Abstract
Background
Multiple Sclerosis (MS) relapses are episodes of transient disease exacerbation. There are contradictory findings regarding seasonal variation in MS relapses. In this systematic review and meta-analysis, we aimed to investigate the seasonal and monthly variation in relapse rates among patients with MS.
Methods
We systematically queried PubMed, Scopus, and Web of Science for published papers until February 30, 2022.
Results
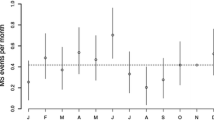
A total of 24 studies were included in this systematic review and meta-analysis with a total of 29,106 patients with MS. We found that the relapse rate was significantly lower in fall compared to the average relapse rate in other seasons with a risk ratio (RR) of 0.97 (95% CI 0.95–0.98). Furthermore, patients with MS experienced a higher number of relapses in April (RR: 1.06, 95% CI 1.01–1.11) and March (RR: 1.08, 95% CI 1.00–1.16) compared to other months. Also, the risk of relapse was lower in August (RR: 0.92, 95% CI.85–0.98), September (RR: 0.97, 95% CI.94–0.99), October (RR: 0.92, 95% CI.89–0.96), and November (RR: 0.93, 95% CI.89–0.97).
Conclusion
Our systematic review and meta-analysis confirm the temporal fluctuations in the relapse of MS through a comprehensive review of the existing literature, with a lower relapse rate during late summer and fall and a higher relapse rate during early spring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple Sclerosis (MS) is a neurological disease caused by chronic, inflammatory processes that lead to demyelination of the central nervous system (CNS), causing a wide range of physical and mental symptoms, including double vision, blindness, ataxia, urination disorders, and cognitive impairment. The pathogenesis of MS is not well known yet, but MS onset is correlated with impaired functioning of the immune system [1]. Both genetic and non-genetic risk factors have been incriminated in the pathogenesis of MS [1]. Episodes of transient exacerbation of MS, called relapses, are a key feature of MS [2]. A relapse is the clinical manifestation of acute inflammation and focal demyelination in a clinically eloquent region of the CNS [3, 4].
The effects of environmental risk factors in the onset of MS have been studied widely. Several environmental risk factors are suggested to contribute to MS, such as smoking, vitamin D deficiency, vascular risk factors, infectious diseases, such as Epstein–Barr virus (EBV) infection, obesity in childhood, and dietary habits [5,6,7,8].
There are clear seasonal physiological responses in animals, but the evidence supporting seasonality in humans is limited [9]. Some seasonal changes are observed in various human physiological processes, including nutrient intake [10], level of plasma cholesterol [11], blood pressure [12], and vitamin D metabolism [13]. Some of these factors also play a role in MS [13]. Moreover, several complex polygenic disorders, such as autoimmune [14], metabolic [15], and infectious diseases [16], exhibit seasonal patterns. At the molecular level, seasonal variation in the expression of a large set of genes in white blood cells is reported [17]. Despite these findings, the underlying mechanism of seasonality affecting human physiology is yet to be discovered.
A seasonal variation in relapses of MS has been demonstrated in some studies [18,19,20]; however, some other studies dispute these findings [21,22,23], where seasonal variation has been identified, peak frequencies in spring and summer, irrespective of latitude, have generally been observed [19, 20, 22, 24], and this pattern has also been supported by imaging findings [25]. The effect of seasonality on vitamin D metabolism might be one of the potential factors that causes seasonal and monthly patterns in relapse rates among patients with MS [26]. Seasonal relapse patterns might point the way for future studies on environmental variables linked to MS, as well as identifying potential pathways for relapse onset. In this systematic review and meta-analysis, we aimed to investigate the seasonal and monthly variation in relapse rates among patients with MS.
Methods
The Preferred Reporting Items for Systemic review and Meta-Analysis checklist was used to report the present systemic review and meta-analysis study [27].
Literature search
The following databases were systematically queried for published papers up to February 30, 2022: PubMed, Scopus, and Web of Science. The reference list of previous review studies was also reviewed to identify citations not captured in the initial search query. The following search strategy was adopted: (Multiple sclerosis) AND (season OR weather OR seasonality). Additional studies were identified through a manual search of the reference list of previous review studies.
Eligibility criteria
We included original research studies that reported the relapse rate of the MS population in different seasons or months. Non-English studies, case reports, case series, and letters were excluded.
Study selection
First, the title and abstract of the studies were reviewed by two independent investigators (M.Y, K.M) to identify relevant citations. Then the same reviewers screened the full text of identified citations for final selection.
Data extraction
The following data were extracted by the two investigators (M.Y, K.M): patient demographics, study design, follow-up duration, number of patients with MS, type of MS, EDSS score, and the relapse rate in different seasons or months.
Quality assessment
The Newcastle–Ottawa scale (NOS) was used as a tool for assessing the quality of the included studies. The quality of included studies was assessed in three domains including selection, comparability, and outcome using NOS which ranges from 0 to 8 [28].
Statistical analysis
We used a risk ratio (RR) with a 95% confidence interval (CI) to assess the risk of relapse in each season or month compared to other seasons or months. To do this, we compare the risk of relapse in a specific month or season versus total all other months or seasons. To measure the heterogeneity, Cochrane’s Q test and I-squared (I2) were used. A P value smaller than (< 0.10) and the I2 value > 75% represent high heterogeneity. The random effects model was applied in statistical models. We used Stata 11.0 (College Station, TX) to perform statistical analysis.
Results
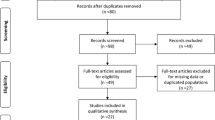
In total, our literature search and manual addition yielded 1121 articles. After title and abstract screening, 104 studies remained (Fig. 1). Finally, 24 studies entered our systematic review and meta-analysis after a full-text review [18,19,20,21,22,23,24, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. A total of 29,106 patients with MS were included in our study (Table 1). Among included citations, 14 were retrospective, two prospective, and two case–controls. Two papers were multicenter studies, three were from Italy, two from the UK, two from Iran, two from Italy, and one each from Japan, Brazil, Cuba, France, Germany, South Korea, Serbia, Israel, Ireland, Scotland, Portugal, Argentina, and Canada. The quality assessment showed that the mean score of NOS was 7.29.
Meta-analysis
Our analysis showed that the relapse rate is significantly lower in fall compared to the average relapse rate in other seasons with a RR of 0.97 (95% CI 0.95–0.98, Q value = 34.12, P = 0.06, I2 = 0.02%) (Figs. 2 and 3). Furthermore, there was no statistically significant higher or lower risk of relapse in total other seasons (Supplementary 1).
Evaluating the risk of relapse in different months revealed that MS patients experienced a higher number of relapses in April (RR: 1.06, 95% CI 1.01–1.11, Q value = 30.18, P = 0.05, I2 = 40.74%) and March (RR: 1.08, 95% CI 1.00–1.16, Q value = 49.16, P = 0.00, I2 = 74.37%) compared to total relapse rate in other months (Figs. 4 and 5). Also, it seems that the risk of relapse is lower in August (RR: 0.92, 95% CI 85–0.98, Q value = 38.25, P = 0.01, I2 = 65.87%), September (RR: 0.97, 95% CI 94–0.99, Q value = 28.01, P = 0.08, I2 = 0.00%), October (RR: 0.92, 95% CI 89–0.96, Q value = 18.82, P = 0.40, I2 = 19.68%), and November (RR: 0.93, 95% CI 89–0.97, Q value = 23.66, P = 0.15, I2 = 24.31%) (Fig. 6). There was no statistically significant difference in risk of relapse in other months versus the total average in all other months (Supplementary 2).
Discussion
It is evident that several environmental factors, including climate and latitude, play a role in the development and activity of MS [6, 46]. Thus, relapse rates in MS may also be subject to seasonal and monthly variations. Studies have found conflicting results regarding this matter, and a more comprehensive investigation is needed before a definitive conclusion can be made. In this systematic review and meta-analysis, we included 24 studies to evaluate the seasonal and monthly fluctuations in the frequency of relapses. Jin et al. performed a meta-analysis on seasonal variation in relapse rate among MS patients [47]. However, our study is the first systematic review and meta-analysis that investigates the seasonal and monthly patterns of relapses based on a comprehensive review of the previous literature.
According to our study, fall shows a significantly lower risk of relapse than other seasons. With a minimum heterogeneity, this finding is in line with most of the existing literature. Moreover, this meta-analysis reveals that relapse rates vary by month, ranging from higher rates in April and March to lower rates in August, September, October, and November compared to other months.
In the previous literature, MS has been frequently associated with seasonal patterns. Although the exact mechanisms underlying seasonal variations in MS outcomes remain unclear, several theories have been proposed to explain this phenomenon. These theories include fluctuations in vitamin D levels, melatonin levels, infections, sodium consumption, and air pollution. Each of these factors is proven to be an independent factor affecting MS relapses, and their potential role in the observed temporal pattern will be discussed later.
Further to MS relapses, other similar neuroinflammatory disorders, including Neuromyelitis Optica Spectrum Disorder (NMOSD), also demonstrate seasonal patterns, suggesting that the seasonal changes could probably have roles in the pathophysiology of these diseases [43, 48]. Interestingly, an association has been observed between the month of birth and MS prevalence [49]. Children born in the winter months are less likely to develop MS later in their life [50]. The epigenetic effects of vitamin D in certain months of gestation might explain this correlation [51]. Moreover, MS is a neuroinflammatory disease affected by the release of cytokines, which also follow a seasonal pattern [52].
Vitamin D levels have been the focus of most studies among the aforementioned potential factors. Excluding supplementary multivitamin consumption, the most crucial factor in serum vitamin D levels is sun exposure, affecting vitamin D levels more than the dietary intake. Vitamin D exerts anti-inflammatory effects by influencing T helper 17 (Th17) cells, IL17 pathways [53], and IFN-beta activity [54, 55], all playing an essential role in the pathophysiology of MS, and are targets for immunotherapies. Several studies have confirmed the effect of vitamin D on MS, stating that higher vitamin D levels are associated with lower incidence and better prognosis of MS [56, 57]. similar findings are observed in neuroimaging studies [58, 59]. Furthermore, in animal studies, vitamin D demonstrates a protective effect on autoimmune encephalitis, an animal model for MS [60]. However, this theory is subject to debate. In most studies, MS relapses are less frequent in autumn and winter, during which sun exposure and vitamin D levels are lower compared to other seasons. This observation is called the “seasonal paradox” [61]. To explain this phenomenon, some researchers state that MS seasonality is mainly attributed to factors other than vitamin D, including melatonin levels [61]. Another theory is that there is a time lag between the maximum and the minimum sun exposure and its effect on MS activity, and the length of this lag varies between 30 and 60 days depending on the geographical latitude [43]. According to this theory, maximum sun exposure occurs in midsummer, creating a protective effect in late summer and early autumn. On the other hand, the minimum sun exposure in winter triggers relapses in early spring. The results of our meta-analysis are more consistent with the second theory.
Moreover, it has been observed that sun exposure itself is a factor affecting MS activity, independent of vitamin D levels [62]. Thus, both vitamin D and non-vitamin D pathways seem to be involved in the protective effects of sun exposure. As stated before, another possible reason for the seasonality is the melatonin level, which is controlled by night darkness duration. Melatonin follows a seasonal pattern, peaking in autumn and winter, and is an independent factor in MS exacerbations and their seasonality [61]. Unlike vitamin D levels, there is no time lag or seasonal paradox in the association between melatonin levels and MS relapses [63]. Melatonin is also associated with lower autoimmune encephalitis activity by regulating Th17 and regulatory T cells type 1 (Treg1) [61].
Furthermore, there is a positive relationship between MS exacerbations and infectious diseases, especially upper respiratory tract infections [64]. Minor viral infections of the upper respiratory tract can trigger MS relapses and are more common in winter [49, 65]. Another possible factor is NaCl ingestion, which affects Th17 cells and might trigger autoimmune diseases [66]. There is evidence of a seasonal pattern in sodium consumption, with a peak in winter [67]. MS has also been linked to air pollution [68]. Particulate matter and NOx pollution are associated with MS relapses and are higher in winter [45, 69], which could be one of the potential explanations for the observed seasonal pattern in the relapses.
Limitations
Our study has some limitations: First, the absence of a specified definition for relapse is potentially a significant source of bias in our results. Of 24 studies included in this meta-analysis, some were retrospective and relied on self-reported data with regard to (or to identify) the relapses, which brings a risk of recall bias, while others relied on physician reports. Second, the geographic diversity among the study locations is another potential confounding factor in our meta-analysis, which can affect the accuracy of our findings. Studies conducted in various locations have demonstrated different relapse patterns based on environmental and climatic factors. According to a multinational study, the temporal variation in the frequency of MS relapses also depends on the latitude of the study center [43]. Due to a low number of studies performing the sub-group analysis based on climate region or northern hemisphere or southern hemisphere was impossible. Thus, future studies should be performed to give a better understanding by adjusting for climate regions. Another limitation was the lack of complete details on the treatments used by the patients and also, several included studies did not report the type of MS among the participating individuals.
Conclusion
Our systematic review and meta-analysis confirm the temporal fluctuations in the relapse of MS through a comprehensive review of the existing literature, with a lower relapse rate during late summer and fall and a higher relapse rate during early spring. Several explanations have been proposed to influence the seasonal patterns observed in MS outcomes, including sun exposure, vitamin D levels, melatonin levels, air pollutants, allergens, and viral infections. However, further studies are required to determine the effect of each factor on the seasonal pattern of MS relapses.
References:
Nabizadeh F, Pirahesh K, Rafiei N, Afrashteh F, Ahmadabad MA, Zabeti A et al (2022) Autologous hematopoietic stem-cell transplantation in Multiple Sclerosis: a systematic review and meta-analysis. Neurol Ther. https://doi.org/10.1007/s40120-022-00389-x
Nabizadeh F, Ramezannezhad E, Kazemzadeh K, Khalili E, Ghaffary EM, Mirmosayyeb O (2022) Multiple sclerosis relapse after COVID-19 vaccination: a case report-based systematic review. J Clin Neurosci 104:118–125
van der Mei I, Lucas RM, Taylor BV, Valery PC, Dwyer T, Kilpatrick TJ et al (2016) Population attributable fractions and joint effects of key risk factors for multiple sclerosis. Mult Scler 22(4):461–469
Dardiotis E, Nousia A, Siokas V, Tsouris Z, Andravizou A, Mentis AA et al (2018) Efficacy of computer-based cognitive training in neuropsychological performance of patients with multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord 20:58–66
Klineova S, Lublin FD (2018) Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med 8:a028928
Olsson T, Barcellos LF, Alfredsson L (2017) Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol 13(1):25–36
Munger KL, Bentzen J, Laursen B, Stenager E, Koch-Henriksen N, Sørensen TI et al (2013) Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler 19(10):1323–1329
Katz SI (2018) The role of diet in Multiple Sclerosis: mechanistic connections and current evidence. Curr Nutr Rep 7(3):150–160
Bronson FH (2004) Are humans seasonally photoperiodic? J Biol Rhythms 19(3):180–192
de Castro JM (1991) Seasonal rhythms of human nutrient intake and meal pattern. Physiol Behav 50(1):243–248
Gordon DJ, Trost DC, Hyde J, Whaley FS, Hannan PJ, Jacobs DR Jr et al (1987) Seasonal cholesterol cycles: the lipid research clinics coronary primary prevention trial placebo group. Circulation 76(6):1224–1231
Brennan PJ, Greenberg G, Miall WE, Thompson SG (1982) Seasonal variation in arterial blood pressure. Br Med J (Clin Res Ed) 285(6346):919–923
Kasahara AK, Singh RJ, Noymer A (2013) Vitamin D (25OHD) serum seasonality in the United States. PLoS ONE 8(6):e65785
Iikuni N, Nakajima A, Inoue E, Tanaka E, Okamoto H, Hara M et al (2007) What’s in season for rheumatoid arthritis patients? Seasonal fluctuations in disease activity. Rheumatol (Oxford) 46(5):846–848
Moltchanova EV, Schreier N, Lammi N, Karvonen M (2009) Seasonal variation of diagnosis of Type 1 diabetes mellitus in children worldwide. Diabet Med 26(7):673–678
Fisman DN (2007) Seasonality of infectious diseases. Annu Rev Public Health 28:127–143
Dopico XC, Evangelou M, Ferreira RC, Guo H, Pekalski ML, Smyth DJ et al (2015) Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun 6:7000
Bamford CR, Sibley WA, Thies C (1983) Seasonal variation of multiple sclerosis exacerbations in Arizona. Neurology 33(6):697–701
Damasceno A, Von Glehn F, de Deus-Silva L, Damasceno BP (2012) Monthly variation of multiple sclerosis activity in the southern hemisphere: analysis from 996 relapses in Brazil. Eur J Neurol 19(4):660–662
Disanto G, Handel AE, Morahan JM, Deluca GC, Kimball SM, Hypponen E et al (2011) Vitamin D and multiple sclerosis hospital admissions in Scotland. QJM 104(11):1001–1003
Fonseca AC, Costa J, Cordeiro C, Geraldes R, De Sá J (2009) Influence of climatic factors in the incidence of multiple sclerosis relapses in a Portuguese population. Eur J Neurol 16(4):537–539
Ogawa G, Mochizuki H, Kanzaki M, Kaida K, Motoyoshi K, Kamakura K (2004) Seasonal variation of multiple sclerosis exacerbations in Japan. Neurol Sci 24(6):417–419
O’Reilly MA, O’Reilly PM (1991) Temporal influences on relapses of multiple sclerosis. Eur Neurol 31:391–395
Salvi F, Bartolomei I, Smolensky MH, Lorusso A, Barbarossa E, Malagoni AM et al (2010) A seasonal periodicity in relapses of multiple sclerosis? A single-center, population-based, preliminary study conducted in Bologna, Italy. BMC Neurol. https://doi.org/10.1186/1471-2377-10-105
Meier DS, Balashov KE, Healy B, Weiner HL, Guttmann CR (2010) Seasonal prevalence of MS disease activity. Neurology 75:799–806
Pierrot-Deseilligny C, Souberbielle JC (2017) Vitamin D and multiple sclerosis: an update. Mult Scler Relat Disord 14:35–45
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Lo CK-L, Mertz D, Loeb M (2014) Newcastle-ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 14:45
Byun S, Myung W, Kim H, Lee H (2020) Association between diurnal temperature range and emergency department visits for multiple sclerosis: a time-stratified case-crossover study. Sci Total Environ 720:137565
CarneroContentti E, Lopez PA, Pettinicchi JP, Criniti J, Pappolla A, Miguez J et al (2021) Seasonal variation in attacks of neuromyelitis optica spectrum disorders and multiple sclerosis: evaluation of 794 attacks from a nationwide registry in Argentina. Mult Scler Relat Disord 58:103466
Fundora-Hernández H, Dorta-Contreras AJ, Socias-López M, Fraga-Santana S, Villatoro-Rodríguez SN, Padilla-Docal B et al (2009) Seasonal distribution and evolving forms of multiple sclerosis patients diagnosed from April 2004 to November 2007. Arq Neuro-Psiquiatr 67:661–663
Goodkin DE, Hertsgaard D (1989) Seasonal variation of multiple sclerosis exacerbations in North Dakota. Arch Neurol 46:1015–1018
Harding K, Tilling K, MacIver C, Willis M, Joseph F, Ingram G et al (2017) Seasonal variation in multiple sclerosis relapse. J Neurol 264:1059–1067
Hopkins CE, Swank RL (1955) Multiple sclerosis and the local weather: a four-year study of correlation between multiple sclerosis exacerbation and local weather factors in montreal. Arch Neurol Psychiatry 74:203–207
Iuliano G (2012) Multiple sclerosis: Long time modifications of seasonal differences in the frequency of clinical attacks. Neurol Sci 33:999–1003
Iuliano G, Boz C, Cristiano E, Duquette P, Lugaresi A, Oreja-Guevara C et al (2013) Historical changes of seasonal differences in the frequency of multiple sclerosis clinical attacks: a multicenter study. J Neurol 260(5):1258–1262
Koziol JA, Feng AC (2004) Seasonal variations in exacerbations and MRI parameters in relapsing-remitting multiple sclerosis. Neuroepidemiology 23(5):217–223
Miclea A, Miclea M, Pistor M, Hoepner A, Chan A, Hoepner R (2017) Vitamin D supplementation differentially affects seasonal multiple sclerosis disease activity. Brain Behav 7:e00761
Saaroni H, Sigal A, Lejbkowicz I, Miller A (2010) Mediterranean weather conditions and exacerbations of multiple sclerosis. Neuroepidemiology 35(2):142–151
Schapira K (1959) The seasonal incidence of onset and exacerbations in multiple sclerosis. J Neurol Neurosurg Psychiatry 22:285–286
Shafa MA, Ebrahimi HA, Khanjani N (2014) A study of the seasonal incidence of multiple sclerosis attacks in Kerman. Iran J Kerman Univ Med Sci 21(5):376–383
Sistani SS, Moghtaderi A, Dashipoor AR, Ghaffarpoor M, Ghahderijani BH (2019) Seasonal variations of 25-OH vitamin D serum levels in multiple sclerosis patients with relapse using MRI. Eur J Transl Myol 29(3):268–275
Spelman T, Gray O, Trojano M, Petersen T, Izquierdo G, Hupperts R et al (2014) Seasonal variation of relapse rate in multiple sclerosis is latitude dependent. Ann Neurol 76(6):880–890
Tataru N, Vidal C, Decavel P, Berger E, Rumbach L (2006) Limited impact of the summer heat wave in France (2003) on hospital admissions and relapses for multiple sclerosis. Neuroepidemiology 27(1):28–32
Vojinović S, Savić D, Lukić S, Savić L, Vojinović J (2015) Disease relapses in multiple sclerosis can be influenced by air pollution and climate seasonal conditions. Vojnosanit Pregl 72(1):44–49
Simpson S, Blizzard L, Otahal P, Van der Mei I, Taylor B (2011) Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry 82(10):1132
Jin Y, de Pedro-Cuesta J, Söderström M, Stawiarz L, Link H (2000) Seasonal patterns in optic neuritis and multiple sclerosis: a meta-analysis. J Neurol Sci 181(1–2):56–64
Chang VTW, Chang HM (2020) Review: Recent advances in the understanding of the pathophysiology of neuromyelitis optica spectrum disorder. Neuropathol Appl Neurobiol 46(3):199–218
Watad A, Azrielant S, Soriano A, Bracco D, Abu Much A, Amital H (2016) Association between seasonal factors and multiple sclerosis. Eur J Epidemiol 31(11):1081–1089
Dobson R, Giovannoni G, Ramagopalan S (2013) The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatry 84(4):427
Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC (2005) Timing of birth and risk of multiple sclerosis: population based study. BMJ 330(7483):120
Killestein J, Rep MHG, Meilof JF, Adèr HJ, Uitdehaag BMJ, Barkhof F et al (2002) Seasonal variation in immune measurements and MRI markers of disease activity in MS. Neurology 58(7):1077
Toghianifar N, Ashtari F, Zarkesh-Esfahani SH, Mansourian M (2015) Effect of high dose vitamin D intake on interleukin-17 levels in multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. J Neuroimmunol 285:125–128
Stewart N, Simpson S, van der Mei I, Ponsonby A-L, Blizzard L, Dwyer T et al (2012) Interferon-β and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 79(3):254
Bianchi N, Emming S, Zecca C, Monticelli S (2020) Vitamin D and IFN-β modulate the inflammatory gene expression program of primary human T lymphocytes. Front Immunol. https://doi.org/10.3389/fimmu.2020.566781
Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL et al (2010) Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol 67(5):618–624
Simpson S Jr, Taylor B, Blizzard L, Ponsonby A-L, Pittas F, Tremlett H et al (2010) Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol 68(2):193–203
Zivadinov R, Treu CN, Weinstock-Guttman B, Turner C, Bergsland N, Connor K et al (2013) Interdependence and contributions of sun exposure and vitamin D to MRI measures in multiple sclerosis. J Neurol Neurosurg Psychiatry 84(10):1075
Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR et al (2012) Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol 72(2):234–240
Pedersen LB, Nashold FE, Spach KM, Hayes CE (2007) 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res 85(11):2480–2490
Farez Mauricio F, Mascanfroni Ivan D, Méndez-Huergo Santiago P, Yeste A, Murugaiyan G, Garo Lucien P et al (2015) Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162(6):1338–1352
Lucas RM, Ponsonby AL, Dear K, Valery PC, Pender MP, Taylor BV et al (2011) Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 76(6):540
YeganehSalehpour M, Mollica A, Momtaz S, Sanadgol N, Farzaei MH (2019) Melatonin and Multiple Sclerosis: from plausible neuropharmacological mechanisms of action to experimental and clinical evidence. Clin Drug Investig 39(7):607–624
Tremlett H, van der Mei IAF, Pittas F, Blizzard L, Paley G, Mesaros D et al (2008) Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology 31(4):271–279
Sibley W, Bamford C, Clark K (1985) Clinical viral infections and multiple sclerosis. Lancet 325(8441):1313–1315
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA et al (2013) Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496(7446):518–522
Marti-Soler H, Pommier C, Bochud M, Guessous I, Ponte B, Pruijm M et al (2017) Seasonality of sodium and potassium consumption in Switzerland. Data from three cross-sectional, population-based studies. Nutr Metab Cardiovasc Dis 27:792–798
Farahmandfard MA, Naghibzadeh-Tahami A, Khanjani N (2021) Ambient air pollution and multiple sclerosis: a systematic review. Rev Environ Health 36(4):535–544
Peng RD, Dominici F, Pastor-Barriuso R, Zeger SL, Samet JM (2005) Seasonal analyses of air pollution and mortality in 100 US Cities. Am J Epidemiol 161(6):585–594
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nabizadeh, F., Valizadeh, P., Yazdani Tabrizi, M. et al. Seasonal and monthly variation in multiple sclerosis relapses: a systematic review and meta-analysis. Acta Neurol Belg 122, 1447–1456 (2022). https://doi.org/10.1007/s13760-022-02103-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-022-02103-y