Abstract
Polyamines (PAs) have been implicated in fruit ripening where they antagonize the action of ethylene: a ripening inducing phytohormone. S-adenosylmethionine decarboxylase (SAMDC) is a key enzyme involved biosynthesis of higher PAs- spermidine and spermine. Here, we report the genetic modification of tomato fruit ripening and quality by over-expressing human-SAMDC driven by fruit-specific promoter (2A11). The PA analysis of ripening fruits from these transgenics showed elevated PA levels in comparison to wild-type (WT). The increased levels of higher PAs are correlated with the accumulation of heterologous SAMDC transcripts in such fruits. Transgenic fruits exhibited reduced levels of ethylene (~50 %) production, ~10 days delay in on-vine ripening and extended post-harvest storage of ~11 days as compared to the WT fruits. As a result, these fruits showed improvement in various ripening traits like enhanced lycopene, vitamin C and total soluble solid. In Lesam fruits, an up-regulated expression of SlySAMDC, SlyEXP1, SlyTBG4, SlyDXS 1 and SlyPSY 1 was observed, while ethylene biosynthesis genes were down-regulated. Here, we have demonstrated the important role of PAs in altering the molecular and biochemical processes underlying fruit ripening by interfering with the ethylene biosynthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato fruit ripening being climacteric in nature is majorly governed by ethylene. This crop is prone to various post-harvest damages which cost up to 40 % yield loss. Excessive softening and over-ripening are the major causal factors for such losses. Efforts have been made to develop crop plants with reduced post-harvest losses by either delaying the ripening process by down-regulating ethylene metabolism or reducing fruit softening by manipulating the cell wall/membrane metabolism. In order to improve the processing attributes of tomatoes, scientists have attempted to reduce the activity of cell-wall degrading enzymes such as cellulase, polygalacturonase (PG), and pectinesterase (PE) through genetic modification (Sheehy et al. 1988; Phan et al. 2007; Meli et al. 2010). Inhibition of membrane lipid catabolism is another alternative for delaying senescence, improving shelf life and quality in ripening fruits (Pinhero et al. 2003). But, all these technologies did not achieve pace, as multigene families govern these enzymes and the isoform of the suppressed enzyme can take up its function. Similarly, by manipulation of ethylene pathway, ripening was delayed but the overall fruit quality was affected (Xiong et al. 2005). These fruits failed to ripen and were inferior in quality with respect to the color, aroma and taste as these characteristics are developed by gradual changes in the metabolism in ripening fruits (Theologis 1992; Hackett et al. 2000). Thus one of the alternate strategies seems to be utilizing the metabolic interactions of pathways related to post-harvest characteristics such as those of polyamines (PAs) and ethylene. The choice of PAs in improving the post-harvest quality of fruits is because of the functional and metabolic intersection of the two biosynthetic pathways. Indeed, the engineering of PA metabolism was considered as an alternate strategy for post-harvest biotechnology (Mehta et al. 2002; Nambeesan et al. 2010).
PAs: putrescine (Put), spermidine (Spd), and spermine (Spm) are low molecular weight, polycationic compounds that affect large number of developmental and physiological responses in a number of organisms, including plants (Kusano et al. 2008; Schwartz et al. 2011). Free PAs are known to act as anti-senescence agents and also influence early fruit development and ripening (Valero et al. 2002). In tomato, both ornithine decarboxylase (ODC) and arginine decarboxylase (ADC) pathways for Put biosynthesis are active (Adiga and Prasad 1985). Put is either synthesized directly from ornithine via the action of ornithine decarboxylase (ODC) or indirectly from arginine via arginine decarboxylase (ADC). Higher PAs (Spd and Spm) are synthesized by the addition of aminopropyl group to Put with the help of biosynthetic enzymes Spd synthase (SPDSYN) and Spm synthase (SPMSYN) respectively. The required aminopropyl groups are provided by the decarboxylated S-adenosylmethionine (dcSAM), a product of SAM decarboxylation (Evans and Malmberg 1989; Tiburcio et al. 1990). SAMDC (SAM decarboxylase) is the enzyme involved in this decarboxylation reaction of SAM. SAM has been established as a common precursor for both PA and ethylene biosynthesis pathways. Numerous physiological effects of ethylene in plants seem to be antagonized by PA treatment (Parra-Lobato and Gomez-Jimenez 2011). PAs along with salicylic acid, an inhibitor of wound-responsive genes in tomato, have been suggested to regulate ethylene biosynthesis at the level of ACC synthase transcript accumulation (Li et al. 1992). The exogenous application of PAs has been demonstrated to delay fruit ripening, impede color change, increase fruit firmness, prolong the shelf life, improve quality characteristics of fruits, increase the fruit size and yield, and also reduced ethylene and respiration rate emissions, induced mechanical stress resistance, and reduced chilling symptoms (Martýnez-Romero et al. 2002). Tomato transgenic lines over-expressing yeast SAMDC showed increase in Spd and Spm along with enhanced lycopene and ethylene levels and better fruit juice viscosity in tomato fruit (Mehta et al. 2002). Similar results were obtained with over-expression of yeast Spd synthase (ySPDSYN) in tomato (Nambeesan et al. 2010). The increased PAs in both these transgenics seem to surpass the senescence effects of higher ethylene levels. Thus, PAs and ethylene while competing for SAM as a common substrate, influence plant growth and senescence antagonistically (Kumar and Rajam 2004).
Apart from their role in improvement of fruit characteristics PA fortification in fruit also make it more nutritious since PAs when supplemented in diets increases feeding efficiency and weight by countering the effect of anti-nutritional factors (Sousadias and Smith 1995; Bardocz et al. 1998).
Thus, it is evident that PA-ethylene nexus plays a crucial role in fruit ripening. Looking at the multifunctional and regulatory aspects of PA and ethylene, it is possible that controlled manipulation of such a key regulator rather than a structural or a regulatory gene operating in a single branch of the biosynthesis pathway would have the advantage of allowing the ripening to occur in a near normal way and may result in better improvement of fruit shelf life and quality traits. By over-expressing PA biosynthesis genes the SAM flux can be diverted towards the PA synthesis, which in turn would retard ethylene biosynthesis. Moreover, this will also provide insight into the metabolic involvement of PAs during fruit development and ripening. Therefore, the present study was undertaken to improve the tomato fruit characteristics by expressing human-SAMDC under the control of fruit-specific promoter (2A11) and also an attempt was made to determine the molecular basis for improved fruit characteristics.
Materials and methods
Plasmid constructs and plant material
SAMDC gene from human source was chosen for the present study as it was accessible during the initiation of this work. The 4.0 kb 2A11 promoter was excised from pGEM-T-2A11 (provided by Dr. V. S. Reddy, ICGEB, New Delhi) by digesting with SmaI and EcoRI and was ligated into the pBinAR, replacing CaMV 35S promoter. Plasmid pGEM3Z-f-human SAMDC (provided by Prof. Tony Pegg, Hershey Medical Center, Hershey, USA), was digested with SacI and XbaI enzymes to release 1.2 kb h-SAMDC gene fragment. This fragment was end filled with klenow fragment (Fermentas, Canada) and was subsequently ligated into the SmaI digested pBin2A11 vector in the sense orientation with respect to the promoter. In addition, the pBin-T-DNA contained the neomycin phosphotransferase II (NPT-II) gene from the Tn5 transposon of E. coli (K12), under the control of the nos promoter from Agrobacterium tumefaciens (Fig. 1). The seeds of tomato (Solanum lycopersicum Mill. cv. Pusa Ruby) were procured from National Seeds Corporation, New Delhi. Fully expanded cotyledons from 10 to 12 days old tomato seedlings grown in pots containing garden soil and vermiculite (1:1) under controlled growth conditions (26 ± 1 °C, 16 h photoperiod with irradiance of 40 μE mol m−2 s−1) were utilized for transformation experiments.
Tomato transformation
The A. tumefaciens (LBA4404) mediated tomato transformation with pBIN-SAMDC binary vector was performed to develop tomato transformants according to a protocol developed by Madhulatha et al. (2007). The expression of NPTII activity was used as a selectable trait to screen transformed plants. Transformed and wild-type (WT) regenerants, with well-developed roots were then transferred to transgenic green-house for further studies.
Transgene integration and expression analysis of tomato transformants
Genomic DNA was isolated from the leaves of WT and tomato transformants using CTAB method (Doyle and Doyle 1990). Utilizing 100 ng of genomic DNA, the transformants were analyzed by PCR for the presence of the transgene. The primer pairs used for the amplification of 750 bp fragment of NPT-II gene at 59 °C annealing, were 5’-TCAGAAGAACTCGTCAAGAA-3’ and 5’-ATGGGGATTGAACAAGATGG-3’ and for 570 bp amplicon of h-SAMDC gene at 53 °C annealing, were 5’-GCTGCACATTTTTTCGAAG-3’ and 5’-AGGTTTGATCTGGCTGAC-3’.
Genomic DNA (10 μg), restricted with XbaI enzyme was subjected to Southern hybridization using radiolabelled h-SAMDC probe. The probe was made using nick translation kit according to manufacturer’s guidelines (Bangalore Genei, India). Blots were prepared using the standard protocol (Sambrook et al. 1989), using nylon membrane (Hybond N, Pharmacia). Pre-hybridization and hybridization were carried out according to Sambrook et al. (1989).
Each of the ~100 mg of Leaf, flower bud (FB), Pericarp tissue from mature green (MG), breaker (BR), pink red (PR) and red ripe (RR) fruits was pulverized to homogenate powder. The homogenate was used for isolation of total RNA with the TriZol reagent following manufacturer’s guidelines (Invitrogen, USA) with subsequent RNase free DNase (Fermentas, Canada) treatment. Semi-Quantitative RT-PCR was performed to check the transcript level of the transgene and various other genes involved in fruit ripening. DNA-free RNA (200 ng) was used for the one-step RT-PCR reaction as specified in the kit manual (Taurus-Scientific, India). The reaction conditions were as follow: 45 °C for 50 min, followed by 94 °C for 5 min, n cycles of 94 °C for 30 s, annealing temperature for 30 s and 72 °C for 30 s and finally 72 °C for 15 min. The products were analyzed on ethidium bromide stained 1.5 % agarose gel. The reaction conditions and probes are detailed in Table S1. All semi-quantitative-RT-PCR experiments were carried out thrice. In each independent experiment, the pulverized tissue of three fruits from same plant was pooled to get 100 ng RNA.
Transgene segregation analysis
The leaves from T1, T2 and T3 progenies (40 each) of transgenic lines along with few WT plants were utilized for segregation analysis studies. Segregation pattern was confirmed by PCR in all the 40 progenies from T1, T2 and T3 generations using transgene specific primers.
Polyamine analysis
PAs were estimated by TLC method with 100 mg of tissues each from leaf, FB and pericarp of the MG, BR, PR and RR fruits. Tissue was homogenized in 1 mL of 10 % perchloric acid. The resultant extract was fractionated, dansylated, chromatographed and quantified as described by Bajaj and Rajam (1996) using dual wavelength fluorometer (Bio-Rad, VersaFluor, USA) with an excitation wavelength of 350 nm and emission wavelength of 495 nm. The PA content was estimated in three independent experiments.
Ethylene estimation
For the determination of ethylene levels, three RR fruits (pre-weighed) were enclosed in 100 mL of air-tight container for about 1 h at room temperature. 3 mL of the headspace atmosphere of the container was withdrawn with a syringe and injected into a gas chromatograph (Model HP 5890, Hewlett Packard, USA) (Singh and Pal 2008). The ethylene estimation was carried out in ten independent experiments with three experimental replicates.
Estimation of respiration rate
RR fruits (pre-weighed) were sealed in an air-tight container for 1 h and their respiration rate was determined by head space gas analysis technique using CO2/O2 analyzer (Model Checkmate 9900 O2/CO2, PBI Dansensor, Denmark). The respiratory activity was measured in ten independent experiments with nine experimental replicates.
On-vine ripening period determination
Flowers were tagged at anthesis and the days were noted for the fruit formation. The MG fruits displaying first sign of colour change were identified as BR stage. Average days for BR to reach RR were noted to determine the on-vine ripening period. The on-vine ripening period was measured for nine fruits as biological replicates each in ten independent sets, in three generations.
Determination of fruit shelf life
The RR fruits were kept at room temperature and the time was noted for the first visual sign of shriveling. The shelf life of fruits in three generations was noted for nine fruits each in ten independent sets.
Determination of physiological loss of water (PLW)
PLW in RR fruits during storage at room temperature was estimated by subtracting the sample weights from their previous recorded weights and was represented as % PLW compared to initial weight. The data was recorded over three generations for nine biological replicates each in ten independent sets.
Total soluble solids (TSS)
Tomato fruit (RR) homogenate was utilized for measuring TSS content by a hand refractrometer (Model: Fisher, Japan). Results were expressed as oBrix and were corrected to 20 °C temperature. TSS content was measured in nine replicates in ten independent experiments.
Determination of sugars
To determine the sugars in tomato fruits, the method described by Hortwitz (1960) was followed. Sugar content was measured in nine fruits in three independent experiments.
Estimation of ascorbic acid (AsA) content
AsA content in the red fruits was estimated titrimetrically using 2, 6-dichlorophenol indophenol as the indicator dye (Singh and Pal 2008). AsA standard was prepared by dissolving 100 mg of L-ascorbic acid in 100 mL of 1 % HPO3 (Singh and Pal 2008). AsA content was measured in nine fruits in three independent experiments.
Quantification of lycopene content
Lycopene fraction of RR fruit pericarp tissue (~2 g) was determined by spectrophotometric method as described by AOAC (2000). Lycopene fractions were estimated in nine fruits in three independent experiments.
Data collection and analysis
For each of the experiment, data from three consecutive generations, with their replicates was collected. The data presented are average (mean) with the standard error from all the experiments. Significant differences were determined by Student’s T-test (p < 0.05).
Results and discussion
Generation of homozygous lines of transgenic tomato plants over-expressing h-SAMDC
Several tomato transformants over-expressing h-SAMDC gene under the control of 2A11-fruit specific promoter were generated. Transgene integration in the transformants was confirmed by PCR with NPT-II and h-SAMDC gene specific primers, while Southern hybridization detailed the transgene copy number (supplementary Fig. 1a, 1b, 1c). Transgene expression in transgenic plants is correlated with copy number (Matzke et al. 1994) and the site of transgene integration (Hobbs et al. 1990). Therefore, several tomato transformants for h-SAMDC over-expression were analyzed for its segregation pattern in three generations. The results revealed Lesam16, Lesam24 and Lesam56 transgenic lines to be homozygous for h-SAMDC integration (supplementary Fig. 1d). To negate the differential expression due to variable copy number, the homozygous lines were proceeded for further analysis.
Stable expression of transgene during fruit ripening
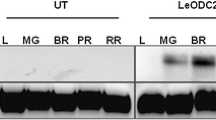
The transgene transcript levels were examined in the T3 fruits from transgenics and WT plants. The stability of transgene expression in the developed transgenics was depicted by the specific presence of h-SAMDC transcripts in transgenic tomato fruits (Fig. 2a). The transgene was found to be exclusively expressed across MG to RR stages with higher expression in BR and PR over MG and RR stage. Such trend in expression was concomitant with the onset of upstream promoter i.e., 2A11 activity. This promoter has been very well characterized from tomato plant where the 2A11 gene expresses strictly in a fruit-specific manner (Pear et al. 1989).
Fruit specific transgene expression in T3 generation of Lesam24 fruits. a Semi-quantitative RT-PCR analysis showing stable expression of human-SAMDC at different stages of fruit ripening in Lesam24; b Semi-quantitative RT-PCR analysis for ACTIN gene as an internal control, L-1 Kb ladder; LF-Leaf; FB-Flower bud; IG-Immature green; MG-Mature green; BR-Breaker; PR-Pink red; RR-Red ripe
Elevated polyamine levels
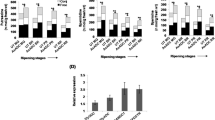
Accumulation of h-SAMDC transcripts influenced the levels of PAs in transgenic fruits. PA titers were significantly higher in the transgenic tomatoes as compared to WT. In WT, the PAs titers, mainly Put levels were higher in the FB followed by MG and BR fruits, and thereafter declined in the later stages of ripening, yet these levels were significantly higher in transgenic line over WT. As expected with the over expression of h-SAMDC, significant increase was observed in the Spd followed by Spm and then Put in the Lesam fruits (Fig. 3). The PA analysis in the transgenic lines revealed increase in all three fractions of Put, Spd and Spm. Put showed about 1.1–2.2 fold increase in the free, 1.1–3.4 fold increase in the conjugated and 1.1–2.3 fold increase in the bound fractions. Free Spd fraction showed ~3 fold increase, whereas the conjugated and bound fractions showed ~1.2–4.1 and 1.1–3 fold increase respectively. Transgenic fruits exhibited ~1.1–3.0 fold increase in free Spm, conjugated fraction ~1.2–3.9 and ~1.1–3 fold increase in bound Spm over WT fruits (Fig. 5a).
PA accumulation in Lesam24 fruits over WT fruits. a Put levels; b Spd levels; c Spm levels; d total PA levels, Bars represent the standard errors of three independent experiments. Asterisk, dagger and double dagger denote significant differences in free, conjugated and bound fractions respectively from WT at (P < 0.05)
However, no significant change in mRNA titers of endogenous genes viz., ODC, ADC, SAMDC and SPDSYN was seen in transgenic fruits over WT (Fig. 7). Also the expression patterns of these genes were observed to be steady during all the stages which implies the possibility of post-translational or metabolic regulation of PA concentration in Lesam transgenics. The increase in Put in the transgenic lines over-expressing h-SAMDC could be attributed to enhanced inter-conversion of Spd/Spm into Put through the acetylation mechanism. The PA acetylating enzyme, Spd/Spm N1-acetyltransferase (SSAT) has the potential to participate in PA pool homeostasis by lowering intracellular PA levels. SSAT can actively respond to PA excess by acetylating Spd and Spm and thereby facilitating their excretion out of the cell and/or promoting their back-conversion to Put which can be catabolized by various Put-directed oxidases (Seiler 2004).
This result is consistent with the work done on different crop plants such as tobacco (Waie and Rajam 2003), rice (Kumria and Rajam 2002) and sweet potato (Kasukabe et al. 2006) where over-expression of different PA genes under constitutive or tissue-specific promoters have shown an increase in the PA metabolism. Similar observations were noted in the transgenic tomato plants over-expressing heterologous ODC, ADC and SPDS genes under the control of fruit-specific promoter, 2A11, developed in our Lab (Unpublished data). All these three-types of transgenics showed an up-regulation of PA metabolism with increase seen in all the three PAs.
Reduced ethylene levels in transgenic fruits
Ethylene has been well established as a ripening inducing hormone while it coordinates the fruit maturation and ripening processes by modulating various ripening specific pathways. In the present study transgenic tomato fruits over-expressing h-SAMDC evolved ~50 % less ethylene as compared to WT fruits (Fig. 4). Further there was a significant dip in transcript titers of ethylene biosynthesis genes viz., fruit specific homologues of ACS and ACO in Lesam fruits specifically from BR to RR stages (Fig. 7). The lower accumulation of ethylene biosynthesis gene transcripts in transgenic fruits in comparison to the WT fruits has lead to decreased evolution of ethylene in transgenic fruits. Numerous reports advocate negative correlation between PA levels and ACS and ACO expression. PAs have been suggested to regulate ethylene biosynthesis at the level of ACS transcript accumulation (Li et al. 1992; Handa and Mattoo 2010). Also, PAs and ethylene intersect biosynthetically at a common point for precursor, SAM (Winer and Apelbaum 1986). Therefore, it would not be irrational to argue that the synthesis of PAs and ethylene in Lesam tomatoes may be competitive and increased PA biosynthesis has caused a dip in ethylene levels, thereby strongly supporting that SAM could be a rate limiting factor for both the pathways. The same is supported by the use of PA inhibitors (Kushad and Dumbroff 1991; Parra-Lobato and Gomez-Jimenez 2011). On the contrary Mehta et al. (2002) reported elevated ethylene levels in the tomato transgenic over-expressing yeast SAMDC, suggesting the co-existence of these two biosynthetic pathways.
Delayed fruit ripening and enhanced shelf life
The pooled data of three generations revealed that the over expression of h-SAMDC in Lesam tomatoes resulted in decreased rate of respiration and reduced physiological loss of water (PLW). A significant decline in CO2 evolution of around 20–30 % was noted in transgenic fruits over WT (Fig. 5a). Results on PLW showed almost similar trends of reduced PLW percentage among the transgenic tomatoes over WT fruits (Fig. 5b). On-vine ripening period taken for MG fruit to reach BR stage and BR to RR showed minimum of 10 days delay as compared to the WT (Fig. 5d). WT red fruits stored at room temperature rotted after 8–10 days whereas the Lesam red fruits had a prolonged shelf life of about 11 days over WT (Fig. 5c). The reduced respiratory activity and PLW possibly explains the delayed on-vine and off-vine ripening period accompanied by enhanced shelf life in transgenic tomato fruits over WT fruits. The enhanced shelf life can also be attributed to low levels of ethylene in transgenic tomato fruits since the rate of ethylene evolution of harvested tomato fruit is negatively correlated with its shelf life (Guillén et al. 2007). Similarly, in many fruits the pre- and post-harvest applications of Put have shown to reduce their respiration rate by suppressing the climacteric ethylene production thereby, delaying the ripening process (Khan et al. 2008; Torrigiani et al. 2012). These results thus establish that increase in PA decrease the fruit ripening rate.
Storage attributes of transgenic tomato. a Reduced rate of respiration in transgenic fruits; b Decreased PLW in transgenic fruits over WT fruits kept at room temperature; c Enhanced shelf life of Lesam transgenic fruits as compared to WT fruits at room temperature; d Transgenic fruits exhibiting delayed fruit ripening (noted as days taken to reach RR stage from BR) in comparison with WT fruits. Bars represent the standard errors of measurements in nine fruits each for ten independent sets carried for three generations. Asterisk values that were determined by the t-test to be significantly different (P < 0.05) from WT
The integrity of cell membrane and cell wall is a major factor determining processing characteristics, firmness, and shelf life of tomato fruits. During ripening, cell wall undergoes substantial disassembly. Expansins (EXP) have been shown to play an important role in fruit softening (Rose et al. 1997), where they are involved in cell wall degradation and simultaneously increasing the accessibility of other cell wall modifying proteins such as polygalaturonase (PG) to cell wall polymers (Rose and Bennett 1999). β-galactosidase (TBG) is reported to be associated with ripening related firmness loss (Smith et al. 2002). Expression analysis of both EXP1 and TBG4 revealed elevated transcript levels, at PR and RR stages of fruit development in Lesam fruit over WT fruits (Fig. 7). On the other hand, PG which is involved in pectin metabolism and thus has been associated with cell wall break down, fruit softening and loss of tissue integrity, showed no difference in transcript abundance at any of the ripening stage in Lesam tomato from WT fruits (Fig. 7). Although our findings on expression of cell wall loosening genes are in conflict with the results of enhanced shelf life, increased PA content in the Lesam fruits seems to justify the enhanced shelf life possibly by stabilizing the membranes. PAs have been reported as powerful modulator of supra-molecular conformation of pectin (Messiaen et al. 1997). PAs can also bind covalently with cell wall and inhibition of PA biosynthesis interferes with the cell wall formation making it amorphous and its exogenous application reverses the changes (Berta et al. 1997). Comparable results for delayed fruit softening has been reported in PA treated peach (Ziosi et al. 2006) and plum fruits (Khan et al. 2008).
Enhanced lycopene content
Lesam fruits are noticeably deeper red in color than the WT fruits. This can be associated with the phytonutrient lycopene in the fruits. The study of the lycopene content of the transgenic showed its significant higher level ranging from 10 to 40 % in transgenic fruits over WT (Fig. 6a). Lesam56 was found to be significantly superior followed by Lesam24 and Lesam16 transgenic lines with 22–40 % increase in lycopene content. Expression pattern of lycopene biosynthesis genes viz., DXS1 and PSY1 revealed higher mRNA titres throughout fruit ripening viz., MG, BR, PR and RR while LES which is involved in catabolism of lycopene (Ronen et al. 1999) did not show any significant difference from WT (Fig. 7). The up-regulated expression of these transcripts thus accounts for the enhanced lycopene content in transgenic fruits over WT fruits. The elevated levels of lycopene biosynthesis gene transcripts in Lesam tomatoes with increased PAs, suggest the role of PAs in regulation or at least stabilization of mRNA turnover of lycopene biosynthesis genes. In has been studied that reduced degradation of membrane phospholipids in fruits tend to reduce the turnover of phospholipids and free fatty acids. Since fatty acid biosynthesis and carotenoid biosynthesis share the common precursor (Acetyl Coenzyme A), reduced demand for acetyl CoA for fatty acid biosynthesis in transgenic fruits may tend to enhance their availability for carotene and lycopene biosynthesis (Oke et al. 2003). This could also explain the enhanced lycopene in Lesam transgenic fruits. Both these arguments are in agreement with the reports by Neily et al. (2011), in transgenic tomato over-expressing SPDSYN. Thus, increase in the PA levels appears to cause an increase in the phytonutrients in fruits.
Improvement in fruit quality traits in transgenic plants a Fruits from Lesam lines were enriched with phytonutrient lycopene, Bars represent the standard errors of measurements in nine fruits each for three independent sets carried for three generations; b Fruits of Lesam lines exhibited enhanced TSS content, Bars represent the standard errors of measurements in nine fruits each for ten independent sets carried for three generations; c Increased AsA contents in Lesam fruits over WT, Bars represent the standard errors of measurements in nine fruits each for three independent sets carried for three generations; d Accumulation of total sugars in Lesam fruits over WT, Bars represent the standard errors of measurements in nine fruits each for three independent sets carried for three generations. Asterisk values that were determined by the t-test to be significantly different (P < 0.05) from WT
Improved fruit quality
Total soluble solid (TSS) content as depicted by ratio of total sugars v/s organic acid, is the key parameter of the tomato fruit quality. Sugars and acids are responsible for the taste of fresh tomatoes and play an important role in determining the overall sensory quality of tomatoes. The Lesam transgenics exhibited up to 35 % increase in TSS with the highest of 6.62 obrix in Lesam24 as against only 5.14 obrix in WT tomatoes (Fig. 5b). The result seems to be another manifestation of enhanced PA accumulation in Lesam fruits and is supported by various other reports (Costa and Bagni 1983; Mitra and Sanyal 1990). Increase in total sugar was observed to be 1.5 times more in Lesam24 over WT (Fig. 5d). There was a considerable rise in reducing sugar percentage in Lesam16 and Lesam56 among the Lesam lines over WT (data not shown). Lesam24 fruits showed remarkably higher levels of AsA as compared to WT (Fig. 5c). In general the AsA levels decline during ripening and senescence and has been correlated with its getting used up in ethylene biosynthesis pathway while acting as a co-factor for ACO (Watada et al. 1976). Therefore, in transgenic fruits with the reduction in ethylene evolution, the accumulation in AsA is apparent. Yahia et al. (2001) have associated the endogenous concentration of Put with higher ascorbic acid in fruits. Asghar et al. (2010) demonstrated increase in ascorbic acid content on Spd application in pomegranate fruits. Thus PAs have a role in influencing AsA content with the direct mechanism still unknown.
Abbreviations
- Put:
-
Putrescine
- SAMDC:
-
S-adenosylmethionine decarboxylase
- Spd:
-
Spermidine
- Spm:
-
Spermine
References
Adiga PR, Prasad GL (1985) Biosynthesis and regulation of polyamines in higher plants. Plant Growth Regul 3:205–226
AOAC (2000) Official methods of analysis. Association of Official Analytical Chemist. EUA
Asghar R, Majid R, Manoochehr M, Kholdebarin B, Saeid E et al (2010) The ameliorative effects of spermidine and calcium chloride on chilling injury in pomegranate fruits after long-term storage. Fruits 665:169–178
Bajaj S, Rajam MV (1996) Polyamine accumulation and near loss of morphogenesis in long term Callus cultures of rice: restoration of plant regeneration by manipulation of cellular polyamine levels. Plant Physiol 112:1343–1348
Bardocz S, Grant G, Brown DS, Pusztai A (1998) Putrescine as a source of instant energy in the small intestine of the rat. Gut 42:24–28
Berta G, Altamura M, Fusconi A, Cerruti F, Capitani F, Bagni N (1997) The plant cell wall is altered by inhibition of polyamine biosynthesis. New Phytol 137:569–1146
Costa G, Bagni N (1983) Effect of polyamines on fruit set of apple. HortSci 18:59–61
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Evans PT, Malmberg RL (1989) Do polyamines have a role in plant development? Annu Rev Plant Physiol Plant Mol Biol 40:235–269
Guillén F, Castillo S, Zapata PJ, Martínez-Romero D, Serrano M, Valero D (2007) Efficacy of 1-MCP treatment in tomato fruit: 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol Technol 43:23–27
Hackett RM, Ho CW, Lin Z, Foote HC, Fray RG, Grierson D (2000) Antisense inhibition of the Nr gene restores normal ripening to the tomato Never-ripe mutant, consistent with the ethylene receptor-inhibition model. Plant Physiol 124:1079–1086
Handa AK, Mattoo AK (2010) Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol Biochem 48:540–546
Hobbs SLA, Kpodar P, DeLong CMO (1990) The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol 15:851–864
Hortwitz W (1960) Official and tentative method of analysis, 9th edn. Association of Official Agriculture Chemists, Washington, pp 314–320
Kasukabe Y, He L, Watakabe Y, Otani M, Shimada T, Tachibana S (2006) Improvement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotechnol 23:75–158
Khan AS, Singh Z, Abbasi NA, Swinny EE (2008) Pre- or postharvest applications of putrescine and low temperature storage affect fruit ripening and quality of ‘Angelino’ plum. J Sci Food Agric 88:1686–1695
Kumar SV, Rajam MV (2004) Polyamine-ethylene nexus: a potential target for post-harvest biotechnology. Indian J Biotechnol 3:299–304
Kumria R, Rajam MV (2002) Alteration in polyamine titers during Agrobacterium-mediated transformation of indica rice with ornithine decarboxylase gene affects plant regeneration potential. Plant Sci 162:769–777
Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Planta 228:367–381
Kushad MM, Dumbroff EB (1991) Metabolic and physiological relationship between the polyamine and ethylene biosynthetic pathways. In: Slocum RD, Flores HE (eds) Biochemistry and physiology of polyamines in plants. CRC Press, Boca Raton, pp 77–92
Li N, Parsons B, Liu D, Mattoo AK (1992) Accumulation of wound-inducible ACC synthase transcript in tomato fruit is inhibited by salicylic acid and polyamines. Plant Mol Biol 18:477–487
Madhulatha P, Pandey R, Hazarika P, Rajam MV (2007) High transformation frequency in Agrobacterium mediated genetic transformation of tomato by using polyamines and maltose in shoot regeneration medium. Physiol Mol Biol Plants 13:191–198
Martýnez-Romero D, Serrano M, Carbonell A, Burgos L, Riquelme F, Valero D (2002) Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J Food Sci 67:1706–1712
Matzke AJM, Neuhuber F, Park YD, Ambros PF, Matzke MA (1994) Homology-dependent gene silencing in transgenic plants: epistatic loci contain multiple copies of methylated transgenes. Mol Gen Genet 244:219–229
Mehta R, Cassol T, Li N, Ali N, Handa AK, Mattoo AK (2002) Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat Biotechnol 20:613–618
Meli VS, Ghosh S, Prabha TN, Chakraborty N, Chakraborty S, Datta A (2010) Enhancement of fruit shelf life by suppressing N-glycan processing enzymes. Proc Natl Acad Sci U S A 107(6):2413–2418
Messiaen J, Cambier P, Van Cutsem P (1997) Polyamines and pectins I. Ion exchange and selectivity. Plant Physiol 113(2):387–395
Mitra SK, Sanyal D (1990) Effect of putrescine on fruit set and fruit quality of Litchi. Gartenbauwiss 55:83–84
Nambeesan S, Datsenka T, Ferruzzi MG, Malladi A, Mattoo AK, Handa AK (2010) Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. Plant J 63:836–847
Neily MH, Matsukura C, Maucourt M, Bernillon S, Deborde C, Moing A et al (2011) Enhanced polyamine accumulation alters carotenoid metabolism at the transcriptional level in tomato fruit over-expressing spermidine synthase. J Plant Physiol 168:242–252
Oke M, Pinhero RG, Paliyath G (2003) The effects of genetic transformation of tomato with antisense phospholipase D cDNA on the quality characteristics of fruits and their processed products. Food Biotechnol 17:163–182
Parra-Lobato MC, Gomez-Jimenez MC (2011) Polyamine-induced modulation of genes involved in ethylene biosynthesis and signalling pathways and nitric oxide production during olive mature fruit abscission. J Exp Bot 62:4447–4465
Pear JR, Ridge N, Rasmussen R, Rose RE, Houck CM (1989) Isolation and characterization of a fruit-specific cDNA and the corresponding clone from tomato. Plant Mol Biol 13:639–651
Phan TD, Bo W, West G, Lycett GW, Tucker GA (2007) Silencing of the major salt-dependent isoform of pectinesterase in tomato alters fruit softening. Plant Physiol 144:1960–1967
Pinhero RG, Almquist KC, Novotna Z, Paliyath G (2003) Developmental regulation of phospholipase D in tomato fruits. Plant Physiol Biochem 41:223–240
Ronen G, Cohen M, Zamir D, Hirschberg J (1999) Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J 17:341–351
Rose JKC, Bennett AB (1999) Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends Plant Sci 4:176–183
Rose JKC, Lee HH, Bennett AB (1997) Expression of a divergent expansin gene is fruit-specific and ripening-regulated. Proc Natl Acad Sci U S A 94:5955–5960
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, New York
Schwartz CE, Wang X, Stevenson RE, Pegg AE (2011) Spermine synthase deficiency resulting in X-linked intellectual disability (Snyder-Robinson syndrome). Methods Mol Biol 720:437–445
Seiler N (2004) Catabolism of polyamines. Amino Acids 26:217–233
Sheehy RE, Kramer M, Hiatt WR (1988) Reduction of polygalacturonase activity in tomato fruit by antisense RNA. Proc Natl Acad Sci U S A 85:8805–8809
Singh S, Pal RK (2008) Response of climacteric-type guava (Psidium guajava L.) to postharvest treatment with 1-MCP. Postharvest Biol Technol 47:307–621
Smith DL, Abbott JA, Gross KC (2002) Down-regulation of tomato β-galactosidase 4 results in decreased fruit softening. Plant Physiol 129:1755–1762
Sousadias MG, Smith TK (1995) Toxicity and growth-promoting potential of spermine when fed to chicks. J Anim Sci 73:2375–2381
Theologis A (1992) One rotten apple spoils the whole bushel: the role of ethylene in fruit ripening. Cell 70:181–185
Tiburcio AF, Kaur-Sawhney R, Galston AW (1990) Polyamine metabolism. In: Miflin BJ, Lea PJ (ed) Intermedatory nitrogen metabolism.16, the biochemistry of plants. Academic Press, pp 283–325
Torrigiani P, Bressanin D, Beatriz Ruiz K, Tadiello A, Trainotti L et al (2012) Spermidine application to young developing peach fruits leads to a slowing down of ripening by impairing ripening-related ethylene and auxin metabolism and signaling. Plant Physiol 146:86–98
Valero D, Martínez-Romero D, Serrano M (2002) The role of polyamines in the improvement of the shelf life of fruit. Trends Food Sci Technol 13:228–232
Waie B, Rajam MV (2003) Effect of increased polyamine biosynthesis on stress response in transgenic tobacco by introduction of human S-adenosyl-methionine gene. Plant Sci 164:727–734
Watada AE, Aulenbach BB, Worthington JT (1976) Vitamins A and C in ripe tomatrres as aifected by’ stage of ripeness at harvest and by supplementary ethylene. J Food Sci 41:856–858
Winer L, Apelbaum A (1986) Involvement of polyamines in the development and ripening of avocado fruits. J Plant Physiol 126:223–456
Xiong AS, Yao QH, Peng RH, Li X, Han PL, Fan HQ (2005) Different effects on ACC oxidase gene silencing triggered by RNA interference in transgenic tomato. Plant Cell Rep 23:639–646
Yahia EM, Contreras-Padilla M, Gonzalez-Aguilar G (2001) Ascorbic acid content in relation to ascorbic acid oxidase and polyamine content in tomato and bell pepper fruits during development, maturation and senescence. Lebensm Wiss Technol 34:452–457
Ziosi V, Bregoli AM, Bonghi C, Fossati T, Biondi S et al (2006) Transcription of ethylene perception and biosynthesis genes is altered by putrescine, spermidine and aminoethoxyvinylglycine (AVG) during ripening in peach fruit (Prunus persica). New Phytol 172:229–238
Acknowledgments
This work was generously supported by grants from the Department of Biotechnology (Govt. of India), New Delhi (Grant Nos. BT/PR/2990/Agr/16/232/2002 and BT/PR8657/PBD/16/738/2007), University Grants Commission–Special Assistance Programme and Department of Science and Technology–FIST programme. Research fellowships to P. Madhulatha and Aarti Gupta by the Council of Scientific and Industrial Research are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Madhulatha and Aarti Gupta contributed equally
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Molecular characterization of tomato primary transformants. (a) PCR with primers specific to NPT II gene: L-1 kb ladder; PC-Plasmid DNA; NC-DNA from untransformed control; Lesam 1–70-DNA from different transgenic tomato lines, (b) PCR with SAMDC gene-specific primers: L-1 kb ladder; PC-Plasmid DNA, NC-DNA from untransformed control; Lesam 1–56-DNA from different tomato transgenic lines, (c) Southern blot analysis of Lesam transgenics for copy number using NPT II gene probe. NC-DNA from untransformed control; Lesam 1–56- DNA from different transgenic tomato lines digested with XbaI enzyme. (d) PCR analysis of Lesam T3 progenies. L-Ladder; PC-Plasmid DNA; NC-DNA from untransformed control; D.1) PCR of Lesam16 D.2) PCR of Lesam24 D.3) PCR of Lesam56. 1–12 DNA from Lesam T3 progenies. (JPEG 85 kb)
Table S1
List of primer sets used in the study (DOC 50 kb)
Rights and permissions
About this article
Cite this article
Madhulatha, P., Gupta, A., Gupta, S. et al. Fruit-specific over-expression of human S-adenosylmethionine decarboxylase gene results in polyamine accumulation and affects diverse aspects of tomato fruit development and quality. J. Plant Biochem. Biotechnol. 23, 151–160 (2014). https://doi.org/10.1007/s13562-013-0194-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-013-0194-x