Abstract
Calcium oscillations are an imperative mode of signaling phenomenon. These oscillations are due to the active interactions taking place between some of the parameters like voltage gated calcium channels (VGCC), sodium calcium exchanger (NCX), calcium binding buffers, endoplasmic reticulum (ER) and mitochondria. The present paper focuses on the problem of higher level of calcium concentration in neurons which may further result into Alzheimer’s Disease (AD). For this, a three-dimensional mathematical model having a system of differential equations depicting the changes in cytosolic calcium (in presence of buffers, VGCC and NCX), ER calcium and mitochondrial calcium, is formulated. A three-dimensional neuronal structure is targeted as the domain which is further discussed and solved using finite element technique in Comsol Multiphysics 5.4. Apposite boundary conditions matching well with the in-situ conditions are assumed. The obtained results clearly show the significance of the lower amount of the buffer and higher calcium mediated activities of VGCC, NCX, ER and mitochondria on calcium profile. These changes may lead to AD. To transit from AD condition to normal, exogenous buffers are added to check their significance. The results thus show that the replenishment of buffer may balance the amount of cell calcium and hence can affect positively on Alzheimer’s affected cells.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
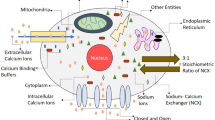
Being a second messenger, calcium plays diversified roles in numerous signaling pathways, thus regulating many cellular functions, gene expression, synaptogenesis, cell differentiation and several fundamental phenomena underlying learning and memory [20]. The spatiotemporal calcium signals are modified and modulated by more than a few mechanisms like calcium extrusion, calcium buffering and sequestration of calcium via internal cellular compartments. These signals arise and start by a passive entry of calcium through several calcium dependent channels like VGCC [2]. Subsequently, calcium is diffused into the cell cytoplasm where it reacts with the calcium binding buffers present at the cell’s periphery. The free calcium is then sequestered at other cellular entities. These free calcium ions move in an oscillatory pattern and further these oscillations are modified due to the calcium interactions taking place between cytosol and endoplasmic reticulum (ER) [3]. Over and above this, mitochondria also play an active role in sequestering the calcium ions and thus affecting and modulating the calcium signals [42, 45]. The shape and amplitude of these signals are distinguished, depending upon the geometries of the cell as well as the distance between the calcium influx source and the intracellular domains like ER and mitochondria [1]. The intracellular calcium is then maintained by several exchnagers like NCX [6]. Thus, the activation of these complex dynamical phenomenon of calcium homeostasis triggers various physiological processes and impacts almost all aspects of cellular functions. As calcium plays a vital and essential role in neuronal physiology, even the slightest of the alterations of calcium homeostasis renders profound and long-lasting functional impairments [5]. These functional impairments are in the form of devastating neuronal disorders like AD, Parkinson’s disease, epilepsy, amyloid lateral sclerosis, etc. [36].
There are different dementias prevailing amongst people, out of which, AD is the utmost common. Across the globe, billions of people are suffering from AD and most of them are the old age group mass who are in their late sixties/ seventies [24]. The characteristic symptom of AD is the loss of memory power. Other than memory loss, deterioration in the learning abilities and abstract thinking, lack of judgmental power, inability in performing regular and routine tasks as well as some linguistic problems arises and are experienced by the people suffering from AD [44]. Although, the severity of these symptoms depends on the stage of the AD developed in the brain and faced by an individual. AD is sporadic in nature but researchers have found several causes which may have an active role in AD, i.e. due to several factors like natural aging process, toxicity rendered due to higher level of calcium concentration, lower level of certain neurotransmitters, genetical changes, certain living lifestyle or environmental factors and/ or combination of these factors [25]. Khachaturian laid the fact that impaired calcium signaling leads to the neurodegenerativity of AD [22]. As discussed, the calcium toolkit parameters have a huge role in preserving cell calcium, hence, the disruption in any of these parameters may directly affect the normal function of neuronal calcium signaling. Due to this higher level of calcium concentration level, the cell experience toxicity which results into the hindrance in memory formation and maintenance and thus, the key symptom of AD prevails [44]. Thus, the study of calcium diffusion is a necessitate, be it computational or experimental.
Computationally, several researchers have tried to estimate calcium diffusion in presence of various parameters of calcium toolkit. Jha and Adlakha in 2014 studied the unsteady state calcium concentration distribution in neurons [12]. Dave and Jha investigated the calcium diffusion in the presence of buffers, VGCC and ER in Alzheimeric cells [8,9,10]. Numerically, finite element method has been used widely and studies have been carried out for various cells and in presence of different parameters [13, 14, 16, 18, 19, 27,28,29,30,31,32,33, 43]. Geometrically, Khalid et al. have studied calcium distribution in astrocytes [23]. Various geometries have been studied by them and results were obtained using finite element technique. Thus, on the basis of the above-mentioned literature survey, it has been found that very few attempts [15] have been done to delineate calcium concentration distribution for irregular domains and in view of neurodegenerative disorders.
Hence, an attempt is therefore made to mathematically model unsteady state 3D calcium concentration distribution model for irregular shaped neuron in presence of buffers, VGCC, NCX, ER and mitochondria in AD. The mathematical formulations for the same and the simulations are discussed in the sections hereafter.
2 Mathematical Formulations
In the present study, spatially homogeneous model which allows the spatial variation of calcium concentration and uses differential equations is formulated. As said by Kirk and Sneyd, the mathematical models are not constructed to be accurate enough but to present it as a useful tool in guiding the complex physiological understanding [4]. Hence, an attempt has been made here to mathematically model calcium concentration in presence of the calcium toolkit parameters. The mathematical formulations of these parameters and the calcium dynamics behind them are discussed in the following subsections.
2.1 Calcium Dynamics in Buffer, VGCC, ER, NCX and Mitochondria
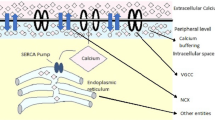
Figure 1 shows the schematic diagram of calcium dynamics in presence of buffers, VGCC, ER, NCX and mitochondria. This figure shows that all the parameters and ions present there works as a syncytium and maintains the cell calcium which helps in regulating various cellular functions.
The mathematical formulations behind the calcium dynamics and working of each of these parameters are discussed here, on the basis of which the problem will be formulated thereafter.
2.1.1 Voltage Gated Calcium Channels
The entry of calcium depends largely on the concentration gradient. This entry takes place via several calcium permeable channels like VGCC. It is the principle route of calcium influx in neurons as neurons are electrically active cells. There are basically five types of VGCC’s, i.e. P, Q, N, L and T [39]. Each of these channels have specified predefined role to perform. Out of them, L type of calcium channel plays an active role in perturbation of normal neuronal calcium signaling cascades. High activities of L-type calcium channels are detected in CNS disorders and specifically AD [38]. Due to this, more amount of calcium enters into the cellular domain and impairs the normal calcium levels in the cell.
The mathematical formulation of VGCC depends on the Goldman– Hodgkin–Katz (GHK) current equation, which is given as [21]:
The description and values of these parameters are mentioned in Table 1. Further, the VGCC mediated flux is obtained by converting the above equation into the form of moles/ second equation. It is stated as:
2.1.2 Sodium Calcium Exchanger
After VGCC, NCX is the second most important source for calcium in/efflux in the cell. It maintains the cell calcium by exchanging the calcium with sodium ions [7]. The stoichiometric ratio of NCX is 3:1 which means that three sodium ions are exchanged against a single calcium ion. In Alzheimeric condition of the cell, the normal functioning of NCX is altered which results into higher calcium concentration levels in the intracellular region [7]. The mathematical formulation of calcium in/efflux via NCX is done as follows [32]:
The values and parameters are descripted in Table 1 .
2.1.3 Calcium Binding Buffers
The buffers play an active role in preserving the cell calcium after the calcium entry into the cell, i.e. after diffusion in the intracellular zone. The free calcium then reacts with the buffers present at the periphery of the cell which results into calcium bound buffers. Thus, the level of calcium is decreased and maintained. The buffering of calcium thus modulates the calcium signals and plays a key role in shaping the calcium oscillations [34]. If the amount of buffer varies, as a result of which calcium signals are also altered. In AD, buffer levels fall significantly and result in a higher level of calcium, which makes normal neuronal cells toxic. [37, 44]. Thus, the role of buffers in maintaining the cell calcium is immense.
The mathematical formulations of the calcium buffering are given below [40, 41]
On the basis of above equation, the change in free calcium, buffers and calcium bound buffers are formulated using Fick’s law as follows [21, 40]:
where \(R_j=-k^+_j\left[ B_j\right] \left[ Ca^{2+}\right] + k^-_j \left[ CaB_j\right]\) is the reaction term. Further, as we have considered immobile buffers, \({D_B}_j={D_{CaB}}_j=0\)
2.1.4 Endoplasmic Reticulum
ER, the largest intracellular domain, is known as the store house of the cell as it contains the highest amount of calcium. As it contains large amount of calcium, it greatly affects the shape and amplitude of the calcium signals [3]. After the buffering process, the remaining free calcium is passed to ER for further sequestration. The ER calcium is exchanged at the cytosolic level from ER lumen via several leak, channel and pumps. Once the ER calcium is sequestered, it is replenished by these pumps and channels. From the literature, it has been observed that high calcium activities in ER takes place in AD [35]. Due to the high calcium, the cell may become toxic and Alzheimeric condition may prevail. For the mathematical modeling of ER calcium, De–Young–Keizer gave the model having most general and detailed kinetics governing the leak, gate and channel [11]. They have not considered the IP3 mediated ER calcium handling. We also have adhered to the same modeling assumptions, i.e. the interactions which solely take place between ER lumen and cell cytoplasm via \(J_{leak}\), \(J_{chan}\) and \(J_{pump}\). The in detailed mathematical formulations for the same are given below The conservation of calcium in ER is given by [17]
The material balance equation is given by
The mathematical formulation of diffusion taking place between ER and cytosol is governed by the following leak, channel and pumps. They are given as:
2.1.5 Mitochondria
Alike ER, mitochondria also functions as calcium stores in cell. It also has some amount of calcium stored in it. Mitochondria sequesters the calcium and release the rest of the free calcium. Thus, the mitochondrion affects the shape, frequency and amplitude of the calcium signals [45]. The alteration thus leads to higher calcium concentration and neurodegenerativity of AD [42]. Thus, the interplay between cytosolic calcium and mitochondrion calcium has been modelled here. The mathematical formulation for the calcium flux mediated by mitochondria is given below.
The flux via mitochondria is mediated by following equation [45]:
where
Using these formulations, the problem is described in the next sub-section.
2.2 Problem Description
In this section, we have mathematically modeled the interplay of calcium advection reaction diffusion taking place between cell cytosol, ER and mitochondria using law of mass action and Fickian diffusion. A system of 3D differential equations has been used to formulate the problem.
2.2.1 Apposite Boundary Conditions
The initial calcium concentration at cytoplasmic level is taken to be
The calcium flux is mediated by VGCC and NCX present as follows:
where n is perpendicular to the surface. Whereas, the same for ER lumen and mitochondria are taken as
3 Simulations Using Finite Element Technique
Finite element modeling has been used on a wide scale due to its beauty of handling the irregular shaped geometry and non-linearity of the raised problem. Hence, in this paper, the problem of a 3D calcium advection reaction diffusion in a typical neuronal cell is treated with finite element technique. The geometry of an irregular shaped neuron and the pre-processing is discussed in the following sub-sections.
3.1 Targeted Domain and Physiological Fluxes
Figure 2a shows the geometry of an irregular shaped neuronal cell which contains dendrites, cell- body and axon terminals. The geometry has been first constructed in Solidworks and then imported in Comsol Multiphysics5.4 for further simulations. Here, the geometry is built up in such a way that the interaction between cytosolic calcium and calcium of intracellular entities like ER and mitochondria can take place. It contains a space dimension of three with three sub-domains. Number of boundaries used for drawing this geometry are 765, edges are 1804 and vertices are 1047. Hence, this complex neuronal structure is created using above mentioned geometry statistics. The calcium flux in this neuron is mediated by VGCC and NCX present on the cell boundaries which is shown in Fig. 2b.
The type of shape function used to solve the above problem is Lagrange having quadratic element order in spatial frame.
3.2 Mesh Analysis and Element Information
In finite element method, meshing of the domain plays a critical role. The solution depends on the type of the mesh generated. Since the geometry is irregular and complicated, extra fine elements are used instead of normal or coarse meshing so that it can easily deal with the irregular boundaries. Figure 3 shows the discretization of the neuron in 515125 tetrahedral as well as 139526 triangular elements. The generated mesh has 1047 vertex elements and 24256 edge elements. Further, the maximum and minimum element sizes are obtained to be 2.59 and 0.000467 respectively. The curvature factor is found to be 0.6 and maximum element growth rate is 1.5. The minimum element quality is 0.01906 whereas average quality is 0.6819. This shows that the mesh generated is highly acceptable.
4 Results and Discussion
The findings obtained are discussed in this section. Table 1 shows the description and values of the physiological parameters needed to achieve the results.
4.1 Temporal Changes in Calcium Concentration
Figure 4 shows the temporal variations taking place near the source, near ER and near mitochondria. Figure 4a shows the three domain point probes, where the temporal changes has been found and Fig. 4b shows the temporal variations in calcium concentration level. From the graph, it is observed that the calcium concentration level near VGCC and NCX mediated source decreases gradually due to calcium buffering. The rest of the calcium is then sequestered at mitochondria and ER as shown in Fig. 4b.
The nature of the spatio-temporal calcium concentration level obtained in the present work is found to have the same nature as that of obtained by Means et al. and Smith [26, 40].
4.2 Spatial Changes in Calcium Concentration
Figures 5 and 6 shows the variations in calcium oscillatory patterns due to alterations in parametric conditions.
In Fig. 5, the calcium oscillations are obtained for normal amount of Calbindin buffer and different influx conditions of VGCC and NCX and varying intracellular fluxes of ER and mitochondria. Fig. 5a shows the oscillations at normal flux conditions and then they are altered in Figs. 5b and 5c. From the graphs, it is observed that if all the parameters are functioning normally then the calcium oscillations starts at around 0.3 and reaches up to the altitude of 1.3. If more number of VGCC’s and NCX are active than more calcium influx takes place. This phenomenon is observed in Fig. 5b. Here the oscillations start at around 0.8, hence the peak of calcium concentration level is affected by the VGCC and NCX fluxes. Also, if the influx and intracellular fluxes increases, then the calcium concentration level, inspite of high influxes, starts at around 0.4 as the rest of the calcium gets sequestered in the domain.
Figure 6 shows the calcium oscillatory patterns due to low amount of buffer and altered VGCC, NCX,ER and mitochondrial fluxes (different conditions- as dicussed in Fig. 5). On comparing Figs. 5 and 6, it is observed that the altitude of calcium concentration level is comparatively higher in Fig. 6. This hike is due to low amount of buffer present in the cell. Due to low amount of buffer, amount of calcium reacting with it also decreases and hence higher peak of calcium oscillations are obtained. This higher calcium concentration level renders toxicity to the cell and the normal functioning cascades of the cell are impaired. This impairment may be in the form of AD as in AD high level of calcium concentration plays a lead role in cell loss phenomenon. Due to toxicity rendered, cell loss takes place which creates hindrance in memory formations and forming linguistic disorders. From the graphs, it is observed that the highest peak is obtained in Fig. 6c, where all the fluxes are higher and which may be the case of Alzheimeric condition.
4.3 Transition from Diseased to Normal State
To deal with the higher amount of calcium concentration level, exogenous buffers can be added which may help in lowering down the raised calcium levels and may affect the Alzheimeric condition in a positive manner. In Figs. 7 and 8, exogenous buffers, EGTA and BAPTA have been added. From the figures, it is clearly observed that there is a significant decrease in the altitude of calcium oscillations. Moreover, it is seen that in-spite of adding the exogenous buffers, the hike levels are different. This is due to the variations in the buffer association rates of both the buffers. BAPTA has higher association rates, hence it decreases the level more significantly even it is added in low amount and may help in Alzheimeric condition. Although, there may be a side effect of low affinity buffer as well, because more amount would be needed to lower down the calcium concentration level. This may lead to some in-situ consequences?? Thus, for more precise and better approximated results, the researchers and biologists may find the buffer having same range of the association rate as that of calbindin that is present in in-situ condition.
5 Conclusion
In the present article, an attempt has been made to mathematically model the transient calcium oscillations taking place in a typical neuronal cell. A system of non- linear different equations has been formulated for the same which shows the change in cytosolic calcium concentration level, endoplasmic reticulum and mitochondria. Thereafter, apposite boundary conditions have been incorporated which matches well with the physiological conditions. To further deal with the irregularity of the neuron and non- linearity of the system, finite element technique is incorporated using COMSOL Multiphysics 5.4 which deals beautifully with both the above mentioned issues. The obtained results clearly signifies the impact of all the assumed parameters, i.e. VGCC, NCX, buffers, ER and mitochondria. The alterations in the influx of calcium mediated via VGCC and NCX results in higher amount of calcium concentration. Also, lower amount of buffer also results in higher calcium level. Moreover, being the calcium reservoirs, alterations in ER and mitochondrial calcium fluxes lead to higher calcium concentration level. These increases in the calcium concentration level leaves profound and long- lasting impact on the cell which may result into neurodegenerative disorder named AD, as high level of calcium is one of the causes for AD to prevail. The altered calcium oscillations interrupt the normal signaling cascades and many of the normal neuronal functions like memory formations, linguistic impairments, etc. To address this problem, we have added exogenous calcium binding buffers, EGTA and BAPTA. EGTA being low affinity buffer, binds comparatively lower amount of calcium even if more amount is added, whereas, BAPTA binds more amount of calcium on lower amount of addition of it and hence the increased levels of calcium oscillations can be controlled. To further get more accurate approximations of the spatio-temporal calcium oscillations in in-vitro conditions, the theoretical scientists and biologists can investigate and find a buffer having same range and properties as that of calbindin in in-situ condition. Moreover, various other physiological parameters can be added to the mathematical model so that a better approximation of calcium profile can be obtained.
References
Augustine GJ, Santamaria F, Tanaka K (2003) Local calcium signaling in neurons. Neuron 40:331–346. https://doi.org/10.1016/s0896-6273(03)00639-1
Benarroch EE (2010) Neuronal voltage-gated calcium channels: brief overview of their function and clinical implications in neurology. Neurology 74:1310–1315. https://doi.org/10.1212/WNL.0b013e3181da364b
Berridge MJ (1998) Neuronal calcium signaling. Neuron 21:13–26. https://doi.org/10.1016/s0896-6273(00)80510-3
Bertram R, Tabak J, Teka W, Vo T, Wechselberger M, Kirk V, Sneyd J (2015) Math Anal Complex Cellular Activity. https://doi.org/10.1007/978-3-319-18114-1
Bezprozvanny I (2011) Calcium signalling and neurodegenerative diseases. Trends Mol Med 15(3):89–100. https://doi.org/10.1016/j.molmed.2009.01.001.Calcium
Blaustein MP, Lederer WJ (1999) Sodium / calcium exchange : its physiological implications. Physiol Rev 79(3):763–854. https://doi.org/10.1152/physrev.1999.79.3.763
Colvin RA, Bennett JW, Colvin SL (1991) Na+-Ca2+ exchange activity is increased in Alzheimer’s Disease brain tissues. Ann N Y Acad Sci 639:325–327. https://doi.org/10.1111/j.1749-6632.1991.tb17320.x
Dave DD, Jha BK (2018) Analytically depicting the calcium diffusion for Alzheimer’s affected cell. Int J Biomath 11(6), https://doi.org/10.1142/S1793524518500882
Dave DD, Jha BK (2018) Delineation of calcium diffusion in alzheimeric brain. J Mech Med Biol 18(2):1–15. https://doi.org/10.1142/S0219519418500288
Dave DD, Jha BK (2020) 3D mathematical modeling of calcium signaling in Alzheimer’s disease. Netw Modeling Anal Health Inform Bioinform 3:1–10. https://doi.org/10.1007/s13721-019-0207-3
De Young GW, Keizer J (1992) A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci USA 89(October):9895–9899. https://doi.org/10.1073/pnas.89.20.9895
Jha A, Adlakha N (2014) Analytical solution of two dimensional unsteady state problem of calcium diffusion in a neuron cell. J Med Imaging Health Inform 4(4):547–553. https://doi.org/10.1166/jmihi.2014.1282
Jha A, Adlakha N (2015) Two-dimensional finite element model to study unsteady state Ca 2+ diffusion in neuron Involving ER, LEAK and SERCA. Int J Biomath 8(1):1–14. https://doi.org/10.1142/S1793524515500023
Jha A, Adlakha N, Jha BK (2015) Finite element model to study effect of Na+-Ca2+ exchangers and source geometry on calcium Dynamics in a neuron cell. J Mech Med Biol 16(2):1–22. https://doi.org/10.1142/S0219519416500184
Jha BK, Dave DD (2020) Approximation of calcium diffusion in Alzheimeric cell. J Multiscale Modell 11(2):1–21. https://doi.org/10.1142/S1756973720500018
Jha BK, Jha A (2015) Two dimensional finite volume model to study the effect of ER on cytosolic calcium distribution in astrocytes. Proc Comput Sci 46:1285–1293. https://doi.org/10.1016/j.procs.2015.01.052
Jha BK, Adlakha N, Mehta MN (2011) Finite Volume Model to Study the Effect of ER flux on Cytosolic Calcium Distribution in Astrocytes. J Comput 3(11):74–80, https://sites.google.com/site/Journal of Computing
Jha BK, Adlakha N, Mehta M (2013) Two-dimensional finite element model to study calcium distribution in astrocytes in presence of VGCC and excess buffer. Int J Modeling Simul Sci Comput 4(2): 1250030-1-1250030-15 https://doi.org/10.1142/S1793962312500304
Jha BK, Jha A, Adlakha N (2019) Three-dimensional finite element model to study calcium distribution in astrocytes in presence of VGCC and Excess. Differ Equ Dyn Syst. https://doi.org/10.1007/s12591-019-00502-x
Kawamoto EM, Vivar C, Camandola S (2012) Physiology and pathology of calcium signaling in the brain. Front Pharmacol 3(April):1–17. https://doi.org/10.3389/fphar.2012.00061
Keener J, Sneyd J (2009) Mathematical Physiology, 2nd edn. Springer, Berlin
Khachaturian Z (1989) Introduction and Overview. Annals New York Academy of Sciences pp 4–7, https://doi.org/10.1111/j.1749-6632.1989.tb12485.x
Khalid MU, Tervonen A, Korkka I, Hyttinen J, Lenk K (2018) Geometry-based Computational Modeling of Calcium Signaling in an Astrocyte. In: IFMBE Proceedings 65, Springer Nature Singapore Pvt Ltd., Tampere, Finland, pp 1–4, https://doi.org/10.1007/978-981-10-5122-7
Laferla FM (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer’s Disease. Nat Rev Neurosci 3:862–872. https://doi.org/10.1038/nrn960
Magi S, Castaldo P, Macrì ML, Maiolino M, Matteucci A, Bastioli G, Gratteri S, Amoroso S, Lariccia V (2016) Intracellular calcium dysregulation : implications for Alzheimer ’ s Disease. Biomed Res Int 2016:1–14. https://doi.org/10.1155/2016/6701324
Means S, Smith AJ, Shepherd J, Shadid J, Fowler J, Wojcikiewicz RJH, Mazel T, Smith GD, Wilson BS (2006) Reaction diffusion modeling of calcium dynamics with realistic ER geometry. Biophys J 91:537–557. https://doi.org/10.1529/biophysj.105.075036
Naik P, Pardasani KR (2017) Three-dimensional finite element model to study calcium distribution in oocytes. Netw Modeling Anal Health Inform Bioinform. https://doi.org/10.1007/s13721-017-0158-5
Naik PA, Pardasani KR (2015) Two-dimensional finite element model to study calcium distribution in oocytes. J Multiscale Model 6(1):1–15. https://doi.org/10.1142/S1756973714500024
Naik PA, Pardasani KR (2016) Finite element model to study calcium distribution in oocytes involving voltage gated Ca 2 + channel, ryanodine receptor and buffers. Alexandria J Med 52(1):43–49. https://doi.org/10.1016/j.ajme.2015.02.002
Naik PA, Pardasani KR (2018) Three-dimensional finite element model to study effect of RyR calcium channel, ER leak and SERCA pump on calcium distribution in oocyte cell. Int J Comput Methods 15(3):1–19. https://doi.org/10.1142/S0219876218500913
Naik PA, Zu J (2020) Modeling and simulation of spatial-temporal calcium distribution in T lymphocyte cell by using a reaction-diffusion equation. J Bioinform Comput Biol Article. https://doi.org/10.1142/S0219720020500134
Panday S, Pardasani KR (2013) Finite Element Model to Study Effect of Advection Diffusion and Na+/Ca2+ Exchanger on Ca2+ Distribution in oocytes. J Med Imaging Health Inform 3(3):374–379. https://doi.org/10.1166/jmihi.2013.1184
Pathak K, Adlakha N (2015) Finite Element Model to Study Calcium Signalling in Cardiac Myocytes Involving Pump, Leak and Excess buffer. J Med Imaging Health Inform 5:1–6. https://doi.org/10.1166/jmihi.2015.1443
Petersen OH (2017) The effects of Ca2+ buffers on cytosolic Ca2+ signalling. J Physiol 10:3107–3108. https://doi.org/10.1113/JP273852
Popugaeva E, Bezprozvanny I, Stutzmann B, Franklin R (2013) Role of endoplasmic reticulum Ca 2+ signaling in the pathogenesis of Alzheimer disease. Front Mol Neurosci 6(September):1–7. https://doi.org/10.3389/fnmol.2013.00029
Rajagopal S, Ponnusamy M (2017) Calcium signaling : from physiology to diseases. Springer Nature Singapore Pvt Ltd
Riascos D, Leon DD, Baker-Nigh A, Nicholas A, Yukhananov R, Bu J, Wu CK, Geula C (2011) Age-related loss of calcium buffering and selective neuronal vulnerability in Alzheimer ’ s disease. Acta Neuropathol 122:565–576. https://doi.org/10.1007/s00401-011-0865-4
Schampel A, Kuerten S (2017) Danger : high voltage–the role of voltage-gated system pathology. Cells 6:1–8. https://doi.org/10.3390/cells6040043
Simms BA, Zamponi GW (2014) Neuronal voltage-gated calcium channels: structure, function, and dysfunction. NEURON 82(1):24–45. https://doi.org/10.1016/j.neuron.2014.03.016
Smith GD (1996) Analytical steady-state solution to the rapid buffering approximation bear an open Ca2+ channel. Biophys J 71:3064–3072. https://doi.org/10.1016/S0006-3495(96)79500-0
Smith GD, Dai L, Miura RM, Sherman A (2001) Asymptotic analysis of buffered calcium diffusion near a point source. SIAM J Appl Math 61(5):1816–1838. https://doi.org/10.1137/S0036139900368996
Supnet C, Bezprozvanny I (2010) Neuronal calcium signaling, mitochondrial dysfunction and Alzheimer’s disease. J Alzheimers Dis 20:S487–S498. https://doi.org/10.3233/JAD-2010-100306
Tewari SG, Pardasani KR (2012) Modeling effect of sodium pump on calcium oscilations in neuron cells. J Multiscale Model 4(3):1–16. https://doi.org/10.1142/S1756973712500102
Turkington C, Mitchell D (2010) The Encyclopedia of Alzheimer’s Disease, 2nd edn. An imprint of Infobase Publishing, Facts On File
Wacquier B, Combettes L, Van Nhieu GT, Dupont G (2016) Interplay between intracellular ca 2+ oscillations and ca 2+-stimulated mitochondrial metabolism. Sci Rep 6(1):1–16. https://doi.org/10.1038/srep19316
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Dave, D.D., Jha, B.K. Mathematical Modeling of Calcium Oscillatory Patterns in a Neuron. Interdiscip Sci Comput Life Sci 13, 12–24 (2021). https://doi.org/10.1007/s12539-020-00401-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-020-00401-8