Abstract
The present paper discusses the estimation of calcium ions in presence of calcium binding buffer, voltage-gated calcium channel (VGCC) and sodium calcium exchanger (NCX) for normal and Alzheimer’s affected neuronal cells. In Alzheimer’s disease (AD), amount of buffer decreases whereas the calcium activities increase in VGCC and NCX. Due to these alterations, the normal calcium diffusion in that area gets disturbed and gets affected by AD. The governing equation of this physiological phenomena is in the form of calcium diffusion equation which is solved along with initial and boundary conditions. The approximate solution to this problem has been obtained using finite element technique in MATLAB. The significance of buffers at cytosolic level has been shown using single and multiple buffering phenomena. Moreover, to check the influence of fluxes mediated by VGCC and NCX at cytosolic level, for normal and Alzheimer’s affected cells, the single and multiple fluxes are assumed and the results are obtained. The obtained results clearly show the significance of the assumed parameters on calcium concentration at the cytosolic level. The hike in the calcium concentration due to decrease in buffer and increase in VGCC and NCX mediated fluxes may lead to neurodegenerativity of AD. This study may help the theoretical scientists in knowing the role of calciumopathy in AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Applied mathematics has widened its horizon in almost all fields of science and engineering. Scientists and biologists prefer to first have the mathematical and computational estimated results of their experiments so that the outputs can be modified with an ease. The computational study helps in estimating the possible pros and cons of the in situ condition. Hence, the mathematical formulation and computation have helped scientists in all fields including neuroscience. Computational neuroscience is an emerging research area gaining much interest nowadays. The computational modelling and simulation have helped a lot in knowing the functioning and working of the brain. This paper focuses on the mathematical modelling of the calcium diffusion phenomenon taking place in normal and Alzheimer’s affected neuronal cells. Over the last few years, the study of Alzheimer’s disease and its possible causes have gained much of the attention from the research community. Still, no breakthrough has been discovered. Mostly being sporadic in nature, the perfect cause for the Alzheimer to occur is still unknown (Turkington and Mitchell 2010). AD is basically the age-oriented disorder which progressively effects and impairs the memory storage (Magi et al. 2016; Turkington and Mitchell 2010). It mostly affects the older age people having 60 plus of age . Till now there were mainly two different hypotheses on which the study of pathogenesis of AD was going on, namely amyloidogenic hypothesis generating amyloid beta plaques and tau hypothesis supporting neurofibrillary tangles (Mattson and Chan 2003; Turkington and Mitchell 2010). Both of these hypotheses assumed to be the key causes of AD to occur and prevail. But the emerging research shows that calciumopathy may have the role in prevailing this dementia (Bezprozvanny 2011; Carafoli and Brini 2007; Green and Laferla 2008; Kawamoto et al. 2012; Magi et al. 2016). It has been found that the alterations and dysregulation of calcium signaling in neurons is the key cause in triggering the neurodegenerativity of AD. The intracellular mishandling of calcium leads to alteration in normal signaling cascades. This fact was laid by Khachaturian in eighties (Khachaturian 1989, 1993). Calcium is known as the second messenger which has its hand in maintaining variety of neuronal functions (Clapham 2007; Kawamoto et al. 2012; Rajagopal and Ponnusamy 2017). The calcium is entered into the neuronal cell via certain channels and pumps like VGCC and NCX. After entering into the cell, the calcium buffer binds the calcium which further results into lowering down the intracellular calcium levels (Rajagopal and Ponnusamy 2017). It has been found that almost amount of calcium gets buffered and only 1% of the calcium gets sequestered further. All the parameters on the whole works as a syncytium and maintains the cell calcium. The deregulation in any of these parameters lead to increasing intracellular calcium. These elevated calcium level directly affects the cell fate. It renders toxicity to the cell which subsequently disturbs the normal physiological processes (Bezprozvanny 2011). Hence the study of this physiological phenomena is focused here. In this paper we have considered a two-dimensional partial differential equation delineating the calcium diffusion. Moreover, we have considered the calcium flux via VGCC and NCX, followed by calcium binding buffers which maintains the intracellular cell calcium. The initial and boundary conditions are adopted such that it matches well with the in situ conditions.
Retrospective survey suggests that several computational attempts have been made to portray the calcium diffusion in Alzheimer’s affected cells. Analytical, traditional finite element method and various numerical methods have been extensively used by the researchers in past to obtain the approximate solution. Jha et al. (2012) have found the analytical solution of calcium advection diffusion in astrocytes, whereas, Jha and Adlakha (2014) have found the significance of excess buffering phenomenon on calcium concentration level using Laplace and Fourier transforms. Dave and Jha have analytically found the solution to calcium advection reaction diffusion in presence of several parameters like buffers, VGCC and ER, taking place in normal and Alzheimer’s affected neuron cells (Dave and Jha 2018a, b, 2020). Traditional finite element methodology has been applied on a large scale to estimate the calcium diffusion taking place in various cells. Tewari and Pardasani have used FEM to check the significance of sodium pumps in neurons (Tewari and Pardasani 2012). Panday and Pardasani and Naik and Pardasani have used FEM to study the role of some parameters like NCX, VGCC, SERCA pumps, etc., in calcium concentration distribution in oocytes (Naik and Pardasani 2015, 2017, 2018; Panday and Pardasani 2013). Jha et al. have used FEM to delineate calcium concentration distribution in presence of excess buffers in astrocytes (Jha et al. 2014). Also, the significance of NCX and source geometry at cytosolic level of neuron were checked by Jha et al. (2015). Although researchers have worked in knowing the impact of several parameters like buffers, VGCC, ER, NCX, etc., on calcium diffusion in various cell (Pathak and Adlakh 2015a, b), very less work has been done on irregular neuronal cell and neuronal disorders (Jha and Dave 2020). Also, artificial intelligence, pattern recognition, rough set method, etc., have helped in visioning the object tracking algorithms, several skin diseases and in prediction of some targeted proteins as well (Liu 2020; Sinha and Namdev 2020; Sinha et al. 2020, 2018). Hence, on the basis of this literature survey, we have adopted finite element technique to estimate calcium diffusion in irregular shaped normal and Alzheimer’s affected neuronal cell.
2 Mathematical formulation
The mathematical modeling of the mechanisms underlying these physiological parameters help in knowing that on what factors it depends and how it can be used to obtain the computational results. Hence, in this section mathematical formulation of the calcium buffering phenomenon is shown. Also, the calcium flux via VGCC and NCX is formulated and stated mathematically using several equations. Also, the role of these parameters in AD has been mentioned and discussed here.
2.1 Calcium buffering
Calcium binding buffers are present at the peripheral area of the cell. Once the calcium gets into the cell, most of the calcium gets buffered (Schwaller 2010). This results into lowering down and maintenance of the intracellular calcium. The elevation in the intracellular calcium level results into neurodegenerativity of AD (Riascos et al. 2011). This neurodegenerativity is toxic to that area of the cell. In AD, different areas are affected which have different results. here, we have considered the hippocampal area which is highly affected in AD. Calmodulin is the buffer responsible for maintaining the cell calcium in the hippocampal area. It has been found that during AD, the amount of buffer reduces greatly which directly lead to hike in intracellular cell calcium. The amount of buffer decreases to round about of 20–30% of the total amount present in normal hippocampal neurons (Turkington and Mitchell 2010). Thus, we have considered calcium binding buffering phenomenon. The mathematical formulation of this is stated as below. Calcium on reaction with calcium binding buffers results into calcium bound buffers. Mathematically, it is stated as (Smith 1996):
With the help of standard Fickian diffusion, the resulting partial differential equations are obtained as (Smith 1996; Keener and Sneyd 2009):
where
Here, \(D_{Ca}\) is the diffusion coefficient for calcium, whereas \(D_{Bj}\) and \(D_{CaB}\) are the diffusion coefficients of calcium binding buffers and calcium bound buffer respectively. \(k_j^+\) and \(k_j^-\) are the association and the dissociation rates respectively for buffer j. In this paper, we have obtained the results for single and multiple buffers to understand the role and impact of buffer on cytoplasmic calcium concentration level. We have considered, endogenous buffer Calmodulin which have its active role in hippocampal area and widely known exogenous buffers like EGTA and BAPTA.
2.2 Voltage-gated calcium channel (VGCC)
In electrically excitable cells like neurons, the calcium entry is mainly regulated by voltage dependent calcium channels. There are different types of VGCC’s depending upon there localization namely P, Q, N, T and L types (Yagami et al. 2012). The calcium channels that perturb normal neuronal calcium homeostasis and gets affected in AD are L type of voltage gated calcium channels having family Cav 1.1– Cav 1.4. L type calcium channels are mainly located at the area of dendritic spines and cellular body area. It regulates the calcium influx and maintains several intracellular phenomena depending upon their residing area. In AD, the normal functioning of the L type- VGCC gets impaired and hence the normal calcium homeostasis gets impaired (Yagami et al. 2012; Schampel and Kuerten 2017). This impairment leads to increase in intracytoplasmic calcium levels which further generates toxicity and hence AD. The mathematical formulation of the calcium influx through VGCC has been stated as below using Goldman-Hodgkin-Katz (GHK) current equation as (Keener and Sneyd 2009; Jha et al. 2013):
The values and the parameters are stated in Table 1. The above current equation is converted into voltage gated calcium flux equation using (Keener and Sneyd 2009),
The mathematical formulation of the calcium influx in the cytoplasmic region regulation is governed by above equation.
2.3 Sodium calcium exchanger (NCX)
The NCX is found in the plasma membrane. After VGCC, NCX maintains and regulates the influx of intracellular cell calcium. It plays significant role in maintaining cell calcium in neuronal and glial cells (Colvin et al. 1994). The stoichiometric ratio of the NCX is found to be 3:1, i.e., three sodium ions are exchanged instead of one calcium ion. Also, it has been observed that this ratio can vary depending upon the intracellular sodium and calcium levels (Annunziato et al. 2004). In this paper, the standard stoichiometry has been followed. In AD, the NCX activities are altered which results into elevated calcium concentration level (Colvin et al. 1991). The rest of the values were mostly found to be unchanged. The mathematical formulation of the NCX regulated calcium influx is stated below (Panday and Pardasani 2013). We have considered 3:1 ratio of sodium: calcium via NCX.
The values and parameters are descripted in Table 1.
3 Statement of the problem
The two dimensional transient calcium diffusion phenomenon in presence of calcium binding buffers, VGCC and NCX is stated in the form of linear partial differential equation. Using above mentioned mathematical formulations of these physiological phenomena, the statement of the problem having the governing differential equation is stated as:
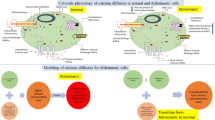
Physiologically, calcium binding buffers reduces the cell calcium whereas VGCC and NCX increases the level of intracellular cell calcium. Hence, the balance of cell calcium depends on normal functioning and working of these parameters. Schematically, the physiological phenomenon of calcium diffusion in presence of buffer, VGCC and NCX is shown in Fig. 1.
3.1 Boundary conditions
The statement of the problem is treated with appropriate initial and boundary conditions which physiologically matches the actual in situ conditions. Thus, the initial and boundary conditions which are incorporated to obtain the desired results are stated as (Panday and Pardasani 2013):
where \(\eta\) is perpendicular to the surface.
The background calcium concentration is taken to be \([Ca^{2+} ]=0.1 \mu M\). In the next section we have discussed the solution technique which is used to estimate the calcium flow.
The following flow chart depicts entire methodology of obtaining the solution to the problem.

4 Methodology
4.1 Traditional finite element method
Since decades, finite element method has been widely opted by the researchers to obtain the solution of their problems. It gives the best possible approximate solution to the irregular shaped domain. Due to this beauty of the finite element method, it has not been confined to engineering branches only. Theoretical scientists and many mathematicians have opted for it to get the estimated solutions. The steps of traditional finite element method has been described hereby (Rao 2011):
-
1.
Discretization of the domain
-
2.
Selection of appropriate interpolation function
-
3.
Derivation of element matrices and vectors using variational principle or weighted residual approach
-
4.
Assemble the element equations to obtain overall equilibrium equations
-
5.
Solve the system for unknown values
Since the following paper focuses on the irregular domain, instead of traditional finite element method, finite element technique has been used in MATLAB.
4.2 Finite element technique
It has been found that, finite element method has been widely used in the field of biology, be it using the traditional method approach or by advanced software approach due to its capacity of handling the irregular structures well. Thus, in this paper, we have adopted finite element technique to estimate the calcium diffusion in irregular shaped hippocampal neuron. Further in this section, the domain of the problem with the calcium fluxes and domain discretization is discussed.
4.2.1 Approximate geometry
The shape and size of the neuronal cells depend on the residing region of it. The approximated geometry of the hippocampal neuron which is highly damaged in AD is shown in Fig. 2. It consists of dendrites, soma and the axon terminals. It is pyramidal in nature. The number of dendrites and axon terminals are taken approximately to obtain the computational results.
4.2.2 Physiological boundary conditions
Figures 3 and 4 shows the calcium influx in the targeted domain of the hippocampal neuron via single and multiple boundary conditions. Neumann boundary conditions have been incorporated to delineate the calcium flux in the intracellular region of the hippocampal neuron. By considering the multiple boundary conditions, the actual flux condition in in situ can be estimated well. Mathematically, line flux has been incorporated but the multiple fluxes so as to obtain more precise results is incorporated here for the irregular shaped hippocampal neuron.
4.2.3 Domain discretization
The pyramidal hippocampal neuron has been discretized into elements having nodes. This discretization yields to the approximate solution of the problem. Figure 5 shows the discretization of the targeted domain of the neuron. The mesh has been refined by improving the triangle quality and sensitivity so as to obtain better approximate results. Further, the mesh has not been refined because no significant change has been observed in the obtained results. The results obtained are discussed in the next section.
5 Results and discussion
Table 1 shows the values of the physiological parameters which are used to obtain the approximated calcium concentration in presence of buffer, VGCC and NCX.
Figure 6 shows the calcium diffusion to differentiate the obtained estimated results for different meshes. Figure 6a shows the initialization of the mesh for hippocampal neuron. The calcium diffusion and the calcium concentration level is around 0.2. Further in Fig. 6b, the calcium diffusion is shown for refined mesh. Here, we can observe that the altitude of the calcium concentration has increased from 0.2. Lastly, more refined results are obtained in Fig. 6c, where the change in altitude level and the change in the nature of the calcium flow is observed. Hence, it is observed that the refinement of the mesh generated yields a significant change on the calcium concentration level. Thus, now onwards all the results are obtained for refined mesh having most significant flow.
Figure 7 shows the calcium diffusion in presence of calmodulin. Literature survey suggests that the level of calmodulin decreases significantly in hippocampal area for Alzheimer’s affected cells (Turkington and Mitchell 2010). Also, it has been found from the research that, in Alzheimer’s affected condition of the cell, the activities of VGCC and NCX are highly increased (Yagami et al. 2012; Colvin et al. 1994). The decrease in buffer and increase in calcium via VGCC and NCX results in increase in calcium concentration level. The increased calcium, which is more than the normal level has adverse effect on the cell. It renders toxicity to the cell which further results in cell death. Due to the loss of cell in that area, normal calcium signaling is altered and disturbed. The significant change in the calcium diffusion for normal and Alzheimer’s affected is clearly observed from Fig. 7a and b. The calcium concentration for normal cell is estimated and obtained as 0.5, whereas the same for Alzheimer’s affected condition of the cell is obtained to be at around 1.6. Hence, the significant role of calmodulin in hippocampal area is observed from Fig. 7. Also, it has been observed from the results obtained that the graphs here has the same nature of flow as obtained in previous studies (Jha et al. 2014; Smith 1996).
Figure 8 shows the calcium diffusion in presence of multiple buffers, VGCC and NCX. Here we have considered calmodulin, EGTA and BAPTA as three different buffers. Calmodulin is the endogenous buffer whereas, EGTA and BAPTA comes under exogenous buffers category. Due to multiple buffers, the calcium flow in presence of other buffers, if any, in in situ condition can be estimated. Here, we have obtained the results for normal and Alzheimer’s affected condition of the brain. Also, on comparing the results obtained from Figs. 7 and 8 that, the level of calcium is lower in Fig. 8 due to presence of multiple calcium binding buffers.
Figure 9 shows the calcium diffusion in presence of multiple entries of calcium via NCX and VGCC and calcium binding buffers present at the peripheral area of the neuron. In this figure, the impact of calcium flux through VGCC and NCX is shown which signifies the impact of both the parameters on cytosolic calcium concentration distribution. Due to multiple entries, the in situ calcium flow can be estimated. Here, the results are obtained for normal and Alzheimer’s affected condition of the cell. The significant change in Alzheimer’s affected condition of the cell is seen from Fig. 9b. The normal calcium concentration level is around 0.8, whereas, for Alzheimer’s affected cell it is found to be more than 2.5. The reason for higher calcium level in Alzheimer’s affected cell in Fig. 9 in comparison to Fig. 7 is multiple entries of calcium. Due to the multiple entries of calcium, there is hike in calcium concentration level. Hence, the calcium flux is estimated in presence of calmodulin, VGCC and NCX.
Figure 10 shows the calcium concentration distribution in presence of multiple entries of calcium, multiple buffers, VGCC and NCX. Here also, the results are obtained for normal and Alzheimer’s affected conditions of the hippocampal neuron. The values of all the parameters taken into consideration are mentioned in Table 1. The calcium concentration level for normal cell is found to be around 0.8, whereas for Alzheimer’s affected cell it is around 2.5 and after that it gradually attains the background calcium concentration level.
Figure 11 shows the spatio-temporal calcium concentration distribution in presence of single and multiple buffers for normal and Alzheimer’s affected cells. From the figure, it can be observed that initially the calcium concentration increases and then decreases gradually in a normal condition. Moreover, it has been observed that in Alzheimer’s affected case, the calcium concentration level remains high and then decreases gradually and finally attains the background level. The level of calcium concentration is comparatively low in Fig. 11b due to multiple buffering phenomenon. Similarly, the spatio-temporal distribution can be obtained for multiple fluxes and multiple buffering phenomenon also.
6 Conclusion
Till date, very less attempts have been made by the researchers to study calcium concentration distribution for diseased cells using irregular neuronal cells and finite element technique. Hence, in this paper, an attempt has been made to interpret the calcium diffusion in normal and Alzheimer’s affected neuronal cells using 2D transient computational model. Calcium binding buffers, VGCC and NCX have been considered as the parameters affecting the cytoplasmic calcium concentration level. It is found that all these parameters have significant impact on the cytosolic calcium concentration level. Also, the irregular shaped geometry of neuron of hippocampal area is considered, as this area gets highly affected in AD. The neuron which is pyramidal in nature having dendrites, soma and axons, is estimated in this paper. To delineate this physiological phenomenon of the calcium diffusion in hippocampal neuron, we have considered a two-dimensional linear partial differential equation. The results are obtained using initial and boundary conditions which matches well with the in situ condition of a typical neuronal cell of the brain. Further, to obtain the estimated calcium diffusion, finite element estimation has been used. Initial and refined meshes are shown to show the significant impact of the refinement on the domain of the targeted geometry. The effect of buffer concentration on calcium is found significant in presence of single and multiple buffers. Exogenous buffers are considered in case of multiple buffering phenomena. Moreover, to find the impact of the fluxes of calcium at cytosolic level, we have incorporated single and multiple boundary conditions. It has been found that, the cell having multiple fluxes have higher level of calcium concentration levels which clearly shows the impact of VGCC and NCX on cytosolic calcium concentration level. The fact that the lower amount of calcium binding buffers and increased activities of VGCC and NCX results in higher level of calcium concentration level is verified from the results obtained. These results clearly signify the impact of normal amount of buffers, VGCC and NCX on cytosolic calcium concentration level. Thus, the impact of the calcium toolkit parameters has been found on normal and Alzheimer’s affected cells using finite element technique. Further, this kind of computational models can be extended by incorporating more physiological parameters and more rigorous boundary conditions which may further help the theoretical scientists and biologists to estimate the in situ calcium concentration level in healthy and diseased conditions.
References
Annunziato L, Pignataro G, Renzo GFDI (2004) Pharmacology of brain \(Na^+/Ca^{2+}\) exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev 56(4):633–654
Bezprozvanny I (2011) Calcium signalling and neurodegenerative diseases. Trends Mol Med 15(3):89–100
Carafoli E, Brini M (eds) (2007) Calcium signalling and disease. Springer, Berlin
Clapham DE (2007) Calcium signaling. Cell 131:1047–1058
Colvin RA, Bennett JW, Colvin SL (1991) \(Na^{+}-Ca^{2+}\) exchange activity is increased in Alzheimer’s disease brain tissues. Ann NY Acad Sci 639:325–327
Colvin RA, Davis N, Wu A, Murphy CA, Levengood J (1994) Studies of the mechanism underlying increased \(Na^+/Ca^{2+}\) exchange activity in Alzheimer’s disease brain. Brain Res 665:192–200
Dave DD, Jha BK (2020) 3D Mathematical modeling of calcium signaling in Alzheimer’s disease. Netw Model Anal Health Inform Bioinform 9(1):1–10
Dave DD, Jha BK (2018) Delineation of calcium diffusion in Alzheimeric brain. J Mech Med Biol 18(2):1–15
Dave DD, Jha BK (2018) Analytically depicting the calcium diffusion for Alzheimer’s affected cell. Int J Biomath 11(6):1–13
Green KN, Laferla FM (2008) Linking Calcium to \(A\beta\) and Alzheimer’s Disease. Neuron 59:190–194
Jha BK, Dave DD (2020) Approximation of calcium diffusion in Alzheimeric cell. J Multiscale Model:11(2):2050001
Jha A, Adlakha N (2014) Analytical solution of two dimensional unsteady state problem of calcium diffusion in a neuron cell. J Med Imaging Health Inform 4(4):547–553
Jha BK, Adlakha N, Mehta MN (2012) Analytic solution of two-dimensional advection diffusion equation arising in cytosolic calcium concentration distribution. Int Math Forum 7(3):135–144
Jha BK, Adlakha N, Mehta MN (2013) Two-dimensional finite element model to study calcium distribution in astrocytes in presence of VGCC and excess buffer. Int J Model Simul Sci Comput 4(2):12500301–12500315
Jha BK, Adlakha N, Mehta MN (2014) Two-dimensional finite element model to study calcium distribution in astrocytes in presence of excess buffer. Int J Biomath 7(3):1–11
Jha A, Adlakha N, Jha BK (2015) Finite element model to study effect of \(Na^+/ Ca^{2+}\) exchangers and source geometry on calcium dynamics in a neuron cell. J Mech Med Biol 16(2):1–22
Kawamoto EM, Vivar C, Camandola S (2012) Physiology and pathology of calcium signaling in the brain. Front Pharmacol 3:1–17
Keener J, Sneyd J (2009) Mathematical physiology, 2nd edn. Springer, Berlin
Khachaturian ZS (1989) Introduction and overview. Ann NY Acad Sci. 568(1):1–4
Khachaturian ZS (1994) Calcium hypothesis of Alzheimer’s disease and brain aging. Ann NY Acad Sci:747(1):1–11
Liu S et al (2020) Overview of correlation filter based algorithms in object tracking. Complex Intell Syst. https://doi.org/10.1007/s40747-020-00161-4
Magi S, Castaldo P, Macrì ML et al (2016) Intracellular calcium dysregulation: implications for Alzheimer’s disease. Biomed Res Int. 2016:1–14
Mattson MP, Chan SL (2003) Neuronal and glial calcium signaling in Alzheimer’s disease. Cell Calcium 34:385–397
Naik P, Pardasani KR (2015) Two-dimensional finite element model to study calcium distribution in oocytes. J Multiscale Model 6(1):1–15
Naik PA, Pardasani KR (2016) Finite element model to study calcium distribution in oocytes involving voltage gated \(Ca^{2+}\) channel, ryanodine receptor and buffers. Alexandria J Med 52(1):43–49
Naik PA, Pardasani KR (2017) Three-dimensional finite element model to study calcium distribution in oocytes. Netw Model Anal Health Inform Bioinform. https://doi.org/10.1007/s13721-017-0158-5
Naik PA, Pardasani KR (2018) 2D finite-element analysis of calcium distribution in oocytes. Netw Model Anal Health Inform Bioinform. https://doi.org/10.1007/s13721-018-0172-2
Panday S, Pardasani KR (2013) Finite element model to study effect of advection diffusion and \(Na^+/ Ca^{2+}\) exchanger on \(Ca^{2+}\) distribution in oocytes. J Med Imaging Heal Inform 3(3):374–379
Pathak K, Adlakh N (2015) Finite element model to study calcium signalling in cardiac myocytes involving pump. Leak and excess buffer. J Med Imaging Health Inform 5:1–6
Pathak K, Adlakh N (2015) Finite element model to study one dimensional calcium dyanmics in cardiac myocytes. J Multiscale Model 6(2):1–12
Rajagopal S, Ponnusamy M (2017) Calcium signaling: from physiology to diseases. Springer Nature Singapore Pvt Ltd., Singapore
Rao SS (2011) The finite element method in engineering, 5th edn. Elsevier, Amsterdam
Riascos D, De LD, Baker-Nigh A et al (2011) Age-related loss of calcium buffering and selective neuronal vulnerability in Alzheimer’s disease. Acta Neuropathol 122:565–576
Schampel A, Kuerten S (2017) Danger: high voltage—the role of voltage-gated system pathology. Cells 6:1–8
Schwaller B (2010) Cytosolic \(Ca^{2+}\) buffers. Cold Spring Harb Perspect Biol 2(11):1–20
Sinha AK, Namdev N, Kumar A (2020) A mathematical model of adiponectin resistance. J Theor Biol 494:110246
Sinha AK, Namdev N (2020) Feature selection and pattern recognition for different types of skin disease in human body using the rough set method. Netw Model Anal Health Inform Bioinform 9:27
Sinha AK, Namdev N, Kumar A (2018) Rough set method accurately predicts unknown protein class/family of Leishmania donovani membrane proteome. Math Biosci 301:37–49
Smith GD (1996) Analytical steady-state solution to the rapid buffering approximation near an open \(Ca^{2+}\) channel. Biophys J 71:3064–3072
Tewari SG, Pardasani KR (2012) Modeling effect of sodium pump on calcium oscilations in neuron cells. J Multiscale Model 4(3):1–16
Turkington C, Mitchell D (2010) The encyclopedia of Alzheimer’s disease, second. Facts on file: an imprint of Infobase Publishing
Yagami T, Kohma H, Yamamoto Y (2012) L-type voltage-dependent calcium channels as therapeutic targets for neuro- degenerative diseases. Curr Med Chem 1:4816–4827
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dave, D.D., Jha, B.K. 2D finite element estimation of calcium diffusion in Alzheimer’s affected neuron. Netw Model Anal Health Inform Bioinforma 10, 43 (2021). https://doi.org/10.1007/s13721-021-00322-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13721-021-00322-6
Keywords
- Calcium diffusion
- Buffer
- Voltage-gated calcium channel
- Sodium calcium exchanger
- Alzheimer’s disease
- Finite element technique