Abstract
Purpose of Review
Agitation is a serious complication of dementia for many patients and can be a challenging clinical problem for medical providers. With the information provided in this review, it is hoped that readers are equipped with up-to-date knowledge to implement effective treatments for their patients with this significant medical problem.
Recent Findings
This review provides an overview of the assessment and management of dementia-related agitation, including nonpharmacologic and pharmacologic approaches. These include guidelines on antipsychotic use in patients with dementia, two recently published medication algorithms, and research on agents such as prazosin, nabilone, and gabapentin. Overall, findings are mixed but promising, and a framework to guide providers exists to effectively treat dementia-related agitation.
Summary
While evidence is mixed and there is variability in published recommendations, broad principles can be applied. These include a careful assessment and systematic approach towards nonpharmacologic interventions, as well as balancing benefit, risk, and patient goals of care in medication decision-making. Treatment for dementia-related agitation also continues to be a field of active research, with promising directions to further guide future practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia-related agitation is frequently listed as a primary cause for caregiver strain and patient distress and can risk harm to patients and those around them [1]. Safety risks subsequently precipitate ER visits, psychiatric and medical inpatient hospitalizations, and institutionalization [2•]. As health care providers, having the knowledge to apply effective treatments for dementia-related agitation therefore not only improves the quality of lives of patients and their caregivers but can enhance safety and potentially reduce burden on hospital and long-term care systems.
The goal of this review is to provide an overview of the current literature around nonpharmacologic and pharmacologic treatments for dementia-related agitation, with a focus on patients living at home with a caregiver. The evidence presented is mixed and suggests that treatment planning can require time and careful effort; however, with the information provided in this review, it is hoped that readers will be equipped with the up-to-date knowledge needed to successfully implement effective treatment for their patients.
Terminology
The language used in studies investigating dementia-related agitation is highly variable. This variability lies in the fact that neurodegenerative disorders cause global injury, so almost any psychiatric symptom can occur as a complication of these disorders. An illustration of this phenomenon is that the Neuropsychiatric Inventory (NPI), a rating scale commonly used in dementia research, assesses psychiatric symptoms across 12 broad domains, which include psychotic symptoms, such as delusions and hallucinations; what many consider agitated behaviors, such as agitation, irritability, disinhibition and motor disturbance; and other symptoms including depression, anxiety, elation, apathy, nighttime behaviors, and changes in eating behaviors [3]. In the literature, the term Behavioral and Psychological Symptoms of Dementia (BPSD) is often used to encompass this broad range of psychiatric complications in dementia.
Distinct from BPSD is wording used to describe agitation specifically. Different terms include agitation and aggression, disruptive agitation, and dementia-related agitation. Some studies will distinguish between verbal and physical agitation [4]. More recently, the International Psychogeriatric Association published a provisional definition in 2015 [5]. Criteria include a diagnosis of dementia; one of the following three symptoms: motor activity, verbal aggression, and physical aggression; and an association of these symptoms with emotional distress. These symptoms are to occur for at least two weeks, contribute to disability, and cannot be solely attributed to another cause.
For the purposes of this review on agitation, studies that use BPSD as a primary outcome measure are included in addition to those that focus specifically on dementia-related agitation. This reflects the observation that while agitation is recognized as a symptom that requires targeted attention, agitation frequently co-occurs with other psychiatric symptoms [6], and studies usually include a measure of BPSD to capture response to treatments broadly. Additionally, seminal studies prior to 2015 did not maintain a consistent definition for agitation leading to the use of variable outcome measures. This review will describe when possible if clinical trials are describing agitation specifically or broader behavioral disturbances.
Assessment
When assessing behavioral disturbances associated with dementia, it is important to rule out causes unrelated to dementia, such as delirium, another psychiatric disorder, pain, and medication side effects. A good history, physical, and laboratory and imaging studies if indicated can clue practitioners as to whether these are occurring in their patients [7].
When other causes are ruled out and a diagnosis of BPSD is made, it is helpful to obtain quantitative measures [8]. These allow practitioners to identify the severity of the symptom present, as well as measure response when interventions are implemented. Basic questions for the patient and caregiver on the frequency, length, and severity of behaviors can be relatively quick to obtain and a good start. If a specific rating scale is of interest, for consideration is the Neuropsychiatric Inventory Questionnaire (NPI-Q), which is an abbreviated version of the full NPI [9]. This brief questionnaire can be filled out by caregivers and provides a frequency and severity rating of a broad range of symptoms. Another quantitative measure that may be easy to obtain for those working in skilled nursing facilities is the Minimum Data Set Section E (MDS) which includes standard nursing assessments documented by skilled nursing facilities to maintain compliance with regulatory agencies [10].
Treatment guidelines also recommend ratings scales validated by research [8]. One of these is the Brief Psychiatric Rating Scale [11], which is not dementia specific but has the advantage of being administered by a clinician rather than the patient or caregiver, providing objective findings. Another scale is the Cohen Mansfield Agitation Inventory which is specific to dementia-related agitation and has the advantage of documenting individual behaviors, such as physical or verbal aggression [12].
Nonpharmacologic Approaches
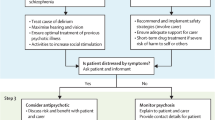
After a careful assessment, nonpharmacologic measures are considered the next appropriate treatment-planning step. Because of the side effects, risks, and uncertainty over efficacy with medications, nonpharmacologic approaches are guideline recommended for any severity of disease, and are considered first-line (before medications) when symptoms are mild to moderate in severity [8]. A one-sized-fits all approach does not exist, however, and therefore require time and “detective work” on the part of treatment providers and caregivers to design a tailored treatment plan.
One framework to consider is the “A-B-C’s” which has basis in functional analysis [13]. This approach can be easily taught to caregivers and provides a way to gather data and promote problem-solving a tailored approach to a specific challenging behavior. “A” stands for “Antecedent,” “B” for “Behavior,” and “C,” for “Consequence.” Consider a case of restlessness and pacing in a patient with dementia while watching television in a common area in skilled nursing facility. Using the A-B-C framework to gather history from the patient’s caregiver, the “Antecedent” is identified as the patient being in a common area where news coverage is playing on the television. The “Behavior” is pacing, restlessness, and a look of distress, but no verbal or physical aggression. The “Consequence” in this example could be the observation that behaviors persist if the patient stays in the common area, but distress resolves after movement to a quieter room. Going through this exercise allows providers and caregivers an opportunity to talk through a scenario and identify areas of intervention, which in this case could include monitoring television programming or moving a patient to a quiet area quickly when symptoms emerge.
Another useful framework is the DICE ApproachTM, which stands for Describe, Investigate, Create, and Evaluate [14]. After contextualizing and characterizing behaviors in the Describe step, this approach encourages incorporating patient, caregiver, and environmental factors while examining possible causes (i.e., Investigate), and while collaboratively implementing a treatment plan with caregivers or other team members (i.e., Create). At each step of the algorithm there is also a consideration to include psychotropic medications, particularly if there is a safety concern present. The Evaluate step reinforces the importance of assessing results and revising treatment plans as needed. Not only useful for healthcare providers, this approach has also been developed for direct-training to caregivers [15].
Another approach is to use a biopsychosocial formulation, which is often applied by psychiatrists to clinical problems [16]. This thought-process helps identify, in a systematic manner, contributors to symptoms and interventions tailored to the patient. For example, when considering biological contributors to agitation, these could include the dementia process itself, medication side effects, pain, and medical illness. Psychological contributors could include premorbid personality or psychiatric illness. There are also several psychological theoretical models around dementia-related behaviors that can fit in this category. One is a reduced threshold for stress model, in which it is postulated that patients with dementia are less able to accommodate stressful stimuli due to decreased coping abilities; therefore, stimuli that are tolerated by others instead lead to frustration, irritability, or distress in patients with dementia [17]. In the above case example, distress caused by television news programming fits this conceptualization. Another conceptualization that may fit in either the psychological or social category is the unmet needs model [18]. In this model, it is postulated that patients with cognitive impairment are less able to express their needs, both physical and psychological, therefore resulting in an expression of distress through agitation. Examples can include boredom, physical needs (e.g., pain), or need for social interaction. As for the social and environmental aspect of this biopsychosocial formulation, additional examples can include environmental triggers, presence or lack of structured activities, or caregiver interactions.
There are also specific modalities that have been studied for BPSD or agitation and can be options to consider [19,20,21,22,23,24,25,26,27,28,29,30]. Some of these are listed in Table 1. Studies tend to have mixed results or are limited by small numbers of participants, and therefore results difficult to generalize. This also likely reflects the heterogeneity behind agitated behaviors, and how a tailored approach to treatment is more likely to be successful. Despite limited evidence, these approaches can have intuitive face value (e.g., aromatherapy as a relaxation approach), are relatively easy to try, and do not have adverse effects associated with them. Many memory care units or long-term care facilities have instituted similar programming (e.g., pet therapy, exercise therapy) that allow for easy access for their residents. Therefore, these approaches are worth a consideration for many.
Caregiver Support and Education
Caregiver education and support are not just important interventions for caregivers in and of themselves but can also improve dementia-related agitation. Some examples of evidence-based approaches for caregiver training are occupational therapist interventions [31] and the Resources for Enhancing Alzheimer's Caregiver Health (REACH) Intervention [32], which is a protocol including sessions with a coach or therapist working with caregivers on self-care and training in behavioral management.
In addition, there is general advice that providers can pass along to caregivers [33••, 34]. Communication advice includes the use of simple language and one-step directions, with a calm tone while maintaining good eye contact. Offering guided choices with just two options to invite patients’ preferences can be helpful. Another communication strategy is avoiding raising one’s one voice, criticizing, or arguing and instead addressing underlying feelings and providing reassurance (“You’re safe, I am here with you.”) In addition, advice on adjusting the environment includes decreasing noise and distractions to keep the area calm, focusing on pleasant events, and offering simple exercise options or activities; music and art are examples. Pragmatic advice that may be worth reminding caregivers is to keep dangerous objects out of reach and to avoid caffeine and alcohol.
There are also several organizations with caregiver resources that can be recommended to family members. The first is the Alzheimer's Association, which is a national grassroots organization that not only focuses on advocacy and promotion of research, but also has extensive educational materials, support with a 24/7 helpline, and facilitation of support groups [33••]. The Family Caregiver Alliance also has online educational materials, including information specifically on BPSD [34]. There may be other resources available through local Area Agencies on Aging [35]. Many senior centers and hospital systems offer caregiver conferences and educational courses.
Respite programs may be of benefit in many cases. While studies are limited, there is a suggestion that adult day health programs may reduce BPSD [36]. Forms of respite to discuss with caregivers not only include adult day health programs but could also involve asking other family members to take shifts with caregiving and hiring paid caregivers or companions. Overnight respite programs in skilled nursing and assisted living facilities also allow caregivers time for extended leave to attend to their own medical needs or important travel.
Pharmacologic Approaches
Overview
There are several things to consider when reviewing research around pharmacotherapy for agitation related to dementia. First, there is only one FDA approved medication at the time of this writing for behavioral disturbances specifically associated with dementia, so most medications are prescribed off-label. Also, a study population may be different than patients in real-world clinics, hospitals, and emergency room settings. For example, most studies occur over a duration of 10 or more weeks, so may not generalize to severe symptoms seen in emergency settings. There may also be ethnic, gender, and socioeconomic differences in study versus real-world setting practices.
Findings are often mixed or complicated by serious side effects, and some agents have evidence limited to pilot studies or case series. Therefore, choosing which medications is not clear-cut, and done on a case-by-case basis weighing potential side effects risks versus risks of undertreatment, with patient and family goals of care factoring heavily. For example, for a patient with severe agitation and for which family are choosing a comfort-care approach, it may be more appropriate to trial an agent with stronger quality evidence but with significant side effect risk. This is in contrast with a patient with mild severity agitation for whom family wishes to minimize side effects, where an agent with pilot study level data but benign side effect profile may be preferred. Despite these complexities and uncertainties, in most cases providers do improve the quality of lives of their patients with the help of medications, so there is a role for pharmacotherapy in treatment.
Atypical Antipsychotics
Atypical antipsychotics tend to be more commonly used and are the most rigorously studied class of medications for dementia-related behavioral disturbances [37,38,39,40,41, 42••]. A major caveat with this class is the black box warning for increased risk of mortality in older adults treated for dementia-related psychosis. The FDA black box warning describes a 1.6 to 1.7 increased mortality risk in patients who were enrolled in clinical trials assessing efficacy for dementia-related psychosis. This number involves 4.5% mortality in those taking active drug, compared to 2.6% mortality in patients taking placebo in these clinical trials [43]. Subsequent analyses have replicated these findings [44, 45]. It is advisable to inform patients and families of these numbers, where the increased mortality risk may or may not be acceptable to them as they weigh factors such as severity of agitation, the patient’s life expectancy, and goals of care for comfort versus length of life. It is also in large part because of this mortality risk that APA guidelines recommend these agents be limited to those with severe agitation where there is risk of harm or significant distress to the patient [8].
There are at this time 5 atypical antipsychotics for which large scale randomized placebo-controlled studies have been conducted: risperidone, olanzapine, quetiapine, aripiprazole, and brexpiprazole. Risperidone has a larger number of clinical trials and a slightly more consistent demonstration of response than the other agents [8]. Brexpiprazole is the most recent agent studied in this class and includes agitation (and not psychosis) as the primary outcome measure in these studies. At the time of this writing, findings for 2 studies for brexpiprazole have been published with mixed findings [42], but an as yet unpublished 3rd study was completed resulting in FDA approval in May of 2023 [46].
For all these agents, dose ranges tend to start at the lowest available dose, with the maximum dose being less than half of the younger adult maximum dose indicated in those with schizophrenia or other psychotic disorders [37]. When considering which agent to choose, side effect profiles can be a consideration. For example, risperidone has a stronger evidence base but a greater risk for extrapyramidal side effects, while quetiapine has the weakest evidence base for efficacy but the least likely in this class to cause extrapyramidal side effects.
Selective Serotonin Reuptake Inhibitors
The CitAD study was a multi-site randomized controlled trial of citalopram that demonstrated, at 30mg daily, superiority over placebo for treatment of agitation in patients with Alzheimer's dementia [47]. Secondary analysis found efficacy was more prominent in patients living at home, with moderate (and not severe) agitation, and with milder severity dementia [48]. In other words, this study suggests that citalopram can be a preferred choice in those with milder degrees of cognitive impairment or behavioral disturbance. This study was done prior to the FDA recommending a dose limit of 20 mg per day in older adults due to risk of prolonged QT; therefore, it is not entirely clear that 20 mg per day is as efficacious as the 30 mg dose studied in this clinical trial. At the time of this review, a study of escitalopram, which is an enantiomer of citalopram, is underway and could shed light on this alternative agent [49]. Although other SSRIs have not been as rigorously studied, they are often considered for this indication as well [50]. A commonly used SSRI is sertraline, as its favorable side effect profile and relatively few drug interactions make it an attractive choice in older adults.
Cholinesterase Inhibitors and Memantine
Cholinesterase inhibitors and memantine can also be considerations in some cases. Studies with positive results are limited to secondary analysis, where primary outcome measures were cognitive measures and study participants did not have significant behavioral disturbances at baseline. However, neuropsychiatric profiles were also assessed as secondary measures, and those who were assigned active drug tended to have more favorable neuropsychiatric profiles than those taking placebo [51, 52]. This likely mirrors the modest positive symptomatic effect in cognitive profiles associated with these medications, as opposed to a specific mechanism that addresses behavioral disturbances. Subsequent studies enrolling patients with behavioral disturbances for which these symptoms were primary outcome measures did not find superiority over placebo [53,54,55].
Therefore, cholinesterase inhibitors and memantine may be worthwhile considerations for those with mild degrees of agitation. The cholinesterase inhibitors include donepezil, rivastigmine, and galantamine. Donepezil tends to be commonly used, as it is administered once daily and FDA approved for all stages of Alzheimer's dementia, from mild to severe; rivastigmine and galantamine have FDA approval for mild Alzheimer’s dementia. Rivastigmine has an additional FDA approval for Parkinson’s Disease Dementia and has a patch formulation which makes this an attractive option when patients have difficulty with oral medications. Memantine is FDA approved for moderate to severe Alzheimer’s dementia.
Anticonvulsant Medications
Another class studied for dementia-related agitation is the anticonvulsant class. Results for valproic acid have mixed results for agitation, where smaller studies suggest efficacy, but a single larger multi-site study of divaloproex did not find superiority over placebo [56,57,58]. Evidence behind carbamazepine includes small studies, which were positive, but without a published follow-up larger trial [59, 60]. Both valproic acid and carbamazepine are limited by need for laboratory monitoring of blood levels. Evidence around gabapentin include only case studies at the time of this writing, but because of its more benign side effect profile than other anticonvulsants and lack of need for laboratory monitoring, this is often considered for dementia-related agitation as well [61].
Other Medications
Prazosin is an alpha-1 adrenoceptor antagonist, FDA approved for hypertension, but also frequently used off-label for post-traumatic stress disorder sleep disturbance. The rationale behind this agent for consideration in dementia-related agitation is that noradrenergic system abnormalities have been observed in Alzheimer's disease, for which an alpha-1 adrenoreceptor antagonist may be uniquely suited. Even in early-stage disease, neurodegeneration of the locus coeruleus results in fewer norepinephrine producing neurons, but without a decrease in the amount of CSF norepinephrine produced, suggestive of increased compensatory upregulation of remaining noradrenergic neurons [62, 63]. There is also an upregulation in alpha-1 adrenoreceptors in the frontal cortex, clinically correlated with agitation severity [64, 65]. Clinical studies have also shown an association between increased noradrenergic outflow and an increase in agitated behaviors in patients with Alzheimer’s dementia [66]. Given these findings that suggest a compensatory noradrenergic system upregulation in part mediated by the alpha-1 adrenoreceptor, it is postulated that prazosin could be effective for this specific etiology for agitation in some Alzheimer's disease patients. A small double-blind placebo-controlled pilot study demonstrated superiority over placebo with good tolerability [67]. Because prazosin is an older and now generic medication, it has a long-known relatively safe side effect profile compared to atypical antipsychotics, avoiding adverse effects such as EPS and sedation. The pilot study published for this medication used a dose range of 1 mg to 6 mg total per day, in divided doses.
Another promising agent is nabilone, which is a synthetic cannabinoid used as an antiemetic in the setting of chemotherapy. A pilot randomized double-blind study involving 39 patients found greater symptom reduction compared to placebo [68]. The dose range in the study was 1 to 2 mg, and nabilone was noted to be well-tolerated, with sedation more common in patients taking active drug compared to placebo. A larger study is currently recruiting to investigate this agent further.
Another agent for consideration is dextromethorphan-quinidine, which is currently FDA approved for pseudobulbar affect. In a Phase II multicenter double-blind placebo-controlled trial for agitation and aggression related to Alzheimer's dementia, results indicated greater symptom reduction compared to placebo [69]. Dose ranges included a starting dose of dextromethorphan-quinidine 20 mg/10mg once daily, increasing up to 30 mg/10mg. Side effects included gastrointestinal symptoms and the potential for a prolonged QT interval. At the time of this writing, a Phase III clinical trial has not been published.
Lastly benzodiazepines are commonly used, although the appropriateness for use is controversial. It is typically recommended these medications be avoided by older adults, given the concern for cognitive impairment, oversedation, falls, and risk for delirium. Studies indicate that benzodiazepines are being prescribed long-term in contrast to these guidelines, however [70]. If a benzodiazepine is felt to be clinically needed, lorazepam may be the best choice because of its relatively short half-life and lack of hepatic metabolism.
Overall Approach to Medication Selection
Given the above studies show uncertain efficacy or significant side effects, there can be different ways to apply these findings into clinical practice. Table 2 summarizes the severity of symptoms, the timeframe involved, and proposes an appropriate choice in each scenario. In situations where the risk of undertreatment is high (i.e., agitation is severe and could result in safety concerns) a more aggressive approach utilizing agents with higher side effect risk, but stronger evidence for efficacy (e.g., atypical antipsychotics) would be a higher priority first choice. In the opposite scenario, milder symptoms could potentially be addressed with agents that have less proven efficacy, but safer side effect profiles. If a first choice does not result in significant improvement, switching to an agent in a different class is a next step. However, if there is partial improvement with an initial agent, some may prefer adding a second agent in an augmentation approach to avoid losing partial benefit from the first agent, with the caution that there is polypharmacy risk and need for careful monitoring of side effects.
There are also two recently published algorithms to guide medication decisions around pharmacotherapy. One of these is a sequential algorithm developed and adopted by a university health system in Canada for use in patients hospitalized for dementia-related agitation [71]. This algorithm emphasizes first a washout of medications upon admission, followed by adequate trials of 6 agents to be given in sequence and as monotherapy; the writers start with risperidone, followed by aripiprazole/quetiapine, carbamazepine, citalopram, gabapentin, and then prazosin. If these are not effective, a combination of these agents or ECT is suggested.
A second algorithm published by the Harvard South Shore Algorithm Project recommends medication trials based on severity of symptoms [72••]. For symptoms deemed emergent and requiring intramuscular administration of medications, olanzapine is recommended first, followed by haloperidol and then lorazepam. For urgent symptoms, the order of trials is aripiprazole, then risperidone, followed by prazosin. ECT is also a consideration if these trials fail. For non-emergent symptoms, first removing anticholinergic medications and addressing pain is recommended, followed by sleep optimization, donepezil and memantine, an SSRI, an atypical antipsychotic, prazosin, and finally carbamazepine.
Deprescribing
It is important also to note that behavioral disturbances associated with dementia can wax and wane, and that long term treatment may not be necessary for everyone. A randomized placebo-controlled discontinuation trial of risperidone demonstrated that 60% of volunteers switched to placebo experienced a relapse in symptoms after discontinuation, compared to 33% who continued risperidone [73]. This suggests that discontinuation may be tolerated by many, but risk for recurrence exists and is important to consider on a careful basis. American Psychiatric Association guidelines for antipsychotic use in patients with dementia provide specific recommendations on deprescribing this class of medications, where after 4 months of response to treatment, guidelines recommend offering a taper or discontinuation, followed by monthly assessments for 4 months to monitor need for restarting the medication [8]. In skilled nursing facilities, federal regulations also dictate regular attempts at gradual dose reductions of psychiatric medications in residents.
Conclusion
Agitation is a serious complication of dementia for many patients and can be a daunting clinical problem for medical providers. However, using a systematic approach as discussed above, meaningful care and improvement for patients and their caregivers is achievable. To summarize this systematic approach, key components are an assessment of the symptoms involved, utilizing a framework to identify nonpharmacologic approaches, and utilizing a stepwise approach to medication decision-making which balances severity of symptoms, side effect risk, and patient goals of care. While evidence is mixed and there is variability in recommended algorithms and in practice, these broad principles can still be applied. Treatment for dementia-related agitation also continues to be a field of active research, with promising directions to further guide future practice.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Patrick KS, Gunstad J, Martin JT, Chapman KR, Drost J, Spitznagel MB. Specific agitation behaviours in dementia differentially contribute to aspects of caregiver burden. Psychogeriatrics. 2022;22(5):688–98.
• Jones E, Aigbogun MS, Pike J, Berry M, Houle CR, Husbands J. Agitation in Dementia: Real-World Impact and Burden on Patients and the Healthcare System. J Alzheimers Dis. 2021;83(1):89-101. This survey-based study documents high degrees of health care utilization for patients with agitation in dementia. The authors estimate monetary costs per-patient and emphasize the real-world burden agitation in dementia has on health-care systems.
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14.
Watt JA, Goodarzi Z, Veroniki AA, Nincic V, Khan PA, Ghassemi M, et al. Comparative Efficacy of Interventions for Aggressive and Agitated Behaviors in Dementia: A Systematic Review and Network Meta-analysis. Ann Intern Med. 2019;171(9):633–42.
Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand DP, et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr. 2015;27(1):7–17.
Kroenke K, Gao S, Mosesso KM, Hickman SE, Holtz LR, Torke AM, et al. Prevalence and Predictors of Symptoms in Persons with Advanced Dementia Living in the Community. J Palliat Med. 2022;25(9):1376–85.
Walaszek A. Behavioral and Psychological Symptoms of Dementia. 2019.
Reus VI, Fochtmann LJ, Eyler AE, Hilty DM, Horvitz-Lennon M, Jibson MD, et al. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia. Am J Psychiatry. 2016;173(5):543–6.
Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–9.
Center for Medicare and Medicaid Services. Minimum Data Set (MDS) 3.0 for Nursing Homes and Swing Bed Providers. 2022. [updated 10/12/2022. Available from: https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/nursinghomequalityinits/nhqimds30.
Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale: "The drift busters.". International Journal of Methods in Psychiatric Research. 1993;3:221-44.
Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44(3):M77-84.
Moniz Cook ED, Swift K, James I, Malouf R, De Vugt M, Verhey F. Functional analysis-based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012(2):Cd006929.
Gerlach LB, Kales HC. Managing Behavioral and Psychological Symptoms of Dementia. Clin Geriatr Med. 2020;36(2):315–27.
Kales HC, Kern V, Kim HM, Blazek MC. Moving Evidence-Informed Assessment and Management of Behavioral and Psychological Symptoms of Dementia into the Real World: Training Family and Staff Caregivers in the DICE Approach. Am J Geriatr Psychiatry. 2020;28(12):1248–55.
Spector A, Orrell M. Using a biopsychosocial model of dementia as a tool to guide clinical practice. Int Psychogeriatr. 2010;22(6):957–65.
Pickering CEZ, Yefimova M, Wang D, Maxwell CD, Jablonski R. Dynamic structural equation modelling evaluating the progressively lowered stress threshold as an explanation for behavioural symptoms of dementia. J Adv Nurs. 2022;78(8):2448–59.
Cohen-Mansfield J, Dakheel-Ali M, Marx MS, Thein K, Regier NG. Which unmet needs contribute to behavior problems in persons with advanced dementia? Psychiatry Res. 2015;228(1):59–64.
Abraha I, Rimland JM, Trotta FM, Dell’Aquila G, Cruz-Jentoft A, Petrovic M, et al. Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series BMJ Open. 2017;7(3): e012759.
Scales K, Zimmerman S, Miller SJ. Evidence-Based Nonpharmacological Practices to Address Behavioral and Psychological Symptoms of Dementia. Gerontologist. 2018;58(suppl_1):S88-s102.
Lam HL, Li WTV, Laher I, Wong RY. Effects of Music Therapy on Patients with Dementia-A Systematic Review. Geriatrics (Basel). 2020;5(4).
Sheikh AB, Javed N, Leyba K, Khair AH, Ijaz Z, Dar AA, et al. Pet-Assisted Therapy for Delirium and Agitation in Hospitalized Patients with Neurocognitive Impairment: A Review of Literature. Geriatrics (Basel). 2021;6(4).
Leng M, Liu P, Zhang P, Hu M, Zhou H, Li G, et al. Pet robot intervention for people with dementia: A systematic review and meta-analysis of randomized controlled trials. Psychiatry Res. 2019;271:516–25.
Ball EL, Owen-Booth B, Gray A, Shenkin SD, Hewitt J, McCleery J. Aromatherapy for dementia. Cochrane Database Sys Revs. 2020;(8).
Abraha I, Rimland JM, Lozano‐Montoya I, Dell'Aquila G, Vélez‐Díaz‐Pallarés M, Trotta FM, et al. Simulated presence therapy for dementia. Cochrane Database Sys Revs. 2017;(4).
Kouloutbani K, Venetsanou F, Markati A, Karteroliotis KE, Politis A. The effectiveness of physical exercise interventions in the management of neuropsychiatric symptoms in dementia patients: a systematic review. Int Psychogeriatr. 2022;34(2):177–90.
Onega LL, Pierce TW. Use of bright light therapy for older adults with dementia. BJPsych Advances. 2020;26(4):221–8.
Cusic E, Hoppe M, Sultenfuss M, Jacobs K, Holler H, Obembe A. Multisensory Environments for Outcomes of Occupational Engagement in Dementia: A Systematic Review. Physical & Occupational Therapy In Geriatrics. 2022;40(3):275–94.
Liu Y-C, Liao C-N, Song C-Y. Effects of manual massage given by family caregivers for patients with dementia: A preliminary investigation. Geriatric Nursing. 2022;46:112–7.
Senderovich H, Gardner S, Berall A, Shultz R, Grant B, Santaguida V. Therapeutic Touch in the Management of Responsive Behaviors in Patients with Dementia. Dementia and Geriatric Cognitive Disorders. 2022;51(2):142–9.
Oliveira AM, Radanovic M, Mello PCH, Buchain PC, Vizzotto ADB, Harder J, et al. Adjunctive Therapy to Manage Neuropsychiatric Symptoms in Moderate and Severe Dementia: Randomized Clinical Trial Using an Outpatient Version of Tailored Activity Program. J Alzheimers Dis. 2021;83(1):475–86.
Elliott AF, Burgio LD, Decoster J. Enhancing caregiver health: findings from the resources for enhancing Alzheimer’s caregiver health II intervention. J Am Geriatr Soc. 2010;58(1):30–7.
•• Alzheimer's Association. Help and Support. 2023. Available from: https://www.alz.org/help-support. The Alzheimer's Association is an invaluable resource for patients with dementia and their families, including informative materials on their website, sponsorship of caregiver trainings and conferences, a network of support groups, opportunities for advocacy, and support of research.
Logan B. Caregiver’s Guide to Understanding Dementia Behaviors: Family Caregiver Alliance; 2016. Available from: https://www.caregiver.org/resource/caregivers-guide-understanding-dementia-behaviors/.
Administration on Aging. Area Agencies on Aging. Available from: https://eldercare.acl.gov/Public/About/Aging_Network/AAA.aspx.
Vandepitte S, Van Den Noortgate N, Putman K, Verhaeghe S, Verdonck C, Annemans L. Effectiveness of respite care in supporting informal caregivers of persons with dementia: a systematic review. Int J Geriatr Psychiatry. 2016;31(12):1277–88.
Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525–38.
Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group J Clin Psychiatry. 1999;60(2):107–15.
Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, Tamura RN, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry. 2000;57(10):968-76.
Streim JE, Porsteinsson AP, Breder CD, Swanink R, Marcus R, McQuade R, et al. A randomized, double-blind, placebo-controlled study of aripiprazole for the treatment of psychosis in nursing home patients with Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(7):537–50.
Zhong KX, Tariot PN, Mintzer J, Minkwitz MC, Devine NA. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res. 2007;4(1):81–93.
•• Grossberg GT, Kohegyi E, Mergel V, Josiassen MK, Meulien D, Hobart M, et al. Efficacy and Safety of Brexpiprazole for the Treatment of Agitation in Alzheimer's Dementia: Two 12-Week, Randomized, Double-Blind, Placebo-Controlled Trials. Am J Geriatr Psychiatry. 2020;28(4):383-400. This publication describes 2 Phase III trials of brexpiprazole for the treatment of agitation in Alzheimer's Dementia, revealing mixed results but a signal for efficacy at higher doses.
Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957–70.
Kales HC, Kim HM, Zivin K, Valenstein M, Seyfried LS, Chiang C, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71–9.
Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438–45.
FDA. FDA approves first drug to treat agitation symptoms associated with dementia due to Alzheimer’s disease. May 11, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-agitation-symptomsassociated-dementia-due-alzheimers-disease.
Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–91.
Schneider LS, Frangakis C, Drye LT, Devanand DP, Marano CM, Mintzer J, et al. Heterogeneity of Treatment Response to Citalopram for Patients With Alzheimer’s Disease With Aggression or Agitation: The CitAD Randomized Clinical Trial. Am J Psychiatry. 2016;173(5):465–72.
Ehrhardt S, Porsteinsson AP, Munro CA, Rosenberg PB, Pollock BG, Devanand DP, et al. Escitalopram for agitation in Alzheimer’s disease (S-CitAD): Methods and design of an investigator-initiated, randomized, controlled, multicenter clinical trial. Alzheimers Dement. 2019;15(11):1427–36.
Seitz DP, Adunuri N, Gill SS, Gruneir A, Herrmann N, Rochon P. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011(2):Cd008191.
Gauthier S, Loft H, Cummings J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer’s disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry. 2008;23(5):537–45.
Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry. 2008;69(3):341–8.
Fox C, Crugel M, Maidment I, Auestad BH, Coulton S, Treloar A, et al. Efficacy of memantine for agitation in Alzheimer’s dementia: a randomised double-blind placebo controlled trial. PLoS One. 2012;7(5): e35185.
Herrmann N, Gauthier S, Boneva N, Lemming OM. A randomized, double-blind, placebo-controlled trial of memantine in a behaviorally enriched sample of patients with moderate-to-severe Alzheimer’s disease. Int Psychogeriatr. 2013;25(6):919–27.
Howard RJ, Juszczak E, Ballard CG, Bentham P, Brown RG, Bullock R, et al. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med. 2007;357(14):1382–92.
Herrmann N, Lanctôt KL, Rothenburg LS, Eryavec G. A placebo-controlled trial of valproate for agitation and aggression in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23(2):116–9.
Porsteinsson AP. Divalproex sodium for the treatment of behavioural problems associated with dementia in the elderly. Drugs Aging. 2006;23(11):877–86.
Tariot PN, Raman R, Jakimovich L, Schneider L, Porsteinsson A, Thomas R, et al. Divalproex sodium in nursing home residents with possible or probable Alzheimer Disease complicated by agitation: a randomized, controlled trial. Am J Geriatr Psychiatry. 2005;13(11):942–9.
Tariot PN, Erb R, Leibovici A, Podgorski CA, Cox C, Asnis J, et al. Carbamazepine treatment of agitation in nursing home patients with dementia: a preliminary study. J Am Geriatr Soc. 1994;42(11):1160–6.
Tariot PN, Erb R, Podgorski CA, Cox C, Patel S, Jakimovich L, et al. Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry. 1998;155(1):54–61.
Supasitthumrong T, Bolea-Alamanac BM, Asmer S, Woo VL, Abdool PS, Davies SJC. Gabapentin and pregabalin to treat aggressivity in dementia: a systematic review and illustrative case report. Br J Clin Pharmacol. 2019;85(4):690–703.
Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26(2):467–78.
Elrod R, Peskind ER, DiGiacomo L, Brodkin KI, Veith RC, Raskind MA. Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatry. 1997;154(1):25–30.
Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: evidence of compensatory changes. Neuroscience. 2007;146(1):471–80.
Sharp SI, Ballard CG, Chen CP, Francis PT. Aggressive behavior and neuroleptic medication are associated with increased number of alpha1-adrenoceptors in patients with Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(5):435–7.
Peskind ER, Wingerson D, Murray S, Pascualy M, Dobie DJ, Le Corre P, et al. Effects of Alzheimer’s disease and normal aging on cerebrospinal fluid norepinephrine responses to yohimbine and clonidine. Arch Gen Psychiatry. 1995;52(9):774–82.
Wang LY, Shofer JB, Rohde K, Hart KL, Hoff DJ, McFall YH, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009;17(9):744–51.
Herrmann N, Ruthirakuhan M, Gallagher D, Verhoeff N, Kiss A, Black SE, et al. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. Am J Geriatr Psychiatry. 2019;27(11):1161–73.
Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharre DW, et al. Effect of Dextromethorphan-Quinidine on Agitation in Patients With Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA. 2015;314(12):1242–54.
Markota M, Rummans TA, Bostwick JM, Lapid MI. Benzodiazepine Use in Older Adults: Dangers, Management, and Alternative Therapies. Mayo Clin Proc. 2016;91(11):1632–9.
Davies SJ, Burhan AM, Kim D, Gerretsen P, Graff-Guerrero A, Woo VL, et al. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509–23.
•• Chen A, Copeli F, Metzger E, Cloutier A, Osser DN. The Psychopharmacology Algorithm Project at the Harvard South Shore Program: An update on management of behavioral and psychological symptoms in dementia. Psychiatry Res. 2021;295:113641. The Psychopharmacology Algorithm Project at Harvard South Shore published this set of algorithms providing guidance around medication selection for dementia-related agitation, based on symptom severity. This guidance can be helpful for practitioners uncertain how to apply the mixed evidence-base around medication efficacy and side effect risk.
Devanand DP, Mintzer J, Schultz SK, Andrews HF, Sultzer DL, de la Pena D, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497–507.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Competing Interests
No funding was received to assist with the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L.Y. Assessment and Management of Dementia-Related Agitation. Curr Geri Rep 12, 103–111 (2023). https://doi.org/10.1007/s13670-023-00388-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-023-00388-2