Abstract
In this report, synthesis, characterization and application of nano-sphere silica sulfuric acid as a novel and heterogeneous catalyst for the synthesis of β-acetamido ketone derivatives at room temperature are described. The catalyst has been identified using various techniques (XRD, SEM, TEM, EDX, TGA, FT-IR), and results show that it has a spherical shape and its particle size is between 60 and 90 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, considerable emphasis has been placed on improvement in the environmental impact of industrial chemical processes. The development of heterogeneous catalysts for fine chemical synthesis has become a major area of research. The inherent advantages of heterogeneous catalyst system in liquid phase over their homogeneous counterparts lie mainly on their easy separation, avoids formation of inorganic salts, recyclable, non-toxic, easy to handle, safe to store, long life time, easy and inexpensive removal from reaction mixture by filtration or centrifugation, tolerates a wide range of temperatures and pressures, and easy and safe disposal. These type of catalysts have been used for various support materials. One useful example of support material is silica gel [1]. Recently, various types of mesoporous silica have been synthesized. Silica nano-spheres with uniform size have gained considerable attention in scientific research due to their unique properties such as high specific surface area, narrow pore size distribution, large pore volumes, good thermal and chemical stability, and low toxicity [2–4]. Studies found that functionalization and modification of silica nano-spheres made them suitable for future technical applications [5, 6]. For example, mesoporous silica compounds that have been functionalized by sulfonic acid offer simpler, less costly, more reactive and more environmentally benign alternatives than their homogeneous counterparts. These materials are of great interest for use as novel heterogeneous catalysts [7–9].
Multi-component reactions (MCRs), defined as one-pot reactions in which at least three functional groups join through covalent bonds, have been steadily gaining importance in synthetic organic chemistry. The reagents employed may be different molecules or functional groups of the same reagent. Speed, diversity, efficiency, and environmental amiability are some of the key features of this class of reactions [10, 11]. One of the most representative examples of multi-component reactions is preparation of β-acetamido ketone derivatives. These are the precursors of molecules such as 1,3-amino alcohols, and structural scaffolds found in natural nucleoside peptide antibiotics such as nikkomycins or neopolyoxins. Moreover, it is reported that β-acetamido ketones can act as a glucosidase inhibitors [12–14]. The structural and bioactive properties of β-acetamido carbonyl compounds led to the generation of some processes employing some catalysts such as CoCl2 [15], TMSCl [16], BiCl3 [17], I2 [18], ZnO [19], select fluor™ [20], and ZrOCl2·8H2O [21].
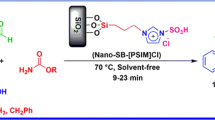
Along the course of our investigations in the development of new nano-structured catalysts [22, 23], we describe synthesis, characterization, and application of nano-sphere silica sulfuric acid (NS-SSA) as new heterogeneous catalyst for one-pot multi-component synthesis of β-acetamido carbonyl compounds at room temperature. The primary step of this objective was achieved by the synthesis and functionalization of silica nanoparticles (Scheme 1).
First, the catalysis was synthesized by the reaction of nano-sphere silica with chlorosulfonic acid in CH2Cl2 for 0.5 h resulting in an excellent yield. Nano-sphere silica and the corresponding heterogeneous catalysts characterized by transmission electron microscopy (TEM), FT-IR, XRD, EDS, N2 adsorption–desorption techniques, and thermal analysis.
Experimental section
Preparation of the catalyst
Monodispersed nano-sphere silica were synthesized according to the literature procedure [13]. Nano-sphere silica (1.0 g) was dispersed in CH2C12 (10 mL) in a flask. Chlorosulfonic acid (0.638 gr, 5.5 mmol) was dissolved in CH2C12 (10 mL) and added to the nano-sphere silica suspension through a constant-pressure dropping funnel under stirring over a period of 30 min at room temperature. After the addition was completed, the mixture was stirred for another 30 min at room temperature. The pale brown solid was collected by filtration and washed with methyl t-buthyl ether 50 mL. Then it was dried at room temperature. Finally, the acidity (the number of H+) of nano-sphere silica sulfuric acid was determined by titration with NaOH. The amount of H+ for heterogeneous catalyst was 9.4 mmol H+ g−1.
Characterization of the catalyst
TEM investigation provides the direct observation of the morphology. nano-sphere silica and nano-sphere silica sulfuric acid were characterized by transmission electron microscopy (TEM). The results confirm the formation of the catalyst, with spherical morphology and a size range of 60–90 nm (Fig. 1).
The XRD pattern for the prepared samples before and after functionalization are shown in Fig. 2. As shown in Fig. 2, the prepared samples show the same positions with different intensities. The results show that the nano particles have spherical shape after functionalization.
The surface areas, pore volumes, and pore size distributions of the prepared sample materials were analyzed by nitrogen adsorption–desorption techniques. The N2 adsorption–desorption isotherm of nano-sphere silica sample exhibited high surface area (1,411 m2/g), pore volume (1.406 cc/g) and large pore size (ca. 3.98 nm). As is predictable, after the loading of –SO3H group on nano-sphere silica caused a significant decrease in surface area (65.55 nm2/g), pore volume (0.061 cc/g) and pore diameter (ca. 3.77 nm).
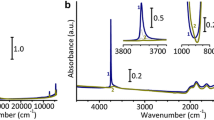
The IR Spectra of nano-sphere silica sulfuric acid showed some peaks in region of 474, 675, 800, 1,011, 1,173, 1,286 and 3,408 cm−1. The band of the Si–O rocking vibrations appears about 474 cm−1. The band at 800 cm−1 is assigned to symmetric vibrations (Si–O–Si) and the band at 1,011 cm−1 is assigned to the asymmetric vibrations of (Si–O–Si) of nano-sphere silica sulfuric acid (Fig. 3). The band of the S–O vibrations appears about 675 cm−1. The bands which are at 1,173 and 1,286 cm−1 are assigned to symmetric and asymmetric stretching of S=O respectively. A very broad IR absorption band centered at 3,408 cm−1 is assigned to hydroxyl groups (Fig. 3). The EDS spectrum of the catalyst was shown in Fig. 3. As shown in spectra, the peaks of Si and S are obviously observed, and no other impurities occur.
Nano-sphere silica and nano-sphere silica sulfuric acid were characterized by thermal analysis. In general, two distinct weight loss TGA profiles were found, including a small weight loss due to the desorption of water and large weight loss due to the decomposition of the sulfonic acid groups. As shown in TG/DTG curves of nano-sphere silica and nano-sphere silica sulfuric acid, the weight loss below 150 °C is due to the desorption of water. Decomposition of the sulfonic acid groups takes place below 380 °C.
General procedure for the synthesis of β-acetamido carbonyl compounds
To a mixture of compounds consisting of methyl ketone (1 mmol), aldehyde (1 mmol), acetonitrile (3 mL) and acetyl chloride (0.3 mL) in a 10 mL round bottomed flask was added NS-SSA (0.01 g, 0.094 mmol H+), and the resulting mixture was stirred at room temperature. After completion of the reaction, as monitored with TLC, the reaction mixture was centrifuged for 5 min. Then, the clear liquid was separated and crushed ice (15 mL) was added to the reaction mixture and stirred thoroughly. On solidification, the crude product was filtered, dried, and purified by short column chromatography on silica gel eluted with EtOAc/n-hexane (1/4).
Result and discussion
After characterization of nano-sphere silica sulfuric acid, experiments were performed to optimized reaction conditions for the synthesis of β-acetamido carbonyl compounds (Scheme 2).
To obtain the optimized reaction conditions, the reaction of 4-bromoacetophenone (1 mmol) with benzaldehyde (1 mmol), acetonitrile (3 mL) and acetyl chloride (0.3 ml) was selected as a model reaction to provide compound 1j, and its behavior was studied in the presence of different molar ratios of NS-SSA at room temperature. The results are summarized in Table 1. As Table 1 indicates, in the absence of catalyst, when 4-bromo acetophenone (1 mmol) was treated with benzaldehyde (1 mmol), acetonitrile (3 mL) and acetyl chloride, reaction was not active (entry 1). As the results indicate, higher yields of the product and shorter reaction times were obtained when the reaction was carried out using 0.01 g (0.09 mol % H+) of nano-sphere silica sulfuric acid.
In another study, to recognize generality and efficacy of the catalyst, different enolizable carbonyl compounds were reacted with structurally and electronically diverse aldehydes, acetonitrile and acetyl chloride under the optimized reaction conditions; the respective results are summarized in Table 2. As it can be seen in Table 2, the protocol was general and efficient; all reactions proceeded efficiently and the desired products were obtained in good to excellent yields in relatively short reaction times. As Table 2 shows, electron-releasing groups increased the reaction yields (Table 2, compounds 1a and 1c); electron-withdrawing substituents slightly decreased the yields (Table 2, compounds 1f and 1i). Moreover, the method worked well, when 2-naphthaldehyde was used instead of benzaldehydes (Table 2, compounds 1b, 1e, 1i, and 1l). Various enolizable ketones including acetophenones bearing electron-releasing substituents, electron-withdrawing substituents, and halogens on the aromatic ring were also condensed with aldehydes, acetonitrile and acetyl chloride in the presence of nano-sphere silica sulfuric acid to afford the corresponding β-acetamido ketones in high yields within relatively short reaction times.
For practical applications of heterogeneous systems, the recovery of the catalyst is an important aspect for the synthesis of fine chemicals. To clarify this issue, we established a set of experiments for the synthesis of N-(3-(4-bromophenyl)-3-oxo-1-phenylpropyl)acetamide (1j) using the nano-sphere silica sulfuric acid catalyst. After the completion of the first reaction, to afford the corresponding β-acetamido ketone compound, the reaction mixture was centrifuged for 5 min. Then, the clear liquid was separated and the residue was finally dried at 50 °C. A new reaction was then performed with fresh enolizable ketone, aldehyde, acetonitrile, and acetyl chloride under the same conditions. The catalyst could be used at least 5 times without any change in the activity.
To recognize efficiency and importance of the catalysts in the synthesis of β-acetamido carbonyl compounds, the model reaction was also examined using H2SO4 as well as ClSO3H, separately (Table 2, entries 3 and 4). As it can be seen in Table 3, H2SO4 and ClSO3H afforded the product in 39 and 27 % yields within 3.5 h, respectively. In these conditions, a large amount of the starting materials remained and also some by-products were obtained. This observation confirmed that to increase the efficiency of ClSO3H in the synthesis of β-acetamido ketones, it is necessary to combine them to inorganic compounds (here, nano-sphere silica). If SiO2 (amorphous) was directly used as catalyst, the yield of product was 15 % (entry 1). The use of nano-sphere silica was also examined whereby a low yield of product was obtained and it needs a long reaction time (entries 2).
Conclusion
In conclusion, we have shown that the nano-sphere silica sulfuric acid can be easily prepared from commercially available materials. This catalyst efficiently affects the synthesis of β-acetamido ketone derivatives at room temperature with excellent yields.
References
M.R. Maurya, A. Kumar, J.C. Pessoa, Coord. Chem. Rev. 255, 2315–2344 (2011)
B. Sreedhar, P. Radhika, B. Neelima, N. Hebalkar, J. Mol. Catal. A Chem. 272, 159–163 (2007)
B. Sreedhar, D. Yada, P. Surendra Reddy, Adv. Synth. Catal. 353, 2823–2836 (2011)
B. Sreedhar, D. Keerthi Devi, Deepthi Yada, Catal. Commun. 12, 1009–1014 (2011)
P. Dutta, A. Sarkar, Adv. Syn. Catal. 353, 2814–2822 (2011)
S. Verma, M. Nandi, A. Modak, S.L. Jain, A. Bhaumik, Adv. Syn. Catal. 353, 1897–1902 (2011)
T.M. Suzuki, T. Nakamura, E. Sudo, Y. Akimoto, K. Yano, J. Catal. 258, 265–272 (2008)
T.M. Suzuki, T. Nakamura, E. Sudo, Y. Akimoto, K. Yano, Micro. Meso. Mater. 111, 350–358 (2008)
T.M. Suzuki, M. Yamamoto, K. Fukumoto, Y. Akimoto, K. Yano, J. Catal. 251, 249–257 (2007)
A. Dömling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168–3321 (2000)
V. Nair, C. Rajesh, A.U. Vinod, S. Bindu, A.R. Sreekanth, J.S. Mathen, L. Balagopal, Acc. Chem. Res. 36, 899–907 (2003)
D. Enders, M. Moser, G. Geibel, M.C. Laufer, Synthesis 2040–2046 (2004)
B. Das, M. Krishnaiah, K. Laxminarayana, K. Ravinder Reddy, J. Mole. Catal. A Chem. 270, 284–288 (2007)
A.K. Tiwari, R.M. Kumbhare, S.B. Agawane, A.Z. Ali, K.V. Kumar, Bioorg. Med. Chem. Lett. 18, 4130–4132 (2008)
I.N. Rao, E.N. Prabhakaran, S.K. Das, J. Iqbal, J. Org. Chem. 68, 4079–4082 (2003)
M.M. Heravi, L. Ranjbar, F. Derikvand, F.F. Bamoharram, Catal. Commun. 8, 289–291 (2007)
E. Rafiee, F. Shahbazi, M. Joshaghani, F. Tork, J. Mole, Catal. A Chem. 242, 129 (2005)
B. Das, K.R. Reddy, R. Ramu, P. Thirupathi, B. Ravikanth, Synlett 11, 1756–1758 (2006)
M.T. Maghsoodlou, A. Hassankhani, H.R. Shaterian, S.M. Habibi- Khorasani, E. Mosaddegh, Tetrahedron Lett. 48, 1729–1734 (2007)
V.S. Shinu, B. Sheej, E. Purushothaman, D. Bahulayan, Tetrahedron Lett. 50, 4838–4843 (2009)
R. Ghosh, S. Maiti, A. Chakraborty, S. Chakraborty, A.K. Mukherjee, Tetrahedron 62, 4059–4064 (2006)
M.A. Zolfigol, Tetrahedron 57, 9509–9511 (2001)
N. Koukabi, E. Kolvari, A. Khazaei, M.A. Zolfigol, B. Shirmardi-Shaghasemi, H.R. Khavasi, Chem. Commun. 47, 9230–9232 (2011)
Acknowledgments
The authors gratefully acknowledge partial support of this work by the Research Affairs Office of Bu-Ali Sina University and Center of Excellence in Development of Chemical Method (CEDCM), Hamedan, I.R. Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khazaei, A., Zolfigol, M.A., Mokhlesi, M. et al. Nano-sphere silica sulfuric acid: novel and efficient catalyst in the one-pot multi-component synthesis. J IRAN CHEM SOC 10, 1297–1301 (2013). https://doi.org/10.1007/s13738-013-0272-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-013-0272-y