Abstract
Purpose of Review
Agitation is common among older adults with dementia; its origin may be multi-factorial, and it is often difficult to treat. In this paper, we summarize current knowledge and offer considerations on pharmacologic management of behavioral and psychological symptoms of dementia (BPSD).
Recent Findings
We reviewed human studies published from 2013 to 2018 evaluating pharmacologic management of BPSD manifestations including depressive symptoms, mania, psychosis, and other BPSD, as well as severe agitation without determination of underlying cause. After non-pharmacological management is exhausted, the choice of pharmacological options depends on patient comorbidities, specific BPSD presentation, and patient tolerance of medications.
Summary
Depending on manifestations of BPSD, low- to moderate-quality evidence supports the use of anti-depressants, anti-psychotics, or anti-epileptics in conjunction with cholinesterase inhibitors. The current evidence base needs to be augmented with future research that focuses on real-world medication use alongside head-to-head evaluation of medication effectiveness rather than comparison to placebo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia is expected to affect at least 13 million Americans by 2050, the majority of whom will be over 85 years old [1]. While some studies have pointed to a potential decline in future dementia diagnoses [2, 3], dementia remains one of the most common and challenging diseases to manage among older adults. Most patients with dementia experience behavioral and psychological symptoms of dementia (BPSD) [4], ranging from “nuisance behaviors” that are not necessarily harmful to the patient or caregivers, such as calling out, repetitive questions, dysphoric mood, and delusions, to behaviors that pose immediate potential or actual harm to patients and caregivers, such as wandering and physical or sexual aggression towards others.
BPSD is a geriatric syndrome with multiple potential causes. Accordingly, based on expert opinion, comprehensive geriatric assessment is indicated in the evaluation and management of this condition. This should include assessment of the potential causes of agitation or aggressive behavior and utilization of non-pharmacological management strategies before proceeding to pharmacological management of BPSD. Clinicians should assess patient-level factors such as pain, onset of new illness or impairment, side effects from new or established medications, and sensory deficits. It is also important to consider caregiver and environmental factors, such as antecedents to the patient behavior and a need for caregiver education or support. Additionally, as many patients with dementia have difficulty eating or swallowing and often develop insomnia, it is important to assess whether patients have sufficient nourishment and sleep. Different non-pharmacologic approaches have been found to improve behavior, reduce harm to patients and caregivers, and avoid use of medications, specifically communication skills training, group activities, music therapy, massage, pet therapy visits, and physical activity [5, 6]. Additional information regarding non-pharmacological considerations and approaches is available elsewhere [7].
The consequences of BPSD are often significant for patients and caregivers, ranging from patient/caregiver injuries to emergency department visits and hospitalization or institutional placement [8, 9]. Thus, when non-pharmacological approaches are insufficient, based on robust assessment of potential triggers, pharmacological management of BPSD is appropriate. While medications may help avoid hospitalization or institutionalization for patients, medications may have limited efficacy in controlling target behaviors. Additionally, medications may entail significant risks, including increased mortality among patients with dementia receiving anti-psychotic medications. Ultimately, a trial of medication is appropriate when non-pharmacological interventions have not been effective, when patient behavior is interfering with the ability to provide care to them, when there is a safety risk to patient and/or caregiver, or if the patient’s behavior risks placement in an institutional setting. In this review, we offer an overview of recently published research regarding pharmacological options for managing the various manifestations of BPSD and clinical considerations for the use of each class of agents. We have organized our approach to BPSD management based on presenting manifestations, in accordance with our conceptualization of BPSD as a geriatric syndrome, first to identify and treat potentially remediable causes and contributors to BPSD prior to turning to non-specific pharmacotherapeutic management.
Methods
For this review, we consulted multiple databases to retrieve and assess available evidence regarding pharmacological management of agitation in dementia published in the last 5 years. We searched PubMed, EMBASE, CINAHL, PsychInfo, and the Cochrane Collaboration for studies published in English from January 2013 to the present, conducted in humans, considering Alzheimer’s disease (AD), Lewy body dementia (LBD), vascular dementia, and frontotemporal dementia, using the terms “agitation,” “aggression,” and/or “behavioral and psychological symptoms.” We included studies published prior to 2013 if they were influential studies or provided information on medications for which there are little available data. We prioritized randomized clinical trials (RCT) as the highest quality of available evidence, but also included observational studies and review articles to augment available evidence as needed. We also reviewed references of included articles to ensure that we performed a comprehensive review of available evidence. For this review, we offer a geriatrics and gero-pharmacology standpoint from which to optimize treatment of BPSD, focusing on deprescribing when possible and optimizing the treatment of co-occurring medical conditions that may cause BPSD.
Results
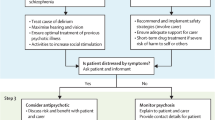
Our initial search generated 1448 potentially pertinent articles. We reviewed titles of each article and pulled 231 abstracts for further review to assess relevance to our research question. We included 66 original research articles, meta-analyses, and systematic reviews herein. Of note, as of 2018, there is no medication specifically approved by the US Food and Drug Administration for the treatment of BPSD; thus, all medications in this review are used in an off-label capacity. In Table 1, we present different medication options for BPSD, arranged by class. Table 2 includes recommendations for medication use by BPSD manifestation. Figure 1 is a flowchart for assessment and pharmacological management of BPSD.
Medication Options
Cholinesterase Inhibitors
Cholinesterase inhibitors have a generally favorable safety profile and are generally well tolerated. They may be used long-term until a patient experiences decline to terminal dementia, in which case the continued use of cholinesterase inhibitors may be of little benefit. If a patient has not started a cholinesterase inhibitor, then guidelines recommend beginning donepezil for mild-moderate disease and considering high-dose donepezil in conjunction with memantine for moderate to severe disease [10].
The strongest evidence for use of cholinesterase inhibitors in BPSD management is in AD and LBD, where their use may help delay the onset of BPSD [11]. An earlier RCT found that rivastigmine reduces hallucinations in patients with LBD [12]; this robust response to a cholinesterase inhibitor may be because those with LBD have a greater cholinergic deficit than people with AD [13]. Overall, evidence for the utility of cholinesterase inhibitors to treat established BPSD is conflicting. A 2015 systematic review of memantine and cholinesterase inhibitors found no clinically significant impact on BPSD [14]. However, a 2014 crossover, randomized, open-label study among patients with mild-to-moderate AD found that over 12-month time, patients receiving memantine or rivastigmine had more improvement in BPSD than those receiving donepezil or galantamine [15], although BPSD was not eliminated. Given the limits of neurobiological categorization, using a cholinesterase inhibitor might produce benefits in a psychotic or delusional behavior that is mediated to some degree through the dopaminergic system, or potentially have benefits on a broad range of behavioral disturbances. If a patient is using cholinesterase inhibitors, yet is experiencing new-onset aggression, non-pharmacologic methods should be utilized. If after this, the patient is still experiencing BPSD, then the patient may need an increased dose of cholinesterase inhibitors or additional medications.
Recent studies have focused on combining cholinesterase inhibitors with other medications. In a double-blinded RCT of 113 patients with mild-moderate AD and cerebrovascular injury, those receiving donepezil plus choline alphoscerate, a phospholipid found in the brain that is a precursor to acetylcholine, showed marked decrease in depression, anxiety, or apathy compared to those receiving donepezil plus placebo [16••]. Memantine may be effective as an add-on therapy for BPSD. One study recruited 240 patients who were receiving maximum doses of donepezil, galantamine, or rivastigmine yet were experiencing BPSD or worsening cognitive function. The patients received 20 mg/day of memantine in addition to their baseline medication. While 80% of patients experienced a reduction in agitation per the neuropsychiatric inventory (NPI) score, the remaining 20% experienced increased agitation [17]. This retrospective open-label study may be prone to treatment selection bias. However, a recent meta-analysis found that gradual titration of memantine and donepezil in tandem reduces aggression as measured by the NPI in patients with moderate to severe AD [18•].
A different trial explored the utility of rivastigmine patches in conjunction with memantine to treat aggression in 147 patients with mild to moderate AD. The study found no improvement in aggressive behaviors, defined as inappropriate disrobing, aggressive vocalization, and wandering, but significant improvement for those with non-aggressive agitation, such as hoarding objects or repetitive sentences/questions [19]. For patients with LBD and relapsed BPSD, a small study of 24 patients noted a decrease in BPSD with increase of 10 mg/day of donepezil above the dose of donepezil the patients were already receiving. Of note, some patients experienced significant gastrointestinal upset with the increase in donepezil dose, but no other adverse events were noted [20].
Anti-depressants and Anxiolytics
An anti-depressant may help decrease agitation among patients who are also exhibiting depressed mood, anxiety, or paranoia. Unfortunately, tricyclic anti-depressants and selective serotonin reuptake inhibitors (SSRIs) are consistently associated with increased fall risk in older adults, so starting at a low dose and titrating these medications slowly are of paramount importance to reduce fall risk [21].
The largest evidence base for the use of anti-depressants in patients with dementia is for SSRIs, such as citalopram or sertraline [22]. In the 2014 CitAD study [23••], a RCT of 186 patients who received citalopram (n = 94) or placebo (n = 92) in conjunction with behavioral therapy found a statistically significant decrease in agitation and caregiver burden. However, patients receiving citalopram were more likely to experience QT prolongation and worsening of cognition at 30 mg/day dosing. While a different RCT did not find improvement in depression among AD patients receiving sertraline or mirtazapine, a secondary data analysis suggested that mirtazapine might be helpful for BPSD [24]. Overall, a narrative review found little evidence that anti-depressants improve depression in patients with dementia. These agents may decrease aggression yet should be used with caution due to the potential for significant side effects in these patients [25].
Buspirone was evaluated in a retrospective observational study of BPSD, using an average dose of 25.7 mg ± 12.50 mg [26]. Among 179 patients, 68% had improved scores on the Clinical Global Impression scale, which assesses patient response to medication in relation to baseline severity of mental illness. These study results should be interpreted with caution, as there was no comparator arm and patients were not randomized to receive buspirone. Serotonin-norepinephrine reuptake inhibitors (SNRIs) may reduce BPSD and address co-occurring pain, but the evidence base for use is again limited. One small observational study showed that milnacipran may reduce depressive symptoms, such as depressed mood and loss of interest, in older adults with dementia [27]. A different randomized study recruited 59 patients with moderate AD and major depressive disorder to assess the impact of sertraline, venlafaxine, or desipramine on depressive symptoms and cognition. Investigators observed improvement across domains with use of sertraline across all time periods. However, improvement with venlafaxine or desipramine was seen in the first 4–8 weeks of the study then waned [28]. Bupropion is another potential option for patients who are smoking and experiencing depression, but this is based only on expert opinion and a case report of reduced apathy in a patient with frontotemporal dementia receiving bupropion [29].
Trazodone or benzodiazepines are commonly prescribed for older adults who are experiencing sleep disturbances. Limited evidence supporting the use of trazodone for BPSD exists. Trazodone may help to lessen insomnia [30, 31], but it should be used with caution given the risk of orthostatic hypotension. As benzodiazepines raise the risk of falls, delirium, and behavior disinhibition among patients with dementia, they are generally not recommended in this population. A systematic review of RCTs found no support for routine use of benzodiazepines to treat BPSD [32]. A different review noted some evidence that lorazepam and alprazolam may reduce agitation in AD patients; however, benzodiazepines have also been linked to increased cognitive decline among patients with dementia [33].
Anti-psychotics
Anti-psychotics—especially risperidone, aripiprazole, and olanzapine—have been evaluated in multiple studies and demonstrated improvement in severe agitation, aggression, and psychosis (e.g., delusions, hallucinations) among patients with AD [34]. However, the effect size observed in these trials was modest and placebo effects common; thus, a careful assessment of benefit and risks of treatment is important prior to starting therapy. The adverse effect profile of anti-psychotics is significant compared to those of other agents, as falls, sedation, and hypotension are common. Patients should be monitored for extrapyramidal manifestations (e.g., tardive dyskinesia, akathisia, medication-induced Parkinsonism), especially if using first-generation anti-psychotics. Clozapine is an alternative agent that may be used for patients who do not respond to other medications; however, a rigorous monitoring regimen is required due to the risk of agranulocytosis; this may not be feasible for all patients. Clinicians may find the Abnormal Involuntary Movement Scale (AIMS) helpful for detecting and monitoring the development of tardive dyskinesia for patients taking anti-psychotics [35]. Finally, the US Food and Drug Administration has issued black box warnings for patients with dementia treated with first- and second-generation anti-psychotic agents given the elevated risk of sudden cardiac death, stroke, and other adverse effects associated with these medications. Accordingly, it is generally advisable to use anti-psychotics in the lowest dose needed to control symptoms, for the shortest possible duration, with monitoring for the development of adverse effects.
While clinicians should consider tapering or discontinuing anti-psychotics after control of BPSD to mitigate risk associated with these medications, there is limited evidence regarding patient outcomes after discontinuation of anti-psychotics. A recent Cochrane review noted that patients with severe baseline symptoms at anti-psychotic initiation may experience worsening symptoms after discontinuation, and those with less severe symptoms may see no change following discontinuation. There is insufficient evidence to indicate whether discontinuation impacts mortality or other side effects associated with anti-psychotics [36]. Tjia and colleagues proposed a two-phase gradual dose reduction of anti-psychotics based on pharmacokinetic principles that harmonizes evidence from discontinuation trials [37•]. A small study of 36 patients suggested that adding citalopram to a medication regimen may facilitate subsequent discontinuation of anti-psychotics for older adults with AD [38]. The Halting Antipsychotic Use in Long-term Care (HALT) study was a single-arm longitudinal study conducted in Australian long-term care facilities among patients taking anti-psychotics, 98.5% of whom had dementia. Of the 93 patients who completed the study, 69 (74%) had anti-psychotics successfully deprescribed without reinitiating anti-psychotics or experiencing increase in BPSD [39••].
Anti-epileptics
For patients experiencing BPSD, the duration of anti-epileptic therapy necessary to see a reduction in BPSD will vary by patient. Like anti-psychotics, we recommend these medications be used for as short a duration as possible. Divalproex sodium (valproate) has been investigated as a preventative agent for BPSD. A 24-month multi-site RCT found no difference between onset of BPSD in subjects receiving valproate or placebo, but those taking valproate had significantly higher incidence of toxicity, such as somnolence, diarrhea, and gait disturbance [40]. As valproate is not effective in preventing onset of aggression, and a previous Cochrane review noted its side effects likely outweigh any benefits [41], other agents are likely a better choice.
A 2014 review [42] noted that while some data exist for the value of carbamazepine to diminish agitation, sample sizes are small, and many patients cannot tolerate carbamazepine as older adults are more likely to experience side effects such as weight change, sedation, gait disturbance, and gastrointestinal problems. The carbamazepine monitoring regimen to evaluate liver enzymes and complete blood count may also be onerous for patients and caregivers. Since that review, Suzuki and colleagues [43] conducted a 2015 open-label trial of lamotrigine in conjunction with risperidone or diazepam among 40 hospitalized patients with severe AD. While subjects receiving lamotrigine did not need as much risperidone or diazepam compared to those not receiving lamotrigine, the findings of this 16-week, open-label study are not widely generalizable given the small study size and heterogeneous mix of different medications participants received. In a small case series, gabapentin reduced aggression among seven patients with vascular dementia or mixed vascular/AD, using daily doses ranging from 200 to 600 mg daily. Three of the seven patients were able to discontinue anti-psychotics after gabapentin initiation [44].
Dextromethorphan-Quinidine
Investigators studied the efficacy of dextromethorphan-quinidine to reduce aggression in AD in a 10-week RCT [45••] (this use is off-label, as the FDA-approved indication is for treatment of pseudobulbar affect). Patients receiving dextromethorphan-quinidine had significantly lower NPI agitation/aggression scores, which was the primary endpoint of the study. Those receiving dextromethorphan-quinidine also had a significantly higher incidence of falls and a higher incidence of serious adverse events (7.9% vs 4.7%). Of note, the medication regimens that subjects were receiving were heterogeneous, including cholinesterase inhibitors, memantine, anti-psychotics, anti-depressants, and benzodiazepines. Given that this was a parallel crossover design, patients were not matched one to one with respect to concomitant medications to allow for a comparison between dextromethorphan-quinidine and placebo. Furthermore, while patients may benefit from dextromethorphan-quinidine, its high price point may limit its use due to high out-of-pocket costs.
Special Considerations and Other Classes of Medications
Patients may exhibit BPSD because they are experiencing pain, but no longer have the capacity to verbalize their discomfort. Data from RCTs support the use of analgesics to reduce BPSD. A recently published review noted three RCTs where acetaminophen, morphine, or long-acting oxycodone reduced symptoms of BPSD over 8-week time among nursing home residents [46]. Additionally, investigators in a clustered site study in Norwegian nursing homes [47] found that among 352 patients with moderate to severe dementia, verbal aggression, pacing, and restlessness responded to personalized pain therapy, using a combination of pregabalin, acetaminophen, buprenorphine patches, or extended-release morphine.
Various agents have been explored in case series and clinical trials to afford more options for clinicians and patients. Mania is common among patients with dementia experiencing aggression, and lithium may hold promise as a potential treatment for this manifestation of BPSD. As of August 2018, an ongoing clinical trial is investigating whether lithium is an effective treatment for aggression among older adults with moderate to severe AD [48]. In a different study utilizing allopurinol, among eight veterans with dementia experiencing refractory aggression, six of the eight receiving allopurinol demonstrated reduced aggressive episodes [49].
Insomnia and sleep disturbances are ubiquitous among patients with dementia, which then contribute to BPSD symptoms. A RCT found that patients with mild-to-moderate AD and insomnia experienced improved sleep and cognition with 2 mg daily melatonin compared to patients receiving placebo [50]. Anti-histamines such as diphenhydramine may be used by patients or caregivers to improve sleep, but the risks of falls and residual sleepiness as well as anti-cholinergic symptoms such as constipation and dry mouth make diphenhydramine a poor choice. Ramelteon, trazodone, or zolpidem may be safer options for patients with dementia experiencing sleep disturbance based on a recent review [51]. However, as noted by a 2016 Cochrane review, no studies regarding zolpidem have been performed in this population; a study of 74 participants evaluating ramelteon was not published but did not show significant side effects, and one small RCT with 30 participants randomized to trazodone showed increased sleep efficiency with no serious adverse events [52].
A recent study investigated the use of tumor necrosis factor α-inhibitor etanercept as a potential treatment option for AD. In this double-blinded RCT, investigators found no difference in behavioral symptoms between patients receiving placebo versus those receiving etanercept, although those receiving etanercept had more infections due to the immunosuppressive nature of the medication [53].
Sexual disinhibition is common among patients with dementia, which may endanger patients and caregivers. The available evidence regarding treatment is comprised of case reports and case series, as there are no RCTs addressing inappropriate sexual behavior among patients with dementia. Briefly, non-pharmacological methods, such as patient distraction and redirection, should first be employed. If such methods are not effective in reducing or eliminating inappropriate sexual behavior, then clinicians may consider pharmacotherapy including anti-depressants, anti-psychotics, anti-convulsants, cholinesterase inhibitors, and beta-blockers. Hormonal agents may also be used, but some clinicians and caregivers may be reticent to utilize these agents (medroxyprogesterone acetate, estrogen, leuprolide) as they are associated with “chemical castration” [54].
Only for use in the last days or weeks of life, palliative sedation (defined as the use of sedative medication to relieve intolerable suffering from refractory symptoms by a reduction in patient consciousness) may be necessary as a treatment of last resort to control severe BPSD that does not respond to other pharmacotherapeutic and non-pharmacotherapeutic interventions [55]. A case series reported that among older adults dying with dementia in Dutch long-term care facilities, pain and agitation were the most common cause of distress, and 21% received palliative sedation before dying [56]. Even among experts, there is significant disagreement as to whether palliative sedation is appropriate for severe agitation among patients with dementia and limited life expectancy, with only 22% of clinicians agreeing that in such circumstances, palliative sedation is appropriate although 100% agreed with palliative sedation for refractory delirium [57]. Agents that may be used for palliative sedation include midazolam, chlorpromazine, levomepromazine (unavailable in the USA), phenobarbital, and propofol [58]. A detailed discussion of palliative sedation is beyond the scope of this review; readers are referred to recent reviews and guidelines for more information [55, 59, 60].
Complementary and Alternative Medicine
Various studies have considered the use of complementary and alternative medicines to treat aggression, including cannabis, which is legal in some parts of the USA (although still illegal under federal statute). As caregivers may ask about these modalities, we offer a brief synopsis herein. A 2015 RCT [61] evaluated tetrahydrocannabinol (THC), the active ingredient in cannabis, compared to placebo for control of aggression symptoms among 50 patients with AD, vascular, or mixed dementia, with 24 receiving THC and 26 receiving placebo. There were no significant differences in neuropsychiatric symptoms, activities of daily living, agitation, or quality of life at 21 days; however, THC was well tolerated with no difference in mild or moderate adverse events. There is some evidence that gingko biloba may help reduce aggression in dementia. In a meta-analysis of randomized studies, patients receiving 22–24 weeks of gingko biloba experienced a reduction in BPSD, except for psychosis [62]. Multiple investigators have evaluated whether aromatherapy, specifically lavender spray, reduces BPSD, but no significant reduction in agitation has been found [63,64,65]. Another study [66] found that aromatherapy or aromatherapy + acupuncture was superior to placebo for reducing aggression, but participants were not blinded to the intervention of interest and may have been biased as a result. Additionally, those receiving aromatherapy started with higher agitation scores, thus had more potential change in behavior compared to the control group.
Multiple studies have evaluated the efficacy of yokukusan, a combination of seven dried herbs, to reduce agitation among patients with dementia. A 2017 double-blinded placebo-controlled multi-site randomized study [67] found no significant difference at 16 weeks between yokukusan and placebo for BPSD reduction. However, people with 20 or fewer points on the mini-mental state examination had a statistically significant reduction in aggression compared to people receiving placebo. A 2013 single-center study [68] compared yokukusan, fluvoxamine, or risperidone for reduction in BPSD; 30 patients were assigned to each group. Across all groups, investigators noted a reduction in BPSD symptoms, but higher extrapyramidal symptoms with risperidone. Study enrollment was limited to subjects with few comorbidities, limiting the generalizability of study findings.
Conclusion
BPSD often precipitates substantial distress and burden in patients and caregivers. In this review, we have described the various pharmacological options to treat BPSD, focusing on literature published 2013 to 2018. We offer a geriatric viewpoint whereby first non-pharmacologic intervention should be utilized, then other etiologies for BPSD should be explored, and only after optimization of current medications and conditions should pharmacotherapy for BPSD be added. Ultimately, medications should be used as part of a comprehensive treatment plan to identify and remediate potential causes of BPSD and ensure the safety of patients and caregivers, while avoiding hospitalization and/or institutionalization for patients with dementia.
The mixed and limited study findings we present here underscore the need for comparative effectiveness research for the best ways to address BPSD among multi-morbid, older patients who are often taking a combination of medications. Even among experts, recommendations and treatment algorithms for BPSD vary. A recent review proposed risperidone as a first-choice agent for agitation and aggression among dementia patients [69•], whereas a Delphi panel suggested citalopram and analgesia [70•]. Unfortunately, despite RCTs of agents to treat BPSD, and the development of novel agents, currently there is no well-tolerated medication that has shown clinically meaningful benefits, with minimal side effects, for preventing or treating BPSD [71].
As there is still no FDA-approved medication to treat agitation and aggression among older adults with dementia, future studies are needed to build the evidence base regarding real-world effectiveness and observed side effects of these commonly used medications. Given the challenge of BPSD for patient and caregiver safety, plus quality of life, research progress in this area is needed to address the challenge of effectively treating BPSD and to improve patient quality of life and that of their caregivers.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–83.
Derby CA, Katz MJ, Lipton RB, Hall CB. Trends in dementia incidence in a birth cohort analysis of the Einstein aging study. JAMA Neurol. 2017;74(11):1345–51.
Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80–93.
van der Linde RM, Dening T, Stephan BC, Prina AM, Evans E, Brayne C. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry. 2016;209(5):366–77.
Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry. 2014;205(6):436–42.
de Oliveira AM, Radanovic M, de Mello PC, Buchain PC, Vizzotto AD, Celestino DL, et al. Nonpharmacological interventions to reduce behavioral and psychological symptoms of dementia: a systematic review. Biomed Res Int. 2015;2015:218980.
Brasure M, Jutkowitz E, Fuchs E, Nelson VA, Kane RA, Shippee T, et al. Nonpharmacologic interventions for agitation and aggression in dementia. Rockville (MD): AHRQ Comparative Effectiveness Reviews; 2016.
Maust DT, Kales HC, McCammon RJ, Blow FC, Leggett A, Langa KM. Distress associated with dementia-related psychosis and agitation in relation to healthcare utilization and costs. Am J Geriatr Psychiatry. 2017;25(10):1074–82.
Suehs BT, Shah SN, Davis CD, Alvir J, Faison WE, Patel NC, et al. Household members of persons with Alzheimer’s disease: health conditions, healthcare resource use, and healthcare costs. J Am Geriatr Soc. 2014;62(3):435–41.
Cummings JL, Geldmacher D, Farlow M, Sabbagh M, Christensen D, Betz P. High-dose donepezil (23 mg/day) for the treatment of moderate and severe Alzheimer’s disease: drug profile and clinical guidelines. CNS Neurosci Ther. 2013;19(5):294–301.
Cummings J, Lai TJ, Hemrungrojn S, Mohandas E, Yun Kim S, Nair G, et al. Role of donepezil in the management of neuropsychiatric symptoms in Alzheimer’s disease and dementia with Lewy bodies. CNS Neurosci Ther. 2016;22(3):159–66.
McKeith I, Del Ser T, Spano P, Emre M, Wesnes K, Anand R, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031–6.
Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003;60(12):1745–8.
Wang J, Yu JT, Wang HF, Meng XF, Wang C, Tan CC, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86(1):101–9.
Cumbo E, Ligori LD. Differential effects of current specific treatments on behavioral and psychological symptoms in patients with Alzheimer’s disease: a 12-month, randomized, open-label trial. J Alzheimers Dis. 2014;39(3):477–85.
•• Carotenuto A, Rea R, Traini E, Fasanaro AM, Ricci G, Manzo V, et al. The effect of the association between donepezil and choline alphoscerate on behavioral disturbances in Alzheimer’s disease: interim results of the ASCOMALVA trial. J Alzheimers Dis. 2017;56(2):805–15 Interim results suggest addition of choline alphoscerate may result in reduced BPSD and decreased accompanying caregiver distress in patients with mild-moderate AD.
Gareri P, Putignano D, Castagna A, Cotroneo AM, De Palo G, Fabbo A, et al. Retrospective study on the benefits of combined memantine and cholinesterase inhibitor treatMent in AGEd patients affected with Alzheimer’s disease: the MEMAGE study. J Alzheimers Dis. 2014;41(2):633–40.
• Chen R, Chan PT, Chu H, Lin YC, Chang PC, Chen CY, et al. Treatment effects between monotherapy of donepezil versus combination with memantine for Alzheimer disease: a meta-analysis. PLoS One. 2017;12(8):e0183586 Analysis notes greater improvement in BPSD for patients with severe AD receiving combination therapy vs donepezil only.
Yoon SJ, Choi SH, Na HR, Park KW, Kim EJ, Han HJ, et al. Effects on agitation with rivastigmine patch monotherapy and combination therapy with memantine in mild to moderate Alzheimer’s disease: a multicenter 24-week prospective randomized open-label study (the Korean EXelon patch and combination with mEmantine comparative trial study). Geriatr Gerontol Int. 2017;17(3):494–9.
Manabe Y, Ino T, Yamanaka K, Kosaka K. Increased dosage of donepezil for the management of behavioural and psychological symptoms of dementia in dementia with Lewy bodies. Psychogeriatrics. 2016;16(3):202–8.
Seppala LJ, Wermelink A, de Vries M, Ploegmakers KJ, van de Glind EMM, Daams JG, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics. J Am Med Dir Assoc. 2018;19(4):371 e11–7.
Seitz DP, Adunuri N, Gill SS, Gruneir A, Herrmann N, Rochon P. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;2:CD008191.
•• Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–91 RCT demonstrated efficacy of citalopram for management of BPSD.
Banerjee S, Hellier J, Romeo R, Dewey M, Knapp M, Ballard C, et al. Study of the use of antidepressants for depression in dementia: the HTA-SADD trial--a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine. Health Technol Assess. 2013;17(7):1–166.
Farina N, Morrell L, Banerjee S. What is the therapeutic value of antidepressants in dementia? A narrative review. Int J Geriatr Psychiatry. 2017;32(1):32–49.
Santa Cruz MR, Hidalgo PC, Lee MS, Thomas CW, Holroyd S. Buspirone for the treatment of dementia with behavioral disturbance. Int Psychogeriatr. 2017;29(5):859–62.
Mizukami K, Hatanaka K, Tanaka Y, Sato S, Asada T. Therapeutic effects of the selective serotonin noradrenaline reuptake inhibitor milnacipran on depressive symptoms in patients with Alzheimer’s disease. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33(2):349–52.
Mokhber N, Abdollahian E, Soltanifar A, Samadi R, Saghebi A, Haghighi MB, et al. Comparison of sertraline, venlafaxine and desipramine effects on depression, cognition and the daily living activities in Alzheimer patients. Pharmacopsychiatry. 2014;47(4–5):131–40.
Lin CP, Chu CP, Liu HC. Bupropion improved apathy in behavioral variant frontotemporal dementia: a case report. Neurocase. 2016;22(5):466–8.
Martinon-Torres G, Fioravanti M, Grimley EJ. Trazodone for agitation in dementia. Cochrane Database Syst Rev. 2004;4:CD004990.
Camargos EF, Louzada LL, Quintas JL, Naves JO, Louzada FM, Nobrega OT. Trazodone improves sleep parameters in Alzheimer disease patients: a randomized, double-blind, and placebo-controlled study. Am J Geriatr Psychiatry. 2014;22(12):1565–74.
Tampi RR, Tampi DJ. Efficacy and tolerability of benzodiazepines for the treatment of behavioral and psychological symptoms of dementia: a systematic review of randomized controlled trials. Am J Alzheimers Dis Other Demen. 2014;29(7):565–74.
Defrancesco M, Marksteiner J, Fleischhacker WW, Blasko I. Use of benzodiazepines in Alzheimer’s disease: a systematic review of literature. Int J Neuropsychopharmacol. 2015;18(10):pyv055.
Tampi RR, Tampi DJ, Balachandran S, Srinivasan S. Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Ther Adv Chronic Dis. 2016;7(5):229–45.
Kane JM, Correll CU, Nierenberg AA, Caroff SN, Sajatovic M, Tardive Dyskinesia Assessment Working G. Revisiting the Abnormal Involuntary Movement Scale: Proceedings From the Tardive Dyskinesia Assessment Workshop. J Clin Psychiatry. 2018;79(3).
Van Leeuwen E, Petrovic M, van Driel ML, De Sutter AI, Vander Stichele R, Declercq T, et al. Withdrawal versus continuation of long-term antipsychotic drug use for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2018;3:CD007726.
• Tjia J, Reidenberg MM, Hunnicutt JN, Paice K, Donovan JL, Kanaan A, et al. Approaches to gradual dose reduction of chronic off-label antipsychotics used for behavioral and psychological symptoms of dementia. Consult Pharm. 2015;30(10):599–611 Synthesizes data supporting proposed de-prescribing algorithm for patients receiving anti-psychotics.
Mathys M, Fang S, John J, Carter J. Antipsychotic discontinuation after the initiation of selective serotonin reuptake inhibitors therapy for the treatment of behavioral and psychological symptoms associated with dementia. Ment Health Clin. 2018;8(3):122–6.
•• Brodaty H, Aerts L, Harrison F, Jessop T, Cations M, Chenoweth L, et al. Antipsychotic deprescription for older adults in long-term care: the HALT study. J Am Med Dir Assoc. 2018;19(7):592–600 e7 Demonstrated that anti-psychotics may be safely withdrawn without increase in BPSD or adverse outcomes.
Tariot PN, Schneider LS, Cummings J, Thomas RG, Raman R, Jakimovich LJ, et al. Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry. 2011;68(8):853–61.
Lonergan E, Luxenberg J. Valproate preparations for agitation in dementia. Cochrane Database Syst Rev. 2009;3:CD003945.
Gallagher D, Herrmann N. Antiepileptic drugs for the treatment of agitation and aggression in dementia: do they have a place in therapy? Drugs. 2014;74(15):1747–55.
Suzuki H, Gen K. Clinical efficacy of lamotrigine and changes in the dosages of concomitantly used psychotropic drugs in Alzheimer’s disease with behavioural and psychological symptoms of dementia: a preliminary open-label trial. Psychogeriatrics. 2015;15(1):32–7.
Cooney C, Murphy S, Tessema H, Freyne A. Use of low-dose gabapentin for aggressive behavior in vascular and mixed vascular/Alzheimer dementia. J Neuropsychiatry Clin Neurosci. 2013;25(2):120–5.
•• Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharre DW, et al. Effect of dextromethorphan-quinidine on agitation in patients with alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242–54 In short-term 10 week trial, authors found reduced agitation among AD patients taking dextromethorphan-quinidine.
Tampi RR, Hassell C, Joshi P, Tampi DJ. Analgesics in the management of behavioral and psychological symptoms of dementia: a perspective review. Drugs Context. 2017;6:212508.
Habiger TF, Flo E, Achterberg WP, Husebo BS. The interactive relationship between pain, psychosis, and agitation in people with dementia: results from a cluster-randomised clinical trial. Behav Neurol. 2016;2016:7036415.
Devanand DP, Strickler JG, Huey ED, Crocco E, Forester BP, Husain MM, et al. Lithium treatment for agitation in Alzheimer’s disease (lit-AD): clinical rationale and study design. Contemp Clin Trials. 2018;71:33–9.
Carr CN, Straley CM, Baugh TB. Allopurinol for the treatment of refractory aggression: a case series. Pharmacotherapy. 2017;37(6):748–54.
Wade AG, Farmer M, Harari G, Fund N, Laudon M, Nir T, et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging. 2014;9:947–61.
Schroeck JL, Ford J, Conway EL, Kurtzhalts KE, Gee ME, Vollmer KA, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340–72.
McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev. 2016;11:CD009178.
Butchart J, Brook L, Hopkins V, Teeling J, Puntener U, Culliford D, et al. Etanercept in Alzheimer disease: a randomized, placebo-controlled, double-blind, phase 2 trial. Neurology. 2015;84(21):2161–8.
De Giorgi R, Series H. Treatment of inappropriate sexual behavior in dementia. Curr Treat Options Neurol. 2016;18(9):41.
Quill TE, Lo B, Brock DW, Meisel A. Last-resort options for palliative sedation. Ann Intern Med. 2009;151(6):421–4.
Hendriks SA, Smalbrugge M, Hertogh CM, van der Steen JT. Dying with dementia: symptoms, treatment, and quality of life in the last week of life. J Pain Symptom Manag. 2014;47(4):710–20.
Benitez-Rosario MA, Morita T. Palliative sedation in clinical scenarios: results of a modified Delphi study. Support Care Cancer. 2018.
Cherny NI, Group EGW. ESMO Clinical Practice Guidelines for the management of refractory symptoms at the end of life and the use of palliative sedation. Ann Oncol. 2014;25(Suppl 3):iii143–52.
Gurschick L, Mayer DK, Hanson LC. Palliative sedation: an analysis of international guidelines and position statements. Am J Hosp Palliat Care. 2015;32(6):660–71.
Bodnar J. A review of agents for palliative sedation/continuous deep sedation: pharmacology and practical applications. J Pain Palliat Care Pharmacother. 2017;31(1):16–37.
van den Elsen GA, Ahmed AI, Verkes RJ, Kramers C, Feuth T, Rosenberg PB, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: a randomized controlled trial. Neurology. 2015;84(23):2338–46.
Savaskan E, Mueller H, Hoerr R, von Gunten A, Gauthier S. Treatment effects of Ginkgo biloba extract EGb 761(R) on the spectrum of behavioral and psychological symptoms of dementia: meta-analysis of randomized controlled trials. Int Psychogeriatr. 2018;30(3):285–93.
Fu CY, Moyle W, Cooke M. A randomised controlled trial of the use of aromatherapy and hand massage to reduce disruptive behaviour in people with dementia. BMC Complement Altern Med. 2013;13:165.
Yoshiyama K, Arita H, Suzuki J. The effect of aroma hand massage therapy for people with dementia. J Altern Complement Med. 2015;21(12):759–65.
O’Connor DW, Eppingstall B, Taffe J, van der Ploeg ES. A randomized, controlled cross-over trial of dermally-applied lavender (Lavandula angustifolia) oil as a treatment of agitated behaviour in dementia. BMC Complement Altern Med. 2013;13:315.
Yang MH, Lin LC, Wu SC, Chiu JH, Wang PN, Lin JG. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
Furukawa K, Tomita N, Uematsu D, Okahara K, Shimada H, Ikeda M, et al. Randomized double-blind placebo-controlled multicenter trial of Yokukansan for neuropsychiatric symptoms in Alzheimer’s disease. Geriatr Gerontol Int. 2017;17(2):211–8.
Teranishi M, Kurita M, Nishino S, Takeyoshi K, Numata Y, Sato T, et al. Efficacy and tolerability of risperidone, yokukansan, and fluvoxamine for the treatment of behavioral and psychological symptoms of dementia: a blinded, randomized trial. J Clin Psychopharmacol. 2013;33(5):600–7.
• Davies SJ, Burhan AM, Kim D, Gerretsen P, Graff-Guerrero A, Woo VL, et al. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509–23 Proposes treatment algorithm for BPSD, considering various manifestations of BPSD.
• Kales HC, Lyketsos CG, Miller EM, Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: an international Delphi consensus. Int Psychogeriatr. 2018;1–8. Expert consensus regarding treatment considerations for BPSD.
Soto M, Andrieu S, Nourhashemi F, Ousset PJ, Ballard C, Robert P, et al. Medication development for agitation and aggression in Alzheimer disease: review and discussion of recent randomized clinical trial design. Int Psychogeriatr. 2014:1–17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Cara McDermott and David Gruenewald declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclosure
The contents of this article do not represent the views of the US Department of Veterans Affairs or the US Government.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Palliative Care
Rights and permissions
About this article
Cite this article
McDermott, C.L., Gruenewald, D.A. Pharmacologic Management of Agitation in Patients with Dementia. Curr Geri Rep 8, 1–11 (2019). https://doi.org/10.1007/s13670-019-0269-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-019-0269-1