Abstract
A new series of the anti-inflammatory drug ketoprofen derivatives bearing aryl chalcone-amide congeners were synthesized. The structures of the synthesized compounds were identified by the 1H NMR, 13C NMR, and EIMS spectroscopic methods. The inhibitory activity of the synthesized compounds on cholinesterase enzymes was investigated. Biological results revealed that five compounds displayed moderate activities against acetylcholinesterase (AChE) with IC50 values below 10 μM. Among the tested compounds, (BTPhP) was found to be the most potent against AChE (IC50 0.98 ± 0.02 μM), while the chalcone-amide analogues (MeOPh), (HydPh), (FPh), and (ChPh) exhibited moderate activities with IC50 values ranged between 5.19–9.61 μM. Molecular docking study showed that compound (BTPhP) could combine with the active site of acetylcholinesterase by the π–π between the ketoprofen phenyl groups is embedded in a cavity surrounded by two aromatic residues of Tyr334 and Trp279. The present results strongly suggest that the para-position of the D-ring should be a preferred modification site for further structural optimization design. Thus, compound (BTPhP) emerged as a promising lead for the development of new acetylcholinesterase inhibitor agent. The preliminary quantum structure-activity relationship (QSAR) among the newly synthesized congeners was obtained by Genetic Function Approximation (GFA).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Alzheimer’s disease (AD) is a multifactorial neurodegenerative dementia, characterized by deterioration of memory and cognition in elder patients [1, 2]. The main cause of the loss of cognitive functions in AD patients is a continuous decline of the cholinergic neurotransmission in cortical and other regions of the human brain [3]. Cholinergic neurotransmission is mediated by the neurotransmitter acetylcholine (ACh), which is released and carried out the effect followed by rapidly hydrolysis via acetylcholinesterase (AChE) to acetate and choline [4, 5]. One of successful therapeutic strategies for treatment of AD is based on the cholinergic hypothesis [6]. This hypothesis assumes that in AD, the level of acetylcholine (ACh) is reduced due to the loss of the cholinergic neurons and decreased synthesis of ACh [3, 7]. The major therapeutic target in the AD treatment strategies is the inhibition of brain AChE to increase the ACh level in brain, causing increase in the cholinergic neurotransmission in AD patients [8, 9]. At present, there are four FDA-approved AC\hE inhibitor-type drugs, tacrine (I), donepezil (II), galantamine (III) and rivastigmine (IV) (Fig. 1) for treatment of cognitive dysfunction and memory loss associated with mild-to-moderate AD [10]. Recently, various potential analogues have been synthesized and evaluated for their activity against AChE such as indoles [11, 12], pyrroles [13, 14] chalcones [15–17], thiophene analogues [18], coumarin derivatives [19–21] and other potential compounds [22]. Design strategy and advantages of AChE inhibitors have been extensively reviewed [23–25].
Chalcones exhibited diverse biological activities [26], but few investigations were carried out by pharmaceutical researchers for examining their AChE inhibition activity. Based on the design experiences from existing AChE inhibitors, we synthesized a series of new chalcone-amide derivatives derived ketoprofen with evaluation of their AChE inhibition activity. Furthermore, molecular docking study was carried out for compound as well as donepezil for comparison purposes and to study the binding mode and selectivity of this analogue against AChE.
RESULTS AND DISCUSSION
The anti-inflammatory drug ketoprofen (2-(3-benzoylphenyl)propanoic acid, (V)) has been selected as a key intermediate for the synthesis of new substituted chalcone-amide derived ketoprofen derivatives, aiming at the evaluation of their AChE inhibition activity, via three steps. Thus, treatment of (V) with PCl5 at 50°C for 30 min. gave the acyl chloride analogue (VI), followed by simultaneous addition of 4‑aminoacetophenone to the amide derivative. Treatment of the crude product with various substituted aryl benzaldehydes in the presence of NaOH afforded, after chromatographic purification, the chalcone-amide analogues (VIII–XVII) in 60–73% yield (Scheme 1).

Scheme 1. Synthesis of some novel ketoprofen–chalcone-amide hybrides (VIII)–(XVII).
The structures of compounds (VIII)–(XVII) were confirmed by the IR, 1H, and 13NMR spectra. The IR spectra of (VIII)–(XVII) were characterized by the appearance of the absorption band at 1631–1705 cm–1, attributed to the (C=O) stretching, while the strong absorption bands in the range 1585–1600 cm–1, were assigned to (C=C) stretching group. In addition, the spectra showed weak absorption bands appeared in the range 3036–3053 cm–1, were due to the (CH) stretching. The medium absorption bands at the regions 3455 and 3228–3335 cm–1, were assigned to the OH and NH2 groups of compounds (XI) and (XII). In the 1H NMR spectra of compounds (VIII)–(XVII), the olefinic proton of chalcone moiety H-7 appeared as a broad singlet at the regions δ 8.54–7.90 ppm, while H‑8 of the same group overlapped with the aromatic protons at the regions δ 8.10–7.88 and 7.38–7.25 ppm. H-18 appeared as a broad singlet at the regions δ 3.46–3.31 ppm, whereas the singlet at the regions δ 1.39–1.30 ppm assigned to Me-31.
The aliphatic protons were fully analysed (c.f. Experimental section). In the 13C NMR spectra of compounds (VIII)–(XVII), the carbonyl carbon atoms of the chalcone scaffold C9=O resonated at the regions δ 172.3–170.0 ppm, whereas the carbonyl carbon atoms C17=O and C24=O were appeared at δ 191.9–189.7 ppm. C-7 and C-13 resonated together at the regions δ 146.6–143.9 ppm, while C-21 appeared at the regions δ 139.0–136.5 ppm. The resonances at the regions δ 137.1–133.3 assigned to carbon atoms C-19 and C-25 as overlapped signals, whereas C-8 appeared at the regions δ 126.7–121.9 ppm. The signals at the regions δ 44.0–40.1 ppm attributed to the carbon atom C-18. Carbon atom C-F of compound (XV) appeared as a doublet at δ 157.6 (JC,F = 247 Hz). The other aromatic and aliphatic substituents carbon atoms were fully analysed (c.f. Experimental section). Compound (XII) was selected for further NMR experiments. From a gradient HMBC [27] NMR spectrum, the carbonyl carbon atom of the chalcone moiety C9=O at δ 189.7 ppm showed three 3JC,H couplings with H-7 proton at δ 7.96 ppm as well as with H-11 together with H‑15 at δ 7.88 ppm, respectively. Further, H-18 at δ 3.44 ppm exhibited two 2JC,H couplings: one with carbonyl carbon atom C17=O at δ 171.5 ppm and the other coupling with methyl carbon atom Me-31 at δ 19.8 ppm, respectively (Fig. 2).
The mass spectra of the prepared compounds showed the correct molecular ions suggested by their molecular formulas.
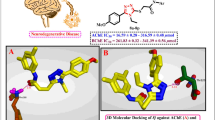
In vitro AChE inhibition activity. Acetylcholinesterase (AChE) is an enzyme that catalyzes the breakdown of acetylcholine and of some other choline esters that function as neurotransmitters. AChE is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. An acetylcholinesterase inhibitor (often abbreviated AChEI) or anti-cholinesterase is a chemical or a drug that inhibits the acetylcholinesterase enzyme from breaking down acetylcholine, thereby increasing both the level and duration of action of the neurotransmitter acetylcholine. In the present work, compounds (VIII)–(XVII) were screened for their inhibitory potential against acetylcholinesterase, following the Ellman method [28] and using galantamine as the positive control. The results are presented in Table 1 with varying degrees of inhibition pattern, where the IC50 values of compounds are in the micromolar range. The results indicated that all compounds exhibited good to moderate inhibitory activities against AChE. Interestingly, compound (IX), exhibited the strongest AChE inhibitory activity (IC50 = 0.98 μM) of the series, whereas compounds (X), (XI), (XIII), (XV), and (XVI) showed a moderate activity against AChE with IC50 values of 5.19, 12.19, 8.18, 9.61, and 6.61 μM, respectively. From these results it is evident that the methyl group as a substituent on the phenyl-chalcone scaffold has a vital role in the inhibition of acetylcholinesterase. The replacement of methyl to methoxy group, as in the case of product (X), will make the compound activity approximately 5-fold less effective against AChE. The least activity was observed in compound (XIV), which has the 4-nitro substituent on the phenyl-chalcone moiety with IC50 of 58.94 μM against AChE, while the 4-chloro or 4‑fluoro substituents on the same moiety, as electron-withdrawing groups, led to marked improvement in the activity against AChE (IC50 = 6.61 and 9.61 μM, respectively). Further, it was found that the 4-bromo substituted compound (XVII) is less active (IC50 = 21.27 μM) than the chloro and fluoro derivatives. However, compound (XI) with 4-hydroxyl substituent exhibited a moderate activity against AChE. The structure-activity relationship study revealed that the activity is greatly dependent on the type and nature of the substituents. Figure S1 (Supplementary Material) shows the inhibition (%) of the acetylcholinesterase versus the concentrations of compounds (VIII–XVII).
Molecular modelling analysis. Docking studies were performed in order to gain more insight into the binding mode of the most active compounds of the chalcone amide derivatives derived ketoprofen to the AChE enzyme. The structures of the studied ligands were prepared with the MOE interface and minimized using the default procedure in MOE (Molecular Operating Environment (MOE), 2015.10 [29]; Chemical Computing Group Inc.: Montreal, QC, Canada) with the Amber10:EHT force field. The crystal structure of the receptor with ID 1EVE was retrieved as a PDB file from the PDB base. The co-crystallized waters and the NAG ligands were stripped out before docking. The protein structure was corrected and hydrogen atoms were added and the protein-native ligand structure was then energy minimized using the Amber10:EHT force field. In docking studies were used the Stochastic search method and London dG scoring function, which is implemented in MOE. Root Mean Squared Distance (RMSD) corresponding atoms in an initial conformer and a docked pose was used to evaluate the quality of self-docking and cross-docking studies. The satisfactory values for RMSD were those lower than or equal to 2 Å.
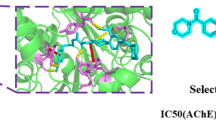
In our search for new lead derivatives as acetylcholinesterase (AChE) inhibitor, we have selected compound (IX) for molecular docking study. Thus, molecular docking of conjugate (IX) into the three-dimensional AChE (PDB ID: 1EVE) was performed using the MEO program [29]. The prospective ligands were ranked according to the highest energy of the best conforms. The calculated binding energy scores for compound (IX) is –9.51 kcal mol–1, indicating selectivity binding of this analogue to the active site of the protein receptor pocket (1EVE). The chalcone moiety of ketoprofen backbone oriented in an appropriate position for its binding with the amino acids of AChE via the π–π stacking interaction with Trp279 and Tyr334. Additionally, non-bonded amino acid residues surrounded (IX) were observed. In addition, non-bonded amino acid residues such as Gln74, Tyr84, Tyr70, Tyr130, Leu127, Tyr116, Glu199, Asp72, Ser122, Phe331, Phe330 and Ser386 of the receptor surrounded the compound (IX) were observed, which would enhance its inhibitory potency.
Quantitative structure–activity relationship (QSAR). QSAR plays a crucial role in drug development as it analyzes the properties of the drug. It is a mathematical model that links the structural features of the compounds (i.e. molecular descriptors) to their quantity showing specific biological or chemical activity [30]. Therefore, it is important in QSAR study to establish the relationship relationship between IC50 and numerous parameters by regression models. In this study, we investigated the structural features and conformational behaviour and the optimized geometries of building blocks of chalconyl steroids and their analogues, at the PWC/DNP level of theory using the software Dmol [31] in Materials Studio package. The QSAR study was done with the Materials Studio package using genetic function approximation (GFA) technique [32]. The quantum chemical indices EHOMO, ELUMO, and molecular flexibility were calculated with VAMP software. This will serve as a basis for future theoretical and experimental work on more complex aromatic steroid structures related to their biological activity. A set data of ten ketoprofen carrying chalcone-amide derivatives with selected descriptors has been chosen to study their QSAR using GFA method to generate a linear model with two varied equations (1) and (2) obtained for calculating predictive AChE inhibition activity as shown in Tables 2 and 3.
In conclusion, the binding score for the tested compounds were congruent with their AChE inhibitory activity. A good correlation between the predicted and the experimentally observed inhibitory activities (pIC50) (Tables 2 and 3) of the most ketoprofen-chalcone-amide hybrides suggested that the identified binding conformations of these inhibitors are reliable. The results of docking study provided an insight into the pharmacophoric structural requirements (compound IX) for AChE inhibitory activity of this class of compounds.
EXPERIMENTAL
Melting points are uncorrected and were measured on a Büchi melting point apparatus B-545 (BüchiLabortechnik AG, Switzerland). NMR spectra were recorded on 400 (1H) and on 150:91 MHz (13C) spectrometers (Bruker, Germany) in DMSO-d6 with TMS as internal standard and on the δ scale in ppm. Mass spectra (EI, 70 eV, and FAB) were recorded on MAT 8200 spectrometers (Finnegan MAT, USA). TLC plates 60 F254 were purchased from Merck. The chromatograms were visualized under UV 254-366 nm and iodine. For atom numbering refer to Fig. 2.
N-(4-Acetylphenyl)-2-(3-benzoyl)propenamide amide derivative of ketoprofen (VII). A mixture of ketoprofen (V) (282 mg, 1.0 mmol) and PCl5 (459 mg, 3.0 mmol) was stirred at 50°C, for 30 min, followed by addition of 4-amino acetophenone (270 mg, 2.0 mmol). The mixture was stirred at the same temperature for another 2 h. After cooling, few drops of water were added and stirred for 15 min, then the mixture was partitioned between CHCl3 (3 × 30 mL) and water (30 mL). The combined organic layers were dried (Na2CO3), filtered and evaporated to dryness. The residue was purified on a short SiO2 column (5 g) using, in gradient, MeOH (0–10%) and CHCl3 as eluent to give the desired amide (VII) (204 mg, 55%) as an amorphous.
General procedure for preparation of ketoprofen chalcone derivatives (VIII–XVII). To a stirred solution of ketone derivative (VII) (100 mg, 0.27 mmol) in EtOH (10 mL) was added arylaldehydes (0.37 mmol) and aq. solution of 2 M NaOH (5 mL). After stirring at ambient temperature for 24 h, the mixture was neutralized with 1 M HCl and partitioned with EtOAc (3–15 mL). The combined organic extracts were washed with brine, dried over Na2SO4 and evaporated in vacuo. The residue was purified on a short SiO2 column using the eluent hexane: EtOAc (3 : 2) as eluent to give the chalcone analogues (VIII–XVII).
2-(3-Benzoylphenyl)-N-(4-cinnamoylphenyl)propanamide (VIII). From benzaldehyde (39 mg). Yield: 102 mg (60%) as a yellow amorphous, Rf = 0.64, IR (film, ν, cm–1): 3348 (NH), 1658 (C=O), 1597 (C=C); 1H NMR, δ, ppm: 7.90–7.23 (m, 20H, Ar–H + Holefinic-7 + Holefinic-8), 3.31 (br s, 1H, H-18); 1.30 (s, 3H, Me-31); 13C NMR, δ, ppm: 190.1 (C24=O), 189.8 (C9=O), 172.0 (C17=O), 143.9 (C-7 + C-13), 136.5 (C-21), 134.8 (C-19 + C-25), 132.1. 131.2, 129.0, 128.4, 127.8, 127.1, 126.5, 126.0, 125.4, 124.0 (Carom), 122.4 (C-8), 40.1 (C-18), 19.9 (Me-31); EIMS: m/z, 460 [M + H]+.
2-(3-Benzoylphenyl)-N-(4-(3-(4-tolyl)acryloyl)-phenyl)propanamide (IX). From 4-methylbenzaldehyde (44 mg). Yield: 114 mg (65%) as a brown solid, mp 66–68°C, Rf = 0.69, IR (film, ν, cm–1): 2920 (CΗ), 3298 (NH), 1670 (C=O), 1600 (C=C); 1H NMR, δ, ppm: 9.57 (s, 1H, NH), 8.51 (br s, 1H, Holefinic-7), 8.10–7.26 (m, 18H, Ar–H + Holefinic-8), 3.38 (br s, 1H, H-18), 2.03 (s, 3H, C1-Me), 1.31 (s, 3H, Me-31); 13C NMR, δ, ppm: 190.3 (C24=O), 189.7 (C9=O), 170.0 (C17=O), 146.0 (C-7 + C-13), 137.9 (C-21), 135.9 (C-19 + C-25), 132.4, 132.0, 131.1, 129.5, 128.7, 128.5, 127.7, 126.0, 125.4 (Carom), 124.2 (C-8), 41.9 (C-18), 26.4 (Me-1), 19.2 (C-31); EIMS: m/z, 474 [M + H]+.
2-(3-Benzoylphenyl)-N-(4-(3-(4-methoxyphenyl)-acryloyl)phenyl)propanamide (X). From 4-methoxybenzaldehyde (50 mg). Yield: 110 mg (61%), mp 120–122°C, Rf = 0.70, IR (film, ν, cm–1): 3352 (NH), 2924 (CH), 1697 (C=O), 1593 (C=C); 1H NMR, δ, ppm: 8.60 (br s, 1H, Holefinic-7), 8.28 (br s, 2H, H-11 + H-15), 7.98–7.25 (m, 16H, Ar–H + Holefinic-8), 3.68 (br s, 1H, H-18), 3.21 (s, 3H, OMe), 1.55 (s, 3H, Me-31); 13C NMR, δ, ppm: 191.4 (C9=O + C24=O), 171.7 (C17=O), 153.6 (C-OMe), 145.8 (C-7 + C-13), 137.3 (C-21), 136.4 (C-19 + C-25), 132.4, 132.0, 129.4, 128.4, 127.3, 128.5, 127.7, 126.8, 125.8, 125.4 (Carom), 124.6 (C-8), 115.2 (C-2 + Carom-6), 53.1 (OMe), 42.8 (C-18), 19.9 (Me-31); EIMS: m/z, 490 [M + H]+.
2-(3-Benzoylphenyl)-N-(4-(3-(4-hydroxyphenyl)-acryloyl)phenyl)propanamide (XI). From 4-hydroxybenzaldehyde (45 mg). Yield: 98 mg (56%) as a brown solid, mp 107–109°C, Rf = 0.69, IR (film, ν, cm–1): 3456 (ΟΗ), 3336, (NH), 1674 (C=O), 1597 (C=C); 1H NMR, δ, ppm: 9.57 (s, 1H, OH), 8.54 (br s, 1H, Holefinic-7), 7.98–7.28 (m, 16H, Ar–H + Holefinic-8), 6.56 (br s, 2H, H-2 + H-6), 3.48 (br s, 1H, H-18), 1.37 (s, 3H, Me-31); 13C NMR, δ, ppm: 190.9 (C24=O), 189.7 (C9=O), 171.8 (C17=O), 161.4 (C–OH), 145.9 (C-7 + C-13), 137.4 (C-21), 136.5 (C-19 + C-25), 132.4, 132.1, 129.6, 129.4, 128.5, 127.5, 127.2, 126.9, 125.7, 125.5 (Carom), 124.9 (C-8), 115.6 (C-2 + C-6), 43.0 (C-18), 19.5 (Me-31); EIMS: m/z, 476 [M + H]+.
N-(4-(3-(4-Aminophenyl)acryloyl)phenyl)-2-(3-benzoylpheyl)propanamide (XII). From 4-aminobenzaldehyde (45 mg). Yield: 117 mg (67%), mp 82–84°C, Rf = 0.67, IR (film, ν, cm–1): 3376 (NH), 3335, 3228 (OH and NH2), 3053 (CH), 1676 (C=O), 1589 (C=C); 1H NMR, δ, ppm: 9.64 (br s, 2H, NH2), 7.96 (br s, 1H, Holefinic-7), 7.88–7.37 (m, 16H, H-11 + H-15 + Ar–H + Holefinic-8), 6.64 (m, 2H, H-2 + H-6), 3.44 (br s, 1H, H-18), 1.38 (s, 3H, Me-31); 13C NMR, δ, ppm: 191.2 (C9=O + C24=O), 171.5 (C17=O), 146.6 (C-7 + C-13 + C-NH2), 137.4 (C-21), 136.6 (C-19 + C-25), 132.2, 132.0, 129.8, 129.3, 128.3, 127.7, 127.5 (Carom), 126.8 (C-8), 115.7 (C-2 + C-6), 43.2 (C-18), 19.8 (Me-31); EIMS: m/z, 475 [M + H]+.
2-(3-Benzoylphenyl)-N-(4-(3-(4-dimethylamino)-acryloyl)phenyl)propanamide (XIII). From 4-(N,N-dimethylamino)benzaldehyde (55 mg). Yield: 117 mg (63%), mp 110–112°C, Rf = 0.65, IR (film, ν, cm–1): 3356 (NH), 2897 (CH), 1662 (C=O), 1597 (C=C); 1H NMR, δ, ppm: 9.99 (s, 1H, NH), 7.97 (br s, 1H, Holefinic-7), 7.91–7.38 (m, 16H, Ar–H + Holefinic-8), 6.76 (m, 2H, H-2 + H-6), 3.48 (br s, 1H, H-18), 1.37 (s, 3H, Me-31); 13C NMR, δ, ppm: 191.9 (C9=O + C24=O), 171.7 (C17=O), 146.3 (C-NMe2), 144.9 (C-7 + C-13), 137.6 (C-21), 137.1 (C-19 + C-21 + C-25), 132.5, 132.1, 131.5, 130.0, 129.7, 128.4, 127.1, 127.2 (Carom), 126.7 (C-8), 115.5 (C-2 + C-6), 43.8 (C-18), 19.9 (Me-31); EIMS: m/z, 503 [M + H]+.
2-(3-Benzoylphenyl)-N-(4-(3-(4-nitrophenyl)-acryloyl)phenyl)propenamide (XIV). From 4-nitrobenzaldehyde (56 mg). Yield: 136 mg (73%) as an orange-red solid, mp 186–188°C, Rf = 0.72, IR (film, ν, cm–1): 3387 (NH), 2974 (CH), 1705 (C=O), 1597 (C=C); 1H NMR, δ, ppm: 9.71 (s, 1H, NH), 8.47 (br s, 2H, H-2 + H-6), 8.32 (br s, 1H, Holefinic-7), 7.89–7.32 (m, 16H, Ar–H + Holefinic-8), 6.76 (m, 2H, H-3 + H-5), 3.46 (br s, 1H, H-18), 1.38 (s, 3H, Me-31); 13C NMR, δ, ppm: 190.9 (C9=O + C24=O), 171.9 (C17=O), 147.8 (C-NO2), 145.1 (C-7 + C-13), 137.6 (C-21), 137.0 (C-19 + C-25), 133.4, 132.5, 130.9, 129.8, 128.8, 127.4 (Carom), 121.9 (C-8), 42.3 (C-18), 19.9 (Me-31); EIMS: m/z, 505 [M + H]+.
2-(3-Benzoylphenyl)-N-(4-(3-(4-fluorophenyl)-acryloyl)phenyl)propanamide (XV). From 4-fluorobenzaldehyde (46 mg). Yield: 115 mg (65%) as an oil, Rf = 0.56, IR (film, ν, cm–1): 3348 (NH), 2924 (CH), 1672 (C=O), 1597 (C=C), 829 (C–F); 1H NMR, δ, ppm: 9.53 (s, 1H, NH), 8.51 (br s, 1H, Holefinic-7), 7.98–7.26 (m, 18H, Ar–H + Holefinic-8), 3.41 (br s, 1H, H-18), 1.31 (s, 3H, Me-31); 13C NMR, δ, ppm: 191.2 (C9=O + C24=O), 172.2 (C17=O), 157.6 (d, JC,F = 247 Hz, C–F), 144.8 (C-7 + C-13), 137.4 (C-19 + C-21 + C-25), 132.4, 132.0, 130.6, 129.5, 128.6, 128.4, 127.5, 126.7 (Carom), 122.5 (C-8), 115.8 (C-2 + C-6), 43.0 (C-18), 19.9 (Me-31); EIMS: m/z, 477/479 [M + H]+.
2-(3-Benzoylphenyl)-N-(4-(3-(4-chlorophenyl)-acryloyl)phenyl)propanamide (XVI). From 4-chlorobenzaldehyde (52 mg). Yield: 120 mg (66%) as a yellow solid, mp 176–178°C, Rf = 0.55, IR (film, ν, cm–1): 3171 (NH), 1674 (C=O), 1593 (C=C), 833 (C–Cl); 1H NMR, δ, ppm: 8.79 (s, 1H, NH), 8.36 (br s, 1H, Holefinic-7), 8.07–7.32 (m, 18H, Ar–H + Holefinic-8), 3.38 (br s, 1H, H-18), 1.37 (s, 3H, Me-31); 13C NMR, δ, ppm: 191.5 (C24=O), 189.4 (C9=O), 171.9 (C17=O), 144.9 (C-7 + C-13), 137.1 (C–Cl), 136.5 (C-19 + C-21 + C-25), 131.5, 130.6, 129.8, 128.5, 127.8, 126.5 124.7 (Carom), 122.3 (C-8), 43.8 (C-18), 19.7 (Me-31); EIMS: m/z, 494/496 [M + H]+.
2-(3-Benzoylphenyl)-N-(4-(3-(4-bromophenyl)-acryloyl)phenyl)propanamide (XVII). From 4-bromobenzaldehyde (69 mg). Yield: 122 mg (63%) as a pale yellow solid, mp 152–154°C, Rf = 0.71, IR (film, ν, cm–1): 3335 (NH), 3043 (CH), 1974 (C=O), 1585 (C=C), 594 (C–Br); 1H NMR, δ, ppm: 9.86 (s, 1H, NH), 8.38 (br s, 1H, Holefinic-7), 7.97–7.37 (m, 18H, Ar–H + Holefinic-8), 3.36 (br s, 1H, H-18), 1.39 (s, 3H, Me-31); 13C NMR, δ, ppm: 191.0 (C24=O), 189.9 (C9=O), 172.3 (C17=O), 144.1 (C-7 + C-13), 139.0 (C-21), 133.3 (C-19 + C-25), 133.9, 132.4, 131.1, 130.5, 129.5, 129.3, 128.5, 127.5, 126.6, 124.8 (Carom), 122.4 (C-8), 44.0 (C-18), 19.9 (Me-31); EIMS: m/z, 525/527 [M + H]+.
AChE activity assay. Human serum AChE activity was determined using Ellman et al. [28] method as follows: 5,5'-dithiobis-(2-nitrobenzoic acid (DTNB) solution (50 μL, 0.001 M) was added to sodium phosphate buffer solution (2.25 mL, pH 7.3, 0.2 M), then serum (10 μL) was added, mixed well and 2 mL of the mixture was transferred to a measuring cell (1 cm). This procedure was followed by addition of acetyl thiocholine iodide (ASChI) (34 μL, 0.06 M). The changes in absorbency are measured before and after adding the substrate at (430 nm) for (3 min). The enzyme activity is calculated as concentration in μ mole of the substrate hydrolyzed to each mL of samples in 3 min and expressed as μmole/3 min/mL. The IC50 values were calculated by Bliss method [33] and expressed as mean ± SD of the replicates.
CONCLUSIONS
We have synthesized a new series of ketoprofen-based chalcone derivatives derived ketoprofen as potential acetylcholinesterase (AChE) inhibitors. Compound 2-(3-benzoylphenyl)-N-(4-(3-(4-tolyl)-acryloyl)phenyl) propanamide (IX) bearing methyl substituent at position C-4 of the phenyl group of the chalcone moiety showed significant inhibitory effect against the AChE. In general, substituents such as 4‑methoxyphenyl, 4-hydroxyphenyl, 4-fluorophenyl, 4-fluorophenyl, and 4-chlorophenyl are beneficial for the activity of chalcone-amide derivatives derived ketoprofen. Introduction of 4-methylphenyl substituent in the chalcone group resulted in selective inhibitory effect against AChE. However, introduction of unsubstituted phenyl, 4-aminophenyl, 4-nitrophenyl and 4-bromophenyl moieties on chalcone scaffold does not improve the activity. Therefore, (IX) could be a promising lead as AChE inhibitor due to its potent activity against this enzyme.
REFERENCES
Thies, W. and Bleile, L., Alzheimers Dement., 2013, vol. 9, pp. 208–245. https://doi.org/10.1016/j.jalz.2013.02.003
Prince, M., Ali, G.-C. Guerchet, M., Prina, M., Albanese, E., and Wu, T.-T., Alzheimer’s Res. Ther., 2016, vol. 8, pp. 23–35. https://doi.org/10.1186/s13195-016-0188-8
Hample, H., Mesuam, M.-M., Cuello, A.C., Farlow, M.R., Giacobini, E., Grossberg, G.T., Khachaturian, A.S., Vergallo, A., Cavedo, E., Snyder, P.J., and Khachaturian, Z.S., Brain, 2018, vol. 141, pp. 1917–1933. https://doi.org/10.1093/brain/awy132
Anand, P., Singh, B., and Singh, N., Bioorg. Med. Chem., 2012, vol. 20, pp. 1175–1180. https://doi.org/10.1016/j.bmc.2011.12.042
Schuster, D., Spetea, M., Music, M., Rief, S., Fink, M., Kirchmair, J., Schutz, J., Wolber, G., Langer, T., Stuppner, H., Schmidhammer, H., and Rollinger, J.M., Bioorg. Med. Chem., 2010, vol. 18, pp. 5071–5080. https://doi.org/10.1016/j.bmc.2010.05.071
Coyle, J.T., Price, D.L., and De Long, M.R., Science, 1983, vol. 219, pp. 1184–1190. https://doi.org/10.1126/science.6338589
Francis, P.T., Palmer, A.M., Snape, M., and Wilcock, G.K., J. Neurol. Neurosurg. Psychiatry, 1999, vol. 66, pp. 137–147. https://doi.org/10.1136/jnnp.66.2.137
Lane, R.M., Potkin, S.G., and Enz, A., Int. J. Neuropsychopharmacol., 2006, vol. 9, pp. 101–124. https://doi.org/10.1017/S1461145705005833
Giacobini, E., Pharmacol. Res., 2004, vol. 50, pp. 433–440. https://doi.org/10.1016/j.phrs.2003.11.017
Cacabelos, R., Torrellas, C., Teijido, O., and Carril, J.C., Pharmacogenomics, 2016, vol. 17, pp. 1041–1074. https://doi.org/10.2217/pgs-2016-0031
Goyal, D., Kaur, A., and Goyal, B., ChemMedChem, 2018, vol. 13, pp. 1275–1299. https://doi.org/10.1002/cmdc.201800156
Benek, O., Soukup, O., Pasdiorova, M., Hroch, L., Sepsova, V., Jost, P., Hrabinova, M., and Jun, D., ChemMedChem, 2016, vol. 11, pp. 1264–1269. https://doi.org/10.1002/cmdc.201500383
Tumiatti, V., J. Med. Chem., 2001, vol. 44, pp. 105–109. https://doi.org/10.1021/jm000991r
Sangnoi, Y., Sakulkeo, O., Yuenyongsawad, S., Kanjana-opas, A., Ingkaninan, K., Plubrukarn, A., and Suwanborirux, K., Mar. Drugs, 2008, vol. 6, pp. 578–586. https://doi.org/10.3390/md20080029
Liu, H.-R., Huang, X.-Q., Lou, D.-H., Liu, X.-J., Liu, W.-K., and Wang, Q.-A., Bioorg. Med. Chem. Lett., 2014, vol. 24, pp. 4749–4753. https://doi.org/10.1016/j.bmcl.2014.07.087
Zhao, F.-C., Wu, Y., and Song, X.-J., Med. Sci. Monit., 2017, vol. 23, pp. 3311–3317. https://doi.org/10.12659/MSM.901842
Díaz-Rubio, L., Hernández-Martinez, R., Estolano-Cobián, A., Chávez-Velasco, D., Salazar-Aranda, R., de Torres, N.W., Rivero, I.A., García-González, V., Ramos, M.A., and Córdova-Guerrero, I., App. Sci. 2019, vol. 9, pp. 410–439. https://doi.org/10.3390/app9030410
Ismail, M.M., Kamel, M.M., Mohamed, L.W., Faggal, S.I., and Galal, M.A., Molecules, 2012, vol. 17, pp. 7217–7231. https://doi.org/10.3390/molecules17067217
de Souza, G.A., da Silva, S.J., Del Cistia, C.N., Pitasse-Santos, P., Pires, L.O., Passos, Y.M., Cordeiro, Y., Cardoso, C.M., Castro, R.N., Sant’Anna, C.M.R., and Kümmerle, A.E., J. Enzyme Inhib. Med. Chem., 2019, vol. 34, pp. 631–637. https://doi.org/10.1080/14756366.2019.1571270
Zhou, X., Wang, X.-B., Wang, T., and Kon, L.-Y., Bioorg. Med. Chem., 2008, vol. 16, pp. 8011–8021. https://doi.org/10.1016/j.bmc.2008.07.068
Sonmez, F., Kurt, B.Z., Gazioglu, I., Basile, L., Dag, A., Cappello, V., Ginex, T., Kucukislamoglu, M., and Guccione, S., J. Enzyme Inhib. Med. Chem., 2017, vol. 32, pp. 285–297. https://doi.org/10.1080/14756366.2016.1250753
Guzior, N., Wi ckowska, A., Panek, D., and Malawska, B., Curr. Med. Chem., 2013, vol. 22, pp. 373–404. https://doi.org/10.2174/0929867321666141106122628
Anand, P. and Singh, B., Arch. Pharm. Res., 2013, vol. 36, pp. 375–399. https://doi.org/10.1007/s12272-013-0036-3
McHardy, S.F., Wang, H.-Y.L., McCowen, S.V., and Valdez, M.C., Expert Opin. Ther. Pat., 2017, vol. 27, pp. 455–476. https://doi.org/10.1080/13543776.2017.1272571
Sharma, K., Mol. Med. Rep., 2019, vol. 20, pp. 1479–1487. https://doi.org/10.3892/mmr.2019.10374
Zhuang, C., Zhang, W., Sheng, C., Zhang, W., Xing, C., and Miao, Z., Chem. Rev., 2017, vol. 117, pp. 7762–7810. https://doi.org/10.1021/acs.chemrev.7b00020
Willker, W., Leibfritz, D., Kerssebaum, R., and Bermel, W., Mag. Res. Chem., 1993, vol. 31, pp. 287–292. https://doi.org/10.1002/mrc.1260310315
Ellman, G.L., Courtney, K.D., Andresjr, V., and Featherstone, R.M., Biochem. Pharmacol., 1961, vol. 7, pp. 88–90, IN1, 91–95. https://doi.org/10.1016/0006-2952(61)90145-9
Molecular Operating Environment (MOE), ver. 2015.10, Chemical Computing Group Inc., Montreal, QC, Canada, 2015.
Barril, X. and Morley, S.D., J. Med. Chem., 2005, vol. 48, 4432–4443. https://doi.org/10.1021/jm048972v
DMOL 3 User Guide, San Diego, CA, USA: Accelrys, Inc., 2003.
Rogers, D. and Hopfinger, A.J., J. Chem. Inf. Comput. Sci., 1994, vol. 34, pp. 854–866. https://doi.org/10.1021/ci00020a020
Bliss, C.I., Microbiol. Mol. Biol. Rev., 1956, vol. 20, pp. 243–258. https://doi.org/10.1128/br.20.4.243-258.1956
ACKNOWLEDGMENTS
The authors thank Department of Chemistry, University of Basrah for the technical facilities. This paper is dedicated to the soul of Dr. Suha Al-Mosawi who passed away recently.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving human participants performed by any of the authors and does not contain any studies involving animals performed by any of the author.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Additional information
Abbreviations: BTPhP, 2-(3-benzoylphenyl)-N-(4-(3-(4-tolyl)acryloyl)phenyl) propanamide; MePPh, 2-(3-benzoylphenyl)-N-(4-(3-(4-methoxyphenyl)acryloyl)phenyl)propanamide; HydPh, 2-(3-benzoylphenyl)-N-(4-(3-(4-hydroxyphenyl)acryloyl)phenyl)propanamide; FPh, 2-(3-benzoylphenyl)-N-(4-(3-(4-fluorophenyl)acryloyl)phenyl)propanamide; ChPh, 2-(3-benzoylphenyl)-N-(4-(3-(4-chlorophenyl)acryloyl)phenyl)propanamide.
Supplementary Information
Rights and permissions
About this article
Cite this article
Al-Mosawi, S.K., Al-Hazam, H.A., Abbas, A.F. et al. Synthesis and QSAR of Novel Ketoprofen–Chalcone-Amide Hybrides as Acetylcholinesterase (AChE) Inhibitors for Possible Treatment of Alzheimer Disease. Russ J Bioorg Chem 48, 801–808 (2022). https://doi.org/10.1134/S1068162022040045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022040045