Abstract
We analyze human aging—understood as health deficit accumulation—for a panel of European individuals, using four waves of the Survey of Health, Aging and Retirement in Europe (SHARE data set) and constructing a health deficit index. Results from log-linear regressions suggest that, on average, elderly European men and women develop approximately 2.5 % more health deficits from one birthday to the next. In nonlinear regressions (akin to the Gompertz-Makeham model), however, we find much greater rates of aging and large differences between men and women as well as between countries. Interestingly, these differences follow a particular regularity (akin to the compensation effect of mortality) and suggest an age at which average health deficits converge for men and women and across countries. This age, which may be associated with human life span, is estimated as 102 ± 2.6 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is defined as the intrinsic, cumulative, progressive, and deleterious loss of function (Arking 2006; Masoro 2006). In this study, we investigate human aging for a panel of European individuals, using four waves of the Survey of Health, Aging and Retirement in Europe (SHARE data set) and constructing a health deficit index for men and women aged 50–90 in 10 countries. In contrast to the related literature using the SHARE data, we apply a particular theory-led modeling, which allows us to detect regularities of human aging that have remained unnoticed in the previous literature. Specifically, we show the existence of a compensation effect of deficit accumulation (CEDA)—that is, a linear negative relationship between initial health deficits and the rate of health deficit accumulation. According to CEDA, men start out healthier than women but then develop additional health deficits at a higher rate. Similarly, the rate of health deficit accumulation is higher in countries in which individuals are, on average, initially healthier. CEDA implies convergence of group-specific aging processes such that there exists a species-specific age at which health deficits converge for men and women as well as across countries. The point estimate for this invariant of human aging, which could be conceptualized as human life span, is about 102 years.
Our analysis is inspired by two strong empirical regularities observed for human mortality: the Gompertz-Makeham law and the compensation effect of mortality. Gompertz (1825) proposed that age x and the rate of mortality μ(x) are log-linearly related. Makeham (1860) added a constant for age-unrelated mortality, providing the Gompertz-Makeham law of mortality, μ(x) = A + R exp(α · x). The parameters of this nonlinear relationship have been estimated with great precision for different human populations indexed by i and differentiated, for example, by gender and country of residence (see, e.g., Arking 2006; Olshansky and Carnes 1997). The second regularity describes a strong negative relationship between the Gompertz-parameters R i and α i , which holds across populations, such that ln(R) = ln(M) − Bα. This compensation effect of mortality (Gavrilov and Gavrilova 1991), also known as Strehler and Mildvan (1960) correlation, implies that populations with lower initial mortality (characterized by lower R i ) display a larger increase of mortality with age (characterized by higher α i ). It also means that the estimates M and B provide species-specific invariants of human mortality. To see this more clearly, substitute R to obtain the age-related mortality rate μ(x) − A = M eα(x − B), suggesting that in all populations, there exists an age B at which individuals face the same mortality rate M. The age B has been conceptualized as human life span (Gavrilov and Gavrilova 1991; Strulik and Vollmer 2013).
In this study, we show that a similar compensating law holds for human morbidity. This view is inspired by the reliability theory of human aging, which explains how complex systems consisting of nonaging elements (e.g., atoms) increasingly lose function over time, such that the failure rate—that is, the probability of expiry of the system—increases with age (Barlow and Proschan 1975). Human aging is thus conceptualized as a loss of redundancy in organ reserve over time. Gavrilov and Gavrilova (1991) derived the Gompertz law of mortality as well as the compensation effect of mortality from such a microfoundation of aging at the cellular level (see also refinements by Finkelstein 2008; Novoseltsev 2006). Because reliability theory holds also at the subsystem level (of, e.g., organs), it is easy to see why both mortality and the loss of bodily functions (i.e., health deficit accumulation) follow the Gompertz-Makeham law. In particular, CEDA is easily rationalized with reliability theory: initially healthier individuals possess greater organ redundancy such that organ reserve declines at a higher rate, leading to a faster speed of health deficit accumulation. The notion of aging as accelerated loss of organ reserve is in line with the mainstream view in medical science. For example, initially, in young adults, the functional capacity of human organs is estimated to be 10 times higher than needed for survival (Fries 1980).
Our analysis is built on Mitnitski et al. (2002a), who constructed a health deficit index (frailty index) for Canadians and estimated with nonlinear regressions the parameters of the Gompertz-Makeham structure.Footnote 1 They estimated a yearly rate of health deficits accumulation of 4.3 % for men and 3.1 % for women. Men were found to be initially healthier, a fact that can easily be explained by reliability theory: men are, on average, larger and thus have a larger organ reserve (e.g., more bone mass, more muscle mass) to wear off during aging. Moreover, Mitnitski et al. (2002a) found an intersection of the health deficit trajectories of men and women at age 95, which they associated with human life span estimated from the compensation effect of mortality. Although the particular age of intersection is certainly interesting, their study provided (strictly speaking) only weak support of the compensation effect of health deficit accumulation given that any two nonparallel trajectories have to intersect somewhere. In this study, we estimate the average lifetime trajectories of health deficit accumulation for a panel of 10 European countries and show that the compensation effect of health deficit accumulation holds across countries for both men and women. A common intersection of 20 lifetime trajectories is, of course, much harder to be dismissed as pure coincidence. In this sense, the European compensation effect of health deficit accumulation provides a first piece of evidence for a species-specific invariant of human aging.
The seminal studies on the health deficit index by Mitnitski et al. (2001, 2002a, b) instigated a significant research program with hundreds of studies applying the methodology. One reason for the success of the health deficit index—computed as the ratio of the number of health deficits to the total number of deficits considered—is that it is easily understood and implemented (for methodological background, see Rockwood and Mitnitski 2006, 2007). The convenience of the health deficit index becomes particularly salient when we compare it with health capital—that is, the latent variable in the focus of many studies in health economics (following Grossman 1972). Moreover, as noted earlier, the accumulation of health deficits has a micro-foundation in reliability theory, which facilitates the formulation of theory-led hypotheses and the scientific exchange of the disciplines concerned with human aging.
A few studies have investigated the health deficit index (frailty index) using the SHARE data (Harttgen et al. 2013; Romero-Ortuno 2014; Romero-Ortuno and Kenny 2012; Theou et al. 2013).Footnote 2 These studies have focused mainly on the association of health deficits with mortality as well as with several socioeconomic indicators and on comparing health deficits of Europeans and citizens of selected developing countries. None of the available studies exploited the panel structure of SHARE for longitudinal analysis. Here, we also run fixed- and random-effects panel regressions and demonstrate a log-linear relationship between age and health deficits at the individual level. In fact, the fixed-effects estimates differ slightly from the results obtained from pooled data and readjust the sometimes inconclusive findings regarding the gender gap of health deficit accumulation obtained in previous studies (e.g., Harttgen et al. 2013). According to the log-linear regression results, men and women develop an average of 2.6 % and 2.3 % more health deficits, respectively, from one birthday to the next. Nonlinear regressions, however, suggest a much higher impact of age on health deficit accumulation. When we take the constant (the Makeham term) into account, we estimate a rate of health deficit accumulation of 7.3 % for men and 5.2 % for women as well as a highly significant constant (which does not differ by gender in the full sample).
The article is organized as follows. In the next section, we describe the data set and estimate the relationship between the health deficit index (measured as the proportion of deficits that an individual has) and the age of the individuals. Then we proceed with the estimation of the same relationship but with the average values of the index for each age group (defined as all individuals within a specific age—for example, 50, 51, and so on until 90), for each wave, and for each country. The panel analysis is based on a balanced panel because there is some attrition in the sample (approximately 9 % of the people surveyed at Wave 1). In Online Resource 1, we show that our results barely change when we include individuals who passed away across the different waves (considering attrition by death). This robustness analysis is important given that our dependent variable is health-related. We then proceed with the estimation using the binned data of the individuals in the sample within the age frame under study, by year of age separately for each wave and then for each country. We then estimate the compensation effect of deficit accumulation and discuss the convergence of gender-specific aging across Europe, followed by our conclusion.
Empirical Strategy and Basic Results
Data Description
For the empirical analysis, we used the Survey of Health, Aging and Retirement in Europe (SHARE data set release 5.0.0) and considered the four waves (1, 2, 4, and 5) that provide health-related information (Börsch-Supan and Jürges 2005; Börsch-Supan et al. 2008; Malter and Börsch-Supan 2013, 2015; for methodological details, see Börsch-Supan et al. 2013; Gruber et al. 2014).Footnote 3 Wave 1 took place in 2004; Wave 2, in 2006/2007; Wave 4, in 2011 (in 2012 for Germany); and Wave 5, in 2013. We considered individuals aged 50 and older in the 10 countries that participated in all four waves: Austria, Belgium, Denmark, France, Germany, Italy, the Netherlands, Sweden, Spain, and Switzerland.Footnote 4 We kept only individuals aged 90 or younger given that a significant share of older people show “super healthy” characteristics, presumably because of selection effects. Whereas Mitnitski et al. (2002a) considered individuals up to age 79, Harttgen et al. (2013) considered the whole age span.
For each individual, we constructed a health deficit measure following Mitnitski et al. (2002a) and Harttgen et al. (2013). In line with our definition of aging as the accumulation of health deficits and based on the available data, we considered 38 symptoms, signs, and disease classifications, as summarized in Table S1 in Online Resource 1. Details on how each variable was constructed can be found in Table S2 in Online Resource 1.Footnote 5 We then computed the health deficit index as the proportion of deficits that an individual suffers from. When there were missing data for an individual, we computed the deficit index based on the available information about potential deficits (e.g., if information was not available for x potential health deficit for an individual, the observed health deficits were divided by 38 − x). From the surveyed individuals, we kept only those who had a health deficit index with at least 30 items. Because of missing values in the construction of the health deficit index or because of the lack of sufficient deficits to reach the minimum of 30 items, we lost 2.2 % of the potential data set. In Online Resource 1, Table S3 shows how the observations are distributed in each wave, and Table S4 shows the number of observations by gender and country of residence for the full sample (left side) and the balanced panel (right side).
Panel Estimates
We estimate the log-linear relationship between age and health deficits with the following equation:
where i represents the individual, g is gender (we ran separate regressions for males (m) and females (f)), w represents wave, and ε is the error term. We subsequently add further covariates composed of wave dummy variables and/or country or country-wave dummy variables. In terms of the Gompertz-Makeham methodology, Eq. (1) estimates a Gompertz relationship, without consideration of the Makeham term. Formally, we assume that A g = 0 in the nonlinear equation D igw = A g + R g exp(α g · age igw ), R g = exp(r g ), for gender g = f, m.
Results are shown in Tables 1 and 2. Column 1 shows results for the simple specification (Eq. (1)). Column 2 further includes wave dummy variables, column 3 adds country dummy variables, column 4 adds country-wave dummy variables to the specification of column 2, and column 5 provides results for a random-effects specification that also includes wave dummy variables. Finally, column 6 provides the results of a fixed-effects specification. We conducted the Breusch-Pagan Lagrange multiplier (LM) test (Breusch and Pagan 1980) using age and wave dummy variables as explanatory variables; the test suggests that the random-effects estimator is more appropriate than the pooled ordinary least squares (POLS) for both males and females. We also performed a Hausman test. For women (but not for men), the tests suggest that there is some evidence of unobserved heterogeneity that would be biasing the random-effects results. Nevertheless, for both men and women, the coefficients remain fairly constant. In Table S12 in Online Resource 1, we show similar results when the maximum age of individuals in the sample was restricted to 85 (rather than 90).
The results summarized in Tables 1 and 2 show that the health deficit index increases by approximately 2.5 % to 3 % from one birthday to the next. This stylized fact is obtained across methods, but other features are less robust. In particular, the pooled data and random-effects regressions (1)–(5) suggest that women age faster than men in contrast to the earlier finding of Mitnitski et al. (2002a). The regression considering individual fixed effects, however, reverses this result by estimating a (mildly) higher rate of health deficit accumulation for men. In longitudinal perspective, the rate of deficit accumulation is 2.3 % for women and 2.6 % for men.
The sign of the wave dummy variables is also inconclusive across methods.Footnote 6 The fixed-effect estimates, however, suggest that both men and women became, on average, healthier in Wave 2 and unhealthier in Wave 4. This finding could be explained by a generally positive effect of medical technological progress, which is the dominant force in Wave 2 but is offset and reversed in Wave 4 by the negative repercussions of the Great Recession on health.
We also observe great differences in health across countries. As shown by the country dummy variables in Tables 1 and 2, the health deficit level of Spaniards and Italians is, on average, higher than that of Austrians; the one of Swedes, Danes, and Swiss (also Dutch for males) is, on average, lower than that for Austrians. There seems to be no difference in being Belgian, German, or French versus being Austrian, in terms of health deficit accumulation.
A valid concern is whether these results suffer from a sample selection given that we considered only those people who were alive throughout the waves. To test whether the results are biased due to not considering the people who died across the waves,Footnote 7 we performed the variable addition tests including these extra observations, as suggested by Verbeek and Nijman (1992) and as employed by Contoyannis et al. (2004). We estimated the same baseline regressions as in the previous exercise, and we added three variables, one at a time: (1) wave sum (the number of times a person was present in the sample); (2) balanced (whether this person was present in all the waves); and (3) next wave (whether the person is in the next wave). Although the extra variable is statistically significant, we find no evidence for most of the cases that the size of our coefficients is changed because of the addition of this extra variable that inherently also implies a larger sample size. Results can be found in Online Resource 1 (Tables S10 and S11). Then we also included an interaction between the age variable and the extra variable; in none of the cases was this interaction significant. These exercises suggest that our results are not biased from the consideration of only the people alive.
Nonlinear Estimation Results
We next abandon the log-linear specification and estimate an exponential relationship of the Gompertz-Makeham structure. This approach is inspired by Mitnitski et al. (2002a), who argued in favor of such a specification based on the conceptual similarity of aging understood as health deficit accumulation and aging understood as increasing mortality. If health deficits are accumulated in Gompertz-Makeham fashion, then ignoring the Makeham term would indeed seriously bias the results, as Gavrilov and Gavrilova (1991) showed. We thus proceed by estimating the following equation:
where w is an index for waves in the following exercise and will be replaced by c (country) in the subsequent one; ε is the error term.
For linguistic convenience, we refer to A as the Makeham term and α and R as Gompertz terms. The meaning of these terms, however, is different from the original mortality studies. There, the outcome variable is incidence (mortality risk), whereas the outcome variable here is prevalence (of health deficits). The difference matters in particular for the Makeham term. Diagrammatically, an increase of A implies approximately a parallel upward shift of the health deficit curve. Analogously to the mortality studies, we can think of A as measuring environmental factors that influence health deficits independently from age. Another possibility is that A reflects (country-specific) health care institutions, given that access to and quality of health care is independent from age. Incidence—that is, the occurrence of new health deficits—is independent from A. This can be seen by differentiating the prevalence of health deficits, D(t) = A + R exp(αt), with respect to age t, which provides \( \overset{\cdotp }{D} \) (t) = α R exp(αt). This means that the incidence rate \( \raisebox{1ex}{$\overset{\cdotp }{D}$}\!\left/ \!\raisebox{-1ex}{$D$}\right. \) is declining in A, in contrast to the original mortality studies, where a larger Makeham term increases the incidence rate.
To obtain reliable estimates, we binned individuals in one-year age groups (i index). Despite a significant level of variation across individuals of the same age in their health deficits, as shown in the upcoming analysis, the age and the average level of health deficits of individuals by age group show a stable and consistent relationship. We first analyzed the relationship between the health deficit index and age by gender across all waves and all countries. Results are reported in column 1 of Table 3.
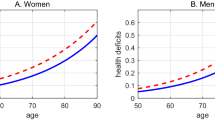
The estimated Makeham term (A) is about the same for both males and females and significantly different from 0. We take this as a first indication that the log-specification Eq. (1), which neglects the Makeham term, is indeed misspecified. Men start out healthier than women (lower R m than R f ) but age subsequently faster (higher α m than α f ). Figure 1 (left side) shows the data points and the predicted association between age and health deficits.
As a precursor of the analysis in the upcoming section, The Compensation Effect of Deficit Accumulation, we show in Fig. 1 (right side) the association between the age-dependent rate of deficit accumulation log(D ig − A g ), and age for gender (g = f, m). The association of adjusted health deficits and age is close to log-linear, and the age-trajectories intersect at age 104. Restricting the sample to individuals aged 85 or younger yields similar results (intersection at age 103; estimates in Online Resource 1 (section 4)).
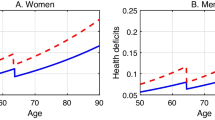
In Table 3, we show the results of the nonlinear regressions by wave. In any wave, women start out less healthy (R f > R m ) and develop new health deficits at a slower pace than men (α f < α m ). The Makeham term A is significantly different from 0 and approximately the same for both genders, aside from Wave 2. In contrast to the OLS results, we now observe clear time trends in the data. For both men and women, the Gompertz term R declines, while the Gompertz term α increases over time (aside of Wave 2 for men). Moreover, the Makeham term A is increases over time (aside of Wave 2 for men). Overall, the model has high predictive power, as indicated by the R2 values above 96 %.
In Fig. 2, we show the association between the log of adjusted health deficits D ig − A g and age per wave. In any wave, men (white dots) start out healthier than women (black dots) such that an intersection of the age trajectories exists at some high age near 103. In Online Resource 1 (section 4), we show these figures for the cutoff of 85 years. Although the precise estimates per country differ somewhat, the general patterns remain the same.
Table 4 shows results by country. In contrast to the results by wave, the coefficient (R) is not always estimated to be significantly different from 0 because the point estimates are close to 0 with only 41 data points. The overall precision of the estimates, however, is high, as indicated by the R2 above 95 % (in most of the cases). In every country, men start out initially healthier (R m < R f ) but age subsequently faster (α m > α f ). The highest rate of health deficit accumulation is observed for men in Switzerland and for women in Sweden; the lowest rate is observed for men in Italy and for women in Spain.
Figure 3 shows the adjusted health deficit index by age for all countries. We see that everywhere, initially unhealthier women (black dots) age more slowly than men (white dots), such that the age trajectories intersect at some high age near 100.Footnote 8
Despite their larger number of health deficits at any age, women die later than men—a feature known as the morbidity-mortality paradox (Kulminski et al. 2008). A potential explanation is that women are more likely to suffer from acute illnesses and nonfatal chronic conditions (such as arthritis, constipation, headaches, and migraines), which lead to poorer health but contribute little to the risk of death (Bird and Rieker 1999; Case and Paxson 2005). Moreover, women engage less in risk-taking health behaviors, eat healthier, and use health care services more frequently than men (Waldron 1993; Wardle et al. 2004). As a result, women suffer more from nonfatal health deficits and develop fatal health deficits later in life (Schünemann et al. 2017; Verbrugge 1985).
The Compensation Effect of Deficit Accumulation
As shown in Tables 3 and 4 and Figs. 1, 2, and 3 (and previously for Canadians by Mitnitski et al. 2002a), men have initially less health deficits than women and age subsequently faster. Formally, for men relative to women, R is estimated to be smaller, and α is estimated to be larger. This means that, diagrammatically, the health deficit trajectories of men and women meet at a certain age. Likewise, countries where men or women start out initially healthier (lower R) display higher rates of health deficit accumulation (higher α); see Tables 3 and 4. As shown in Fig. 4, the negative relationship between α and ln(R) appears to be close to linear across countries for men and women.
We next explored the compensation of better initial health with subsequently faster aging more formally. Inspired by the literature on the compensation effect of mortality (Gavrilov and Gavrilova 1991) and following Mitnitski et al. (2002a), we regressed α on ln(R) across countries using the pooled data:
in which c represents the country. The results are shown in Table 5. The point estimate of B is close to 102 (column 1). Column 2 shows the same regression but controlling for the gender of the original data points. The coefficient on the female dummy variable is not statistically significant, and the point estimate of B (in absolute value) increases slightly, although it is not statistically different from the previous one. Moreover, the adjusted R2 also remains the same, and the Akaike and the Bayesian information criteria (AIC and BIC, respectively) become larger in column 2, suggesting that the model estimated in column 1 is more appropriate for the data. Column 3 builds from the previous model and adds an interaction dummy variable between α and female to see whether there is a difference in the slope. Neither the female dummy variable nor the interaction term is statistically significant. The point estimate for B remains at 102. The adjusted R2 is close to the previously reported ones, and the information criteria suggest that the model estimated in column 1 is the preferred one. Therefore, the estimates imply that men and women across countries share an average lifespan of approximately 102 years with a standard deviation of 2.6 years. The overall precision of the preferred estimate is quite high, as indicated by the R2 higher than 98 %.
The implications of these results can best be seen by inserting Eq. (3) in Eq. (2). Leaving aside the individual subscripts and using the transformation \( \overline{D}=\log \left(\upbeta \right) \), this provides
On the left side of Eq. (4) is the age-dependent part of the health deficit index (D corrected by the age-independent Makeham term A). Equation (4) suggests that a common age exists (B) at which age-dependent health deficits are the same (\( \overline{D} \)) across the European countries. The health deficits \( \overline{D} \) are given by the point estimates of \( \overline{D} \) as exp(−0.504) = 0.604. The point estimate is statistically different from 0 at the 5 % level. Interestingly, our estimates of \( \overline{D} \) correspond with the estimated upper limit of health deficit accumulation found in previous research (Bennett et al. 2013; Rockwood and Mitnitski 2006). To see this, add the average estimated Makeham term A g from Table 3 (of approximately 0.075) to \( \overline{D} \) to obtain 0.68. The previous studies had suggested that there exists an upper (attainable) limit of the health deficit index of approximately 0.7. In light of the literature on human lifespan and the compensation effect of mortality (Gavrilov and Gavrilova 1991), it is thus tempting to identify the common age of deficit accumulation B as an estimate of human life span.
Finally, we explored the implication of our findings for comparative aging across Europe. For that purpose, we fed into Eq. (4) the country- and gender-specific estimates of α from Table 1. The results are shown in Fig. 5 (see the online version of the article to view the figure in color). Along the ordinate, we measure age-dependent health deficits (D − A) in logs. For better visibility, we split the sample between men (right side) and women (left side). The figure shows that Spanish women are the least healthy in our European sample, next to the Italian and Austrians, while Swedish women are the healthiest, next to Belgian and German women. Initially, healthier women age faster subsequently such that all trajectories intersect at a common age of 102.
The figure shows age-dependent health deficits (D − A) across countries, according to Eq. (4), when the country-specific force of aging α ig is fed in from the estimates of Table 4. Structurally, the same picture emerges for men albeit with a somewhat different country ranking. Among women, Italians are the least healthy, next to French and Austrian men. Swiss men are the healthiest next to the Dutch and Swedish. Men in initially healthier countries accumulate additional health deficits faster than women such that a common intersection at age 102 emerges.
Conclusion
In this study, we show in nonlinear regressions the differences and similarities of health deficit accumulation of European men and women. We show that the consideration of a constant (age-independent) Makeham term leads to substantially higher estimates of the rate at which additional health deficits are accumulated with increasing age and that it resolves some previously inconclusive results on aging of men and women. Across countries (and waves of our sample), men are found to be initially healthier than women but to age faster (at a rate of 7.3 % compared with 5.2 %, according to our point estimates). A similar compensation effect of health deficit accumulation is found across the European countries. Countries in which men or women are initially healthier display higher rates of aging in terms of health deficit accumulation. This implies convergence, and we estimate a focal point at age 102 where individuals, on average, age in the same way across gender and across countries. Our sample, however, consists exclusively of fully developed European countries. It would be interesting to see whether the compensation effect of deficit accumulation prevails in a more heterogeneous sample or whether, for example, a North-South gradient of focal points emerges.
Because health deficits at the focal point coincide with estimates of an attainable upper limit of health deficit accumulation, it is tempting to associate the population-invariant age at the focal point with human life span. In this context, it is interesting that previous studies based on mortality rates suggested a shorter human life span of approximately 95 years (Gavrilov and Gavrilova 1991; Strulik and Vollmer 2013). Of course, the estimates from mortality rates are not one-to-one comparable to our estimates from morbidity rates, although a close association exists between biological markers and age as determinants of mortality (see Mitnitski et al. 2002b). Another difference is sample size. Compared with mortality data, which are available for whole populations, the SHARE data are rather limited. Although the huge variability of individual aging (as a stochastic process) can be smoothed out by averaging, we had to omit the oldest-old because of limited representation in the sample. Rather than emphasizing point estimates, it thus seems prudent to conclude with highlighting the qualitative result of structural similarity of health deficit accumulation across the European countries. These results suggest convergence of initially disperse health deficits and the existence of a focal point as a potential invariant of human aging.
Notes
Mitnitski, Rockwood, and coauthors originally established the methodology as the frailty index. Newer studies have also used the term health deficit index (e.g., Mitnitski and Rockwood 2016), which seems to be a more appropriate term when the investigated population consists to a significant degree of nonfrail persons.
On a more general level, our study is also related to the literature on the compensation effect of mortality, also known as the Strehler-Mildvan correlation (Strulik and Vollmer 2013; Yashin et al. 2001; Zheng 2014; Zheng et al. 2011), as well as to the general discussion of human life span (Carey 2003; Carnes and Olshansky 2007; Finch and Pike 1996; Gavrilov and Gavrilova 1991; Oeppen and Vaupel 2002; Wilmoth and Robine 2003).
We used part of the Easyshare release 2.0.0 to compile the data set.
Although the main target is to survey adults aged 50 or older (aiming to create a data set representative of the noninstitutionalized population aged 50+), younger people can also be found in the data because partners are also interviewed. These data were removed because they do not belong to the representative sample.
Here, following Mitnitski et al. (2002a), we coded multilevel deficits using a mapping to the Likert scale in the interval 0–1. We also computed binary deficits using cutoff points, as suggested by Pena et al. (2014); we found that the two indices were not statistically different from each other, across waves and across subgroups.
The SHARE data set provides information on whether people left the sample because they passed away.
In Online Resource 1, we show the figures for the nonlinear estimates and the cutoff of 85 years. Although the precise estimates per country differ, the general patterns remain the same.
References
Arking, R. (2006). The biology of aging: Observations and principles. Oxford, UK: Oxford University Press.
Barlow, R. E., & Proschan, F. (1975). Statistical theory of reliability and life testing: Probability models. New York, NY: Holt, Rinehart and Winston.
Bennett, S., Song, X., Mitnitski, A., & Rockwood, K. (2013). A limit to frailty in very old, community-dwelling people: A secondary analysis of the Chinese Longitudinal Health and Longevity Study. Age and Ageing, 42, 372–377.
Bird, C. E., & Rieker, P. P. (1999). Gender matters: An integrated model for understanding men’s and women’s health. Social Science & Medicine, 48, 745–755.
Börsch-Supan, A., Brugiavini, A., Jürges, H., Kapteyn, A., Mackenbach, J., Siegrist, J., & Weber, G. (Eds.). (2008). First results from the Survey of Health, Ageing and Retirement in Europe (2004–2007). Starting the longitudinal dimension. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging.
Börsch-Supan, A., Brandt, M., Hunkler, C., Kneip, T., Korbmacher, J., Malter, F., . . . Zuber, S. (2013). Data resource profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). International Journal of Epidemiology, 42, 992–1001.
Börsch-Supan, A., & Jürges, H. (Eds.). (2005). The Survey of Health, Ageing and Retirement in Europe—Methodology. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging.
Breusch, T. S., & Pagan, A. R. (1980). The Lagrange multiplier test and its applications to model specification in econometrics. Review of Economic Studies, 47, 239–253.
Brugiavini, A., Cavapozzi, D., Pasini, G., & Trevisan, E. (2013). Working life histories from SHARELIFE: A retrospective panel (SHARE Working Paper Series: 11-2013). Munich, Germany: Max Planck Institute for Social Law and Social Policy.
Carey, J. R. (2003). Life span: A conceptual overview. Population and Development Review, 29, 1–18.
Carnes, B. A., & Olshansky, S. J. (2007). A realist view of aging, mortality, and future longevity. Population and Development Review, 33, 367–381.
Case, A., & Paxson, C. (2005). Sex differences in morbidity and mortality. Demography, 42, 189–214.
Contoyannis, P., Jones, A. M., & Rice, N. (2004). The dynamics of health in the British Household Panel Survey. Journal of Applied Econometrics, 19, 473–503.
Finch, C. E., & Pike, M. C. (1996). Maximum life span predictions from the Gompertz mortality model. Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 51A, B183–B194.
Finkelstein, M. S. (2008). Failure rate modeling for risk and reliability. London, UK: Springer.
Fries, J. F. (1980). Aging, natural death, and the compression of morbidity. New England Journal of Medicine, 303, 130–135.
Gavrilov, L. A., & Gavrilova, N. S. (1991). The biology of human life span: A quantitative approach. London, UK: Harwood Academic Publishers.
Gompertz, B. (1825). On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philosophical Transactions of the Royal Society of London, 115, 513–583.
Grossman, M. (1972). On the concept of health capital and the demand for health. Journal of Political Economy, 80, 223–255.
Gruber, S., Hunkler, C., & Stuck, S. (2014). Generating easy SHARE: Guidelines, structure, content and programming (SHARE Working Paper Series No. 17-2014). Munich, Germany: MEA, Max Planck Institute for Social Law and Social Policy.
Harttgen, K., Kowal, P., Strulik, H., Chatterji, S., & Vollmer, S. (2013). Patterns of frailty in older adults: Comparing results from higher and lower income countries using the Survey of Health, Ageing and Retirement in Europe (SHARE) and the Study on Global AGEing and Adult Health (SAGE). PLoS One, 8(10), e75847. https://doi.org/10.1371/journal.pone.0075847
Kulminski, A. M., Culminskaya, I. V., Ukraintseva, S. V., Arbeev, K. G., Land, K. C., & Yashin, A. I. (2008). Sex-specific health deterioration and mortality: The morbidity-mortality paradox over age and time. Experimental Gerontology, 43, 1052–1057.
Makeham, W. M. (1860). On the law of mortality and the construction of annuity tables. Journal of the Institute of Actuaries, 8, 301–310.
Malter, F., & Börsch-Supan, A. (Eds.). (2013). SHARE Wave 4: Innovations & methodology. Munich, Germany: Mannheim Center for the Economics of Aging, Max Planck Institute for Social Law and Social Policy.
Malter, F., & Börsch-Supan, A. (Eds.). (2015). SHARE Wave 5: Innovations & methodology. Munich, Germany: Mannheim Center for the Economics of Aging, Max Planck Institute for Social Law and Social Policy.
Masoro, E. J. (2006). Are age-associated diseases an integral part of aging? In E. J. Masoro & S. N. Austad (Eds.), Handbook of the biology of aging (6th ed., pp. 43–62). Burlington, MA: Academic Press.
Mitnitski, A. B., Mogilner, A. J., MacKnight, C., & Rockwood, K. (2002a). The accumulation of deficits with age and possible invariants of aging. Scientific World, 2, 1816–1822.
Mitnitski, A. B., Mogilner, A. J., MacKnight, C., & Rockwood, K. (2002b). The mortality rate as a function of accumulated deficits in a frailty index. Mechanisms of Ageing and Development, 123, 1457–1460.
Mitnitski, A. B., Mogilner, A. J., & Rockwood, K. (2001). Accumulation of deficits as a proxy measure of aging. Scientific World, 1, 323–336.
Mitnitski, A., & Rockwood, K. (2016). The rate of aging: The rate of deficit accumulation does not change over the adult life span. Biogerontology, 17, 199–204.
Novoseltsev, V. N. (2006). Mathematical modeling in biology: Systems capable to live and die. Automation and Remote Control, 67, 835–855.
Oeppen, J., & Vaupel, J. W. (2002). Broken limits to life expectancy. Science, 296, 1029–1031.
Olshansky, S. J., & Carnes, B. A. (1997). Ever since Gompertz. Demography, 34, 1–15.
Pena, F. G., Theou, O., Wallace, L., Brothers, T. D., Gill, T. M., Gahbauer, E. A., . . . Rockwood, K. (2014). Comparison of alternate scoring of variables on the performance of the frailty index. BMC Geriatrics, 14, 25. https://doi.org/10.1186/1471-2318-14-25
Rockwood, K., & Mitnitski, A. (2006). Limits to deficit accumulation in elderly people. Mechanisms of Ageing and Development, 127, 494–496.
Rockwood, K., & Mitnitski, A. B. (2007). Frailty in relation to the accumulation of deficits. Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 62, 722–727.
Romero-Ortuno, R. (2014). Frailty index in Europeans: Association with determinants of health. Geriatrics & Gerontology International, 14, 420–429.
Romero-Ortuno, R., & Kenny, R. A. (2012). The frailty index in Europeans: Association with age and mortality. Age and Ageing, 41, 684–689.
Schünemann, J., Strulik, H., & Trimborn, T. (2017). The gender gap in mortality: How much is explained by behavior? Journal of Health Economics, 54, 79–90.
Strehler, B. L., & Mildvan, A. S. (1960). General theory of mortality and aging. Science, 132, 14–21.
Strulik, H., & Vollmer, S. (2013). Long-run trends of human aging and longevity. Journal of Population Economics, 26, 1303–1323.
Theou, O., Brothers, T. D., Mitnitski, A., & Rockwood, K. (2013). Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. Journal of the American Geriatrics Society, 61, 1537–1551.
Verbeek, M., & Nijman, T. (1992). Testing for selectivity bias in panel data models. International Economic Review, 33, 681–703.
Verbrugge, L. M. (1985). Gender and health: An update on hypotheses and evidence. Journal of Health and Social Behavior, 26, 156–182.
Waldron, I. (1993). Recent trends in sex mortality ratios for adults in developed countries. Social Science & Medicine, 36, 451–462.
Wardle, J., Haase, A. M., Steptoe, A., Nillapun, M., Jonwutiwes, K., & Bellisie, F. (2004). Gender differences in food choice: The contribution of health beliefs and dieting. Annals of Behavioral Medicine, 27, 107–116.
Wilmoth, J. R., & Robine, J. M. (2003). The world trend in maximum life span. Population and Development Review, 29, 239–257.
Yashin, A. I., Begun, A. S., Boiko, S. I., Ukraintseva, S. V., & Oeppen, J. (2001). The new trends in survival improvement require a revision of traditional gerontological concepts. Experimental Gerontology, 37, 157–167.
Zheng, H. (2014). Aging in the context of cohort evolution and mortality selection. Demography, 51, 1295–1317.
Zheng, H., Yang, Y., & Land, K. C. (2011). Heterogeneity in the Strehler-Mildvan general theory of mortality and aging. Demography, 48, 267–290.
Acknowledgments
We would like to thank Kenneth Harttgen, Arnold Mitnitski, and Sebastian Vollmer for helpful comments. This article uses data from SHARE Waves 1, 2, 4, and 5 (DOIs: https://doi.org/10.6103/SHARE.w1.500, https://doi.org/10.6103/SHARE.w2.500, https://doi.org/10.6103/SHARE.w4.500, https://doi.org/10.6103/SHARE.w5.500); see Börsch-Supan et al. (2013) for methodological details. The SHARE data collection has been primarily funded by the European Commission through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006-062193, COMPARE: CIT5-CT-2005-028857, SHARELIFE: CIT4-CT-2006-028812) and FP7 (SHARE-PREP: N.211909, SHARE-LEAP: N. 227822, SHARE M4: N.261982). Additional funding from the German Ministry of Education and Research, the U.S. National Institute on Aging (U01-AG09740-13S2, P01-AG005842, P01-AG08291, P30-AG12815, R21-AG025169, Y1-AG-4553-01, IAG-BSR06-11, OGHA-04-064), and from various national funding sources is gratefully acknowledged (see www.share-project.org).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 219 kb)
Rights and permissions
About this article
Cite this article
Abeliansky, A.L., Strulik, H. How We Fall Apart: Similarities of Human Aging in 10 European Countries. Demography 55, 341–359 (2018). https://doi.org/10.1007/s13524-017-0641-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13524-017-0641-8