally, ammonium is metabolized (Miflin and Lea 1975). The nitrogen in the effluent, in the form of nitrate, was metabolized by microalgae with a removal efficiency of over 90%. Cultivation led to the total removal of ammoniacal nitrogen (100%). According to Kumar et al. (2010), ammoniacal nitrogen is the preferred source for microalgae as it is directly metabolized, requiring much less energy for its absorption.
Similarly, microalgae efficiently removed phosphorus, an essential nutrient for their growth and several cellular processes, with removal efficiencies of over 98.8% for C. fusca LEB 111 and 85.3% for Spirulina sp. LEB 18. Yaakob et al. (2021) reported that phosphorus is essential in microalgae cultivation, especially in the phosphate form, which is preferred for microalgal uptake and nucleic acid biosynthesis. In the present study, 89.8% and 82.4% phosphate removal efficiencies were achieved in the cultivation of C. fusca LEB 111 and Spirulina sp. LEB 18, respectively.
Kumar et al. (2019) conducted a study on treating raw dairy effluent using microalgae Ascochloris sp. ADW007. After treating the effluent, the results showed a reduction of 94–96% in COD, 72–80% in nitrate, and 80–97% in total phosphate. In a similar study Hamidian and Zamani (2022) cultivated Chlorella sorokiniana in dairy wastewater and observed a reduction of 59.65% in BOD, 57.17% in COD, 78.82% in nitrate, and 88.17% in phosphate. These results indicate that dairy effluent can be an alternative medium for cultivating several species of microalgae, and the treated effluent and biomass can be utilized in various applications such as animal feed, agriculture, pigments, and biofuels (Costa et al. 2021).
Electrical conductivity is a measurement carried out in effluent to monitor the presence of dissolved substances or impurities in water. Pure water typically exhibits a conductivity of around 5 mS cm−1 (Atlas Scientific 2023). The greater the impurities present in the water, the higher the conductivity. In this study, a removal efficiency of 99.9% in electrical conductivity was achieved, indicating the ability of microalgae to remove impurities from the wastewater.
The raw dairy effluent had a sedimentable material concentration of 23.00 mL L−1 before cultivation. According to the CONAMA resolution, the release condition should be 1 mL L−1 of sedimentable materials in a 1-h test using an Inmhoff cone. After cultivation, a removal efficiency of more than 99% was achieved for this parameter. The treated effluent consisting of a culture supernatant with 100% effluent from the microalgae C. fusca LEB 111 and Spirulina sp. LEB 18, exhibited sedimentable material concentrations of 0.2 and 0.1 mL L−1, respectively.
Based on the characterization of the dairy effluent before and after microalgae cultivation (Table 3), it is evident that although the microalgae C. fusca LEB 111 and Spirulina sp. LEB 18 exhibit excellent nutrient removal efficiency; nutrients remain in the treated effluent, such as nitrogen and phosphorus, which are essential for plant germination and growth, as demonstrated in this study. Furthermore, microalgae can synthesize phytohormones such as auxins and cytokinins during their growth and release them into the culture medium (Ahmed et al. 2010). Therefore, despite obtaining treated effluent through microalgae cultivation, residual nutrients and essential substances for seed germination, such as nitrogen, phosphorus, and phytohormones such as indoleacetic acid, may remain, depending on the harvesting method used, which justifies the results reported in this study (Table 4).
The results obtained in the germination test of this study were promising since the supernatant of microalgae cultivation is often discarded without any application. The treated dairy effluent was used for tomato seed germination, resulting in excellent germination parameters (Table 4). The term "biostimulant" is used in the literature to refer to agents that promote plant development and growth, including products containing amino acids, microbial inoculants, and microalgae extracts. Biostimulants are known to act on plant physiology, increasing crop yields and improving resistance to abiotic and biotic stresses (Braun and Colla 2022). Therefore, this study developed a new type of biostimulant produced from microalgae grown in effluents with potential use in agriculture to replace synthetic products for seed treatment. In addition to using a residue in the cultivations to replace traditional media, reducing their costs, biomass can be used for other purposes, such as the production of different types of biofertilizers, animal feed, biofuels, or even for the extraction of biocompounds with high added value, such as pigments (Costa et al. 2021).
Conclusion
The microalgae Chlorella fusca LEB 111 and Spirulina sp. LEB 18 were able to grow in dairy effluent (DE), showing that it can be used as an alternative source of nutrients in microalgae cultivation. After the cultivations, treated effluent was obtained with BOD removal efficiency of 98.5% and 99.1%, COD 96.5% and 97.7%, total phosphorus 98.8% and 85.3%, ammonia nitrogen 98% and 99%, for C. fusca LEB 111 and Spirulina sp. LEB 18, respectively. In addition, all parameters of conditions for releasing effluents into water bodies were achieved, showing the potential of this biological treatment for dairy effluents. In the condition in which 100% of effluent was used, maximum biomass concentrations of 1.03 g L−1 and 1.98 g L−1 and maximum biomass productivity of 159.70 mg L−1 day−1 and 187.70 mg L−1 day−1 for Chlorella and Spirulina, respectively. The condition in which 25% of effluent and 75% of BG 11 or Zarrouk medium were used proved to be more favorable to the growth and biomass production of C. fusca LEB 111 and Spirulina sp. LEB 18, showing Xmax 1.60 g L−1 and 3.11 g L−1. In this condition, the biomass of Spirulina sp. LEB 1 with 72.6% protein. Furthermore, the C. fusca LEB 111 supernatant grown at 100% DE increased seed germination, contributing to 100% germinated seeds. All supernatants used positively influenced germination, seedling and root length, fresh and dry weight of the plant, and the number of leaves of tomato plants. Thus, this study offers an alternative approach to microalgae cultivation using dairy effluent as a sustainable medium, producing biomass with high potential for use in agriculture and animal feed, as well as treated effluent for sustainable agriculture.
References
Acién Fernández FG, Gómez-Serrano C, Fernández-Sevilla JM (2018) Recovery of nutrients from wastewaters using microalgae. Front Sustain Food Syst 2:59
Ahmed M, Rauf M, Saeed NA (2017) Excessive use of nitrogenous fertilizers : an unawareness causing serious threats to environment and human health. Environ Sci Pollut Res 24:26983–26987
Ahmed M, Stal LJ, Hasnain S (2010) Production of indole-3-acetic acid by the cyanobacterium Arthrospira platensis strain MMG-9. J Microbiol Biotechnol 20:1259–1265
Ammar EE, Aioub AAA, Elesawy AE, Karkour AM, Mouhamed MS, Amer AA, El-Shershaby NA (2022) Algae as bio-fertilizers: Between current situation and future prospective: The role of algae as a bio-fertilizer in serving of ecosystem. Saudi J Biol Sci 29:3083–3096
Andrade CJ, Andrade LM (2017) An overview on the application of genus Chlorella in biotechnological processes. J Adv Res Biotech 2:1–9
AOAC (2000) Official Methods of Analysis, 17th Editi. The Association of Official Analytical Chemists, Gaithersburg, MD, USA
Atlas Scientific (2023) The Importance Of Electrical Conductivity Of Wastewater | Atlas Scientific. https://atlas-scientific.com/blog/electrical-conductivity-of-wastewater/. Accessed 4 Mar 2023.
Bailey JE, Ollis DF (1986) Biochemical engineering fundamentals, 2nd edn. McGraw-Hill, Singapore
Baird RB, Eaton AD, Rice E (2017) Standard Methods for the Examination of Water and Wasterwater, 23rd edn. American Public Health Association, Washington, D.C.
Baldisserotto C, Demaria S, Accoto O, Marchesini R, Zanella M, Benetti L, Pancaldi S (2020) Removal of nitrogen and phosphorus from thickening effluent of an urban wastewater treatment plant by an isolated green microalga. Plants 9:1802
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210
Bezerra PQM, Moraes L, Silva TNM, Cardoso LG, Druzian JI, Morais MG, Nunes IL, Costa JAV (2022) Innovative application of brackish groundwater without the addition of nutrients in the cultivation of Spirulina and Chlorella for carbohydrate and lipid production. Bioresour Technol 345:126543
Brasil (2009) Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Secretaria de Defesa Agropecuária. Mapa/ACS, Brasília
Braun JCA, Colla LM (2022) Use of microalgae for the development of biofertilizers and biostimulants. BioEnergy Res 16:289–310
Coppens J, Grunert O, Van Den Hende S, Coppens J, Grunert O, Van Den Hende S, Vanhoutte I, Boon N, Haesaert G, Gelder L (2016) The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J Appl Phycol 28:2367–2377
Costa JAV, Cruz CG, Rosa APC (2021) Insights into the technology utilized to cultivate microalgae in dairy effluents. Biocatal Agric Biotechnol 35:102106
Costa JAV, Freitas BCB, Rosa GM, Moraes L, Morais MG, Mitchell BG (2019) Operational and economic aspects of Spirulina-based biorefinery. Bioresour Technol 292:121946
Debeni Devi N, Sun X, Hu B, Goud VV (2023) Bioremediation of domestic wastewater with microalgae-cyanobacteria co-culture by nutritional balance approach and its feasibility for biodiesel and animal feed production. Chem Eng J 454:140197
Dineshbabu G, Goswami G, Kumar R, Sinha A, Das D (2019) Microalgae–nutritious, sustainable aqua- and animal feed source. J Funct Foods 62:103545
Duarte JH, Morais EG, Radmann EM, Costa JAV (2017) Biological CO2 mitigation from coal power plant by Chlorella fusca and Spirulina sp. Bioresour Technol 234:472–475
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Ferreira A, Ribeiro B, Ferreira AF, Tavares MLA, Vladic J, Vidović S, Cvetkovic D, Melkonyan L, Avetisova G, Goginyan V, Gouveia L (2019) Scenedesmus obliquus microalga-based biorefinery – from brewery effluent to bioactive compounds, biofuels and biofertilizers – aiming at a circular bioeconomy. Biofuels Bioprod Biorefining 13:1169–1186
Gogoi M, Biswas T, Biswal P, Saha T, Modak A, Gantayet LM, Nath R, Mukherjee I, Thakur AR, Sudarshan M, Chaudhuri SR (2021) A novel strategy for microbial conversion of dairy wastewater into biofertilizer. J Clean Prod 293:126051
Hamidian N, Zamani H (2022) Potential of Chlorella sorokiniana cultivated in dairy wastewater for bioenergy and biodiesel production. BioEnergy Res 15:334–345
Iliopoulou A, Zkeri E, Panara A, Dasenaki M, Fountoulakis MS, Thomaidis NS, Stasinakis AS (2022) Treatment of different dairy wastewater with Chlorella sorokiniana: removal of pollutants and biomass characterization. J Chem Technol Biotechnol 97:3193–3201
Ji L, Xie S, Feng J, Ji LY, Chen L (2012) Heavy metal uptake capacities by the common freshwater green alga Cladophora fracta. J Appl Phycol 24:979–983
Kapoore RV, Wood EE, Llewellyn CA (2021) Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotech Adv 49:107754
Kumar AK, Sharma S, Dixit G, Shah E, Patel A (2020) Techno-economic analysis of microalgae production with simultaneous dairy effluent treatment using a pilot-scale high volume V-shape pond system. Renew Energy 145:1620–1632
Kumar AK, Sharma S, Patel A, Dixit G, Shah E (2019) Comprehensive evaluation of microalgal based dairy effluent treatment process for clean water generation and other value added products. Int J Phytoremed 21:519–530
Kumar K, Sharma AK, Parikh BS, Patel A, Dixit G, Gupta S, Divecha JM (2018) Cultivation of Ascochloris sp. ADW007 - enriched microalga in raw dairy wastewater for enhanced biomass and lipid productivity. Int J Environ Sci Technol 16:943–954
Kumar MS, Miao ZH, Wyatt SK (2010) Influence of nutrient loads, feeding frequency and inoculum source on growth of Chlorella vulgaris in digested piggery effluent culture medium. Bioresour Technol 101:6012–6018
Lachmann SC, Mettler-Altmann T, Wacker A, Spijkerman E (2019) Nitrate or ammonium: Influences of nitrogen source on the physiology of a green alga. Ecol Evol 9:1070–1082
Lowry O, Rosebrough N, Farr AL, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ma M, Yu Z, Jiang L, Hou Q, Xie Z, Liu M, Pei H (2023) Alga-based dairy wastewater treatment scheme: Candidates screening, process advancement, and economic analysis. J Clean Prod 390:136105
Marsh JB, Weinstein DB (1966) Simple charring method for determination of lipids. J Lipid Res 7:574–576
Miflin BJ, Lea PJ (1975) Glutamine and asparagine as nitrogen donors for reductant-dependent glutamate synthesis in pea roots. Biochem J 149:403–409
Morais MG , Vaz BS, Morais EG, Costa JAV (2015) Biologically active metabolites synthesized by microalgae. Biomed Res Int 2015:835761
Ota M, Kato Y, Watanabe H, Watanabe M, Sato Y, Smith RL Jr, Inomata H (2009) Fatty acid production from a highly CO2 tolerant alga, Chlorococcum littorale, in the presence of inorganic carbon and nitrate. Bioresour Technol 100:5237–5242
Peng X, Jiang Y, Chen Z, Osman AI, Farghali M, Rooney DW, Yap PS (2023) Recycling municipal, agricultural and industrial waste into energy, fertilizers, food and construction materials, and economic feasibility: a review. Environ Chem Lett 1:3
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 110:1–61
Schulze PSC, Carvalho CFM, Pereira H, Gangadhar KN, Schüler LM, Santos TF, Barreira L (2017) Urban wastewater treatment by Tetraselmis sp. CTP4 (Chlorophyta). Bioresour Technol 223:175–183
Shiraiwa Y, Goyal A, Tolbert NE (1993) Alkalization of the medium by unicellular green algae during uptake dissolved inorganic carbon. Plant Cell Physiol 34:649–657
Yaakob MA, Mohamed RMSR, Al-Gheethi A, Gokare R, Ambati RR (2021) Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: an overview. Cells 10:393
Zarrouk C (1966) Contribution a l'etude d'une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur la croissance et la photosynthese de Spirulina mixima. Thesis. University of Paris, France
Acknowledgements
The authors thank the CAPES Finance Code 001, CNPq, MCTI, FAPERGS, and the CAPES-PRint FURG Project for supporting this study. The authors also thank the company Geslat Serviços Ltda and Engineer Irineu Scartezini Junior for sending the dairy effluent and performing the analyzes on the effluent.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil – (CAPES) Finance Code 001, CNPq (National Council of Technological and Scientific Development), MCTI (Ministry of Science, Technology, and Innovation), FAPERGS (Fundação de Amparo à pesquisa do Estado do Rio Grande do Sul), and Project CAPES-PRint FURG.
Author information
Authors and Affiliations
Contributions
Camila Gonzales Cruz: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing—original draft. Ana Priscila Centeno da Rosa: Conceptualization, Validation, Formal analysis, Investigation, Writing—review and editing, Supervision. Brenda Rafaela Strentzle: Methodology, Validation, Formal analysis, and Investigation. Jorge Alberto Vieira Costa: Conceptualization, Resources, Writing—review and editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors confirm that there are no conflicts of interest/Competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gonzales Cruz, C., Centeno da Rosa, A.P., Strentzle, B.R. et al. Microalgae-based dairy effluent treatment coupled with the production of agricultural biostimulant. J Appl Phycol 35, 2881–2890 (2023). https://doi.org/10.1007/s10811-023-03091-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03091-z