Abstract
Season of birth is considered to be associated with multiple sclerosis (MS) although some findings opposing to this assumption raise doubts about the seasonality pattern in MS births. The present work synthesizes the evidence of previous published studies aiming at examining whether the month of birth is associated with a higher number of MS births. Pubmed and Scopus were systematically searched and a multivariate meta-analysis of case–control studies was conducted. Data of healthy controls births were retrieved from census reports when not included in the studies. For comparisons, October was set as a reference month and autumn (September–October–November) as a reference season. The meta-analysis included studies that provided the number of MS births for each month or season. Twenty-two eligible studies were included in the meta-analysis involving twenty-four different populations and overall 145,672 MS patients and 75,169,550 healthy controls. The multivariate analysis supports that MS births in spring are higher compared to autumn [odds ratio (OR) 1.14, 95% confidence interval (CI) 1.04, 1.24]. Univariate analyses confirm the same for April (OR 1.12, 95% CI 1.05, 1.21), March (OR 1.05, 95% CI 1.00, 1.11) and May (OR 1.07, 95% CI 1.00, 1.14). A reduction of MS births was found in November (OR 0.96, 95% CI 0.93, 0.99). The month and the season of birth are significantly associated with MS births.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is an inflammatory disorder of the brain and spinal cord that damages myelin (the protective sheath that covers nerve fibers) and axons [1]. This damage causes communication problems between the brain and the rest of the body, resulting in a wide range of signs and symptoms including motor, sensory, visual and autonomic systems. MS is a complex disease of the central nervous system that just in the last decades has given full clinico-pathological characterization whereas its etiology remains unclear [1].
Previous studies suggest that the etiology of MS involves genetic susceptibility, lifestyle, environmental exposure and their interactions [2]. Heritability has been found to increase the relative risk of MS [3, 4]. The HLA complex genes and especially the HLA class I and II genes are most commonly associated with the incidence of MS [2]. Smoking and passive smoking, Epstein–Barr virus (EBV) infection and infectious mononucleosis, night shift work, obesity during adolescent, organic solvent exposure, vitamin D level < 50 nM and low sun exposure have been also suggested to be associated with MS development [2] while smoking has been found to interact with HLA risk alleles [5].
Maternal sun exposure in pregnancy and the month of birth in MS patients have significantly attracted the scientific interest. Evidence shows that people born in spring or in April have increased risk of developing MS later in life [6,7,8]. On the other hand, there are studies supporting that September [9] and December [10] are associated with MS development or studies failing to find any association between month or season of birth and MS development [11, 12]. A relatively recent systematic review and meta-analysis on this topic [13] concluded that people born in April have an increased risk of MS. Contrariwise, October or November presented a reduced risk of MS. These findings altered when analysis performed in studies conducted in latitudes lower than 52°. Nevertheless, the studies included in the meta-analysis had to be published after 2000, to present data of both MS patients and healthy controls and to provide the relative risk of MS for each month of birth compared to the healthy control population. The effect sizes were calculated using the observed and expected MS birth rate for each month. Overall, only ten studies were included in the meta-analysis.
The present systematic review and meta-analysis aims to provide a synthesis of the available evidence on the association of month and season of birth with MS. A multivariate meta-analytic approach was adopted providing effect size estimates and considering the potential pairwise correlations between them.
Materials and methods
Eligibility criteria, information sources and search strategy
The current systematic review and meta-analysis follows PRISMA statement [14]. Eligible studies were those providing the number of MS births in each month or season.
Pubmed and Scopus databases were systematically searched through August 2018 using the following search terms: season of birth, seasonal birth, birthdate, month of birth, timing of birth, and multiple sclerosis. The query of the Pubmed search was ("season of birth" OR "seasonal birth" OR birthdate OR "month of birth" OR "timing of birth") AND ("multiple sclerosis"). Duplicate records on the two databases were removed. Titles and abstracts were screened to exclude the irrelevant articles. Full-text versions of the remaining records were retrieved and verified for eligibility. The references of the eligible studies were also scrutinized to identify additional studies for inclusion. Studies were considered in the meta-analysis when they included sufficient data and examined different populations. In case of suspected duplicate populations, the study involved in the meta-analysis was the one with the largest number of MS births. There were no imposed restrictions related to the language or the quality of the studies.
Data extraction
Data were extracted by two independent reviewers (K.P. and P.B.) who retrieved and examined the articles. Potential discrepancies were resolved by consensus. The retrieved data, if available, included first author’s last name, year of publication, country, city and latitude of population studied, MS patients’ and controls’ characteristics, and the number of MS patients’ and controls’ births in each month or season. There were no study exclusions for low-quality data while quality scoring was not performed [14, 15] to avoid selection bias and subjective assignments of points. Instead we performed subgroup and sensitivity analysis as suggested for reporting in meta-analyses [16].
Additional data and processing
The seasonal number of births of MS patients and controls were estimated by the monthly data. Seasons were defined as winter (December, January, February), spring (March, April, May), summer (June, July, August) and autumn (September, October, November) according to the UK's official weather service definition [17]. For countries in the southern hemisphere, data were adjusted according to warm and cold periods of the northern hemisphere. Data were extracted from graphs where necessary.
When latitudinal data or birth data of controls were not provided, other sources were searched to determine the missing data. Google maps (https://www.maps.google.com) was used to estimate the mean latitude of a country or an extent geographical area. The monthly or seasonal number of births in the general population was retrieved by national statistics services and in accordance with the period of MS patient births where possible. Data of climate annual dry bulb temperature (Tair, °C) and annual mean sunshine duration (tsun, h) were calculated by summing up the respective monthly values and were retrieved mainly from online databases [18,19,20]. The tsun was used as a measure of individual’s exposure to sunlight, to consider the cloudiness of each region.
Statistical analysis
The odds ratio (OR) along with the 95% confidence interval (95% CI) was used as a measure of effect to compare the number of MS births (cases) and control births (general population). A multivariate method for meta-analysis was adopted using the method of moments [21]. October and autumn (September–October–November) were chosen as reference month or season. Previously published studies have reported a lower proportion of MS births in October or spring than in the other months or seasons [13]. Given that all the estimates, i.e., ORs were compared against the same baseline group (October, autumn), we also considered the potential pairwise correlations between them [22].
Table 1 presents the layout of the data. The details of the method used in the present meta-analysis are provided as supplementary material.
The Wald test was used to test whether any of the log(ORs) is significantly different from zero [23]. Meta-regression was used to examine the possible relationship between the estimated risk and longitudinal or climate factors [24].
Univariate methods of meta-analysis were applied to detect the between-studies percentage of variability and publication bias. Each month or season was compared to all the others (i.e., April versus all other months or spring versus all other seasons). Heterogeneity was evaluated by the inconsistency index (I2) (ranging between 0 and 100%). Publication bias was detected based on Egger’s statistical test [25]. Cumulative meta-analysis was used to examine the potential time trend of the combined estimates over the years [26] using the 'first vs. subsequent' method [27] and generalized least squares (GLS) regression-based test [28]. The impact of each study on the overall meta-analysis estimate was examined by sensitivity analysis.
The analysis was performed using Stata 13 (Stata Corporation, College Station, Texas, USA) and the command mvmeta for multivariate meta-analysis and meta-regression. Statistical significance was set at p < 0.05.
Results
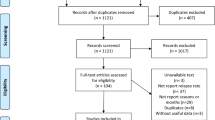
The literature search in Pubmed and Scopus databases generated 80 and 82 results, respectively (Fig. 1). Sixteen papers were additionally identified through the references of articles. After excluding duplicates, the titles and abstracts of 80 articles were screened and 49 full-text articles were assessed for eligibility. Overall, 22 studies were included in the meta-analysis involving 145,672 MS births and 75,169,550 control births of 24 different populations. Of these, fourteen were European and ten non-European populations. The studies of Becker et al. [29] and Fragoso et al. [30] involved populations of the southern hemisphere. Fourteen studies were conducted in latitudes higher than 52°N and eight in lower than 52°N. Two studies presented seasonal data only [31, 32] and one missed data of autumn months [11]. Table S1 (supplementary material) presents information of the studies included in the meta-analysis. Overall, monthly analysis included 19 studies involving 21 different populations. Seasonal analysis included 22 studies involving 24 different populations.
Monthly (Wald test: p value = 0.001) and seasonal (Wald test: p value = 0.007) multivariate meta-analyses using October and autumn as reference month and season produced significant results (Fig. 2). MS births were found to be associated with April (OR 1.12, 95% CI 1.05, 1.21) and spring (OR 1.14, 95% CI 1.04, 1.24). The results were marginally significant indicating also an association with March (OR 1.05, 95% CI 1.00, 1.11), May (OR 1.07, 95% CI 1.00, 1.14) and summer (OR 1.08, 95% CI 1.00, 1.17). Lower risk of MS compared to October was found in November (OR 0.96, 95% CI 0.93, 0.99).
Subgroup analysis by hemisphere or among European and non-European populations yielded similar results with the overall analysis. Exceptions were the northern hemisphere group (OR 1.05, 95% CI 1.00, 1.09; Wald test p = 0.002) and the European populations (OR 1.06, 95% CI 1.00, 1.13; Wald test p < 0.001) in which a marginally significant association was found additionally for June. The results related to the MS births in April (OR 1.11, 95% CI 1.03, 1.19; Wald test p = 0.0013) remained the same when populations in latitudes > 52° were considered. The MS births in November were found to be related to a lower risk of MS development (OR 0.94, 95% CI 0.89, 0.99; Wald test p = 0.0115) at latitudes < 52°.
Meta-regression produced significant effects of Tair in the contrasts of winter (p = 0.042) and summer (p = 0.031) versus autumn (tsun and latitude yield non-significant results). Considering separately studies conducted in populations of the northern hemisphere, we found a significant effect of latitude, Tair and tsun on the number of MS births during all three seasons (− 0.02 ≤ ß ≤ 0.009, 0.004 ≤ p ≤ 0.045). Figure 3 presents the odds ratios for the comparison of MS births in the northern hemisphere in spring versus autumn weighted with the inverse proportion of the variance of an observation in relation to latitude (Fig. 3a), Tair (Fig. 3b) and tsun (Fig. 3c). The meta-regression performed on populations in latitudes > 52° showed an effect of tsun on births during all three seasons (winter ß = − 0.0008, p = 0.023; spring ß = − 0.0091, p = 0.008; summer ß = − 0.0086, p = 0.018).
Logarithm of the odds ratio (LogOR) for the comparison of the number of MS births in populations of the northern hemisphere in spring versus autumn and weighted with the inverse proportion of the variance of an observation versus a latitude, b climate annual dry bulb temperature (Tair, °C) and c annual mean sunshine duration (h)
Based on univariate meta-analysis and the random effect model, an MS patient was 1.11 times (OR 1.11, 95% CI 1.05, 1.18) more likely to be born in April than in the other months (Fig. 4a). None of the individual studies were found to be responsible for this association as indicated by the sensitivity analysis. The I2 of heterogeneity was estimated to be 83.0% (p < 0.001). The Egger’s test failed to detect a publication bias producing non-statistically significant results for the contrast of April versus all other months (p = 0.072). Cumulative meta-analysis indicated a potential trend over time that was confirmed by 'first vs. subsequent' method and GLS regression-based test produced statistically significant trend (p < 0.001).
The contrast November versus all the other months (Fig. 4b) yielded a statistically significant OR 0.93 (95% CI 0.89, 0.97). This result remained unchanged when sensitivity analysis was performed. The heterogeneity observed between the studies was moderate (I2 = 55.5%, p = 0.001). Egger’s test suggested an absence of publication bias (p = 0.181). A time trend was detected according to 'first vs. subsequent' method (p = 0.001) and GLS regression-based test (p = 0.002).
Considering seasonal analysis, univariate meta-analysis showed that a MS patient was 1.06 times (OR 1.08, 95% CI 1.03, 1.14) more likely to be born in spring than all the other seasons (Fig. 5). Sensitivity analysis showed that there is no single study to change this association. The heterogeneity was high (I2 = 90.2%, p < 0.001). Heterogeneity was slightly decreased when only European populations (I2 = 86.7%, p < 0.001) or populations in the northern hemisphere (I2 = 89.3% p < 0.001) were considered. No evidence of publication bias was detected (p = 0.271). A time trend was identified according to both 'first vs. subsequent' method (p = 0.002) and the GLS regression-based test (p < 0.001), suggesting that earlier studies probably over-estimated the effect.
The results of univariate meta-analysis for March and May versus all the other months and summer versus all the other seasons were non-statistically significant.
Discussion
Evidence from twenty-two studies was combined aiming to examine the relationship between month of birth and MS. The results suggest that people born in April or in spring are more likely to develop MS later in life. This relationship became weaker when two studies conducted in the southern hemisphere were involved in the meta-analysis. A significant decrease in births of MS patients was found in November. A marginally significant result was found for March, May and summer that seems to be attributed to populations in latitudes > 52°.
The effect of season of birth was related to latitude, climate annual dry bulb temperature and sunshine duration in the northern hemisphere. This effect was limited to sunshine duration for populations in latitudes > 52°.
Univariate analysis showed that the overall effect was relatively small, and the heterogeneity was from moderate to high which, however, at least partly could be explained by latitude, climate annual dry bulb temperature and sunshine duration variations. No publication bias was detected. Excluding studies one at a time and repeating the meta-analysis, no single study to change the results was found. These results remained the same when European and non-European populations were separately examined for studies conducted in the northern hemisphere or in latitudes > 52°. A significant increase in MS births in April and a decrease in November were also found in the study of Dobson et al. who followed a different method of meta-analysis.
Given that lower sun exposure and consequently lower status of vitamin D is strongly suggested to be related to MS development [33, 34], low maternal sun exposure could be associated with MS development. Vitamin D levels and sun exposure are seasonally varied and intercorrelated making it difficult to determine the effect of each one on the incidence of MS. Vitamin D lowest levels are reached during late winter while early spring is associated with increased activity, clinical severity and relapse rates of various autoimmune diseases including MS [35]. A review on vitamin D, ultraviolet radiation and MS suggests that past high sun exposure may decrease the risk and the severity of MS probably through both vitamin D and non-vitamin D pathways [34]. The latitude has been positively related to MS prevalence among European populations although exceptions existed among Italians and northern Scandinavian populations [36]. This was attributed to gene–environment interactions [36] or some other factors related to latitude and ultraviolet radiation, i.e., temperature [34] highlighting the importance of considering the environmental factors in the incidence of MS.
This work has limitations. The attempt to exclude duplicate data could not be fully successful. The births of MS patients—cases—and general population—controls—were not fully matched for year of birth or regional origin [37]. Only two studies were included in the meta-analysis from the southern hemisphere highlighting the disproportionately high rate of studies conducted in the northern hemisphere. Finally, the exposure to sunlight is related to the duration and type of outdoor activity apart from the sunshine duration. Thus, annual mean sunshine duration could be a crude measure of exposure to sunlight.
Nevertheless, this study summarizes the evidence of the published literature. We conducted a thorough search to identify all eligible studies in the published literature. We neither excluded any study based on the design nor imposed any quality restriction as most recent guidelines advocate against this. An advanced meta-analytic technique was used to produce reliable and robust results.
In conclusion, this meta-analysis supports that the month and season of birth is associated with increased births of MS patients. However, further investigation is needed involving matched samples and new studies at different locations and latitudes, especially in the southern hemisphere. The determination of the environmental factors affecting MS development is indisputably important since it may contribute to the development of preventive measure to decrease the incidence of MS.
References
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517. https://doi.org/10.1016/S0140-6736(08)61620-7
Olsson T, Barcellos LF, Alfredsson L (2017) Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol 13:26–36. https://doi.org/10.1038/nrneurol.2016.187
O’Gorman C, Lin R, Stankovich J, Broadley SA (2013) Modelling genetic susceptibility to multiple sclerosis with family data. Neuroepidemiology 40:1–12. https://doi.org/10.1159/000341902
Westerlind H, Ramanujam R, Uvehag D et al (2014) Modest familial risks for multiple sclerosis: a registry-based study of the population of Sweden. Brain 137:770–778. https://doi.org/10.1093/brain/awt356
Hedström AK, Sundqvist E, Bäärnhielm M et al (2011) Smoking and two human leukocyte antigen genes interact to increase the risk for multiple sclerosis. Brain 134:653–664. https://doi.org/10.1093/brain/awq371
Saastamoinen K, Auvinen M, Tienari PJ (2012) Month of birth is associated with multiple sclerosis but not with HLA-DR15 in Finland. Mult Scler J 18:563–568. https://doi.org/10.1177/1352458511426814
Grytten N, Torkildsen Ø, Aarseth JH et al (2012) Month of birth as a latitude-dependent risk factor for multiple sclerosis in Norway. Mult Scler J 19:1028–1034. https://doi.org/10.1177/1352458512471094
Balbuena LD, Middleton RM, Tuite-Dalton K et al (2016) Sunshine, sea, and season of birth: MS incidence in Wales. PLoS ONE 11:1–11. https://doi.org/10.1371/journal.pone
Tolou-Ghamari Z, Shygannejad V, Ashtari F et al (2015) Preliminary analysis of month of birth in Iranian/Isfahan patients with multiple sclerosis. Adv Biomed Res 14:166. https://doi.org/10.4103/2277-9175.162543
Akhtar S, Alroughani R, Al-shammari A et al (2015) Month of birth and risk of multiple sclerosis in Kuwait: a population-based registry study. Mult Scler J 21:147–154. https://doi.org/10.1177/1352458514541578
Eliasdottir O, Hildeman A, Longfils M, Lycke ONJ (2018) A nationwide survey of the influence of month of birth on the risk of developing multiple sclerosis in Sweden and Iceland. J Neurol 265:108–114. https://doi.org/10.1007/s00415-017-8665-y
Barros P, Marques J, Sá D, José M (2013) Month of birth and risk of multiple sclerosis in a Portuguese population. Clin Neurol Neurosurg 115:1762–1765. https://doi.org/10.1016/j.clineuro.2013.04.007
Dobson R, Giovannoni G, Ramagopalan S (2013) The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatry 84:427–432. https://doi.org/10.1136/jnnp-2012-303934
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341. https://doi.org/10.1136/bmj.b2535
Greenland S (1998) Meta-analysis. In: Rothman KJ, Greenland S (eds) Modern epidemiology. Lippincott Williams & Wilkins, Pennsylvania, pp 643–673.
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies: a proposal for reporting. J Am Med Assoc 283:2008–2012. https://doi.org/10.20959/wjpps20186-11420
Met Office (2018) Meteorological season. https://www.metoffice.gov.uk/learning/seasons/spring/when-does-spring-start. Accessed 31 Aug 2018
UNdata (2018) Sunshine. https://data.un.org/Data.aspx?d=CLINO&f=ElementCode%3A15. Accessed 30 Aug 2018
UNdata (2018) Dry bulb temperature. https://data.un.org/Data.aspx?q=temperature&d=CLINO&f=ElementCode%3A01. Accessed 3 Sep 2018
Weather (2018) Weatherbase. https://www.weatherbase.com. Accessed 3 Sep 2018
Jackson D, White IR, Thompson SG (2010) Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 29:1282–1297
Bagos PG (2012) On the covariance of two correlated log-odds ratios. Stat Med 31:1418–1431. https://doi.org/10.1002/sim.4474
Dimou NL, Pantavou KG, Bagos PG (2017) Apolipoprotein E polymorphism and left ventricular failure in beta-thalassemia: a multivariate meta-analysis. Ann Hum Genet 81:213–223. https://doi.org/10.1111/ahg.12203
White IR (2011) Multivariate random-effects meta-regression: updates to mvmeta. Stata J 11:255–270
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Lau J, Antman E, Jimenez-Silva J et al (1992) Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med 327:248–254
Ioannidis JPA, Trikalinos TA (2005) Early extreme contradictory estimates may appear in published research: the Proteus phenomenon in molecular genetics research and randomized trials. J Clin Epidemiol 58:543–549. https://doi.org/10.1016/j.jclinepi.2004.10.019
Bagos P, Nikolopoulos GK (2009) Generalized least squares for assessing trends in cumulative meta-analysis with applications in genetic epidemiology. J Clin Epidemiol 62:1037–1044
Becker J, Callegaro D, Lana-peixoto MA et al (2013) Season of birth as a risk factor for multiple sclerosis in Brazil. J Neurol Sci 329:6–10. https://doi.org/10.1016/j.jns.2013.03.001
Fragoso YD, Adoni T, Maria S et al (2013) Multiple sclerosis in South America: month of birth in different latitudes does not seem to interfere with the prevalence or progression of the disease. Arq Neuropsiquiatr 71:573–579. https://doi.org/10.1590/0004-282X20130098
Eskandari G, Ghajarzadeh M, Yekaninejad MS et al (2015) Comparison of serum vitamin D level in multiple sclerosis patients, their siblings, and healthy controls. Iran J Neurol 14:81–85
Fernandes de Abreu D, Babron M, Rebeix I et al (2009) Season of birth and not vitamin D receptor promoter polymorphisms is a risk factor for multiple sclerosis. Mult Scler J 15:1146–1152. https://doi.org/10.1177/1352458509106780
Rhead B, Bäärnhielm M, Gianfrancesco M et al (2016) Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet 2:e97. https://doi.org/10.1212/NXG.0000000000000097
Lucas R, Byrne SN, Correale J et al (2015) Ultraviolet radiation, vitamin D and multiple sclerosis. Neurodegener Dis Manag 5:413–424
Watad A, Azrielant S, Bragazzi NL et al (2017) Seasonality and autoimmune diseases: the contribution of the four seasons to the mosaic of autoimmunity. J Autoimmun 82:13–30. https://doi.org/10.1016/j.jaut.2017.06.001
Simpson S, Blizzard L, Otahal P et al (2011) Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry 82:1132–1141. https://doi.org/10.1136/jnnp.2011.240432
Fiddes B, Wason J, Kemppinen A et al (2013) Confounding underlies the apparent month of birth effect in multiple sclerosis. Ann Neurol 73:714–720. https://doi.org/10.1002/ana.23925
Acknowledgements
This research was implemented through IKY scholarships programme and co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the action entitled “Reinforcement of Postdoctoral Researchers”, in the framework of the Operational Programme “Human Resources Development Program, Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) 2014–2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The manuscript does not contain clinical studies or patient data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pantavou, K.G., Bagos, P.G. Season of birth and multiple sclerosis: a systematic review and multivariate meta-analysis. J Neurol 267, 2815–2822 (2020). https://doi.org/10.1007/s00415-019-09346-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09346-5