Abstract
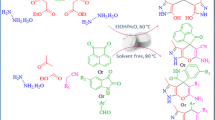
For the first time, alpha-Casein was used as an efficient and eco-friendly catalyst for an effective and facile preparation of dihydropyranopyrazoles and spiropyranopyrazoles. The synthesis of bis (pyrazol-5-ols) derivatives was developed via one-pot, pseudo-five-component condensation, and the target dihydropyrano[2,3-c]pyrazoles and spiropyranopyrazoles were prepared by one-pot four-component reaction. This new method of employing alpha-Casein, which is a green, recyclable, non-toxic and commercially available catalyst, offers advantages such as mild condition, short reaction times, easy work-up, no need for column chromatography, and high yields of the products which make it more economic than other environmentally synthetic protocols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthesis of complex molecules from simple and readily available materials has become one of the important aspects of synthesis of organic compounds. In this context, multicomponent reactions (MCRs) are considered, because three or more simple and flexible starting materials are brought together in a highly convergent approach to immediately make molecular structure and complexity [1,2,3,4]. In modern synthetic chemistry, multicomponent reactions have been proved to be a powerful and useful tool. Due to the compact reactions, easy procedures and high yields, they are in the forefront in bioactive medicinal, combinatorial, synthesis of organic, agro and heterocyclic molecules. Moreover, because of simultaneous formation of two or more bonds and scope for green solvent, MCRs have many considerable advantages, such as reduction in reaction time, simple protocols, inexpensive reactants, green principle, lower costs, high atom-economy, energy saving, expensive purification procedure; Thus, such environmentally benign protocols are opportune and well desired [5,6,7,8,9,10].
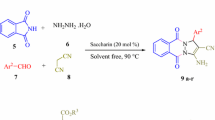
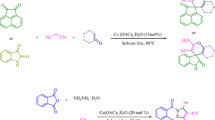
Pyrazoles are considered as an important class of N-heterocycles owing to their wide occurrence, various applications, pharmacological and biological activity, agrochemical, chemical industries and ease of synthesis. They are five-membered ring containing a pyrrole-like N-atom in adjacent positions [8, 11]. Pyrazole ring can be found in natural products including withasomnine, formycin, pyrazofurin, fluviol and drug molecules such as phenazone, Celecoxib, Celebrex, Fomepizole, Ceftolozane, Metamizole, R1530, Viagra, Crizotinib, Lonazolac and Rimonabant (Fig. 1) [12,13,14,15,16,17]. It has been reported that substituted pyrazoles display a wide range of biological properties in medicinal chemistry, for example, as anti-microbial, anti-bacterial, anti-inflammatory, anti-prostate, anti-diabetic, anti-pyretic, anti-depressant, anti-histaminic, anti-biotic, anti-fungal, anti-viral, anti-neoplastic, analgesic, herbicidal, acaricidal and insecticidal [12, 13, 18,19,20]. In coordination chemistry, pyrazoles are used as ligands. They are able to create a bond with metals in different modes and strong bridges [20, 21]. Pyrazole-based dyes are also pharmacological intermediates and the dyestuff industry uses them [20]. The literature survey reveals various ways reported for the synthesis of pyrazole derivatives in the presence of catalysts, namely N-methylimidazolium perchlorate [22], pyridine trifluoroacetate [23], sodium dodecylsulfate [24], triethylbenzylammonium chloride [25], nano-structured diphosphate [26], lemon juice [27], morpholine triflate [28], nanoparticles [29], and triphenylphosphine [30]. Some of these methods have their own weak points such as using toxic reagents, strict reaction condition, costly reagents and catalysts, tedious steps, strong acidic or basic condition, long reaction time and low product yields [7]. However, they have their own advantages too, including operational simplicity, environmental compatibility, non-toxicity, simplified recovery and reusability, low-cost and easy isolation [31,32,33,34]. Development of environmentally benign, efficient, and economic ways for the synthesis of biologically interesting compounds remains a serious challenge in synthetic chemistry [35,36,37,38,39]. The new method of applying α-Casein, which is a green, recyclable, non-toxic and commercially available catalyst, brings advantages such as mild condition, short reaction times, easy work-up, no need for column chromatography, water/ethanol as solvent and high yields of the products which make it more economic than other environmentally synthetic protocols. Herein, we wish to report an efficient one-pot, pseudo-five-component strategy for the rapid synthesis of pyrazole-5-ols 4 from reaction between ethyl acetoacetate 1, hydrazine monohydrate 2, and several aromatic aldehydes 3 and also an efficient one-pot, four-component strategy for effective synthesis of dihydropyrano[2,3-c]pyrazole 6 and spiropyranopyrazoles 8, 10 and 12 by condensation of ethyl acetoacetate 1, hydrazine monohydrate 2, several aromatic aldehydes 3, malononitrile 5 and isatins 7, acenaphthenequinone 9 or ninhydrin 11 in the presence of alpha-Casein as an efficient, green, novel, and eco-friendly catalyst (Fig. 2) in an environmentally benign conditions (Scheme 1) [1, 8, 35].
Experimental
Using an Electro thermal 9100 apparatus and FT-IR-JASCO-460 plus spectrometer, the melting points and IR spectra of all compounds were specified. The 1H and 13C NMR spectra of known compounds were recorded on a Bruker DRX-300 MHz Avance instrument in DMSO at 300 and 75 MHz. All of the reagents were provided from the chemical producer Merck (Darmastadt, Germany), Fluka (Buchs, Switzerland) and Alpha Aesar and used without further purification. TLC was performed on Polygram SILG/UV 254 silica gel plates.
General procedure for the synthesis of 4, 4′-(arylmethylene)bis(1H-pyrazol-5-ol) derivatives (4)
A combination of ethyl acetoacetate (2.0 mmol), hydrazine hydrate (2.0 mmol), and alpha-Casein 20 mmol% (0.157 gr) as catalyst was stirred in EtOH/H2O (2:1) (4 mL). After 5 min, aromatic aldehyde (1.0 mmol) was added, and the mixture was stirred at 60 °C for the time shown in Table 1. The completion of the reaction was observed through thin layer chromatography (TLC). After completion, the reaction mixture was left to cool down to room temperature, and then, water (5 mL) was added to the mixture of reaction, and filtered to separate the product. Finally, the crude product was washed twice (5 mL) with a mixture of water and ethanol (2:1). Then, the solid product was recrystallized from ethanol to obtain the pure product.
General procedure for the synthesis of dihydropyrano[2,3-c]pyrazole (6) and spiropyranopyrazoles derivatives (8, 10 and 12)
A mixture of hydrazine hydrate (1.0 mmol) and ethyl acetoacetate (1.0 mmol) was stirred for 5 min in EtOH/H2O (1:1) until 3-methyl-2-pyrazolin-5-one was precipitated. Then, the α-Casein, malononitrile 5 (1.0 mmol) and aromatic aldehyde 3 (1.0 mmol), isatins 7 (1.0 mmol), acenaphthenequinone 9 (1.0 mmol) or ninhydrin 11 (1.0 mmol) were added, and the mixture was heated at 60 °C. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled down to room temperature, followed by addition of 5 mL water, and subsequent filtration of the mixture for separation of the product. Afterwards, the solid product was washed twice (each time 5 mL) with a mixture of water and ethanol. Then, the resultant product was recrystallized from ethanol to obtain the pure pyranopyrazole derivative. To recover the catalyst, the filtrate was extracted by diethyl ether. The aqueous layer containing α-Casein was separated, followed by evaporation of the solvent component under reduced pressure, resulting in the recovery of α-Casein and reusing it (Fig. 3). As Fig. 3 shows, the catalytic system worked well, up to four catalytic runs and slightly reduced the product yield, which might have been due to the little weight loss of the catalyst during the recovery processes.
Spectral data for the selected compounds
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-nitrophenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4a)
White powder; IR (KBr, cm−1): 3428, 3145, 2968, 1606, 1505; 1H NMR (400 MHz, DMSO-d6): 2.10 (s, 6H, 2CH3), 3.35 (1OH exchanged with water of DMSO-d6), 5.00 (s, 1H, CH), 7.40 (d, J = 8.1 Hz, 2H, H–Ar), 8.14 (d, J = 8.42 Hz, 2H, H–Ar), 11.09 (brs, 3H, 2NH,1OH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(o-tolyl))methyl-3-methyl-1H-pyrazol-5-ol (4d)
Pale orange powder, IR (KBr, cm−1): 3432, 3092, 2926, 1604.58, 1525.05; 1H NMR (400 MHz, DMSO-d6): 1.81 (s, 6H, 2CH3), 2.11 (s, 3H, CH3), 3.38 (2OH exchanged with water of DMSO-d6), 4.92 (s, 1H, CH), 7.02–7.23 (m, 4H, H–Ar), 10.66 (brs, 2H, 2NH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-hydroxy-3-methoxyphenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4e)
White powder; IR (KBr, cm−1): 3373, 3192, 2959, 1610, 1485; 1H NMR (300 MHz, DMSO-d6): δ 2.08 (s, 6H, 2CH3), 3.35 (3OH exchanged with water of DMSO-d6), 3.66 (s, 3H, OCH3), 4.77 (s, 1H, CH), 6.57 (dd, J = 8.1 Hz, J = 1.2 Hz, 1H, H–Ar), 6.65 (d, J = 8.1 Hz, 1H, H–Ar), 6.78 (d, J = 1.5 Hz, 1H, H–Ar), 10.17 (brs, 2H, 2NH). 13C NMR (75 MHz, DMSO-d6): δ 10.9 (CH3), 32.8 (CH), 56.0 (OCH3), 105.0, 112.7, 115.3, 120.4, 134.8, 140.0, 144.9, 147.4, 161.4 (C–Ar).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-hydroxyphenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4h)
White powder; IR (KBr, cm−1): 3415, 3268, 3107, 2928, 1600, 1514; 1H NMR (400 MHz, DMSO-d6): 2.05 (s, 6H, 2CH3), 3.37 (2OH exchanged with water of DMSO-d6), 4.70 (s, 1H, CH), 6.59 (d, J = 8.8 Hz, 2H, H–Ar), 6.90 (d, J = 8.4, 2H, H–Ar), 9.07 (s, 1H, OH), 11.27 (brs, 2H, 2NH).
4-((5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(3-nitrophenyl)methyl)-3-methyl-1H-pyrazol-5-ol (4j)
White powder; IR (KBr, cm−1): 3405, 3096, 2967, 1600, 1527; 1H NMR (400 MHz, DMSO-d6): 2.14 (s, 6H, 2CH3), 3.39 (1OH exchanged with water of DMSO-d6), 5.04 (s, 1H, CH), 7.52–7.63 (m, 2H, H–Ar), 8.00–8.06 (m, 2H, H–Ar), 11.04 (brs, 3H, 2NH,1OH).
3-Hydroxy-3-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)indolin-2-one (4k)
White powder; IR (KBr, cm−1): 3376, 3146, 2876, 1690, 1471; 1H NMR (300 MHz, DMSO-d6): δ 2.15 (s, 3H, CH3), 3.39 (1OH exchanged with water of DMSO-d6), 6.36 (C–OH), 6.82 (d, J = 7.82 Hz, 1H, H–Ar), 6.93 (t, J = 7.52 Hz, 1H, H–Ar), 7.20 (m, 2H, H–Ar), 10.24 (brs, 2H, 2NH). 13C NMR (75 MHz, DMSO-d6): δ 11.9 (CH3), 74.3 (C–OH), 101.1 (C=C–OH), 109.9, 122.0, 125.1, 129.3, 133.7 (C–Ar), 138.1 (CN), 142.2 (C–Ar), 159.1 (OH–C=C), 178.7 (C=O).
5-Bromo-3-hydroxy-3-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)indolin-2-one (4l)
White powder; IR (KBr, cm−1): 3443, 3327, 3071, 1704, 1617,1531; 1H NMR (300 MHz, DMSO-d6): δ 2.26 (s, 3H, CH3), 6.35 (C–OH), 6.78 (d, J = 8.12 Hz, 1H, H–Ar), 7.26 (s, 1H, H–Ar), 7.37 (d, J = 7.52 Hz, 1H, H–Ar), 10.36 (brs, 3H, 2NH, 1OH). 13C NMR (75 MHz, DMSO-d6): δ 12.09 (CH3), 74.3 (C–OH), 101.0 (C=C–OH), 111.9, 113.5, 127.7, 131.8, 136.4 (C–Ar), 138.6 (H3C–C=N), 141.5 (C–Ar), 158.8 (C=C–OH), 178.2 (C=O).
2-Hydroxy-2-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-1H-indene-1,3(2H)-dione (4m)
White powder; IR (KBr, cm−1): 3516, 3370, 3019, 1768, 1748,1710; 1H NMR (300 MHz, DMSO-d6): δ 2.38 (s, 3H, CH3), 3.47 (1OH exchanged with water of DMSO-d6), 6.41 (C–OH), 7.98 (s, 4H, H–Ar), 10.84 (brs, 1H, 1NH). 13C NMR (75 MHz, DMSO-d6): δ 16.8 (CH3), 80.8 (C–OH), 102.7 (CH), 128.7 (C–Ar), 141.5 (H3C–C=N), 145.3 and 145.7 (C–Ar), 163.0 (C=C–OH), 204.1 (C=O).
6-Amino-1,4-dihydro-3-methyl-4-(4-nitrophenyl)pyrano[2,3-c]pyrazole-5-carbonitrile (6b)
White powder; IR (KBr, cm−1): 3476, 3229, 3113, 2975, 2195, 1623, 1648, 1598, 1519, 1402, 1350, 748; 1H NMR (400 MHz, DMSO-d6): δ 1.83 (s, 3H, CH3), 4.85 (s, 1H, CH), 7.08 (s, 2H, NH2), 7.49 (d, J = 7.52 Hz, 2H), 8.22 (d, J = 7.52 Hz, 2H), 12.27 (s, 1H, NH).
6-Amino-1,4-dihydro-3-methyl-4-(4-chlorophenyl)pyrano[2,3-c]pyrazole-5-carbonitrile (6d)
White powder; IR (KBr, cm−1): 3476, 3227, 3094, 2191, 1641, 1592, 1488, 1394, 1082, 797, 744, 499; 1H NMR (400 MHz, DMSO-d6): δ 1.82 (s, 3H, CH3), 4.66 (s, 1H, CH), 6.96 (s, 2H, NH2), 7.22 (d, J = 8.42 Hz, 2H), 7.39 (d, J = 8.42 Hz, 2H), 12.17 (s, 1H, NH).
6-Amino-1,4-dihydro-3-methyl-4-(4-bromophenyl)pyrano[2,3-c]pyrazole-5-carbonitrile (6e)
White powder; IR (KBr, cm−1): 3470, 3227, 3120, 2195, 1651, 1595, 1560, 1401, 1353, 1107, 883, 810, 744, 543; 1H NMR (400 MHz, DMSO-d6): δ 1.80 (s, 3H, CH3), 4.63 (s, 1H, CH), 6.96 (s, 2H, NH2), 7.15 (d, J = 8 Hz, 2H), 7.52 (d, J = 8 Hz, 2H), 12.16 (s, 1H, NH).
6-Amino-1,4-dihydro-3-methyl-4-(4-fluorophenyl)pyrano[2,3-c]pyrazole-5-carbonitrile (6f)
White powder; IR (KBr, cm−1): 3479, 3232, 3117, 2195, 1648, 1595, 1508, 1492, 1398, 1230, 1218, 1156, 807, 560; 1H NMR (400 MHz, DMSO-d6): δ 1.81 (s, 3H, CH3), 4.66 (s, 1H, CH), 6.95 (s, 2H, NH2), 7.09–7.26 (m, 4H),12.18 (s, 1H, NH).
6-Amino-1,4-dihydro-3-methyl-4-(4-hydroxy-3-methoxyphenyl)pyrano[2,3-c]pyrazole-5-carbonitrile (6i)
White powder; IR (KBr, cm−1): 3491, 3412,3274, 3221, 2195, 1657, 1617, 1604, 1512,1399, 1265, 1028; 1H NMR (400 MHz, DMSO-d6): δ 1.85 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 4.53 (s, 1H, CH), 6.58 (dd, J = 8.1 Hz, J = 1.8 Hz, 1H, H–Ar), 6.73–6.76 (2d,2H), 6.83 (s, 2H, NH2), 12.10 (s, 1H, NH).
6-Amino-1,4-dihydro-3-methyl-4-(2,6-dichlorophenyl)pyrano[2,3-c]pyrazole-5-carbonitrile (6j)
White powder; IR (KBr, cm−1): 3405, 3368, 3309,3153, 2183, 1653, 1614, 1599, 1406, 1293, 1072, 826, 789; 1H NMR (400 MHz, DMSO-d6): δ 1.80 (s, 3H, CH3), 5.60 (s, 1H, CH), 7.04 (s, 2H, NH2), 7.33 (t, J = 8.12 Hz, 1H), 7.40 (dd, J = 7.82 Hz, J = 1.5 Hz, 1H), 7.55 (dd, J = 8.82 Hz, J = 1.5 Hz, 1H), 12.13 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6): δ 9.80 (CH3), 32.98 (CH), 53.29 (C–NH2), 95.27 (O–C–CN),120.67, 128.93, 130.06, 131.33, 134.93, 135.43, 135.63, 136.39, 156.0 (C–Aro), 162.56 (CN); MS m/z (%):321.1 (100), 304.3 (51), 282.3 (41), 221.1 (40), 219.1 (56), 185.1 (32) (M+, 1).
Results and discussion
At first, we focused our attention on the synthesis of 4,4ʹ-(arylmethylene)bis(1H-pyrazol-5-ols) and the reaction between ethyl acetoacetate (2.0 mmol), hydrazine hydrate (2.0 mmol), and 4-nitrobenzaldehyde (1.0 mmol) was chosen as a model reaction for preliminary experiments. First, to optimize the reaction temperature, the model reaction was performed using 10, 15 and 20 and 25 mol% of the catalyst at different temperatures. It was revealed that 60 °C is an efficient temperature in terms of reaction time and yield obtained (Table 1, entry 8). When the mixture was stirred at room temperature for 180 min, the product was gained 57% (Table 1, entry 4). Next, the reaction was investigated with different amounts of catalyst and the results were summarized in (Table 1, entries 1–12). The best result was obtained, 94% yield within 15 min, with 20 mol% of catalyst at 60 °C (Table 1, entry 8). Higher amounts of catalyst did not result in significant change in the reaction yields (Table 1, entry 12). If the amount of catalyst was reduced from 20 to 10 mol%, the reaction time increased but the yield decreased (Table 1, entry 5). As it can be seen in Table (1, entry 7), in the absence of catalyst at 60 °C the reaction time improved and offered 52% yield of the expected product. Finally, the effect of solvents, water, ethanol, 1:1 EtOH/H2O, 2:1 EtOH/H2O and 1:2 EtOH/H2O was evaluated and it was found that the rate 2:1 EtOH/H2O is better than other rates (Table 1, entry 8) [1, 37]. In another study for the synthesis of dihydropyrano[2,3-c]pyrazoles, we chose reaction of ethyl acetoacetate (1.0 mmol), hydrazine hydrate (1 mmol), malononitrile (1.0 mmol), and 4-nitrobenzaldehyde (1.0 mmol) as a model reaction. The reaction was investigated with different amounts 10, 15 and 20 mol% of the catalyst at 60, 70 and 80 °C, and also room temperature in water, ethanol and the mixture of them. The summary of results is shown in Table 2. As shown in Table (2, entry 1), the optimization of the reaction conditions demonstrated that the best result was gained 95% when the reaction was performed within 15 min, at 60 °C in the presence of α-Casein (15 mol%) in the rate of 1:1 EtOH/H2O. The yield of the product did not change significantly with the increased amount of catalyst from 15 to 20 mol% (Table 2, entry 6), but with decreased amount of catalyst from 15 to 10 mol% the yield of the product decreased (Table 2, entry 5). In the absence of catalyst, a low yield of the product was achieved after 24 h (Table 2, entry 7) [1, 29, 37, 38].
Interestingly, a variety of aromatic aldehydes including electron-donating and electron-withdrawing groups on the rings (ortho-, meta-, and para-substituted) participated well in this reaction and gave the 4,4ʹ-(arylmethylene)bis(1H-pyrazol-5-ols) 4a–j, 4k–m and dihydropyrano[2,3-c]pyrazoles 6a–j derivatives in good to excellent yield. In the synthesis of compounds 4 and 6, substitution of NO2 group in the fourth position of aromatic aldehyde led to a relatively faster rate and higher yield than the substitution of other groups in various positions of aromatic ring (Tables 3, 4). Then, under this optimized reaction conditions, we used isatins 7, acenaphthenequinone 9 or ninhydrin 11 as a substrate to react with malononitrile 5 and 3-methyl-1H-pyrazol-5(4H)-one. As expected, the reaction developed well to afford spiro[indoline-3,4ʹ-pyrano[2,3-c]pyrazol] 8a-b, spiro[acenaphthylene-1,4ʹ-pyrano[2,3-c]pyrazol] 10a and spiro[ninhydrin-3,4ʹ-pyrano[2,3-c] pyrazole] 12a in good to excellent yields [5, 6, 37] (Table 4).
The probable reaction mechanism for the synthesis of 4,4ʹ-(arylmethylene)bis(1H-pyrazol-5-ols), dihydropyrano[2,3-c]pyrazoles and spiropyranopyrazoles was suggested in Scheme 2. At first, 3-methyl-1H-pyrazol-5(4H)-one 13 was formed from the reaction between ethyl acetoacetate 1 and hydrazine hydrate 2. For the synthesis of 4,4ʹ-(arylmethylene)bis(1H-pyrazol-5-ol) 4, 3-methyl-1H-pyrazol-5(4H)-one by α-Casein via Knoevenagel condensation with the activated carbonyl group of the aromatic aldehydes 3 to create the intermediated 14 where through Michael addition to another 3-methyl-1H-pyrazol-5(4H)-one to give desirable products 4a–j. For the synthesis of dihydropyrano[2,3-c]pyrazoles 6 is proposed the arylidene malononitrile 15 to generate in situ via Knoevenagel condensation by adding malononitrile 5 and active aromatic aldehyde 3. Michael addition of compounds 13 and 15 gives the acyclic adduct products 16, which undergoes intramolecular cyclization and tautomerization to afford the corresponding products 6a-j. On the other hand, for the synthesis of spiropyranopyrazoles, ninhydrine 11, malononitrile 5 and 3-methyl-1H-pyrazol-5(4H)-one 13, undergo Michael addition with Knoevenagel adduct, followed by intramolecular cyclization 17 and 18 and provides the target spiro compounds 12a. α-Casein can catalyze the probable reactions activated by hydrogen bonds [36, 39, 40] (Scheme 2).
To show the capability and efficiency of this work with respect to the reported catalysts for the preparation of 4,4ʹ-(arylmethylene)bis(1H-pyrazol-5-ols) and dihydropyrano[2,3-c]pyrazoles, Table 5 compares our results obtained from the synthesis of compounds 4a and 6b from the reaction of ethyl acetoacetate, hydrazine monohydrate, aromatic aldehyde and malononitrile in the presence of α-Casein with different catalyst and conditions. As it is evident from Table 5, our method was more efficient.
Conclusion
In summary, this paper describes a convenient and highly efficient and green process for the synthesis of functionalized pyrazole-5-ols 4 from reaction between ethyl acetoacetate 1, hydrazine monohydrate 2, and several aromatic aldehydes 3 and also synthesis of dihydropyrano[2,3-c]pyrazole 6 and spiropyranopyrazoles 8, 10 and 12 by condensation of ethyl acetoacetate 1, hydrazine monohydrate 2, several aromatic aldehydes 3, malononitrile 5 and isatins 7, acenaphthenequinone 9 or ninhydrin 11, using α-Casein as a catalyst at 60 °C. The advantages of the present work can be described as: efficiency, simplicity, high generality, short reaction time, high yield and ease of handling of the catalyst. In addition, the catalyst is green, recyclable, non-toxic and removed from the reaction mixture, which make it a useful and attractive procedure for the synthesis of pyran derivatives.
References
M. Fatahpour, S.F. Noori, N. Hazeri, M.T. Maghsoodlou, M.S. Hadavi, S. Mohnaei, J. Saudi Chem. Soc. 21, 998–1006 (2017)
P. Prasanna, S. Perumal, J.C. Menéndez, Green Chem. 15, 12921299 (2013)
P.A. Clarke, S. Santos, W.H.C. Martin, Green Chem. 9, 438–440 (2007)
E. Ruijter, R. Scheffelaar, R.V. Orru, Angew. Chem. Int. Ed. 50, 6234–6246 (2011)
Y. Zou, Y. Hu, H. Liu, D. Shi, ACS. Comb. Sci. 14, 38–43 (2012)
S.A. Padvi, Y.A. Tayade, Y.B. Wagh, D.S. Dalal, Chin. Chem. Lett. 27(5), 714–720 (2016)
S. Maddila, S. Rana, R. Pagadala, S. Kankala, S. Maddila, S.B. Jonnalagadda, Catal. Commun. 61, 26–30 (2015)
M.A. Zolfigol, F. Afsharnadery, S. Baghery, S. Salehzadeh, F. Maleki, RSC Adv. 5(92), 75555–75568 (2015)
M. Srivastava, P. Rai, J. Singh, S. Singh, New J. Chem. 38, 302–307 (2014)
J. Rakhtshah, S. Salehzadeh, E. Gowdini, F. Maleki, S. Baghery, M.A. Zolfigol, RSC Adv. 6, 104875–104885 (2016)
S. Saueressig, J. Tessmann, R. Mastelari, L.P.D. Silva, J. Buss, N.V. Segatto, K.R. Begnini, B. Pacheco, C.M.P.D. Pereira, T. Collares, F.K. Seixas, Biomed. Pharmacother. 98, 390–398 (2018)
D. Nair, P. Pavashe, I.N.N. Namboothiri, Tetrahedron. 74(22), 2716–2724 (2018)
A. Çetin, I. Bildirici, J. Saudi Chem. Soc. 22(3), 279–296 (2018)
S.G. Kucukguzel, S. Senkardes, Eur. J. Med. Chem. 97, 786–815 (2015)
K.M. Kasiotis, E.N. Tzanetou, S.A. Haroutounian, Front. Chem. 2, 1–7 (2014)
A.W. Brown, Adv. Heterocycl. Chem. 126, 55 (2018)
A. Mosallanejad, O. Lorthioir, Tetrahedron Lett. 59, 1708–1710 (2018)
N. Panda, S. Ojha, J. Org. Chem. 861, 244–251 (2018)
G.H. Sayed, M.E. Azab, K.E. Nawer, M.A. Raouf, N.A. Negm, J. Mol. Liq. 252, 329–338 (2018)
C. Bustos, L. Alvarez-Thon, E. Molins, L. Moreno-Villoslada, G. Vallejos-Contreras, C. Sanchez, X. Zarate, D. Mac-leod, E. Carey, Schott, J. Mol. Struct. 1171, 349–361 (2018)
I.D. Alshakova, I. korobkov, L.G. Kuzmina, G.I. Nikonov, J. Org. Chem. 853, 68–73 (2017)
N.G. Khaligh, S.B.A. Hamid, S.J. Titinchi, Chin. Chem. Lett. 27, 104–108 (2016)
E. Soleimani, S. Ghorbani, M. Taran, A.C. Sarvary, C. R. Chimie 15, 955–961 (2012)
W. Wang, S.X. Wang, X.Y. Qin, J.T. Li, Synth. Commun. 35, 1263–1269 (2005)
D. Shi, J. Mou, Q. Zhuang, L. Niu, N. Wu, X. Wang, Synth. Commun. 34, 4557–4563 (2004)
B. Maleki, N. Nasiri, R. Tayebee, A. Khojastehnezhed, H.A. Akhlaghi, RSC Adv. 6, 79128–79134 (2016)
R.H. Vekariya, K.D. Patel, H.D. Patel, Res. Chem. Intermed. 42, 7559–7579 (2016)
C.F. Zhou, J.J. Li, W.K. Su, Chin. Chem. Lett. 27, 1686–1690 (2016)
A. Saha, S. Payra, S. Banerjee, Green Chem. 17, 2859–2866 (2015)
A.K. Imene, F. Amine, L. Oumeima, B. Raouf, B. Boudjemaa, Lett. Org. Chem. 13, 85–91 (2016)
A.H. Chughtai, N. Ahmad, H.A. Younus, A. Laypkov, F. Verpoort, Chem. Soc. Rev. 44, 6804–6849 (2015)
Z. Zhang, F. Xiao, Y. Guo, S. Wang, Y. Liu, ACS. Appl. Mater. Interfaces 5, 2227–2233 (2015)
G. Pal, S. Paul, A.R. Das, New J. Chem. 38, 2787–2791 (2014)
B. Sreedhar, A.S. Kumar, P.S. Reddy, Tetrahedron Lett. 51, 1891–1895 (2010)
N. Hazeri, M.T. Maghsoodlou, F. Mir, M. Kangani, H. Saravani, E. Molashahi, Chin. J. Catal. 35, 391–395 (2014)
K. Niknam, N. Borazjani, R. Rashidian, A. Jamali, Chin. J. Catal. 34, 2245–2254 (2013)
M. Fatahpour, S.F. Noori, N. Hazeri, M.T. Maghsoodlou, M. Lashkari, J. Iran. Chem. Soc. 14, 9, 1945–1956 (2017)
M. Kangani, N. Hazeri, M.T. Maghsoodlou, S.M. Habibi-Khorassani, S. Salahi, Res. Chem. Intermed. 41(4), 2513–2519 (2015)
M. Kangani, N. Hazeri, M.T. Maghsoodlou, A. Ebrahimi, Org. Chem. Res. 2(1), 81–87 (2016)
M.R. Mousavi, N. Hazeri, M.T. Maghsoodlou, S. Salahi, S.M. Habibi-Khorassani, Chin. Chem. Lett. 24(5), 411–414 (2013)
J. Xu-dong, D. Hai-Feng, L. Ying-Jie, C. Jun-Gang, L. Da-Peng, W. Mao-Cheng, Chem. Res. Chin. Univ. 28, 999–1002 (2012)
J. Safaei-Ghomi, B. Khojastehbakht-Koopaei, H. Shahbazi-Alavi, RSC Adv. 4, 46106–46113 (2014)
M.A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, V. Khakyzadeh, S. Azizian, J. Mol. Catal. A Chem. 418–419, 54–67 (2016)
Y. Zou, Y. Hu, Y.H. Liu, D. Shi, ACS Comb. Sci. 14, 38–43 (2011)
M.N. Elinson, A.I. Ilovaisky, V.M. Merkulova, P.A. Belyakov, F. Barba, B. Batanero, Tetrahedron. 68, 5833–5837 (2012)
M.S. Raafat, F.M. Alaa, F.A.L. Fathy, J. Chin. Chem. Soc. 52, 563–567 (2005)
H. Mecadon, M.R. Rohman, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52(19), 2523–2525 (2011)
Y. Zou, H. Wu, Y. Hua, H. Liu, X. Zhao, H. Ji, D. Shi, Ultrason. Sonochem. 18(3), 708–712 (2011)
A. Siddekha, A. Nizam, M.A. Pasha, Spectochim. Acta A. 81, 431–440 (2011)
M.A. Zolfigol, M. Navazeni, M. Yarie, R. Ayazi-Nasrabadi, Appl. Organochem. Chem. 31(6), 1–7 (2017)
M.A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, V. Khakyzadeh, S. Azizian. J. Mol. Catal. A Chem. 54, 418–419 (2016)
S. Paul, K. Pradhan, S. Ghosh, S.K. De, A.R. Das, Tetrahedron. 70(36), 6088–6099 (2014)
Acknowledgements
The authors would like to acknowledge the financial support received from the Research Council of Sistan and Balouchestan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milani, J., Maghsoodlou, M.T., Hazeri, N. et al. Alpha-Casein: an efficient, green, novel, and eco-friendly catalyst for one-pot multi-component synthesis of bis (pyrazol-5-ols), dihydro-pyrano[2,3-c]pyrazoles and spiropyranopyrazoles in an environmentally benign manner. J IRAN CHEM SOC 16, 1651–1664 (2019). https://doi.org/10.1007/s13738-019-01641-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01641-2