Abstract
Purpose of Review
Chronic kidney disease (CKD) is a common condition and a major cause of morbidity and mortality in adults, but children and adolescents are also at risk for early kidney injury and development of CKD. Obesity contributes both directly and indirectly to the development of CKD. The purpose of this review is to describe obesity-related kidney disease (ORKD) and diabetic kidney disease (DKD) and their impact in the pediatric population.
Recent Findings
Although obesity-related CKD in childhood and adolescence is uncommon, nascent kidney damage may magnify the lifetime risk of CKD. Glomerular hyperfiltration is an early phenotype of both ORKD and DKD and typically manifests prior to albuminuria and progressive decline in GFR. Novel treatments for obesity and type 2 diabetes exerting protective effects on the kidneys are being investigated for use in the pediatric population.
Summary
It is important to understand the impact of obesity on the kidneys more fully in the pediatric population to help detect injury earlier and intervene prior to the onset of irreversible progression of disease and to guide future research in this area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a leading cause of death and affects more than 800 million people worldwide [1]. The prevalence of CKD in adults in the USA between 2015 and 2018 was 13.3% [2]. The prevalence of CKD increases with age: 0.36% in children (ages 12–17), 6% in adults 18–44, 12.4% in adults 45–64, and 38.1% in adults over 65 years [3, 4]. However, the initial impact of risk factors such as hypertension, diabetes, and obesity on the kidneys often manifest early in life before the onset of overt kidney disease. Additionally, a large majority of adults with CKD are unaware that they have the disease [4]. A better understanding of the impact of risk factors for the development of CKD in childhood and adolescence and earlier identification of kidney injury may help prevent progression of kidney disease in adulthood.
CKD is defined as abnormal kidney structure or function for greater than 3 months (unless an infant < 3 months old) as represented by impaired GFR (<60 ml/min/1.73 m2) or a marker of kidney damage (albuminuria, histologic abnormality, structural abnormality, urine sediment abnormality, or electrolyte disturbance secondary to tubular disorders) [3, 5]. Diabetic kidney disease (DKD) and hypertension are the leading causes of CKD in the adult population. While clinically evident CKD from diabetes or hypertension are rare in children, the early onset of obesity predisposes children to develop these conditions later in life [3, 6].
Obesity is known to be a major risk factor for CKD and worsens outcomes in patients with pre-existing kidney disease [7, 8]. The metabolic syndrome is a known risk factor for development of CKD, and it is well-established that obesity increases the risk of co-morbid conditions that affect kidney function such as hypertension and diabetes [9,10,11]. Obesity has a direct adverse impact on kidney function as well, as higher BMI has been shown to increase the risk of kidney failure even after adjusting for blood pressure and diabetes [12]. In the USA, an estimated 24% of kidney disease in men and 34% in women is attributed to overweight and obesity [13]. While the evidence is more sparse in the pediatric population, children undergoing kidney transplant have an increased risk of graft failure if the recipient has obesity [14]. Higher BMI is also associated with an increased risk of kidney cancer, stone formation, worse outcomes in IgA nephropathy, and urinary tract infection risk in children [15,16,17,18,19,20]. In a recent systemic review of the pediatric population, 6 of 8 prospective studies (follow-up duration 8–64 years) reported a positive association between higher BMI levels in early life and greater kidney disease risk in later life [21].
A new entity, obesity-related kidney disease (ORKD), is now recognized as a distinct cause of CKD and though it shares similarities and overlap with DKD, it is important to understand its unique characteristics to guide management and prevention strategies. Historically ORKD was rarely diagnosed in pediatrics, but in response to the obesity epidemic, it is an emerging and likely underrecognized and understudied complication of pediatric obesity. Therefore, the purpose of this review is to describe both ORKD and DKD and summarize current research in the pediatric population (Table 1).
Obesity-Related Kidney Disease
ORKD is classically defined as a secondary form of focal segmental glomerulosclerosis (FSGS). In addition to the pathognomonic finding of scarring in a portion of the glomerular tuft, it is characterized by glomerular hypertrophy and proteinuria without hematuria. The diagnosis is established in patients with a BMI meeting criteria for obesity and without evidence of other underlying primary kidney diseases or known cause of FSGS [22]. However, the effects of obesity are not constrained to secondary focal segmental glomerulosclerosis, nor to the glomerulus alone. Indeed, modern studies have defined ORKD histologically as glomerulomegaly with obesity with and without evidence of focal segmental glomerulosclerosis, while functionally the effects of obesity on tubular metabolism have recently gained increasing recognition. Therefore, we contend that the definition of ORKD is likely broad and characterized by heterogeneity in both structural and functional features.
Epidemiology
The prevalence of obesity in children and adolescents 2–19 years old in the United States is nearly 20%, and these children are also more likely to remain overweight or obese as adults [23,24,25]. According to a meta-analysis in 2016, children and adolescents who are obese were five times more likely to be obese in adulthood [26].
Obesity accentuates the risk of hypertension and diabetes, the two leading causes of CKD. Epidemiologic data also support the effect of obesity on the development of kidney disease in the absence of these conditions. A meta-analysis found that obesity was associated with a 28% increased risk of impaired eGFR and a 51% increased risk in albuminuria in adults without prior CKD and that the risk of impaired eGFR persisted even in those without metabolic syndrome suggesting obesity itself is an independent driver of development of CKD [27]. Obesity as a risk factor for kidney disease has been seen in the young adult population as well. One prospective cohort of young adults found that both obesity and poor diet quality were associated with onset of moderate albuminuria after controlling for hypertension and diabetes and another cohort found that BMI ≥35 kg/m2 in young adults was associated with albuminuria [28, 29].
The data in pediatric populations are more limited. In one large cohort of adolescents, having obesity at age 17 was associated with a 19-fold increased risk of kidney failure secondary to diabetes and a 3.4-fold increased risk of kidney failure for reasons beyond diabetes illustrating the impact of obesity both through increasing risk of DKD but also on non-diabetic kidney disease [30]. Another study in children and adolescents found an association between high BMI and glomerular hyperfiltration, and an inverse association of duration of obesity and the homeostatic model of insulin resistance (HOMA-IR) with eGFR. This is consistent with the observation that ORKD starts with a glomerular hyperfiltration phase followed by a slow decrease in GFR over time, though the impact on GFR can be seen as early as childhood [31]. For example, in a cross-sectional study of children aged 5–14 years, being overweight or obese was associated with an increased risk of mildly impaired estimated GFR [32].
The exact prevalence of ORKD is difficult to ascertain. However, it is currently thought to be the underlying cause in up to 30% of patients with CKD. A study of biopsy samples suggested an incidence of 2.7% from 2001–2015 using the definition of BMI ≥30 kg/m2 and the presence of glomerulomegaly with or without FSGS. About half of the patients diagnosed with ORKD by biopsy had a BMI of 40 or higher and the other half had a BMI of 30–40 suggesting an increased risk at any class of obesity [33, 34]. Further work is needed to define the optimal measurement of adiposity to define obesity and the prevalence of ORKD.
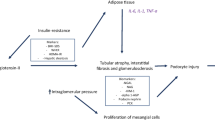
Obesity may not magnify the risk of kidney injury similarly across individuals given the complex range of factors contributing to disease (Fig. 1). People with greater visceral fat, diagnosed with components of the metabolic syndrome, other co-morbid conditions such as sleep apnea, a lower nephron number (which can be related to low birth weight or preterm birth), or a lower nephron mass from nephrectomy or congenital anomalies are at increased risk for experiencing kidney injury secondary to obesity [35]. In the area of cardiovascular risk, a concept of “pound-years” (defined as excess adiposity multiplied by years of exposure) has been described to better quantify the risk of long-term obesity exposure [36]. Obesity starting in childhood and therefore increasing the number of “pound-years” is likely a much stronger risk factor for lifetime CKD risk, but more studies are needed to quantify this risk.
Multi-factorial etiology of obesity-related kidney disease. Created with BioRender.com
Pathogenesis
Though the exact pathophysiology of ORKD is not clearly defined, obesity has been shown to impact the kidneys through multiple mechanisms including hemodynamic changes and hyperfiltration, inflammation, insulin resistance, activation of the renin-angiotensin-aldosterone-system (RAAS), and mitochondrial damage. The defining characteristic of ORKD is an increase in glomerular pressure that causes basement membrane distension and glomerulomegaly and an increased filtration rate leading to hypertrophy by the podocytes and eventual podocyte apoptosis, detachment, and glomerulosclerosis [33]. The 3 major pathways through which obesity leads to these changes include hyperfiltration secondary to increased body weight causing higher glomerular pressures, inflammation from cytokines released from adipose tissues, and changes in metabolic pathways (Fig. 2). Hemodynamic changes occur in obesity that result in increased GFR, renal plasma flow (RPF), and filtration fraction (FF). The mechanisms proposed for this glomerular hyperfiltration include afferent arteriole dilation and an increase in the proximal tubule resorption of sodium and water [37, 38]. Obesity leads to the activation of the RAAS which can also cause hyperfiltration through a vasoconstrictive effect on the efferent arteriole by angiotensin II and aldosterone and an increase in sodium reabsorption by angiotensin II. Glomerular hyperfiltration and increased glomerular pressure then leads to glomerular injury, podocyte loss, and tubulointerstitial inflammation and fibrosis [33, 39]. Angiotensin II contributes to podocyte injury by increasing intracellular calcium causing depolarization.
Pathophysiological pathways promoting kidney injury in obesity. Created with BioRender.com
Adipose tissue itself is associated with an increase in inflammation in patients with obesity through the release of proinflammatory cytokines such as IL-1, IL-6, TNF-\(\alpha\), and a decrease in more beneficial adipokines such as adiponectin which has a protective role for podocytes [40]. Lipotoxicity also contributes to kidney damage as excess free fatty acids and triglycerides are deposited in the kidney leading to mitochondrial dysfunction, generation of reactive oxygen species, and podocyte damage [41,42,43]. Furthermore, physical compression of renal vessels and parenchyma by fat could promote sodium reabsorption and arterial hypertension [44].
Insulin resistance in obesity is associated with kidney dysfunction through multiple mechanisms. Patients with moderate albuminuria have been shown to be more insulin resistant and have higher fasting insulin concentrations than those without moderate albuminuria even in the absence of diabetes and hypertension [45]. Animal studies have shown that onset of podocyte injury and proteinuria occur secondary to insulin resistance independent of hyperglycemia [46]. Hyperinsulinemia can also cause kidney injury through oxidative stress secondary to generation of free radicals and a decrease in antioxidant enzymes. Finally, it can magnify the effect of angiotensin II in the kidney [47].
Structure
Otherwise, healthy children with obesity have significantly larger kidneys than those with a normal BMI [48]. ORKD is histologically marked by glomerular hypertrophy or glomerulomegaly and low glomerular density with or without evidence of FSGS. Glomerulomegaly in obese patients can present early in life and has been reported in children as young as 3 years old [49]. A review of 6818 kidney biopsies found that compared to idiopathic FSGS, patients with ORKD had more glomerulomegaly, less segmental sclerosis, and less extensive foot process effacement [34]. If there is FSGS present in ORKD, the histopathological extent tends to be milder than primary FSGS. The sclerosis is more commonly present in the perihilar region of the glomerular tuft [50, 51]. The FSGS is thought to occur in ORKD due to podocyte hypertrophy that occurs as an adaptation to glomerulomegaly. Ultimately, the podocyte adaptations cannot accommodate the glomerular expansion due to constrained proliferative capacity, and they eventually detach and fail. Other common findings include increased mesangial matrix, mesangial cell proliferation, and irregular podocyte dropout [41, 52]. Lipid accumulation may also be seen in both podocytes and tubular cells [53, 54]. Tubular hypertrophy also occurs in obese patients. In a biopsy study of 11 adult patients with obesity without diabetes, there was an increase in proximal tubular volume secondary to hypertrophy. This is thought to be due to an increase in metabolic load that needs to be reabsorbed in the setting of hyperfiltration [55].
Function
ORKD may be clinically silent and not recognized for several years after onset of the initial kidney insult. The typical progression of disease is marked by initial glomerular hyperfiltration and histological changes. This is followed by onset of albuminuria, which is slowly progressive, and finally impaired GFR and in some cases, progression to kidney failure. Since the early kidney injury often goes undetected, the most common initial clinical presentation is isolated proteinuria or albuminuria. It is typically in a sub-nephrotic range though some patients may have higher degrees of proteinuria. Though albuminuria/proteinuria are the typical markers used to detect ORKD, they may not be manifest until later in the disease course and the adverse effects on the kidneys from obesity can be seen on biopsy before the manifestation of albuminuria [52]. Clinical symptoms of nephrotic syndrome are not typical in ORKD even with nephrotic-range proteinuria [50, 56]. In addition to the less frequent presence of overt nephrotic syndrome, when compared to primary FSGS, ORKD has a more indolent course. There are limited data on the long-term outcomes of ORKD. In one study of 15 adults with biopsy proven obesity-related FSGS, almost 50% advanced to kidney failure with an estimated probability of kidney survival of 77% after 5 years and 51% after 10 years [56]. In one retrospective cohort, children with obesity born at term had a similar course as described in adults while obese children born pre-term had a more rapid deterioration [57].
Screening
Reconsideration of screening guidelines to detect kidney disease may be warranted in children and adolescents with an increased BMI to facilitate identification of ORKD. Serum creatinine and urine albumin to creatinine ratio are not routinely checked in patients with obesity, especially not in youth. Moreover, altered body composition may impact on creatinine production in obese individuals and affect the accuracy of serum creatinine concentration as an index of kidney function. Studies of kidney function using alternative markers such as cystatin C may be required to assess the prevalence of ORKD.
Formulas to estimate GFR based on height and serum creatinine concentration in patients with obesity often do not reflect true filtration function, though using absolute eGFR in lieu of eGFR normalized to body surface area (BSA) may more readily detect the early glomerular hyperfiltration phase of ORKD [58, 59]. Though not specific for ORKD, in order to facilitate earlier detection of kidney damage and better track progression of disease, several novel biomarkers are being investigated. In a study of adolescents with severe obesity undergoing bariatric surgery, they were found to have significantly higher prevalence of subclinical evidence of kidney injury with elevated urine neutrophil gelatinase-associated lipocalin (NGAL), Interleukin-18 (IL-18), and kidney injury molecule-1 (KIM-1) compared to lean subjects despite having no microalbuminuria or evidence of decreased kidney function [60].
There may be a role for kidney imaging in the initial detection and evaluation of progression of ORKD. The accumulation of lipids in the kidney can be seen on ultrasound, computerized tomography (CT), and magnetic resonance imaging (MRI). Different measures that could be derived from ultrasound imaging include the resistance index that can indicate renal perfusion changes and early signs of kidney injury, pararenal and perirenal ultrasonographic fat thickness (PUFT) to measure visceral fat, elastography to evaluate for fibrosis. CT and MRI can be used to measure renal sinus fat (RSF), and whether MRI can be used to detect ectopic lipid deposition remains under investigation. There are not clear guidelines on the use of imaging for detecting ORKD or the usefulness of these markers in the adult or pediatric population, but several studies of adults have found associations between PUFT, RSF and increasing albuminuria and decreasing eGFR [50, 61,62,63,64].
Diabetic Kidney Disease
Epidemiology
Diabetic kidney disease is the leading cause of CKD in adults accounting for about a third of adults with CKD [25]. In children and adolescents, there has been a relative increase in the prevalence of type 1 diabetes (45% increase) and of type 2 diabetes (95% increase) over the past 16 years [65]. There has also been an increase in DKD in children and adolescents over a similar time period (2002–2013) with a rise in prevalence from 1.16 to 3.44% among those with diabetes [66]. Teenagers and young adults with type 2 diabetes have a higher prevalence of DKD than those with type 1 diabetes and are more likely to develop renal failure [67, 68].
There is a strong association between overweight or obesity in adolescence and the development of type 2 diabetes. Having a higher BMI during the adolescent years increases risk of developing diabetes at a younger age and having higher rates of albuminuria. Thus, addressing obesity during childhood and adolescence plays an important role in preventing diabetic kidney disease [69]. The importance is highlighted by the increased incidence of kidney failure in adults with onset of type 2 diabetes in childhood compared to those with onset of diabetes in adulthood [70]. Indeed, younger age at diabetes onset, being overweight or obese, having a positive family history, genetic factors, poor glycemic control, high glucose variability, hypertension, smoking, intrauterine exposure to maternal diabetes or obesity, and low birth weight all exacerbate developing DKD [71].
Pathogenesis
Chronic hyperglycemia is the major driver in the development of DKD leading to glomerular and tubular injury, inflammation, and oxidative stress. Trials have demonstrated that intensive glycemic control (targeting a hemoglobin A1C (HbA1C) level ≤7%) delays the development of and slows the progression of DKD in both type 1 and 2 diabetes. This more intensive glycemic control has lasting beneficial effects on the development of microvascular complications including development of albuminuria and loss of eGFR. In the Diabetes Control and Complications Trial (DCCT), after the intensive glycemic control intervention ended and glycemic control worsened, the intervention group still had less microvascular complications over a decade later [72,73,74,75,76]. While more exposure to hyperglycemia does increase the risk of DKD, not all patients with poorly controlled diabetes develop DKD and not all patients with well-controlled diabetes are spared this complication. This underscores the importance of non-glycemic risk factors, including genetic susceptibility and epigenetics.
Hyperglycemia induces kidney damage through several mechanisms. Like ORKD, glomerular hyperfiltration is an early feature driving kidney damage in DKD [71]. Glomerular hyperfiltration is in part due to dilation of the afferent arteriole. Hyperglycemia contributes to this dilation by release of insulin-like growth factor 1, nitric oxide, vascular endothelial growth factor, and prostaglandins. Additionally, there is an increase in the reabsorption of glucose and sodium in the proximal tubule, which decreases delivery of sodium to the macula densa, reduces tubuloglomerular feedback, and contributes to dilation of the afferent arteriole [77]. Hyperglycemia activates the RAAS system, and increased angiotensin II leads to preferential constriction of the efferent arteriole further contributing to increase intraglomerular pressure [78]. Ultimately hyperfiltration and increased glomerular pressure result in mechanical stress and glomerulosclerosis. Hyperglycemia activates pathways such as the polyol pathway, hexosamine pathway, protein kinase C pathway, and the advanced glycation end-product (AGE)-related pathway resulting in reactive oxygen species (ROS) generation ultimately leading to oxidative stress and cell dysfunction. Other activated intracellular pathways, such as Janus kinase-signal transducers and activators of transcription and nuclear factor kappa-B, contribute to inflammation in DKD [79].
Hypoxia due to the mismatch of oxygen supply and consumption is another important contributor to diabetic kidney disease. Hyperglycemia and glomerular hyperfiltration drive renal energy expenditure and oxygen demand, but due to microvascular injury and interstitial fibrosis, oxygen supply is often impaired [79].
Structure
Advanced stages of DKD are not commonly seen in childhood but structural changes can be seen before development of clinical disease. There is greater frequency of structural abnormalities with increasing duration of disease, but changes have been noted as early as 2 years after diagnosis of diabetes [80,81,82]. Early abnormalities found on kidney biopsies include thickening of the glomerular, tubular, and capillary basement membranes. The tubular basement membrane is one of the earliest sites of change, which may occur prior to development of albuminuria or clinical renal disease [71].
Mesangial expansion is another common finding in DKD and is characterized by an increase in mesangial cells and increased production of matrix proteins. Both mesangial matrix accumulation and mesangial cell hypertrophy have been seen in response to hyperglycemia [83]. Other classic findings include effacement of foot processes and loss of podocytes, mesangial nodule formation, afferent and efferent glomerular arteriolar hyalinosis, and finally global glomerular sclerosis. Type 2 diabetes tends to have more varied findings on biopsy compared to type 1 diabetes [76, 84].
Function
Generally, the progression of DKD is similar to that described in ORKD with initial hyperfiltration, followed by a normalization of eGFR, and finally progression into overt CKD with a fall in eGFR. Albuminuria can develop at any point in this progression.
In prior literature, the stages of DKD have been described as follows. Stage 1 of DKD is characterized by preserved GFR or glomerular hyperfiltration. An increase in kidney size and increased renal plasma flow has also been reported. Stage 2 is described by changes in structure including thickening of the glomerular basement membrane and mesangial expansion and can occur as early as 1.5–2 years after onset of diabetes, and often prior to overt kidney dysfunction. At this stage, GFR, blood pressure, and albuminuria are all typically normal. At stage 3, there is moderate albuminuria (30-300 mg/g) and potentially a modest decrease in GFR. In stage 4, there is severely increased albuminuria (>300 mg/g), impaired GFR (<60 ml/min/1.73 m2), and hypertension. Finally, there is progression to stage 5 with GFR < 15 ml/min/1.73 m2 [71].
Screening
It is recommended that children are screened with a urine microalbumin/creatinine ratio annually starting at age 10 or puberty and after having type 1 diabetes for 5 years or at time of diagnosis for those with type 2 diabetes. Higher rates of moderate albuminuria are seen in adolescents with type 2 diabetes compared to those with type 1 diabetes and an increasing HbA1c increases the risk of moderate albuminuria [85]. Annual screening of eGFR is advised to enable earlier detection of DKD as glomerular hyperfiltration may be found prior to clinical presentation of overt nephropathy [86].
Despite being the key screening method, there are several limitations to albuminuria as a marker of DKD. It can be influenced by hydration, infection, stress, menstrual cycles, glucose levels, and physical activity. Thus, there is significant day-to-day variation. It has a low sensitivity and specificity, and development of albuminuria may not occur until later in disease progression [71]. Albuminuria can also be transitory and patients with albuminuria may never progress to CKD and alternatively patients may develop kidney impairment without albuminuria (i.e., non-albuminuric DKD). In the UK Prospective Diabetes Study, 51% of the study population did not have albuminuria prior to developing renal impairment (defined as an eGFR of <60 ml/min/1.73 m2) [87].
There are several biomarkers being investigated that could help predict DKD earlier than albuminuria, such as NF-\(\alpha\), transferrin, type IV collagen, L-PGDS, IgG, ceruloplasmin, laminin, GAGs, fibronectin, podocalyxin, and VEGF. Additionally, tubular injury precedes glomerular injury so tubular biomarkers may help detect DKD earlier (\(\alpha\) -1-microglobulin CysC, KIM-1, NGAL, nephrin, NAG, L-FABP, VDBP, CypA, s-Klotho). There may also be a role of markers of inflammation and oxidative stress in predicting DKD development and progression [71].
Identifying which patients with obesity have insulin resistance could also play a role in recognizing children most at risk for developing diabetes and kidney disease given its association with hyperfiltration. Adolescents in the TODAY study demonstrated that over time youth with type 2 diabetes experienced rising eGFR and albumin excretion rates, which associated with decreasing insulin sensitivity. In the same study, worsening insulin resistance was associated with glomerular hyperfiltration, and hyperglycemia was associated with increased albumin excretion [86]. However, there has been data in the adult population to show no association between insulin resistance and CKD in non-diabetic patients, and further study is needed to clarify the relationship between insulin resistance and kidney disease risk in the pediatric population [88].
Blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) is a technique that can be used to estimate kidney oxygen availability and has been used to visualize the effects of kidney oxygenation on risk of kidney disease [79].
Management of ORKD and DKD
The primary form of management and prevention for ORKD is to address the underlying cause, obesity, through weight loss and maintenance of a healthy weight. For weight loss, a multifactorial approach should be considered including diet, exercise, pharmacological treatment, and in some cases metabolic bariatric surgery. It is also important to maintain a healthy blood pressure as hypertension can further contribute to kidney injury. Given that ORKD may be silent for many years, it is important for pediatric providers to focus on treatment and prevention of obesity prior to the diagnosis of kidney disease to prevent progression to irreversible disease. Weight loss has been shown to significantly attenuate glomerular hyperfiltration seen in patients with obesity who do not yet have overt kidney disease [89]. A Cochrane review of randomized controlled trials in adults with obesity and existing CKD did not demonstrate clear improvement in proteinuria following weight loss, but did show that lifestyle interventions were effective in weight loss [90].
As detailed in the American Academy of Pediatrics (AAP) “Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity,” lifestyle treatment should be child-focused and family-centered with a focus on understanding the underlying genetic, biologic, environmental, and social determinants that are contributing. The most effective intensive health behavior and lifestyle treatment occurs over 3–12 months with over 26 h of face-to-face counseling on nutrition and physical activity with parent involvement [91].
Specific to nutrition in patients with CKD, the Kidney Disease: Improving Global Outcomes (KDIGO) group recommends limiting daily proteins, restriction of sodium intake in children with hypertension or pre-hypertension, and controlled or reduced phosphorus and potassium intake [5]. There are not clear guidelines for patients with both CKD and obesity.
The traditional mainstays of protecting against the development and progression of DKD include strict glycemic control (goal HbA1c < 7%), blood pressure control, and weight loss if having pre-obesity or obesity. Fewer treatment options exist for children with type 2 diabetes compared to adults. Current standard treatment of type 2 diabetes in children and adolescents consists of metformin and insulin, but high failure rates to metformin have been demonstrated and youth with type 2 diabetes tend to have lower insulin sensitivity than adults [92, 93]. Recently, new therapies have gained approval for use in children.
Pharmacologic therapy may have a role in both ORKD and DKD to help protect the kidneys and halt progression of disease, contribute to weight loss, improve blood pressure, and maintain glycemic control. There are several medications that can be used for nephroprotection in ORKD and DKD. ACE-inhibitors play an important role in both ORKD and DKD as an adjunctive treatment for both blood pressure control and albuminuria reduction. ACE-inhibitors have been shown in a randomized, placebo-controlled trial of adults to prevent the rise of proteinuria that occurs in patients with obesity and reduce the rate of kidney failure by 86% in patients with obesity and 45% in patients with pre-obesity (overweight) [7]. As shown in the ESCAPE trial, blood pressure control with ACE-inhibitors in children with CKD and hypertension improves kidney function [94, 95]. However, there is limited data on use of ACE-inhibitors to specifically help prevent ORKD in the pediatric population. An animal study in pre-pubescent rats showed that lisinopril attenuated glomerular hyperfiltration and reduced glomerular injury, proteinuria, and kidney inflammation in obese rats [96]. More studies are needed in ORKD to determine when to intervene in children with obesity but not diabetes. For DKD, it is recommended to initiate an ACE-inhibitor or ARB if two morning urine samples have a urine microalbumin/Cr ratio >30 mg/g over 6 months despite trying to improve glycemic control and blood pressure [97]. It is important to note, however, that studies in adults have demonstrated a dissociation between antiproteinuric and renoprotective action of ACEI or ARB therapy. Continued study is warranted in pediatric patients with DKD.
Pharmacologic therapy may also be helpful to achieve weight loss in children and adolescents who have not been able to achieve a healthy weight with lifestyle therapy alone. Phentermine, topiramate, and glucagon-like peptide-1 receptor agonists (GLP-1RA) have been used in adolescents and demonstrated significant decreases in BMI [98, 99]. For children with type 2 diabetes, additional medications may be needed to achieve glycemic control. GLP-1RAs have been shown to have beneficial effects on glycemic control, but data on cardiorenal benefits are limited in youth with type 2 diabetes [100,101,102,103,104].
GLP-1 receptor agonists may protect the kidneys and heart indirectly by improving hyperglycemia, hypertension, obesity, and dyslipidemia in addition to direct action on the kidneys by decreasing inflammation, inducing natriuresis, and decreasing glomerular hypertension [105]. Systemic review and meta-analysis of randomized controlled trials of GLP-1RAs in adults found they resulted in weight loss in patients with and without diabetes and several of these medications are now also available to children [106]. In the adolescent population, liraglutide is superior to placebo for reduction of BMI [99]. Liraglutide (daily injection) is FDA approved for children 12 years and older with or without diabetes. Exenatide (weekly injection) is approved for children 10 years and older who also have type 2 diabetes (T2D) and has also been shown to decrease BMI in the pediatric population [91]. Semaglutide (weekly injection) is approved for weight loss in children 12 years and older with obesity or excess weight and has been shown to result in a greater reduction in BMI than lifestyle intervention alone [107]. An oral version of semaglutide has also been approved for adults with type 2 diabetes, but has not yet been approved for children [108]. Other GLP-1 receptor agonists are being studied in children including a recent trial of dulaglutide, a once weekly injection, that demonstrated improved glycemic control but did not impact BMI [100]. There have been randomized trials of GLP-1 receptor agonists in adults that included renal outcomes and found a decrease in new-onset macroalbuminuria, but trials examining hard renal endpoints remain in progress [105].
New dual GLP-1/glucose-dependent insulinotropic polypeptide (GIP) receptor agonists and even triple GLP-1/GIP/glucagon agonists are also under investigation to treat type 2 diabetes and obesity. A dual GLP-1/GIP receptor agonist, tirzepatide, was approved for treatment of type 2 diabetes in 2022 and has been shown to be more effective for glycemic control and weight loss than GLP-1RAs. These drugs are not yet approved for the pediatric population, but trials are undergoing. They have promise in addressing both diabetes and obesity and therefore decreasing risk of kidney failure [109].
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) increase glucosuria and natriuresis through the inhibition of SGLT2 in the proximal tubule causing a decrease in serum glucose, improved blood pressure, and decreased hyperfiltration and therefore help protect the kidneys. Several randomized controlled trials in adults have demonstrated the protective effect of these medications on the kidneys in patients with diabetes and that the benefit to the kidneys occurs even in the absence of diabetes [110, 111]. Most recently, sotagliflozin demonstrated a significant reduction in cardiovascular mortality and hospitalization and received FDA approval for adults with heart failure, type 2 diabetes, chronic kidney disease, or other cardiovascular risk factors [112, 113]. Though the data is more limited in the pediatric population, several SGLT2i’s are under investigation. Dapagliflozin is approved for use in children 10 years and older in Europe. In a recent large phase 3 trial of dapagliflozin in children and young adults with type 2 diabetes (ages 10–24), a decrease in HbA1c was not demonstrated in the intention to treat group though was seen in the protocol compliant participants [102]. In another recent trial, Empagliflozin showed a significant reduction in HbA1c with children ages 1–17 with type 2 diabetes in a multi-center trial across 15 countries. It has been submitted to the FDA for approval in children 12 years and older with type 2 diabetes [104]. Protection against kidney disease was not specifically examined in these trials. Overall, further studies are needed to determine if SGLT2 inhibitors will demonstrate kidney protection benefits in the pediatric population. They could also be beneficial for ORKD through several mechanisms including blood-pressure improvement, weight loss, reduction in renal fat, reduction of pro-inflammatory cytokines, and afferent arteriole vasoconstriction to decrease hyperfiltration [114,115,116].
Metabolic bariatric surgery is a treatment option to consider for severe obesity that could positively impact or prevent both ORKD and DKD [117]. Among adolescents with severe obesity, there is a growing body of evidence suggesting the effectiveness of bariatric surgery to achieve long lasting reduction of BMI and improvement or remission of obesity-related co-morbid conditions [91]. A systemic review of long-term follow-up results from adults undergoing bariatric surgery demonstrated remission from type 2 diabetes at a rate of 66.7% from gastric bypass and 38.6% for gastric band [118]. Additionally, bariatric surgery is more effective at achieving glycemic control in uncontrolled type 2 diabetes than medical therapy alone [119]. Benefits have been seen in the adolescent population as well with a remission rate of 95% for type 2 diabetes and 76% for prediabetes at 3-year post-surgery. These adolescents experienced other health benefits apart from diabetes as well including a mean weight reduction of 27%, 86% remission of abnormal kidney function, and 74% remission of elevated blood pressure at 3-year post-surgery [120].
Conclusion
Childhood and adolescent obesity can result in renal damage even before the onset of clinical disease, increasing the likelihood of chronic kidney disease and, ultimately, kidney failure. However, our current understanding of the impact of obesity on the kidneys in pediatric patients remains limited. To fully comprehend the ramifications of pediatric obesity on the kidneys, further research is imperative. We must investigate the most effective screening methods to detect early kidney injury and prioritize the development of evidence-based interventions during childhood and adolescence. By dedicating resources toward a more comprehensive understanding of the effects of obesity on the kidneys in young patients, we may facilitate the creation of strategies to mitigate the long-term consequences of this growing epidemic.
References
Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11.
Kibria GMA, Crispen R. Prevalence and trends of chronic kidney disease and its risk factors among US adults: An analysis of NHANES 2003–18. Prev Med Rep. 2020;20:101193.
United States Renal Data System. 2020 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020.
Chronic Kidney Disease Surveillance System—United States: Centers for Disease Control and Prevention. 2021. Available from: https://nccd.cdc.gov/ckd/.
Eknoyan G, Lameire N, Eckardt K, Kasiske B, Wheeler D, Levin A, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(1):5–14.
Harada R, Hamasaki Y, Okuda Y, Hamada R, Ishikura K. Epidemiology of pediatric chronic kidney disease/kidney failure: learning from registries and cohort studies. Pediatr Nephrol. 2022:1–15.
Mallamaci F, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, et al. ACE inhibition is renoprotective among obese patients with proteinuria. J Am Soc Nephrol. 2011;22(6):1122–8.
Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17(6):1695–702.
Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16(7):2134–40.
Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140(3):167–74.
Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G, et al. Metabolic mediators of the eff ects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. 2014.
Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–8.
Wang Y, Chen X, Song Y, Caballero B, Cheskin L. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73(1):19–33.
Ladhani M, Lade S, Alexander SI, Baur LA, Clayton PA, McDonald S, et al. Obesity in pediatric kidney transplant recipients and the risks of acute rejection, graft loss and death. Pediatr Nephrol. 2017;32(8):1443–50.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78.
Trinchieri A, Croppi E, Montanari E. Obesity and urolithiasis: evidence of regional influences. Urolithiasis. 2017;45(3):271–8.
Wu C, Wang AY, Li G, Wang L. Association of high body mass index with development of interstitial fibrosis in patients with IgA nephropathy. BMC Nephrol. 2018;19(1):381.
Berthoux F, Mariat C, Maillard N. Overweight/obesity revisited as a predictive risk factor in primary IgA nephropathy. Nephrol Dial Transplant. 2013;28 Suppl 4:iv160–6.
Yim HE, Han KD, Kim B, Yoo KH. Impact of early-life weight status on urinary tract infections in children: a nationwide population-based study in Korea. Epidemiol Health. 2021;43: e2021005.
Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293(4):455–62.
Pourghazi F, Mohammadi S, Eslami M, Zoshk MY, Asadi S, Ejtahed HS, et al. Association between childhood obesity and later life kidney disorders: a systematic review. J Ren Nutr. 2023.
Wei L, Li Y, Yu Y, Xu M, Chen H, Li L, et al. Obesity-related glomerulopathy: from mechanism to therapeutic target. Diabetes Metab Syndr Obes. 2021;14:4371–80.
Freedman DS, Lawman HG, Galuska DA, Goodman AB, Berenson GS. Tracking and variability in childhood levels of BMI: the Bogalusa Heart Study. Obesity. 2018;26(7):1197–202.
Singh AS, Mulder C, Twisk JW, Van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–88.
Stierman B, Afful J, Carroll MD, Chen TC, Davy O, Fink S, Fryar CD, Gu Q, Hales CM, Hughes JP, Ostchega Y. National health and nutrition examination survey 2017–March 2020 prepandemic data files development of files and prevalence estimates for selected health outcomes. 2021.
Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17(2):95–107.
Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017;91(5):1224–35.
Chang A, Van Horn L, Jacobs DR Jr, Liu K, Muntner P, Newsome B, et al. Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis. 2013;62(2):267–75.
Ferris M, Hogan SL, Chin H, Shoham DA, Gipson DS, Gibson K, et al. Obesity, albuminuria, and urinalysis findings in US young adults from the Add Health Wave III Study. Clin J Am Soc Nephrol. 2007;2(6):1207–14.
Vivante A, Golan E, Tzur D, Leiba A, Tirosh A, Skorecki K, et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172(21):1644–50.
Marzuillo P, Grandone A, Di Sessa A, Guarino S, Diplomatico M, Umano GR, et al. Anthropometric and biochemical determinants of estimated glomerular filtration rate in a large cohort of obese children. J Ren Nutr. 2018;28(5):359–62.
Di Bonito P, Licenziati MR, Campana G, Chiesa C, Pacifico L, Manco M, et al. Prevalence of mildly reduced estimated GFR by height- or age-related equations in young people with obesity and its association with cardiometabolic risk factors. J Ren Nutr. 2021;31(6):586–92.
D’Agati VD, Chagnac A, De Vries AP, Levi M, Porrini E, Herman-Edelstein M, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–71.
Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–509.
Tsuboi N, Okabayashi Y, Shimizu A, Yokoo T. The renal pathology of obesity. Kidney Int Rep. 2017;2(2):251–60.
Joshi PH, Hill JA. Pound-Years. Circ Res. 2017;120(10):1533–4.
Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol-Renal Physiol. 2000;278(5):F817–22.
Yim HE, Yoo KH. Obesity and chronic kidney disease: prevalence, mechanism, and management. Clin Exp Pediatr. 2021;64(10):511.
Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M. Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron. 2019;143(1):38–42.
Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118(5):1645–56.
Wang M, Wang Z, Chen Y, Dong Y. Kidney damage caused by obesity and its feasible treatment drugs. Int J Mol Sci. 2022;23(2):747.
Mangat G, Nair N, Barat O, Abboud B, Pais P, Bagga S, et al. Obesity-related glomerulopathy in children: connecting pathophysiology to clinical care. Clin Kidney J. 2022.
Rüster C, Wolf G. Adipokines promote chronic kidney disease. Nephrol Dial Transplant. 2013;28 Suppl 4:iv8–14.
Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S58-65.
Mykkänen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47(5):793–800.
McPherson KC, Taylor L, Johnson AC, Didion SP, Geurts AM, Garrett MR, et al. Early development of podocyte injury independently of hyperglycemia and elevations in arterial pressure in nondiabetic obese Dahl SS leptin receptor mutant rats. Am J Physiol Renal Physiol. 2016;311(4):F793-f804.
Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. 2006;26(3):232–44.
Pantoja Zuzuárregui JR, Mallios R, Murphy J. The effect of obesity on kidney length in a healthy pediatric population. Pediatr Nephrol. 2009;24:2023–7.
Cohen AH. Massive obesity and the kidney. A morphologic and statistical study. Am J Pathol. 1975;81(1):117–30.
Martínez-Montoro JI, Morales E, Cornejo-Pareja I, Tinahones FJ, Fernández-García JC. Obesity-related glomerulopathy: Current approaches and future perspectives. Obes Rev. 2022;23(7): e13450.
Tsuboi N, Utsunomiya Y, Kanzaki G, Koike K, Ikegami M, Kawamura T, et al. Low glomerular density with glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc Nephrol. 2012;7(5):735–41.
Serra A, Romero R, Lopez D, Navarro M, Esteve A, Perez N, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73(8):947–55.
Kim JJ, Wilbon SS, Fornoni A. Podocyte Lipotoxicity in CKD. Kidney360. 2021;2(4):755–62.
Zeitler EM, Jennette JC, Flythe JE, Falk RJ, Poulton JS. High-calorie diet results in reversible obesity-related glomerulopathy in adult zebrafish regardless of dietary fat. Am J Physiol-Renal Physiol. 2022;322(5):F527–39.
Tobar A, Ori Y, Benchetrit S, Milo G, Herman-Edelstein M, Zingerman B, et al. Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS One. 2013;8(9):e75547.
Praga M, Hernández E, Morales E, Campos AP, Valero MA, Martínez MA, et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16(9):1790–8.
Abitbol CL, Chandar J, Rodríguez MM, Berho M, Seeherunvong W, Freundlich M, et al. Obesity and preterm birth: additive risks in the progression of kidney disease in children. Pediatr Nephrol. 2009;24(7):1363–70.
López-Martínez M, Luis-Lima S, Morales E, Navarro-Díaz M, Negrín-Mena N, Folgueras T, et al. The estimation of GFR and the adjustment for BSA in overweight and obesity: a dreadful combination of two errors. Int J Obes (Lond). 2020;44(5):1129–40.
Bielopolski D, Singh N, Bentur OS, Renert-Yuval Y, MacArthur R, Vasquez KS, et al. Obesity related glomerulopathy in adolescent women: the effect of body surface area. Kidney360. 2022;3(1):113.
Xiao N, Devarajan P, Inge TH, Jenkins TM, Bennett M, Mitsnefes MM. Subclinical kidney injury before and 1 year after bariatric surgery among adolescents with severe obesity. Obesity (Silver Spring). 2015;23(6):1234–8.
Sun X, Han F, Miao W, Hou N, Cao Z, Zhang G. Sonographic evaluation of para-and perirenal fat thickness is an independent predictor of early kidney damage in obese patients. Int Urol Nephrol. 2013;45:1589–95.
Shen FC, Cheng BC, Chen JF. Peri-renal fat thickness is positively associated with the urine albumin excretion rate in patients with type 2 diabetes. Obes Res Clin Pract. 2020;14(4):345–9.
Lamacchia O, Nicastro V, Camarchio D, Valente U, Grisorio R, Gesualdo L, et al. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant. 2011;26(3):892–8.
Geraci G, Zammuto MM, Mattina A, Zanoli L, Geraci C, Granata A, et al. Para-perirenal distribution of body fat is associated with reduced glomerular filtration rate regardless of other indices of adiposity in hypertensive patients. J Clin Hypertens (Greenwich). 2018;20(10):1438–46.
Lawrence JM, Divers J, Isom S, Saydah S, Imperatore G, Pihoker C, et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA. 2021;326(8):717–27.
Li L, Jick S, Breitenstein S, Michel A. Prevalence of diabetes and diabetic nephropathy in a large US commercially insured pediatric population, 2002–2013. Diabetes Care. 2016;39(2):278–84.
Dabelea D, Stafford JM, Mayer-Davis EJ, D’Agostino R Jr, Dolan L, Imperatore G, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825–35.
Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35(6):1265–71.
Karin A, Jon E, Martin A, Lena B, Martin L, Naveed S, et al. Body mass index in adolescence, risk of type 2 diabetes and associated complications: a nationwide cohort study of men. EClinicalMedicine. 2022;46: 101356.
Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296(4):421–6.
Muntean C, Starcea IM, Banescu C. Diabetic kidney disease in pediatric patients: a current review. World J Diabetes. 2022;13(8):587–99.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Control TD, Group CD. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney Int. 1995;47(6):1703–20.
Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16.
de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365(25):2366–76.
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45.
Lin YC, Chang YH, Yang SY, Wu KD, Chu T-S. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117(8):662–75.
Barrera-Chimal J, Jaisser F. Pathophysiologic mechanisms in diabetic kidney disease: a focus on current and future therapeutic targets. Diabetes Obes Metab. 2020;22(Suppl 1):16–31.
Sugahara M, Pak WLW, Tanaka T, Tang SCW, Nangaku M. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology. 2021;26(6):491–500.
Afkarian M. Diabetic kidney disease in children and adolescents. Pediatr Nephrol. 2015;30(1):65–74.
Østerby R. Morphometric studies of the peripheral glomerular basement membrane: II. Topography of the initial lesions. Diabetologia. 1973;9:108–14.
Drummond K, Mauer M, Group IDNS. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51(5):1580–7.
Thomas HY, Ford Versypt AN. Pathophysiology of mesangial expansion in diabetic nephropathy: mesangial structure, glomerular biomechanics, and biochemical signaling and regulation. J Biol Eng. 2022;16(1):19.
Di Vincenzo A, Bettini S, Russo L, Mazzocut S, Mauer M, Fioretto P. Renal structure in type 2 diabetes: facts and misconceptions. J Nephrol. 2020;33(5):901–7.
Group TS. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36(6):1735–41.
Bjornstad P, Nehus E, El Ghormli L, Bacha F, Libman IM, McKay S, et al. Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: an observational analysis of data from the TODAY clinical trial. Am J Kidney Dis. 2018;71(1):65–74.
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832–9.
Schrauben SJ, Jepson C, Hsu JY, Wilson FP, Zhang X, Lash JP, et al. Insulin resistance and chronic kidney disease progression, cardiovascular events, and death: findings from the chronic renal insufficiency cohort study. BMC Nephrol. 2019;20(1):60.
Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14(6):1480–6.
Conley MM, McFarlane CM, Johnson DW, Kelly JT, Campbell KL, MacLaughlin HL. Interventions for weight loss in people with chronic kidney disease who are overweight or obese. Cochrane Database Syst Rev. 2021;3(3):Cd013119.
Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151(2).
TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–56.
RISE Consortium Investigators. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care. 2018;41(8):1696–706.
Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639–50.
Smeets NJL, Schreuder MF, Dalinghaus M, Male C, Lagler FB, Walsh J, et al. Pharmacology of enalapril in children: a review. Drug Discov Today. 2020;25(11):1957–70.
Brown AK, Nichols A, Coley CA, Ekperikpe US, McPherson KC, Shields CA, et al. Treatment with lisinopril prevents the early progression of glomerular injury in obese dahl salt-sensitive rats independent of lowering arterial pressure. Front Physiol. 2021;12: 765305.
Lopez LN, Wang W, Loomba L, Afkarian M, Butani L. Diabetic kidney disease in children and adolescents: an update. Pediatr Nephrol. 2022;37(11):2583–97.
Kelly AS, Bensignor MO, Hsia DS, Shoemaker AH, Shih W, Peterson C, et al. Phentermine/topiramate for the treatment of adolescent obesity. NEJM Evidence. 2022;1(6):EVIDoa2200014.
Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117–28.
Arslanian SA, Hannon T, Zeitler P, Chao LC, Boucher-Berry C, Barrientos-Pérez M, et al. Once-weekly dulaglutide for the treatment of youths with type 2 diabetes. N Engl J Med. 2022;387(5):433–43.
Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, et al. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med. 2019;381(7):637–46.
Tamborlane WV, Laffel LM, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022;10(5):341–50.
Tommerdahl KL, Nelson RG, Bjornstad P. Dapagliflozin in young people with type 2 diabetes. Lancet Diabetes Endocrinol. 2022;10(5):303–4.
Laffel LM, Danne T, Klingensmith GJ, Tamborlane WV, Willi S, Zeitler P, et al. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023;11(3):169–81.
Yu JH, Park SY, Lee DY, Kim NH, Seo JA. GLP-1 receptor agonists in diabetic kidney disease: current evidence and future directions. Kidney Res Clin Pract. 2022;41(2):136–49.
Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771.
Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387(24):2245–57.
Hughes S, Neumiller JJ. Oral Semaglutide. Clin Diabetes. 2020;38(1):109–11.
Gallwitz B. Clinical perspectives on the use of the GIP/GLP-1 receptor agonist tirzepatide for the treatment of type-2 diabetes and obesity. Front Endocrinol (Lausanne). 2022;13:1004044.
Jiang B, Cheng Z, Liu F, Li Q, Fu H, Mao J. Renoprotection with sodium-glucose cotransporter-2 inhibitors in children: knowns and unknowns. Nephrology. 2022;27(2):126–32.
Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(1):22–31.
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384(2):117–28.
Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2020;384(2):129–39.
Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs. 2019;79(3):219–30.
Yaribeygi H, Butler AE, Atkin SL, Katsiki N, Sahebkar A. Sodium-glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: possible molecular pathways. J Cell Physiol. 2018;234(1):223–30.
Schaub JA, AlAkwaa FM, McCown PJ, Naik AS, Nair V, Eddy S, et al. SGLT2 inhibitors mitigate kidney tubular metabolic and mTORC1 perturbations in youth-onset type 2 diabetes. J Clin Investig. 2023;133(5).
Pratt JSA, Browne A, Browne NT, Bruzoni M, Cohen M, Desai A, et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obes Relat Dis. 2018;14(7):882–901.
Puzziferri N, Roshek TB 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–42.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. N Engl J Med. 2014;370(21):2002–13.
Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–23.
Funding
P.B. receives salary and research support from NIDDK (R01 DK129211, R01 DK132399, R21 DK129720, K23 DK116720, UC2 DK114886, P30-DK116073), NHLBI (R01 HL165433), JDRF (3-SRA-2022-1097-M-B, 3-SRA-2022-1243-M-B, 3-SRA-2022-1230-M-B), Boettcher Foundation, American Heart Association (20IPA35260142), Ludeman Family Center for Women’s Health Research at the University of Colorado, the Department of Pediatrics, Section of Endocrinology and Barbara Davis Center for Diabetes at University of Colorado School of Medicine.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Alexandra Sawyer and Petter Bjornstad and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Petter Bjornstad reports serving or having served as a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli-Lilly, LG Chemistry, Sanofi, Novo Nordisk, and Horizon Pharma. Petter Bjornstad also serves or has served on the advisory boards and/or steering committees of AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, and XORTX. Evan Zeitler reports that his spouse receives research funding from VtV Therapeutics, Novo Nordisk, Rhythm Pharmaceuticals, and Dexcom. All other authors declare no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sawyer, A., Zeitler, E., Trachtman, H. et al. Kidney Considerations in Pediatric Obesity. Curr Obes Rep 12, 332–344 (2023). https://doi.org/10.1007/s13679-023-00522-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-023-00522-3