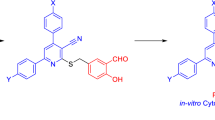
Abstract
GO-Fc@Fe3O4 nanohybrid as a powerful and reusable nanocatalyst was synthesized. The graphene oxide (GO) sheets with meta-chloroperoxybenzoic acid (mCPBA) were treatment, afterward were chemically modified with 4-Fc derivative through the ring-opening reaction between GO nanosheets and 4-ferrocenylbutylamine. Then, the final nanocatalyst was obtained by synthesizing of Fe3O4 nanoparticles onto the modified GO surface. The structure and morphology of GO-Fc@Fe3O4 nanohybrid were characterized using different analysis, such as FT-IR, XRD, FE-SEM, EDX, and VSM techniques. Then, 2-amino-3-cyano-4H-pyran derivatives (4a–l) as a synthetic precursor were synthesized via multi-component reaction in the presence of GO-Fc@Fe3O4 nanohybrid as a novel heterogeneous nanocatalyst. Finally, according to the importance of finding a solution to treat Alzheimer’s disease, 14-aryl-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amines (5a–l) as new tacrine-naphthopyran hybrid analogs were designed and prepared as acetylcholinesterase inhibitors. These compounds were synthesized via Friedländer reaction of 2-amino-3-cyano-4H-pyran derivatives (4a–l) with cyclohexanone. HAChE inhibition assay was carried out in vitro on the synthesized compounds 5a–l. Among them, compound 5f exhibited potent hAChE inhibitors with IC50 values of 0.16 µM. Also, the molecular docking and kinetic studies performed for a better understanding of compound 5f as a representative compound.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is an irreversible and multifactorial neurodegenerative illness and the main cause of dementia in elderly people [1,2,3]. AD begins with memory loss in the early stages and gradually develops in the advanced stages of cognitive impairment, causing severe behavioral abnormalities and eventually leading to death [4,5,6,7]. Due to the increased life expectancy and the number of elderly people, AD has become an important public health issue [8, 9]. AD pathophysiology is not yet fully clear, but factors other than age and heredity are involved as a shortage of acetylcholine (ACh), beta-amyloid (Aβ) aggregates, τ-protein aggregation, oxidative stress, mitochondrial dysfunction, and neuroinflammation [10,11,12,13,14]. There is not a definitive cure for AD, but one effective therapy for AD is increasing ACh levels in the brain by the inhibition of acetylcholinesterase (AChE) [15,16,17]. Acetylcholinesterase inhibitors including donepezil, rivastigmine, tacrine, and galantamine were used to treat AD [18, 19]. Tacrine was the first developed reversible cholinesterase inhibitor (ChEI) that was approved by the US FDA for the therapy for AD in 1993. Nevertheless, due to hepatotoxicity and peripheral side effects, it was approximately removed from the market. Tacrine is synthesized by Friedländer-type cyclocondensation of o-aminobenzonitrile with cyclohexanone [20,21,22,23,24,25]. Currently, research is ongoing on tacrine to design and synthesis new tacrine-based drugs via Friedländer reaction for modification on the structure of tacrine by the exchange of benzene rings in tacrine with other synthetic compounds [7, 26, 27]. The Friedländer reaction has been known for more than a century and is a well-known and simplest method for synthesis of different nitrogen-containing heterocyclic compounds such as quinoline derivatives, tacrine analogs, acridines, pyridines, camptothecins, and phenanthrolines [28,29,30].
Pyran is one of the main core units in several natural and synthetic products. Organic molecules with 4H-pyran ring have biological and potential medical such as anti-allergic, antitumor, antibacterial, anti-HIV, antifungal, anti-coagulant, anti-cancer, anti-inflammatory, and neurodegenerative disorders [31,32,33,34,35,36]. Naphthopyran derivatives show a variety of biological activities as anti-hypertensive, anticoagulant, anti-allergic, anticancer, and antiviral activities. Also, organic photochromic naphthopyrans have an industrial applications of technology and used in manufacturing in plastic photochromic lenses (T-type photochromism). Due to the medicinal properties of pyran derivatives, it is important to use appropriate synthesis methods. One of the methods used is synthesis via a multi-component reaction in the presence of a suitable catalyst [37,38,39,40,41,42,43,44,45]. On the other hand, multi-component, solvent-free, and reusable heterogeneously catalyzed reactions are factors for the principles of green chemistry. Multi-component reactions are very strong and effective bond-forming tools in organic chemistry with high atom efficiency, time and energy saving, friendly environment, and rapid and simple efficiency. Also, the catalyst plays a significant role in selectivity and determining yield. So, the creation of a reusable, mild, inexpensive catalyst for multi-component reactions has attracted interest. The catalysts have been used for multi-component reactions, including ZnAl2O4–Bi2O3, SiO2 NPs, Fe3O4@SiO2@imidazol-bisFc[HCO3], Fe3O4@SiO2-BenzIm-Fc[Cl]/NiCl2, SiO2@Imid-Cl@Fc, and silica-bonded aminoethylpiperazine [33, 46,47,48,49,50,51]. The use of an efficient and stable heterogeneous catalyst as reusable and environmentally friendly substances has a very important role in organic synthesis. Also, the immobilization of catalyst on the solid supports have advantages such as non-toxicity, low solubility, increased reactions selectivity, and easy handling and led to the widespread use of these catalysts. Catalysts immobilized on the nanoparticle supports are more attractive, because of their higher selectivity and activity. Magnetite (Fe3O4) nanoparticle with unique properties such as high surface area, low toxicity, superpara-magnetism, thermal stability, low cost, easy separation from the reaction mixture and recyclability used in the design and synthesis of reusable heterogeneous catalysts [49, 52,53,54,55,56].
Ferrocene derivatives have become very important for their applications such as redox electrochemistry, catalysis, biology, materials science, and nonlinear optical materials. Also, characteristics such as thermal stability, low cost, high tolerance to moisture, and oxygen make these materials attractive [57, 58]. Graphene oxide (GO) is one of the most important derivatives and the oxidized forms of graphene. GO has different oxygen groups such as hydroxyl, carboxyl, epoxide and carbonyl on its surface. Also, it has properties such as good chemical and mechanical stability, high conductivity and a special surface, and various applications such as energy storage materials, polymer nanocomposites and electrocatalysis [59,60,61].
In this work, GO-Fc@Fe3O4 nanohybrid as a new, powerful, and reusable nanocatalyst was synthesized. The modified GO nanosheets provide a large available surface area with several acidic and basic sites including carboxylic acid, hydroxyl, Fc rings on the GO-Fc surface. This nanocatalyst was used to synthesize of 2-amino-3-cyano-4H-pyran derivatives (4a–l) under solvent-free conditions (Fig. 1). The desired products were obtained with good yield and in a short time. Compounds 4a–l were used as precursors in the Friedländer reaction with cyclohexanone and under the usual experimental conditions were obtained 14-aryl-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amines derivatives (5a–l) as new tacrine analogs for inhibition of acetylcholinesterase enzyme (Fig. 1). The amount of hAChE and hBuChE enzymes inhibitory activity was investigated for these compounds and the results showed that this series of compounds have good inhibition only against hAChE enzyme.
Experimental
Materials and instruments
Commercially available compounds were bought from Merck and Sigma-Aldrich. Commercial compounds contain: graphene oxide (GO), sodium nitrate (NaNO3) (CAS number: 7631-99-4), potassium permanganate (KMnO4) (CAS number: 7722-64-7), sulfuric acid (H2SO4) (CAS number: 7664-93-9), hydrogen peroxide solution (H2O2) (CAS number: 7722-84-1), meta-chloroperoxybenzoic acid (mCPBA) (CAS number: 937-14-4), glacial acetic acid (CAS number: 64-19-7), iron(II) chloride tetrahydrate (FeCl2.4H2O) (CAS number: 13478-10-9), iron(III) chloride hexahydrate (FeCl3.6H2O) (CAS number: 10025-77-1), 2-naphthol (CAS number: 135-19-3), 6-bromo-2-naphthol (CAS number: 15231-91-1), malononitrile (CAS number: 109-77-3), benzaldehyde (CAS number: 100-52-7), 4-nitrobenzaldehyde (CAS number: 555-16-8), 4-methylbenzaldehyde (CAS number: 104-87-0), 4-isopropylbenzaldehyde (CAS number: 122-03-2), 4-chlorobenzaldehyde (CAS number: 104-88-1), 4-bromobenzaldehyde (CAS number: 1122-91-4), 2-chlorobenzaldehyde (CAS number: 89-98-5), 3-bromobenzaldehyde (CAS number: 3132-99-8), 4-fluorobenzaldehyde (CAS number: 459-57-4), thiophene-2-carbaldehyde (CAS number: 98-03-3), cyclohexanone (CAS number: 108-94-1), Aluminum chloride (AlCl3) (CAS number: 7446-70-0), 1,2-dichloroethane (CAS number: 107-06-2). The reaction progress was controlled by TLC and detected by UV light (254 nm). 1H NMR and 13C NMR spectra were registered on Bruker Spectrospin Avance 400 and 100 MHz spectrometers in DMSO-d6 solvent, respectively. All chemical shifts were reported as δ (ppm), and coupling constants (J) were given in Hz. The Bruker Tensor 27 tool was used to record the FT-IR spectra of the synthesized compound on the KBr pellets and expressed in cm−1. Using an electric apparatus MEL-TEMP model 1202 was measured the melting point. Elemen-tar Vario EL III tool was used for Elemental analysis (C, H, N). FESEM (TESCAN MIRA3), EDX spectroscopy (TESCAN MIRA3), and XRD analysis (PANalytica X pertPRO (Germany) instrument with Cu-Ka radiation (0.15406 nm) at accelerating voltage of 45 kV) were used to study the morphology and structure of the nanoparticles. Magnetization measurement was performed with a model 155 alternative gradient force magnetometer at room temperature.
Preparation of GO
Hummer’s method was used to prepare GO nanosheets by oxidizing graphite flakes [62]. In brief, a round bottom flask was charged with graphite flake (2.5 g) and NaNO3 (1.25 g). The flask was then immersed in an ice bath and 65 ml of concentrated H2SO4 was added to a flask and stirred for 1 h. Then, finely ground KMnO4 was added in small portions into the above mixture under vigorous stirring at 0 °C. Vigorous stirring was continued for a further 72 h at 25 °C. After adding 175 ml of distilled water, 100 ml of HCl (10%) and 30 ml of H2O2 (30%) were slowly added to the above mixture and stirred for 2 h. After that, the mixture was centrifuged and washed with a large amount of deionized water to give GO powders.
Preparation of over-oxidized GO (GO-Epo)
An amount of 150 mg of mCPBA was added to a suspension of GO in DI water (25 ml, 3 mg/ml) and stirred at ambient temperature for 24 h. After this time, the reaction mixture was washed with dichloromethane to remove all the unreacted mCPBA. Then, the aqueous phase was centrifuged to give the over-oxidized GO nanosheets (GO-Epo) [63].
Functionalization of GO-Epo with 4-ferrocenylbutylamine (GO-Fc)
An amount of 0.3 g of 4-ferrocenylbutylamine [64] was dissolved in 35 ml of ethanol and the resulted solution was then added to a suspension of GO-Epo in water (0.1 g in 30 ml water). The resulting mixture was refluxed for 72 h [60]. At the end of the reaction, the mixture was centrifuged and washed with water and dichloromethane, respectively. After vacuum drying, the GO-Fc was obtained as a black powder.
Synthesis of GO-Fc@Fe3O4 nanohybrid
A round bottom two necked flask was charged with FeCl3·6H2O (1.76 g, 6.5 mmol) and FeCl2·4H2O (0.65 g, 3.25 mmol). A mixture of glacial acetic acid (1.5 ml) and water (50 ml) was added to the above mixture under vigorous stirring. The appropriate amount of GO-Fc suspension was then added to the above flask and the temperature was raised from 25 to 80 °C. After the temperature reached to 80 °C, 10 ml of ammonia was added into the flask and allowed to stir for an additional 15 min at 80 °C. Finally, GO-Fc@Fe3O4 nanohybrid was separated by an external magnetic field and was washed with distilled water and methanol, respectively [65].
General procedure for the preparation of 2-amino-3-cyano-4H-pyran derivatives (4a–l)
A reaction vial was charged with 2-naphthol (1a) or 6-bromo-2-naphthol (1b) (1 mmol), aromatic aldehyde (2a–j) (1 mmol), malononitrile (3) (1.2 mmol), and GO-Fc@Fe3O4 (10 mg) as magnetic nanocatalyst. The mixture was heated at 100 °C under solvent-free conditions. After completion of the reaction (monitored by TLC), an appropriate amount of ethanol was added and the mixture heated for 5 min. Then, the magnetic nanocatalyst was separated by applying an external magnet. The crude solid was collected by filtration and then recrystallized from ethanol to give the pure compound.
General procedure for the synthesis of 14-aryl-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amines derivatives (5a–l)
AlCl3 (1.5 equiv) and dry 1,2-dichloroethane (10 ml) were mixed at ambient temperature under argon atmosphere for 30 min. Then, compounds 4a–l (1 equiv) and cyclohexanone (1.5 equiv) were added to them and hated at reflux for 24 h. Then, using a rotary evaporator was evaporated the solvent and NaOH 10% was added to the residual sediment and stirred for 30 min. The resulted mixture was filtered and washed with EtOH. The synthesized compounds were obtained as solids.
14-(Phenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5a)
Yield 92%; white solid; m.p. > 250 °C (mp. > 260 °C [22]).
14-(4-Nitrophenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5b)
Yield 73%; white solid; m.p. > 250 °C (mp. 258–260 °C [66]).
14-(4-Methylphenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5c)
Yield 59%; white solid; m.p. 223–227 °C (mp. 224–226 °C [66]).
14-(4-Isopropylphenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5d)
Yield 57%; white solid; m.p. 239–244 °C. FTIR (KBr) ν 3492, 3355, 2931, 1617, 1444, 1227 cm−1; 1H NMR: 1.04 (d, 3H, 3J = 0.9 Hz, CH3), 1.05 (d, 3H, 3J = 0.9 Hz,, CH3), 1.71 (br, 4H, CH2), 2.21–2.25 (m, 1H, CH2), 2.38–2.42 (m, 1H, CH2), 2.63–2.64 (m, 2H, CH2), 2.65–2.70 (m ,1H, CH), 6.17 (s, 1H, CH), 6.70 (br, 2H, NH2), 7.04 (d, 2H, 3J = 8.2 Hz, Ar–H), 7.42–7.47 (m, 4H, Ar–H), 7.55–7.59 (m, 1H, Ar–H), 7.91 (d, 2H, 3J = 9.1 Hz, Ar–H), 8.36 (d, 1H, 3J = 8.5 Hz, Ar–H); 13C NMR: 21.5, 21.6, 22.7, 23.6, 29.8, 32.8, 34.0, 99.7, 112.5, 117.3, 118.0, 123.4, 124.7, 126.2, 126.9, 127.5, 128.5, 129.0, 130.5, 130.6, 141.4, 146.5, 147.8, 149.1, 153.0, 153.3; Anal. Calcd. for C29H28N2O (420.55): C 82.82, H 6.71, N 6.66; Found: C 82.89, H 6.70, N 6.59%.
14-(4-Chlorophenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5e)
Yield 65%; white solid; m.p. > 250 °C. FTIR (KBr) ν 3418, 3206, 2940, 1648, 1478, 1226 cm−1; 1H NMR: 1.69–1.72 (m, 4H, CH2), 2.21–2.25 (m,1H, CH2), 2.40–2.45 (m,1H, CH2), 2.72–2.73 (m, 2H, CH2), 6.53 (s, 1H, CH), 7.26 (d, 2H, 3J = 8.5 Hz, Ar–H), 7.44 (t,1H, 3J = 7.8 Hz, Ar–H), 7.49 (d,1H, 3J = 8.9 Hz, Ar–H), 7.55 (t, 1H, 3J = 7.8 Hz, Ar–H), 7.63 (d, 2H, 3J = 8.4 Hz, Ar–H), 7.92–7.99 (m, 2H, Ar–H), 8.36 (d, 1H, 3J = 8.5 Hz, Ar–H); 13C NMR: 22.1, 22.3, 23.0, 32.0, 34.4, 99.0, 112.3, 115.8, 116.5, 117.8, 119.3, 124.3, 126.1, 128.7, 129.3, 129.6, 129.9, 130.5, 131.6, 143.0, 148.0, 148.8, 151.2, 152.3, 154.8. Anal. Calcd. for C26H21ClN2O (412.91): C 75.63, H 5.13, N 6.78; Found: C 75.52, H 5.09, N 6.74%.
14-(2-Cholorophenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5f)
Yield 92%; white solid; m.p. > 250 °C. FTIR (KBr) ν 3466, 3332, 2926, 1625, 1445, 1228 cm−1; 1H NMR: 1.70 (S, 4H, CH2), 2.20–2.24 (m, 1H, CH2), 2.32–2.39 (m, 1H, CH2), 2.53–2.58 (m, 2H, CH2), 5.66-5.67 (br, 2H, NH2), 6.16 (s, 1H, CH), 7.10–7.14 (m,1H, Ar–H), 7.16–7.20 (m, 1H, Ar–H), 7.35 (dd, 1H, 3J = 7.8, 1.2 Hz, Ar–H), 7.39–7.46 (m, 3H, Ar–H), 7.52–7.55 (m, 1H, Ar–H), 7.89–7.92 (m, 2H, Ar–H), 8.25 (d, 1H, 3J = 8.0 Hz, Ar–H); 13C NMR: 22.0, 22.2, 22.9, 31.9, 34.1, 98.2, 112.4, 116.0, 117.9, 122.7, 124.3, 126.9, 127.0, 128.5, 128.6, 129.4, 129.5, 130.2, 130.4, 130.8, 131.5, 141.6, 149.0, 151.3, 152.7, 154.5; Anal. Calcd. for C26H21ClN2O (412.91): C 75.63, H 5.13, N 6.78; Found: C 75.70, H, 5.15, N 6.81%.
14-(4-Bromophenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5 g)
Yield 81%; white solid; m.p. > 250 °C. FTIR (KBr) ν 3453, 3406, 2928, 1629, 1442, 1231 cm−1; 1H NMR: 1.70 (br, 4H, CH2), 2.19–2.23 (m, 1H, CH2), 2.37–2.42 (m, 1H, CH2), 2.571–2.578 (m, 2H, CH2), 6.05 (s, 2H, NH2), 6.13 (s, 1H, CH), 7.35–7.46 (m, 6H, Ar–H), 7.55 (t, 1H, 3J = 7.2 Hz, Ar–H), 7.87–7.90 (m, 2H, Ar–H), 8.29 (d, 1H, 3J = 8.5 Hz, Ar–H); 13C NMR: 22.1, 22.2, 23.0, 32.0, 34.5, 98.8, 112.3, 117.3, 117.8, 119.3, 123.0, 124.3, 126.8, 128.5, 128.9, 129.7, 130.2, 130.6, 131.0, 144.1, 148.8, 151.2, 152.3, 154.8; Anal. Calcd. for C26H21BrN2O (457.36): C 68.28, H 4.63, N 6.12; Found: C 68.22, H 4.61, N 6.15%.
3-Bromo-14-(3-bromophenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno [2,3-b]-quinolin-13-amine (5h)
Yield 78%; white solid; m.p. > 250 °C. FTIR (KBr) ν 3425, 3152, 2934, 1651, 1412, 1230 cm−1; 1H NMR: 1.73-1.74 (m, 4H, CH2), 2.25 (br, 1H, CH2), 2.40 (br, 1H, CH2), 2.73 (br, 2H, CH2), 6.39 (s, 1H, CH), 7.19 (t, 1H, 3J = 7.8 Hz, Ar–H), 7.34 (dd, 1H, 3J = 7.1, 0.7 Hz, Ar–H), 7.44 (d, 1H, 3J = 7.8 Hz, Ar–H), 7.61 (d, 1H, 3J = 9.0 Hz, Ar–H), 7.82 (dd, 2H, 3J = 7.0, 1.9 Hz, Ar–H), 7.89 (s, 1H, Ar–H), 8.02 (d, 1H, 3J = 9.1 Hz, Ar–H), 8.23 (d, 1H, 3J = 9.1 Hz, Ar–H), 8.30 (d, 1H, 3J = 1.0 Hz, Ar–H);13C NMR: 22.5, 22.7, 23.5, 32.4, 35.4, 99.3, 111.4, 113.5, 117.6, 121.8, 122.2, 124.3, 126.2, 128.4, 128.9, 130.2, 130.5, 130.9, 131.4, 131.9, 132.3, 147,6, 149.3, 152.1, 153.1, 155.4. Anal. Calcd. for C26H20 Br2N2O (536.26): C 58.23, H 3.76, N 5.22; Found: C 58.10, H 3.72, N 5.18%.
14-(3-Bromophenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5i)
Yield 72%; white solid; m.p. > 250 °C. FTIR (KBr) ν 3482, 3348, 2933, 1624, 1439, 1231 cm−1; 1H NMR: 1.71 (br,4H, CH2), 2.21–2.25 (m,1H, CH2), 2.37–2.41(m, 1H, CH2), 2.53–2.58 (m, 2H, CH2), 6.08 (s, 2H, NH2), 6.11 (s, 1H, CH), 7.12–7.16 (t, 1H, 3J = 7.8 Hz, Ar–H), 7.24–7.26 (m, 1H, Ar–H), 7.41–7.45 (m, 3H, Ar–H), 7.57–7.61(m, 1H, Ar–H), 7.75 (br, 1H, Ar–H), 7.89–7.92 (m, 2H, Ar–H), 8.29 (d, 1H, 3J = 8.5 Hz, Ar–H); 13C NMR: 22.5, 22.7, 23.5, 32.3, 35.4, 99.4, 113.2, 117.6, 118.4, 122.2, 123.3, 125.2, 127.1, 127.7, 129.3, 129.9, 130.0, 130.5, 130.9, 131.0, 131.2, 147.6, 149.3, 152.0, 153.2, 155.3; Anal. Calcd. for C26H21BrN2O (457.36): C 68.28, H 4.63, N 6.12; Found: C 68.31, H 4.57, N 6.16%.
3-Bromo-14-(4-fluorophenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno [2,3-b]-quinolin-13-amine (5j)
Yield 87%; white solid; m.p. > 250 °C. FTIR (KBr) ν 3480, 3381, 2929, 1618, 1442, 1233 cm−1; 1H NMR: 1.70 (br, 4H, CH2), 2.19–2.23 (m, 1H, CH2), 2.32-2.37 (m, 1H, CH2), 2.57 (br, 2H, CH2), 6.04 (s, 2H, NH2), 6.12 (s, 1H, CH), 7.00 (t, 2H, 3J = 8.8 Hz, Ar–H), 7.46–7.50 (m, 3H, Ar–H), 7.70 (dd, 1H, 3J = 6.9, 2.0 Hz, Ar–H), 7.88 (d, 1H, 3J = 9 Hz, Ar–H), 8.19 (d, 1H, 3J = 2 Hz, Ar–H), 8.24 (d, 1H, 3J = 9.1 Hz, Ar–H); 13C NMR: 22.1, 22.3, 23.1, 32.0, 34.2, 99.8, 111.4, 113.5, 114.6, 115.3, 116.8, 122.0, 124.3, 127.4, 129.0, 129.8, 130.2, 132.6, 131.1, 131.6, 140.5, 148.8, 151.4, 152.2, 154.7. Anal. Calcd. for C26H20BrFN2O (475.35): C 65.69, H 4.24, N 5.89; Found: C 65.54, H 4.23, N 5.83%.
14-(4-Fluorophenyl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5k)
Yield 50%; white solid; m.p. > 250 °C. FTIR (KBr) ν 3488, 3354, 2926, 1617, 1439, 1225 cm−1; 1H NMR: 1.69 (br, 4H, CH2), 2.20-2.24 (m, 1H, CH2), 2.38-2.42 (m, 1H, CH2), 2.57 (br, 2H, CH2), 6.05 (s, 2H, NH2), 6.13 (s, 1H, CH), 7.00 (t, 2H, 3J = 8.7 Hz, Ar–H), 7.38–7.44 (m, 2H, Ar–H), 7.51–7.57 (m, 3H, Ar–H), 7.86-7.90 (m, 2H, Ar–H), 8.32 (d, 1H, 3J = 8.5 Hz, Ar–H); 13C NMR: 22.1, 22.3, 23.0, 32.0, 34.2, 99.2, 112.3, 114.8, 115.0, 117.80, 117.86, 123.0, 124.3, 126.7, 128.5, 128.8, 129.2, 129.3, 130.2, 130.6, 140.9, 148.8, 151.1, 152.2, 154.8; Anal. Calcd. for C26H21FN2O (396.46): C 78.77, H 5.34, N 7.06; Found: C 78.84, H, 5.37, N 7.07%.
14-(Thiophen-2-yl)-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amine (5l)
Yield 52%; white solid; m.p. > 250 °C. FTIR (KBr) ν 3464, 3356, 2932, 1622, 1444, 1235 cm−1; 1H NMR: 1.72 (br, 4H, CH2), 2.30–2.32 (m, 1H, CH2), 2.39–2.43 (m, 1H, CH2), 2.53–2.58 (m, 2H, CH2), 6.15 (s, 2H, NH2), 6.50 (s, 1H, CH), 6.76–6.78 (m, 1H, Ar–H), 7.12–7.15 (m, 2H, Ar–H), 7.39 (d, 1H, 3J = 8.8 Hz, Ar–H), 7.44 (t, 1H, 3J = 7.2 Hz, Ar–H), 7.57–7.61 (m, 1H, Ar–H), 7.88–7.93 (m, 2H, Ar–H), 8.40 (d, 1H, 3J = 8.4 Hz, Ar–H); 13C NMR: 22.1, 22.32, 23.0, 30.6, 32.0, 98.8, 112.2, 117.5, 117.8, 124.2, 125.9, 126.7, 128.4, 128.9, 130.2, 130.7, 148.4, 148.7, 151.3, 152.3, 154.9; Anal. Calcd. for C24H20N2OS (384.50): C 74.97, H 5.24, N 7.29 S 8.34; Found: C 74.87, H, 5.21, N 7.24 S 8.29%.
hAChE and hBuChE inhibition assay
Using Ellman’s method [67] was measured inhibitory capacity of new compounds on ChE activity. Fresh blood was used to make the enzymes used in this procedure [68]. Synthetic compounds were solved in DMSO and they were then diluted with buffer (0.1 M, pH = 8) to reach final concentrations. Phosphate buffer (0.1 M, pH 8, 550 µl), DTNB (3.5 mM, 150 µl), the substrate (acetylthiocholine or butylthiocholine) (7 mM, 150 µl) and different concentrations of inhibitors (150 µl) and phosphate buffer (0.1 M, 700 µl), DTNB (3.5 mM, 150 µl) and different concentrations of inhibitors (150 µl) were dumped in test cuvettes and control cuvettes, respectively. After 5 min, it reached to 37 °C, enzyme (50 µl) was added. All experiments were performed by spectrophotometer Helios-Zeta (Thermospectronic, Cambridge, U.K.) at 412 nm in 5 min.
Kinetic assay
Using Ellman’s method, kinetic studies of inhibition on hAChE were performed to obtain the inhibition model and value Ki. In this study, the enzyme relative speed on various concentrations of the substrates (0.1–1 mM) and two various concentrations of inhibitor 5f (5 × 10−7–1 × 10−6 mM) was determined. Linear regression was used for the calculation of Lineweaver–Burk plots, and all Data analysis was done using Microsoft Excel 2013 [69].
Docking studies
HAChE Structure (pdb ID: 4Ey7) was taken of the Protein Data Bank [70, 71]. To set up and perform blind docking calculations between drug and hAChE were used AutoGrid 4 and AutoDock 4 [72, 73]. At first, water molecules were deleted. Lamarckian genetic algorithms were applied to carry out docking calculations as implemented in AutoDock. The results from AutoDock was rendered with Discovery studio 4.5.
Results and discussion
Synthesis and characterization of GO-Fc@Fe3O4 nanohybrid
GO-Fc@Fe3O4 nanohybrid as a powerful and reusable nanocatalyst for the synthesis of 2-amino-4H-pyran derivatives was synthesized according to scheme 1. Firstly, over oxidized graphene oxide (GO-Epo) was prepared by the treatment of GO with mCPBA in an aqueous medium at room temperature [63]. This step provides a much reactive site for surface modification of GO nanosheets. Afterward, GO-Epo nanosheets were chemically modified with Fc derivative through the ring-opening reaction between GO nanosheets and 4-ferrocenylbutylamine as a nucleophile [60]. Then, Fe3O4 nanoparticles were synthesized onto the modified GO surface via a simple co-precipitation of Fe2+ and Fe3+ under an alkaline solution at 80 °C [65]. The final magnetic nanocatalyst denoted as GO-Fc@Fe3O4.
FT-IR spectra of GO, GO-Fc, and GO-Fc@Fe3O4 nanohybrid are depicted in Fig. 2. As shown in Fig. 2, GO nanosheets show several characteristic peaks at about 3400 cm−1, 2900 cm−1, 2800 cm−1, 1700 cm−1,1600 cm−1 which are related to the OH, aromatic C–H bond, aliphatic C–H bond, C=O, and C=C bonds, respectively [74]. As indicated in this figure, in the FT-IR spectrum of GO-Fc, the appearance of a new peak at about 480 cm−1 can be assigned to the Fe-Cp bond in Fc rings. This new peak proved the success of the grafting of Fc groups onto GO nanosheets. Also, the FT-IR spectrum of GO-Fc@Fe3O4 nanohybrid exhibits a strong IR peak at around 570 cm−1 which is related to the stronger bending vibration of Fe–O in Fe3O4 nanoparticles [75]. These findings indicate that the Fe3O4 nanoparticles were successfully synthesized by the in situ nanoparticle growth method onto the GO-Fc surface.
The XRD patterns of GO, GO-Fc, GO-Fc@Fe3O4 nanohybrid are shown in Fig. 3. The XRD pattern of GO exhibits a sharp diffraction peak at 2θ = 11.5° which is attributed to the (001) peak (d ~ 0.77 nm). After modification of GO surface with Fc moieties, the XRD peak position and shape of GO-Fc changes compared to the XRD peak of GO. These changes may be due to the presence of Fc rings onto the GO surface which disrupts the crystalline structure of GO nanosheets.
The XRD pattern of GO-Fc@Fe3O4 nanohybrid confirmed the presence of Fe3O4 nanoparticles and GO-Fc nanosheets related diffraction peaks. Broad XRD peaks of GO-Fc@Fe3O4 nanohybrid compared to GO-Fc nanosheets can be attributed to the growth of Fe3O4 nanoparticles between GO layers.
FE-SEM micrographs of GO, GO-Fc, GO-Fc@Fe3O4 nanohybrid are depicted in Fig. 4 that tangible changes are observed in the FE-SEM micrographs of them. The FE-SEM image of GO (Fig. 4a) exhibits a layered structure with wrinkles visible. The wrinkled structure of GO can be due to the presence of oxygenic functional groups on its surface. After modification of GO surface with Fc moieties, the GO-Fc nanosheets show rich-wrinkle morphology and also are increased surface turbidity that is due to the entry of Fc into the GO surface (Fig. 4b). Also the presence of aggregated nearly spherical Fe3O4 nanoparticles on the GO-Fc surface is shown in Fig. 4c. FE-SEM images demonstrate that the GO-Fc@Fe3O4 nanohybrid was successfully synthesized. Furthermore, the EDX analysis of GO-Fc@Fe3O4 nanohybrid shows the elements in the structure of nanocatalyst.
EDX analysis is a powerful tool for evaluating the elements of the synthesized compounds. From EDX curves (Fig. 5), they are confirmed that the related elements of GO including C and O were present in the synthesized GO (Fig. 5a). The EDX analysis of the over-oxidized form of GO (GO-Epo) shows a significant increase of O element (Fig. 5b). The presence of the abundance of epoxy rings on the GO surface provides an option of using these active sites for the covalently grafted of Fc moieties on the GO surface through ring-opening reaction. The presence of Fe element in the EDX analysis of GO-Fc (Fig. 5c) confirms the successful modification of GO-Epo surface with Fc moieties. The EDX analysis of GO-Fc@Fe3O4 nanohybrid (Fig. 5d) confirms that the Fe3O4 nanoparticles are successfully grown onto GO layers.
Also, the magnetic properties of GO-Fc@Fe3O4 nanocatalyst have been studied by vibrating sample magnetometer (VSM) technique at room temperature (Fig. 6). As shown in Fig. 6, the saturation magnetization (Ms) values of Fe3O4 and GO-Fc@Fe3O4 nanohybrid are 64 emu/g and 43 emu/g, respectively. The saturation magnetization value is decreased due to the intercalation of Fe3O4 nanoparticles into GO-Fc layers. However, the GO-Fc@Fe3O4 nanocatalyst exhibited enough magnetization value that can be easily separated from the reaction mixture by an external magnet.
Catalytic test of GO-Fc@Fe3O4 nanohybrid
To check the catalytic performance of the GO-Fc@Fe3O4 nanohybrid, firstly, a model reaction between 2-naphthol (1a), benzaldehyde (2a), and malononitrile (3) was chosen. According to previous studies, the presence of acidic and basic sites in the synthesized nanocatalyst can facilitate a coupling reaction between the above mentioned three components [51, 76,77,78]. Also, based on the catalytic activity of Fc, GO-Fc@Fe3O4 nanohybrid has several acidic and basic sites including carboxylic acid, hydroxyl, Fc rings on the GO-Fc surface (Fig. 7). Also, GO nanosheets provide a large available surface area. These mentioned reasons can promote the formation of desired products.
To determine the optimum reaction conditions, the model reaction was performed under various conditions including various solvents, temperature, and amount of catalyst (Table 1). At first, the model reaction was carried out in various solvents and solvent-free systems in the presence of 40 mg of synthesized nanocatalyst at ambient temperature (Table 1, Entries 1–4). Among these conditions, the best yield was achieved under solvent-free conditions. Afterwards, the influence of catalyst amount and various temperature on the yield of the model reaction was investigated by using different amounts of the synthesized nanocatalyst (40, 30, 20, 10, and 5 mg) in the model reaction. As indicated in Table 1, the best results were obtained during 40 min under solvent-free conditions at 100 °C in the presence of 10 mg of the magnetic synthesized nanocatalyst (Entry 13).
After finding the optimum conditions, to explore the catalytic activity, the reaction between 2-naphthol (1a) and 6-bromo-2-naphthol (1b) with various aromatic aldehydes (2a–j), and malononitrile (3) (Scheme 2) were tested under optimum conditions (Table 2). Moreover, the existence of electron-donating and electron-withdrawing functional groups on the aromatic aldehyde ring has no remarkable difference in the yield of the reactions. These findings demonstrate the higher activity of the synthesized nanocatalyst.
Finally, we tested the recyclibility of GO-Fc@Fe3O4 as a magnetic nanocatalyst for the coupling of three components in the model reaction. Upon completion of the reaction, the crude product was dissolved in hot ethanol and the magnetic nanocatalyst was separated using an external magnet. Then, the separated magnetic nanocatalyst was washed several times with hot ethanol and dried. The recovered magnetic nanocatalyst can be reused 7 times without any significant changes in reaction yield (Fig. 8).
We propose a possible mechanism of GO-Fc@Fe3O4 catalyzed reaction as depicted in scheme 3. After activation of the aldehyde groups, a Knoevenagel condensation was carried out between aromatic aldehyde and malononitrile, and arylidenemalononitrile was formed as intermediate. Afterward, the arylidenemalononitrile was coupled with 2-naphthol through Michael’s addition reaction followed by electrophilic cyclization to form the desired product [70].
Preparation of tacrine-naphthopyran hybrid derivatives (5a–l)
14-Aryl-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amines (5a–l) as new tacrine hybrids analogs and hAChE inhibitors have been synthesized by the AlCl3 promoted Friedländer-type reaction between 2‐amino‐3‐cyano‐4H‐pyrans derivatives (4a–l) and cyclohexanone as the selected ketone in dry 1,2-dichloroethane (DCE) at 83 °C, in good yields (50–92%) (Scheme 4) [26, 66].
hAChE inhibition
One way to treat AD is inhibition of ChE enzymes. The cholinesterase inhibition activity of compounds 4a–l and compounds 5a–l was investigated in vitro and using Ellman’s spectrophotometric method against hAChE and hBuChE [67]. Fresh blood was used to prepare the enzymes [68]. The amount of inhibition of compounds 4a–l against hAChE and hBuChE was not sufficient to obtain IC50 for them and these compounds did not show inhibitory activity. Tacrine and galantamine are used as a positive control and the outcome was illustrated as IC50 amount of synthesized compounds in Table 3. The obtained results have shown that compounds 5a–l had medium inhibition activity of hAChE at micro-molar. However, the amount of inhibition of these compounds (5a–l) for hBuChE was not sufficient to obtain IC50 for them and it can be said that these compounds do not show hBuChE inhibitory activity. The range of IC50 values was obtained from 0.16 µM to 37.48 µM for hAChE. Among them, compound 5f with IC50 value equal 0.16 µM displayed good inhibitory activity against hAChE which shows more inhibitory activity of tacrine and galantamine.
Kinetic analysis
The mechanism of hAChE inhibition for compounds 5a–l was investigated using compound 5f. So, kinetic studies of compound 5f as a representative compound with the greatest inhibit hAChE activity between other synthesized compounds was carried out to characterize a better understanding of the mechanism of enzyme inhibition. Due to the structural similarity of the synthesized compounds, a similar practical method was predicted. Using the Lineweaver–Burk plots was determined the type of inhibition (Fig. 9). According to the results, compound 5f was showed mixed-type inhibition. This analysis gives inhibition constants with Ki value equal to 0.8 µM and KI value equal to 0.3 µM. According to available reports, compound 5f is presumably binding simultaneously to the CAS and PAS of hAChE [17, 22].
Molecular docking
The use of computational methods is interest to design new compounds nowadays and the use of molecular docking studies can be obtained interaction affinity between molecules and hAChE enzyme. So, molecular docking studies on the selective compound 5f was performed, to get more information of interact with hAChE on their active site. According to the results, the derivatives can inhibit the enzyme, by binding to the active site of hAChE. The results obtained from the docking of compound 5f (Fig. 10) show that this compound has a better interaction than tacrine (Fig. 11). The binding energy get for compound 5f and tacrine was equal to − 11.61 kcal/mol and − 7.17 kcal/mol, respectively. The compound of 5f and tacrine do not show any hydrogen bonding. The results showed tacrine interacted with TYR341, PHE338, PHE295, TYR124, SER293, TRP286, and VAL294. So, the results were obtained for compound 5f show that TRP86, GLY448, SER203, HIS447, and TYR337 placed around of naphthopyran moiety, GLY121, ASP74 and SER125 are around of 2-Cl-ph and PHE297, PHE295, TYR124,TYR341, and TRP286 placed around of 5,6,7,8-tetrahydroquinolin-4-amine moiety. The compound 5f interacted with the peripheral anionic site (PAS) and catalytic active site (CAS) of hAChE.
Conclusion
In summary, the chemically modified GO-Epo by Fc moiety was synthesized successfully. Then, the novel GO-Fc@Fe3O4 nanohybrid was formed by synthesized of Fe3O4 nanoparticles onto the GO-Fc sheets. Various analyses such as FT-IR, FESEM, EDX, XRD, and VSM analysis were used for the evaluation of all of the intermediates to final nanocatalyst. GO-Fc@Fe3O4 nanohybrid was used as an efficient heterogeneous catalyst for the synthesis 2‐amino‐3‐cyano‐4H‐pyran derivatives (4a–l). Also, the results of its catalytic activity investigation showed that the presence of acidic and basic sites in the synthesized nanocatalyst increased catalytic activity in this reaction. The relative simplicity of separation, high yields, recyclability and reusability are the advantages of using GO-Fc@Fe3O4 as a catalyst in this multi-component reaction. Also, 14-aryl-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]-quinolin-13-amines derivatives (5a–l) were synthesized as tacrine-naphthopyran hybrid derivatives by Friedländer reaction between compounds 4a–l and cyclohexanone. In the following, in vitro anti-hAChE and anti-hBuChE inhibitory activities of compounds 5a–l were investigated. These compounds showed selectively good inhibition of hAChE and among them, compound 5f was a stronger inhibitor of hAChE with IC50 value equal to 0.16 µM. But these series did not show any inhibition for hBuChE.
Abbreviations
- AD:
-
Alzheimer’s disease
- ACh:
-
Acetylcholine
- AChE:
-
Acetylcholinesterase
- BuChE:
-
Butyrylcholinesterase
- ChEI:
-
Cholinesterase inhibitor
- PAS:
-
Peripheral anionic site
- CAS:
-
Catalytic active site
- GO:
-
Graphene oxide
- mCPBA:
-
meta-Chloroperoxybenzoic acid
References
S. Mozaffarnia, F. Parsaee, E. Payami, H. Karami, S. Soltani, M.R. Rashidi, R. Teimuri-Mofrad, ChemistrySelect 4, 9376 (2019)
K. Czarnecka, M. Girek, K. Maciejewska, R. Skibiński, J. Jończyk, M. Bajda, J. Kabziński, P. Sołowiej, I. Majsterek, P. Szymański, J. Enzyme Inhib. Med. Chem. 33, 158 (2018)
L. Peauger, R. Azzouz, V. Gembus, M.L. Ţintas, J. Sopková-de Oliveira Santos, P. Bohn, C. Papamicaël, V. Levacher, J. Med. Chem. 60, 5909 (2017)
S. Mozaffarnia, R. Teimuri-Mofrada, M.-R. Rashidi, Eur. J. Med. Chem. 191, 112140 (2020)
A. Dorababu, Bioorg. Chem. 93, 103299 (2019)
C. Zhang, Q.-Y. Du, L.-D. Chen, W.-H. Wu, S.-Y. Liao, L.-H. Yu, X.-T. Liang, Eur. J. Med. Chem. 116, 200 (2016)
X. Zhang, X. He, Q. Chen, J. Lu, S. Rapposelli, R. Pi, Bioorg. Med. Chem. 26, 543 (2018)
L. Monjas, M.P. Arce, R. León, J. Egea, C. Pérez, M. Villarroya, M.G. López, C. Gil, S. Conde, M.I. Rodríguez-Franco, Eur. J. Med. Chem. 130, 60 (2017)
R. Azzouz, L. Peauger, V. Gembus, M.-L. Ţînţaş, J. Sopková-de Oliveira Santos, C. Papamicael, V. Levacher, Eur. J. Med. Chem. 145, 165 (2018)
Z.-Q. Cheng, K.-K. Zhu, J. Zhang, J.-L. Song, L.A. Muehlmann, C.-S. Jiang, C.-L. Liu, H. Zhang, Bioorg. Chem. 83, 277 (2019)
K.S.T. Dias, C.T. de Paula, T. dos Santos, I.N. Souza, M.S. Boni, M.J. Guimarães, F.M. da Silva, N.G. Castro, G.A. Neves, C.C. Veloso, M.M. Coelho, Eur. J. Med. Chem. 130, 440 (2017)
M. Eghtedari, Y. Sarrafi, H. Nadri, M. Mahdavi, A. Moradi, F.H. Moghadam, S. Emami, L. Firoozpour, A. Asadipour, O. Sabzevari, A. Foroumadi, Eur. J. Med. Chem. 128, 237 (2017)
H. Farrokhi, S. Mozaffarnia, K. Rahimpour, M.R. Rashidi, R. Teimuri-Mofrad, J. Iran. Chem. 17, 593 (2020)
Z. Haghighijoo, O. Firuzi, B. Hemmateenejad, S. Emami, N. Edraki, R. Miri, Bioorg. Chem. 74, 126 (2017)
M. Shidore, J. Machhi, K. Shingala, P. Murumkar, M.K. Sharma, N. Agrawal, A. Tripathi, Z. Parikh, P. Pillai, M.R. Yadav, J. Med. Chem. 59, 5823 (2016)
E. Sawatzky, S. Wehle, B. Kling, J. Wendrich, G. Bringmann, C.A. Sotriffer, J. Heilmann, M. Decker, J. Med. Chem. 59, 2067 (2016)
J.-S. Lan, T. Zhang, Y. Liu, J. Yang, S.-S. Xie, J. Liu, Z.-Y. Miao, Y. Ding, Eur. J. Med. Chem. 133, 184 (2017)
A. Iraji, M. Khoshneviszadeh, O. Firuzi, M. Khoshneviszadeh, N. Edraki, Bioorg. Chem. 97, 103649 (2020)
H.J. Tseng, M.H. Lin, Y.J. Shiao, Y.C. Yang, J.C. Chu, C.Y. Chen, Y.Y. Chen, T.E. Lin, C.J. Su, S.L. Pan, L.C. Chen, Eur. J. Med. Chem. 192, 112193 (2020)
J. Li, L. Zhang, D. Shi, Q. Li, D. Wang, C. Wang, Q. Zhang, L. Zhang, Y. Fan, Synlett 2, 233 (2008)
J. Korábečný, E. Nepovimova, T. Cikankova, K. Špilovská, L. Vašková, E. Mezeiova, K. Kuča, J. Hroudova, Neuroscience 370, 191 (2018)
A. McEneny-King, W. Osman, A.N. Edginton, P.P. Rao, Bioorg. Med. Chem. Lett. 27, 2443 (2017)
M. Esquivias-Pérez, E. Maalej, A. Romero, F. Chabchoub, A. Samadi, J. Marco-Contelles, M.J. Oset-Gasque, Chem. Res. Toxicol. 26, 986 (2013)
H. Boulebd, L. Ismaili, H. Martin, A. Bonet, M. Chioua, J. Marco Contelles, A. Belfaitah, Future Med. Chem. 9, 723 (2017)
C. Derabli, I. Boualia, A.B. Abdelwahab, R. Boulcina, C. Bensouici, G. Kirsch, A. Debache, Med. Chem. Lett. 28, 2481 (2018)
M. Khoobi, F. Ghanoni, H. Nadri, A. Moradi, M.P. Hamedani, F.H. Moghadam, S. Emami, M. Vosooghi, R. Zadmard, A. Foroumadi, A. Shafiee, Eur. J. Med. Chem. 89, 296 (2015)
J.M. Roldan-Pena, D. Alejandre-Ramos, O. Lopez, I. Maya, I. Lagunes, J.M. Padron, L.E. Pena-Altamira, M. Bartolini, B. Monti, M.L. Bolognesi, J.G. Fernandez-Bolanos, Eur. J. Med. Chem. 138, 761 (2017)
M. Shiri, M.A. Zolfigol, H.G. Kruger, Z. Tanbakouchian, Adv. Hetrocycl. Chem. 102, 139 (2011)
W.S. Hamama, S.M. Waly, S.B. Said, H.H. Zoorob, J. Heterocycl. Chem. 55, 1554 (2018)
J. Quiroga, J. Trilleras, R. Abonía, B. Insuasty, M. Nogueras, J. Cobo, J.M. de la Torre, Arkivoc (2009). https://doi.org/10.3998/ark.5550190.0010.e02
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
J. Jung, B.H. Park, Y.R. Lee, Green Chem. 12, 2003 (2010)
S. Banerjee, A. Horn, H. Khatri, G. Sereda, Tetrahedron Lett. 52, 1878 (2011)
M. Gholamhosseini-Nazari, S. Esmati, K.D. Safa, A. Khataee, R. Teimuri-Mofrad, Appl. Organomet. Chem. 33, e4701 (2019)
A.H.F. Abd El-Wahab, Pharmaceuticals 5, 745 (2012)
N.J. Thumar, M.P. Patel, Arkivoc 13, 363 (2009)
Y. Essamlali, O. Amadine, H. Maati, K. Abdelouahdi, A. Fihri, M. Zahouily, R.S. Varma, A. Solhy, ACS Sustain. Chem. Eng. 1, 1154 (2013)
M. Frigoli, F. Maurel, J. Berthet, S. Delbaere, J. Marrot, M.M. Oliveira, Org. Lett. 14, 4150 (2012)
P.J.J. Huang, T.S. Cameron, A. Jha, Tetrahedron Lett. 50, 51 (2009)
A.A. Hussein, I. Barberena, T.L. Capson, T.A. Kursar, P.D. Coley, P.N. Solis, M.P. Gupta, J. Nat. Prod. 67, 451 (2004)
T.S. Jin, J.S. Zhang, L.B. Liu, A.Q. Wang, T.S. Li, Synth. Commun. 36, 2009 (2006)
A.V. Karnik, A.M. Kulkarni, N.J. Malviya, B.R. Mourya, B.L. Jadhav, Eur. J. Med. Chem. 43, 2615 (2008)
S. Li, Y. Yao, Z. Tang, B. Sun, C. Yu, T. Li, C. Yao, Org. Biomol. Chem. 17, 268 (2019)
S. Verma, S.L. Jain, Tetrahedron Lett. 53, 6055 (2012)
H. Yarahmadi, H.R. Shaterian, J. Chem. Res. 36, 49 (2012)
P. Anastas, N. Eghbali, Chem. Soc. Rev. 39, 301 (2010)
R. Teimuri-Mofrad, S. Esmati, S. Tahmasebi, M. Gholamhosseini-Nazari, J. Organomet. Chem. 870, 38 (2018)
R. Teimuri-Mofrad, S. Esmati, M. Rabiei, M. Gholamhosseini-Nazari, J. Chem. Res. 42, 7 (2018)
R. Mohammadi, S. Esmati, M. Gholamhosseini-Nazari, R. Teimuri-Mofrad, J. Mol. Liq. 275, 523 (2019)
R. Mohammadi, S. Esmati, M. Gholamhosseini-Nazari, R. Teimuri-Mofrad, New J. Chem. 43, 135 (2019)
M. Tajbakhsh, M. Kariminasab, H. Alinezhad, R. Hosseinzadeh, P. Rezaee, M. Tajbakhsh, H.J. Gazvini, M.A. Amiri, J. Iran. Chem. Soc. 12, 1405 (2015)
J. Rakhtshah, B. Shaabani, S. Salehzadeh, N. Hosseinpour Moghadam, Bioorg. Chem. 85, 420–430 (2019)
J. Rakhtshah, F. Yaghoobi, Int. J. Biol. Macromol. 139, 904–916 (2019)
S. Rostamnia, E. Doustkhah, J. Magn. Magn. Mater. 386, 111–116 (2015)
D. Yuan, L. Chen, L. Yuan, S. Liao, M. Yang, Q. Zhang, Chem. Eng. J. 287, 241–251 (2016)
A. Ghorbani-Choghamarani, B. Ghasemi, Z. Safari, G. Azadi, Catal. Commun. 60, 70–75 (2015)
R. Teimuri-Mofrad, Sh Tahmasebi, E. Payami, Appl. Organomet. Chem. 33, e4773 (2019)
R. Teimuri-Mofrad, H. Abbasi, K.D. Safa, B. Tahmasebi, Arkivoc 4, 371 (2016)
C. Liu, F. Su, J. Liang, Appl. Surf. Sci. 351, 889 (2015)
R. Teimuri-Mofrad, E. Payami, I. Ahadzadeh, Electrochim. Acta 321, 134706 (2019)
S. Park, J. An, J.R. Potts, A. Velamakanni, S. Murali, R.S. Ruoff, Carbon 49, 3019 (2011)
W.S. Hummers Jr., R.E. Offeman, J. Am. Chem. Soc. 80, 1339 (1958)
K.C. Mei, N. Rubio, P.M. Costa, H. Kafa, V. Abbate, F. Festy, S.S. Bansal, R.C. Hider, K.T. Al-Jamal, Chem. Commun. 51, 14981 (2015)
R. Teimuri-Mofrad, H. Abbasi, T. Vahedinia, I. Ahadzadeh, J. Inorg. Organomet. Polym Mater. 30, 955 (2020)
M. Heidarizadeh, E. Doustkhah, S. Rostamnia, P.F. Rezaei, F.D. Harzevili, B. Zeynizadeh, Int. J. Biol. Macromol. 101, 696–702 (2017)
E. Maalej, F. Chabchoub, A. Samadi, C. Rios, A. Perona, A. Morreale, J. Marco-Contelles, Bioorg. Med. Chem. Lett. 21, 2384 (2011)
G.L. Ellman, K.D. Courtney, V.J. Andres, R.M. Fesrtherstone, Biochem. Pharmacol. 7, 88 (1961)
G. Karimi, M. Iranshahi, F. Hosseinalizadeh, B. Riahi, A. Sahebkar, Pharmacologyonline 1, 566 (2010)
A. Rampa, A. Bisi, F. Belluti, S. Gobbi, P. Valenti, V. Andrisano, V. Cavrini, A. Cavalli, M. Recanatini, Bioorg. Med. Chem. 8, 497 (2000)
Y. Nicolet, O. Lockridge, P. Masson, J.C. Fontecilla-Camps, F. Nachon, J. Biol. Chem. 278, 41141 (2003)
G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanner, R.K. Belew, D.S. Goodsell, A.J. Olson, J. Comput. Chem. 30, 2785 (2009)
G.M. Morris, R. Huey, A.J. Olson, Curr. Protoc. Bioinform. 1, 8 (2008)
Q. Zhang, Y. Li, Y. Feng, W. Feng, Electrochim. Acta 90, 95 (2013)
Y. Li, H. Lu, Y. Wang, Y. Zhao, X. Li, J. Mater. Sci. 54, 7603 (2019)
R. Teimuri-Mofrad, M. Gholamhosseini-Nazari, E. Payami, S. Esmati, Appl. Organomet. Chem. 32, e3955 (2018)
A.R. Moosavi-Zare, M.A. Zolfigol, O. Khaledian, V. Khakyzadeh, M. Darestani Farahani, M.H. Beyzavi, H.G. Kruger, Chem. Eng. J. 248, 122 (2014)
R. Teimuri-Mofrad, M. Gholamhosseini-Nazari, E. Payami, S. Esmati, Res. Chem. Intermed. 43, 7105 (2017)
Acknowledgements
The authors would like to acknowledge the financial support from Iran National Science Foundation (INSF) (To Project Number: 97015588), Health Ministry of Islamic Republic of Iran, Tabriz University of medical sciences and the University of Tabriz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mozaffarnia, S., Teimuri-Mofrad, R. & Rashidi, MR. Synthesis of 2-amino-3-cyano-4H-pyran derivatives using GO-Fc@Fe3O4 nanohybrid as a novel recyclable heterogeneous nanocatalyst and preparation of tacrine-naphthopyran hybrids as AChE inhibitors. J IRAN CHEM SOC 18, 1455–1470 (2021). https://doi.org/10.1007/s13738-020-02125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02125-4