Abstract
Purpose
To determine whether para- and perirenal fat ultrasonographic thickness (PFUT) is related to increased urinary albumin excretion and whether PFUT is an independent indicator of early kidney damage in obese subjects.
Method
Sixty-seven nonhypertensive, nondiabetic obese patients and 34 age- and sex-matched normal healthy volunteers were involved in this study. Clinical characteristics, blood biochemistry, PFUT, and urinary albumin/creatinine ratio (ACR) of the subjects were measured. The intraoperator and interoperator coefficient of variation was 5.6 and 3.2 %, respectively.
Results
ACR and PFUT were significantly higher in obese patients than those of normal healthy volunteers. PFUT was higher in obese patients with microalbuminuria than those with normoalbuminuria. Correlation analysis showed PFUT had a positive correlation with body mass index (BMI, r = 0.677, P < 0.01), waist circumference (WC, r = 0.686, P < 0.01), plasma free fatty acids (FFAs, r = 0.589, P < 0.01), and ACR (r = 0.610, P < 0.01). ACR had a positive correlation with BMI (r = 0.444, P < 0.01), WC (r = 0.440, P < 0.01), and plasma FFAs (r = 0.496, P < 0.01). Multivariate regression analyses showed that ACR could be predicted by PFUT.
Conclusions
PFUT may be an independent predictor of early kidney damage in nonhypertensive, nondiabetic obese patients, and PFUT could be a useful tool for the assessment of visceral fat and early kidney damage in obese patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is known that obesity is not only closely associated with microalbuminuria, an early manifestation of kidney damage, but that it also has a negative impact on ischemic cardiovascular disease [1–3]. There are several mechanisms to explain why obesity may initiate and exacerbate kidney damage, such as hyperglycemia, hyperinsulinemia, and hypertension. However, the PREVEND study showed that 75 % of microalbuminuria occurred in the nonhypertensive, nondiabetic population [4]. Further studies have shown that body fat distribution and abdominal fat accumulation are closely related to increased urinary albumin excretion and lead to obesity-related kidney damage [5, 6]. Therefore, the convenient evaluation of body fat distribution is very important.
In many studies, body fat distribution was evaluated using a variety of methods, including waist circumstance measurement [7], hip circumference measurement, waist–hip ratio, evaluation of visceral fat by computed tomography (CT) [8, 9], and magnetic resonance imaging (MRI) [10, 11]. Although waist circumstance measurement is noninvasive and convenient, it does not accurately quantify body fat distribution because waist circumstance measurement includes all subcutaneous fat and abdominal contents and subcutaneous fat is more abundant in females than in males. Moreover, subcutaneous fat is usually reduced in advanced old age [12]. CT and MRI present another set of problems, as these imaging modalities are not considered to be desirable measurement options in many cases because CT provides considerable ionizing radiation exposure and MRI is both time-consuming and expensive.
It is worth noting that several noninvasive sonographic methods for measuring body fat distribution have also been reported. Armellini et al. [13] first demonstrated that ultrasonography could be useful in the direct evaluation of intra-abdominal fat deposits. Since then, Kawasaki et al. [14] evaluated a new method of determining visceral fat by measuring para- and perirenal fat thickness on abdominal sonography. Para- and perirenal fat ultrasonographic thickness (PFUT), as part of abdominal visceral fat, is in fact thought to possibly play a significant role in obesity-related microalbuminuria, as it surrounds the kidney directly and demonstrates a closer relationship to kidney function than other intra-abdominal fat deposits [15].
In the present study, we sought to first determine whether PFUT is related to increased urinary albumin excretion and to then ascertain whether PFUT can serve as an independent and robust indicator of early kidney damage in nonhypertensive, nondiabetic obese subjects.
Subjects and methods
Subjects
Nonhypertensive, nondiabetic obese patients (OB group) and normal healthy volunteer (NC group), who are Chinese People, from clinic in our hospital were recruited to this study. Inclusion criteria were as follows: (1) age between 18 and 50 years age; (2) OB group: body mass index ≥25 kg/m2, waist circumference ≥90 cm in men, ≥80 cm in women, adopted the cutoffs as suggested for Asians [16]; NC group: body mass index 18–23 kg/m2, waist circumference ≤80 cm in men, ≤75 cm in women; (3) fasting plasma glucose <6.1 mmol/L and 2 h glucose <7.8 mmol/L; (4) systolic blood pressure ≤130 mmHg, diastolic blood pressure ≤80 mmHg; (5) total serum cholesterol <6.0 mmol/L; (6) normal liver and renal function. None of the subjects were smokers or had potentially confounding disorders such as cardiovascular disease, autoimmune disease, chronic liver disease, secondary obesity, or long-term use of drugs which affects glucose metabolism such as diuretics, glucocorticoids, etc. We then divided the obese patients into two groups according to their levels of microalbuminuria: obese patients with normoalbuminuria group (OB-NA group) included patients who had normoalbuminuria (urinary albumin/creatinine ratio <30 mg/g), and obese patients with microalbuminuria group (OB-MA group) included patients who had overt microalbuminuria (urinary albumin/creatinine ratio ≥30 mg/g) [17]. This study was approved by the ethics committee of our hospital, and informed consent was obtained from all subjects.
Assessment methodology
Height, body weight, waist circumference, and blood pressure were measured using standard methods as described previously [18]. In brief, patients’ height and body weight were measured without shoes after overnight fasting by using an electronic scale to the nearest 1 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Plasma glucose, serum lipids, and serum creatinine were measured using a Beckman CX4 automatic biochemical analyzer. Plasma free fatty acids (FFAs) concentrations were determined using colorimetric assays. Urinary albumin/creatinine ratio (ACR) was used as an index of urinary albumin excretion. Early-morning urine samples (first void) were collected from all subjects. Urinary albumin and urinary creatinine concentration were measured by the immunoturbidimetric and Jaffe methods, respectively. eGFR was calculated with the abbreviated Modification of Diet in Renal Disease (MDRD) formula recalibrated for Chinese [GFR = 186 × (SCr × 0.011)−1.154 × (age)−0.203 × (0.742 if female) × (1.233 if Chinese)].
Sonographic examination
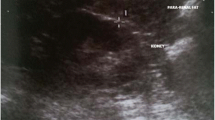
Sonographic evaluation of PFUT was determined using a GE Logiq S6 ultrasound scanner (General Electric, New York, NY, USA) equipped with a 3.5 MHz convex probe as described previously [14]. The patient was positioned in the supine position and the probe was kept perpendicular to the skin on the lateral aspect of the abdomen. Longitudinal scanning was performed, and the probe was moved laterally to the position where the surface of the kidney was almost parallel to the skin (Fig. 1). As light a pressure as possible was exerted on the probe so that the perirenal fat layers were not compressed. PFUT was then determined from the inner side of the abdominal musculature to the surface of the kidney. The average of the ultrasound measurement values on both sides was regarded as the PFUT. PFUT was measured a total of three times by a sonographer. Interoperator repeatability was assessed as difference toward comparison with CT in 45/101 subjects. The intraoperator and interoperator coefficient of variation was 5.6 and 3.2 %, respectively.
Statistical analysis
All data are subjected to statistical analysis by using SPSS 16.0 statistical package. The data are expressed as mean ± standard deviation (SD) for normally distributed variables and median (interquartile range) for skewed variables. Statistical analysis was performed by one-way ANOVA. The number of inter-group differences was detected using Student–Newman–Keuls (SNK) method. Univariate and multivariate analyses were performed to correlate ACR with independent variables. Skewed variables or nonlinear related variables were log-transformed. P value of less than 0.05 was considered statistically significant.
Results
Clinical characteristics
A total of 67 nonhypertensive, nondiabetic obese patients and 34 age- and sex-matched normal healthy volunteers were enrolled as participants in this study. Clinical characteristics of the subjects are described in Table 1. There were no significant differences in age, gender, and blood pressure in the three groups (P > 0.05). Compared with normal healthy volunteers, obese patients had a higher weight, BMI, and waist circumference. However, there were no significant differences in the above indices between obese patients with microalbuminuria and obese patients with normoalbuminuria.
Biochemistry parameters
As expected, serum triglyceride and FFAs levels in obese patients were significantly higher than those observed in normal healthy volunteers (Table 1). There were no significant differences in total cholesterol and fasting plasma glucose levels in the three groups (P > 0.05). In the obese patients, the plasma FFAs level was higher in patients with microalbuminuria than in those with normoalbuminuria. However, no significant difference was found in patients’ serum triglyceride levels in the two obese groups. Urinary albumin excretion was significantly higher in obese patients than in those of normal healthy volunteers (Table 1).
Echographic parameters
As expected, the mean PFUT was significantly higher in obese patients than in normal healthy volunteers (26.54 ± 5.48 vs 7.95 ± 1.57 mm, P < 0.05). In the obese patients, PFUT was higher in patients with microalbuminuria than in those with normoalbuminuria (29.02 ± 4.92 vs 23.98 ± 4.87 mm, P < 0.05). There was no significant difference between the right and left side in each group (NC right vs left 7.86 ± 1.52 vs 7.94 ± 1.63 mm; OB-NA 23.90 ± 4.94 vs 24.06 ± 4.97 mm, OB-MA 29.06 ± 4.82 vs 28.98 ± 5.12 mm, P > 0.05).
Correlation and regression analysis
Correlation analysis showed that PFUT had a positive correlation with BMI (r = 0.677, P < 0.01), waist circumference (r = 0.686, P < 0.01), plasma FFAs (r = 0.589, P < 0.01), and ACR (r = 0.610, P < 0.01). ACR had a positive correlation with BMI (r = 0.444, P < 0.01), waist circumference(r = 0.440, P < 0.01), plasma FFAs (r = 0.496, P < 0.01), and serum triglyceride (r = 0.364, P < 0.01) (Table 2) Multivariate regression analyses showed that ACR could be predicted by PFUT (r 2 = 0.442, P < 0.001) (Table 3).
Discussion
In this study, we demonstrated that PFUT is related to increased urinary albumin excretion and that PFUT may be a good indicator of early kidney damage in nonhypertensive, nondiabetic obese subjects.
Since obesity-related microalbuminuria was first reported by Weisinger in 1974 [2], numerous studies have consistently confirmed the presence of proteinuria in obesity, also known as obesity-related glomerulopathy [19, 20]. Klausen et al. [21] found that there existed a strong association between microalbuminuria and obesity, as based on an observational study of 2,696 subjects. In our study, we also found that urinary albumin excretion was significantly higher in the obese patients than in the normal healthy subjects, which is consistent with our previous study [22]. However, more evidence demonstrated that it was not obesity per se, but rather body fat distribution, that is more closely related to kidney damage [23, 24]. Pinto-Sietsma et al. [24] found that lean subjects with central fat distribution (i.e., excessive visceral fat) are also at high risk of microalbuminuria.
More recently, several studies on the use of sonography for the evaluation of visceral fat volume have been published [25, 26]. Suzuki et al. [27] showed that the abdominal fat index may be an indicator of visceral fat deposition. However, Kawasaki et al. [14] found that the abdominal fat index is not a good indicator of visceral fat due to its inherent measurement difficulty. They used PFUT as a fat-measurement method and found that PFUT correlated significantly with the visceral fat area and that a PFUT of ≥10 mm reflects increased visceral fat deposition [14]. In our study, we found that PFUT was significantly higher in obese patients than in normal healthy volunteers. In the obese patients, PFUT was higher in patients with microalbuminuria than in those with normoalbuminuria. Correlation analysis showed that PFUT had a positive correlation with ACR. Multivariate regression analyses showed that ACR could be predicted by PFUT. Taken together, these findings all suggested that PFUT may be a good indicator of visceral fat area and may serve as an independent and accurate predictor of microalbuminuria in nonhypertensive, nondiabetic obese subjects.
Some mechanisms may account for PFUT as an independent predictor of microalbuminuria in obese patients. Part of the perirenal fat can seep into the renal sinus, compress the renal parenchyma and renal vessels, leading to increased renal interstitial hydrostatic pressure and reduced renal blood flow and, ultimately, to kidney damage [28]. In addition, excessive FFAs released from para- and perirenal fat escape into the kidney and lead to renal lipotoxicity by increasing intracellular fatty acids metabolites. In our study, we found that even in obese patients, plasma FFAs levels were higher in patients with microalbuminuria than in those with normoalbuminuria. Correlation analysis showed that PFUT had a positive correlation with FFAs and that FFAs also had a positive correlation with ACR.
In the present study, we confirmed the association of PFUT and urinary albumin excretion in obese subjects. In fact, Lamacchia et al. [15] found that estimates of the glomerular filtration rate, renal resistance index, and uricemia could be predicted by PFUT in 151 subjects with type 2 diabetes, suggesting that PFUT is an independent predictor of kidney dysfunction in this disease. However, in that study, microalbuminuria was not predicted by PFUT likely because the patients recruited had different characteristics. First of all, they were affected by diabetes which may promote glomerular damage by means of mechanisms which are partly different from those activated by obesity; Moreover, most of the patients were taking antihypertensive therapy which is known to contribute to the reduction in the urinary albumin excretion rate and then it may likely have interfered in the association between PFUT thickness and ACR.
Limitation of our study was that the cross-sectional design in our study helps generate hypotheses, but does not prove the cause–effect relationship between PFUT and early renal damage in obese patients. Therefore, the study design may limit the generalizability of the results but should not influence the internal validity of our findings. However, it should be noted that the study subjects consisted of characterized obese patients with normal glycemia and blood pressure. In addition, the sample size was small, for it is a little difficult to select the obese patients with normal glycemia and blood pressure. The prospective design and larger samples are needed to better clarify the role of PFUT in early kidney damage in obese patients. Admittedly, taking urinary samples for ACR measurement would be much easier and convenient. However, PFUT measurement may improve our estimates of the risk of early kidney damage in obese patients, considering body fat distribution and abdominal fat accumulation are closely related to increased urinary albumin excretion and cardiovascular disease.
In summary, we have shown that urinary albumin excretion was higher in obese patients than in normal healthy subjects. PFUT was higher in obese patients with microalbuminuria than in obese patients without microalbuminuria. Therefore, we believe that PFUT may be an independent predictor of early kidney damage in nonhypertensive, nondiabetic obese patients and that PFUT may be a useful tool for the assessment of visceral fat and early kidney damage in obese patients.
References
Kawar B, Bello AK, El NAM (2009) High prevalence of microalbuminuria in the overweight and obese population: data from a UK population screening programme. Nephron Clin Pract 112(3):c205–c212
Weisinger JR, Kempson RL, Eldridge FL, Swenson RS (1974) The nephrotic syndrome: a complication of massive obesity. Ann Intern Med 81(4):440–447
Zimering MB, Anderson RJ, Ge L, Moritz TE (2011) Increased plasma basic fibroblast growth factor is associated with coronary heart disease in adult type 2 diabetes mellitus. Metabolism 60(2):284–291
de Jong PE, Verhave JC, Pinto-Sietsma SJ, Hillege HL (2002) Obesity and target organ damage: the kidney. Int J Obes Relat Metab Disord 26(Suppl 4):S21–S24
Foster MC, Hwang SJ, Massaro JM, Hoffmann U, Deboer IH, Robins SJ et al (2011) Association of subcutaneous and visceral adiposity with albuminuria: the framingham heart study. Obesity (Silver Spring) 19(6):1284–1289
Tamba S, Nakatsuji H, Kishida K, Noguchi M, Ogawa T, Okauchi Y et al (2010) Relationship between visceral fat accumulation and urinary albumin-creatinine ratio in middle-aged Japanese men. Atherosclerosis 211(2):601–605
Noori N, Hosseinpanah F, Nasiri AA, Azizi F (2009) Comparison of overall obesity and abdominal adiposity in predicting chronic kidney disease incidence among adults. J Ren Nutr 19(3):228–237
Stallone DD, Stunkard AJ, Wadden TA, Foster GD, Boorstein J, Arger P (1991) Weight loss and body fat distribution: a feasibility study using computedtomography. Int J Obes 15(11):775–780
Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S (1983) A novel technique for the determination of body fat by computed tomography. Int J Obes 7(5):437–445
Sohlstrom A, Wahlund LO, Forsum E (1993) Adipose tissue distribution as assessed by magnetic resonance imaging and totalbody fat by magnetic resonance imaging, underwater weighing, and body-waterdilution in healthy women. Am J Clin Nutr 58(6):830–838
Vogt FM, Ruehm S, Hunold P, de Greiff A, Nuefer M, Barkhausen J et al (2007) Rapid total body fat measurement by magnetic resonance imaging: quantificationand topography. Rofo 179(5):480–486
Kirkland JL, Dobson DE (1997) Preadipocyte function and aging: links between age-related changes in celldynamics and altered fat tissue function. J Am Geriatr Soc 45(8):959–967
Armellini F, Zamboni M, Rigo L, Todesco T, Bergamo-Andreis IA, Procacci C et al (1990) The contribution of sonography to the measurement of intra-abdominal fat. J Clin Ultrasound 18(7):563–567
Kawasaki S, Aoki K, Hasegawa O, Numata K, Tanaka K, Shibata N et al (2008) Sonographic evaluation of visceral fat by measuring para- and perirenal fat. J Clin Ultrasound 36(3):129–133
Lamacchia O, Nicastro V, Camarchio D, Valente U, Grisorio R, Gesualdo L et al (2011) Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant 26(3):892–898
Organization WH (2000) The Asia-Pacific perspective: redefining obesity and its treatment. WHO 20, Geneva
Gatling W, Knight C, Mullee MA, Hill RD (1988) Microalbuminuria in diabetes: a population study of the prevalence and anassessment of three screening tests. Diabet Med 5(4):343–347
Pan XR, Yang WY, Li GW, Liu J (1997) Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care 20(11):1664–1669
Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD (2001) Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 59(4):1498–1509
Yasui K, Sumida Y, Mori Y, Mitsuyoshi H, Minami M, Itoh Y et al (2011) Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism 60(5):735–739
Klausen KP, Parving HH, Scharling H, Jensen JS (2009) Microalbuminuria and obesity: impact on cardiovascular disease and mortality. Clin Endocrinol (Oxf) 71(1):40–45
Han F, Hou N, Miao W, Sun X (2012) Correlation of ultrasonographic measurement of intrarenal arterial resistance index with microalbuminuria in nonhypertensive, nondiabetic obese patients. Int Urol Nephrol. doi:10.1007/s11255-012-0300-3
Bonnet F, Marre M, Halimi JM, Stengel B, Lange C, Laville M et al (2006) Larger waist circumference is a predictive factor for the occurrence of microalbuminuria in a non-diabetic population. Arch Mal Coeur Vaiss 99(7–8):660–662
Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE (2003) A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41(4):733–741
Gong W, Ren H, Tong H, Shen X, Luo J, Chen S et al (2007) A comparison of ultrasound and magnetic resonance imaging to assess visceral fat in the metabolic syndrome. Asia Pac J Clin Nutr 16(Suppl 1):339–345
Koda M, Senda M, Kamba M, Kimura K, Murawaki Y (2007) Sonographic subcutaneous and visceral fat indices represent the distribution of body fat volume. Abdom Imaging 32(3):387–392
Suzuki R, Watanabe S, Hirai Y, Akiyama K, Nishide T, Matsushima Y et al (1993) Abdominal wall fat index, estimated by ultrasonography, for assessment of the ratio of visceral fat to subcutaneous fat in the abdomen. Am J Med 95(3):309–314
Rea DJ, Heimbach JK, Grande JP, Textor SC, Taler SJ, Prieto M et al (2006) Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney Int 70(9):1636–1641
Acknowledgments
We would like to thank Dr. Junfang Xu from Center for Diseases Control and Prevention of Jingmen in China for the assistance in statistics. This study was supported by the Science and Technology Innovation Fund of Affiliated Hospital of Weifang Medical University (Grants Number: K12QC1004 and K12CX1007).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, X., Han, F., Miao, W. et al. Sonographic evaluation of para- and perirenal fat thickness is an independent predictor of early kidney damage in obese patients. Int Urol Nephrol 45, 1589–1595 (2013). https://doi.org/10.1007/s11255-013-0404-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-013-0404-4