Abstract
People age at different rates. We have proposed that rates of aging can be quantified by the rate at which individuals accumulate health deficits. Earlier estimates, using cross-sectional analyses suggested that deficits accumulated exponentially, at an annual rate of 3.5 %. Here, we estimate the rate of deficit accumulation using longitudinal data from the Canadian National Population Health Survey. By analyzing age-specific trajectories of deficit accumulation in people aged 20 years and over (n = 13,668) followed biannually for 16 years, we found that the longitudinal average annual rate of deficit accumulation was 4.5 % (±0.75 %). This estimate was notably stable during the adult life span. The corresponding average doubling time in the number of deficits was 15.4 (95 % CI 14.82–16.03) years, roughly 30 % less than we had reported from the cross-sectional analysis. Earlier work also established that the average number of deficits accumulated by individuals (N), equals the product of the intensity of environmental stresses (λ) causing damage to the organism, by the average recovery time (W). At the individual level, changes in deficit accumulation can be attributed to both changes in environmental stresses and changes in recovery time. By contrast, at the population level, changes in the number of deficits are proportional to the changes in recovery time. In consequence, we propose here that the average recovery time, W doubles approximately every 15.4 years, independently of age. Such changes quantify the increase of vulnerability to stressors as people age that gives rise to increasing risk of frailty, disability and death. That deficit accumulation will, on average, double twice between ages 50 and 80 highlights the importance of health in middle age on late life outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
People age at different rates, but how to quantify rates of aging is an important challenge. We have proposed that the rate of aging in individuals and populations is estimated by the rate of health deficit accumulation. Health deficits in this account of aging are broadly defined, and include biological and clinical characteristics, commonly found in most clinical and epidemiological databases (Mitnitski et al. 2001; Rockwood and Mitnitski 2007) and even in laboratory test records (Howlett et al. 2014). The resulting “frailty index” is based on a simple count of deficits, expressed as the ratio of the deficits present in the individual to the total number of available deficits counted (Mitnitski et al. 2001; Woo et al. 2006). When the frailty index (FI) is elevated compared with others of the same age, it suggests an increase in the vulnerability to stresses that defines frailty (Clegg et al. 2013).
In 2013, we further suggested that the origin of deficit accumulation lies in the complex interplay between stochastic damage to the organism, arising from both the internal and the external environment, and its ability to repair/sustain such damage. In so doing, we worked with Little’s Law (N = λW), restated here so that N is the average number of deficits, λ is the intensity of environmental stresses, and W is the average (net) recovery time (Mitnitski et al. 2013, 2015). During the adult lifespan, the number of deficits accumulated in Canadians changes by an order of magnitude, and triples in the last three decades of life, after the age of 65 (Mitnitski et al. 2013; Mitnitski and Rockwood 2015). The quantitative estimates of the changes in the number of deficits were obtained from cross-sectional analysis. These showed that the annual rate of deficit accumulation was about 3 %, an estimate obtained by using the exponential approximation of the age-specific deficit (frailty) trajectories (Mitnitski et al. 2005; Rockwood et al. 2011). These estimates, first reported for people over age of 65 (Mitnitski et al. 2005) varied depending on the data, generally between 2 and 4 % (e.g., Shi et al. 2011, Harttgen et al. 2013). This corresponds to doubling time in the number of deficits between 17 and 34 years. Our most recent estimate for the whole of the adult lifespan gave the value of 3.5 %, towards the upper part of the prior range (Mitnitski et al. 2013). As longitudinal data have become available, we have used such data to model transitions between numbers of deficits up to 16 years (Mitnitski et al. 2012). Of note, the individual trajectories of the number deficits are volatile, showing great stochastic variations (Mitnitski et al. 2012, 2015). At the level of population, however, under the assumption that the damage rate is fairly constant, it appears that fluctuation in the number of deficits can be attributed to the changes in recovery time (Mitnitski et al. 2013). In consequence, although the number of deficits will increase each year, the rate at which the deficits increase would be constant, reflecting a constant degree of lengthening in the recovery time with age.
In this paper, we estimate the rates of changes in the number of deficits in the National Population Health Survey (NPHS) across the adult lifespan, using longitudinal components of the NPHS. In particular, we wanted to address whether the rate of deficit accumulation changes over the adult lifespan (after about 20 years of age) or remains the same.
Methods
We used data from the NPHS, a large Statistics Canadian study which began in 1994/95 and was conducted in nine biannual cycles from 1994 to 2008. Self-reported information was gathered at baseline and every 2 years, as was vital status (Singh et al. 1994). Of 13,668 participants aged between 20 and 92 at baseline, 54 % were women. Thirty-nine self-reported variables (referred to as “deficits”, and dichotomized for this purpose) including medical conditions, disabilities and health history were used to calculate each individual’s health status, combined in a FI (Mitnitski et al. 2013, Supplementary file). Data were coded so that the presence of a deficit was represented by a “1”, and the absence of the deficit by “0”. For any individual, their FI represents the number of deficits present, divided by 39 (the number of deficits considered). Note that even though the theoretical range of the FI is 0 (no deficits) to 1 (all 39 deficits present), in almost every circumstances—including patients admitted with acute medical problems (Evans et al. 2014; Dent et al. 2014), the limit to the FI is seen at an upper value of ~0.7 (Rockwood and Mitnitski 2006; Bennett et al. 2013; Armstrong et al. 2014). Here we report the results using the FI, rather than the deficit count, so as to facilitate comparison with other studies (past and future) which typically will employ varying numbers of deficits.
Statistical analysis
The age-specific trajectories of the number of deficits were fitted using the exponential function; the parameters were estimated using least squares regression. The statistical significance level was set to p = 0.05.
Ethics
The Statistics Canada ethics review process approved the NPHS, and participants provided oral informed consent. Approval for these additional analyses came from the Research Ethics Committee of the Capital District Health Authority, Halifax, Nova Scotia, Canada.
Results
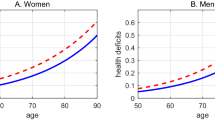
In Fig. 1 (Panel a), age-specific cross-sectional average trajectories for the 9 cycles of the NPHS are shown, over 16 years of follow-up, repeated every 2 years. All follow-up trajectories virtually coincide. The solid line represents the average exponential fit, and demonstrates slope parameter b C = 0.035 (95 % CI 0.033, 0.036) and intercept = −4.163 (95 % CI −4.430 to −3.895). In Fig. 1 (Panel b), longitudinal 16 year trajectories (averaged at each age at baseline) are shown overlaid with the average cross-sectional age-specific trajectory (dashed line).
The dynamics of deficit accumulation. a cross-sectional age-specific trajectories of deficit accumulation for 9 consecutive cycles of the NPHS. b longitudinal age-specific trajectories of deficit accumulations for individuals at the same baseline ages (solid lines) shown for selected 8 baseline ages 22, 31, 41, 52, 62, 72, 80, and 92, overlaid with the cross-sectional average trajectory (dashed line)
Eight longitudinal average trajectories are exemplified in Fig. 1, Panel a for 22, 31, 41, 52, 62, 72, 80, and 92 baseline ages. Longitudinal 16-year trajectories were fitted at each age by exponential curves. The slope parameter for each baseline age are shown in Fig. 2 (Panel a), and the histogram of the slopes is shown In Fig. 2 (Panel b). The average longitudinal slope b L is 0.045 (95 % CI 0.038–0.052) shown by the dashed line, along with the cross-sectional slope =0.035 (the solid line). There is no evidence of a secular trend in the slope parameter. For example, the mean slope for people younger than 60 years at baseline was 0.043 (95 % CI 0.044–0.050) and the slope parameter for those older than 60 years was 0.047 (95 % CI 0.041–0.045). The histogram of the longitudinal slope is presented in Fig. 2 (Panel b).
The distribution of the longitudinal slope of deficit accumulation at each baseline age. a the slope as a function of baseline age. The mean values of the longitudinal slope is shown by the dashed line, the mean value of the cross-sectional slope is shown by the solid line. b the histogram of the longitudinal slope overlaid with the normal curve
The doubling time (the period of time required for a the number of deficits to double is an inverse function of the slope), Td = ln(2)/b. Using the longitudinal estimates, the doubling rate (the time for the number of deficits to increase by a factor of two) is calculated as Td = 15.3 (95 % CI 14.82–16.03) years, whereas the cross-sectional estimate corresponds to Td = 20 years (95 % CI 19.07–20.59). Note that the ratio of doubling of the mean FI score to time is constant across the life span, even though the number of deficits accumulated in any year accelerates with age.
Discussion
In this paper, we estimated the rate of deficit accumulation in a representative sample of Canadians across the adult life span from age 20 years. Using the baseline and eight biannual follow-up cycles of longitudinal data, we found that the annual rate of changes equals 4.5 % (3.75–5.25). This result, obtained over 16 years of follow-up, was significantly higher than the 3.5 % (3.3–3.7) rate of annual deficit accumulation previously estimated by us from cross-sectional analyses from the same dataset. The new doubling time estimate of 15.4 (14.8–16.0) years, significantly less than the estimate from the cross-sectional data, which was 20 years.
The ability to represent the rate of deficit accumulation by a single parameter (i.e., the annual rate or the doubling time) is a consequence of the exponential approximation of the kinetics of deficit accumulation. While we and others reported an exponential pattern of increase in deficit accumulation using cross-sectional analyses (Mitnitski et al. 2005, 2013; Rockwood et al. 2011; Shi et al. 2011; Harttgen et al. 2013), other groups have indicated some leveling off, at older ages, of the age-specific rates at which people accumulate deficits (García-González et al. 2009; Kulminski et al. 2007; Gu et al. 2009). Gu et al. (2009) suggested a logistic fit of the FI to account for saturation of its values at advanced ages. It remains to be seen if this decline is an artifact that might be explained by there being just a small number of people at the oldest ages available for study. Even so, the pattern of deficit accumulation is generally convex, showing age-related acceleration (Mitnitski et al. 2005, 2013, 2015; Rockwood et al. 2011; Kulminski et al. 2007; Theou et al. 2014). Such a nonlinear pattern implies that deficits do not accumulate independently of each other (Taneja et al. 2014).
Our results should be interpreted with caution. The deficit accumulation approach to quantifying aging also extends to characteristics at different levels from the subcellular to the organism (Howlett and Rockwood 2013). Likewise, deficits span between self-reported clinical variables to laboratory abnormalities (Howlett et al. 2014) to cellular biomarkers of immunosenescence (Mitnitski et al. in revision). Here, however, we used only self-reported variables, as these were the only such variables available in this dataset. Another important limitation is that in our assessment of deficit accumulation we ignored mortality. Accounting for mortality is necessary for accurate estimates of longitudinal trajectories of any health characteristics (Yashin et al. 2007, 2012), otherwise the rate of increase of deficit accumulation might be underestimated: people with higher value of frailty die sooner than those with the lower level of deficits. We plan to address this issue in future studies.
We have to acknowledge that our estimates for the rate of deficit accumulation was based on differing numbers of people in each baseline age. As is typical in survey data, people of differing ages are not equally represented in population samples. For example, the number of people in younger group was an order of magnitude higher than the number of people of older ages, monotonically declining after age of 30 year old (from about 350) to less than 30 over age of 80). As the consequence, the variance of the estimates of the rate increases for the older ages but there is no evidence of the secular trend in the rate estimate (Fig. 1a).
Of some interest to studying mortality is that the pattern of deficit accumulation resembles the Gompertz Law of mortality (Milne 2008; Kirkwood 2015). Note too that the mortality rate doubling time (about 8 years) is about two times higher than the doubling rate of deficit accumulation, as might be expected if higher levels of deficit accumulation are relatively lethal.
Our results might shed light on understanding of how increasing vulnerability (or decreasing resilience) of the human organism is related to recovery time from various stresses and insults. At the individual level, the observed changes in deficit accumulation could be attributed to both changes in environmental stresses, λ and changes in recovery time, W. At the level of population, the environmental component of Little’s Law (λ) is approximately constant for the cohort. Accounting for secular trends by considering people of the same age, changes in the average number of deficits can be attributed to changes in recovery time. Such changes might also reflect change in the severity of deficits, as those which are more severe would take more time to recover than those that are less severe, even at the same age. Our results therefore indicate that the average recovery time doubles approximately every 15 year, independently of age. Experimental data on recovery time are sparse but it is known to increase with age (Yanai et al. 2011, 2015; Akushevich et al. 2013; Gould et al. 2015).
Recovery is an enormously complex process, even for very simple conditions such as skin wound healing, and involves many different processes and systems, across many scales—e.g., cellular, mitochondrial, and molecular (Gurtner et al. 2008; Moskalev et al. 2013; Gould et al. 2015). In many instances, these processes can be modulated by medical interventions, and these likely are responsible for much of the life extension observed in developed countries (GBD 2014). It has been suggested that society faces an urgent need “to combat middle-age decline at the stage before incipient old-age problems develop into full-blown diseases” (Wang 2007). Our approach, by accounting for a range of age-related deficits, and not just co-morbidities and functional limitations, allows such changes to be traced systematically, thereby laying the groundwork for its more widespread use in health care and in gerontological research. Indeed, especially with responsive methods to count deficits in animals, (Parks et al. 2012; Whitehead et al. 2014; Feridooni et al. 2015; Kane et al. 2015) which yield FI results comparable to what is seen in humans, this approach allows a tractable means for research on age-related illnesses to quantify the effects of ageing on the whole organism. In so doing, we offer a pragmatic way to get to grips with a compelling critique of much contemporary disease-related research, being its neglect of the fact that “the problems of old age come as a package” (Fontana et al. 2014).
Given that the recovery rate is related to the number of accumulated deficits, it is tempting to pose the question: what causes age-associated changes in recovery time? Does age-related changes in the time of recovery causes accumulation of deficits, or the opposite is true: the accumulation of deficits causes increase in recovery time? In order to answer these questions, we need a more elaborated systems biological model that considers complex network model of the organism where both the interaction between the network nodes corresponding to the different subsystems of the organism and external interactions with the environment are taken into account (Taneja et al. 2014; Mitnitski et al. 2013, 2014). Detailed investigation of such models is motivating further inquiries by our group.
References
Akushevich I, Kravchenko J, Ukraintseva S, Arbeev K, Yashin AI (2013) Recovery and survival from aging-associated diseases. Exp Gerontol 48:824–830
Armstrong JJ, Mitnitski A, Launer LJ, White LR, Rockwood K (2014) Frailty in the Honolulu–Asia aging study: deficit accumulation in a male cohort followed to 90% mortality. J Gerontol A Biol Sci Med Sci 70:125–131
Bennett S, Song X, Mitnitski A, Rockwood K (2013) A limit to frailty in very old, community-dwelling people: a secondary analysis of the Chinese longitudinal health and longevity study. Age Ageing 42:372–377
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381:752–762
Dent E, Chapman I, Howell S, Piantadosi C, Visvanathan R (2014) Frailty and functional decline indices predict poor outcomes in hospitalised older people. Age Ageing 43:477–484
Evans S, Sayers M, Mitnitski A, Rockwood K (2014) The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing 43:127–132
Feridooni HA, Sun MH, Rockwood K, Howlett SE (2015) Reliability of a frailty index based on the clinical assessment of health deficits in male C57BL/6J Mice. J Gerontol A Biol Sci Med Sci 70:686–693
Fontana L, Kennedy BK, Longo VD, Seals D, Melov S (2014) Medical research: treat ageing. Nature 511:405–407
García-González JJ, García-Peña C, Franco-Marina F, Gutiérrez-Robledo LM (2009) A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr 9:47
GBD 2013 Mortality and Causes of Death Collaborators (2014) Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet 385(9963):117–171
Gould L, Abadir P, Brem H, Carter M, Conner-Kerr T, Davidson J, et al. (2015) Chronic wound repair and healing in older adults: current status and future research. J Am Geriatr Soc [Epub ahead of print]
Gu D, Dupre ME, Sautter J, Zhu H, Liu Y, Yi Z (2009) Frailty and mortality among Chinese at advanced ages. J Gerontol B Psychol Sci Soc Sci 64:279–289
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Harttgen K, Kowal P, Strulik H, Chatterji S, Vollmer S (2013) Patterns of frailty in older adults: comparing results from higher and lower income countries using the Survey of Health, Ageing and Retirement in Europe (SHARE) and the Study on Global AGEing and Adult Health (SAGE). PLoS One 8:e75847
Howlett SE, Rockwood K (2013) New horizons in frailty: ageing and the deficit scaling problem. Age Ageing 42:416–423
Howlett SE, Rockwood MR, Mitnitski A, Rockwood K (2014) Standard laboratory tests to identify older adults at increased risk of death. BMC Med 12:171
Kane AE, Hilmer SN, Boyer D, Gavin K, Nines D, Howlett SE, de Cabo R, Mitchell SJ (2015) Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glu315
Kirkwood TB (2015) Deciphering death: a commentary on Gompertz (1825) ‘On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies’. Philos Trans R Soc Lond B Biol Sci 370(1666):20140379
Kulminski A, Ukraintseva SV, Akushevich I, Arbeev KG, Land K, Yashin AI (2007) Accelerated accumulation of health deficits as a characteristic of aging. Exp Gerontol 42:963–970
Milne EMG (2008) The natural distribution of survival. J Theor Biol 255:233–236
Mitnitski A, Rockwood K (2015) Aging as a process of deficit accumulation: its utility and origin. Interdiscip Top Gerontol 40:85–98
Mitnitski AB, Mogilner AJ, Rockwood K (2001) Accumulation of deficits as a proxy measure of aging. Sci World J 1:323–336
Mitnitski A, Song X, Skoog I, Broe GA, Cox JL, Grunfeld E, Rockwood K (2005) Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc 53:2184–2189
Mitnitski A, Song X, Rockwood K (2012) Trajectories of changes over twelve years in the health status of Canadians from late middle age. Exp Gerontol 47:893–899
Mitnitski A, Song X, Rockwood K (2013) Assessing biological aging: the origin of deficit accumulation. Biogerontology 14:709–717
Mitnitski AB, Taneja S, Rutenber A, Rockwood K (2014) The origin of biological age: statistical mechanics of deficit accumulation. Gerontologist 54(suppl 2):164
Mitnitski A, Collerton J, Martin-Ruiz, Jagger C, von Zglinicki T, Rockwood K, Kirkwood TBL (2015) Age-related frailty and its association with biological markers of intrinsic ageing (under review)
Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE (2013) The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev 12:661–684
Parks RJ, Fares E, Macdonald JK, Ernst MC, Sinal CJ, Rockwood K, Howlett SE (2012) A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci 67:217–227
Rockwood K, Mitnitski A (2006) Limits to deficit accumulation in elderly people. Mech Ageing Dev 127:494–496
Rockwood K, Mitnitski A (2007) Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 62:722–727
Rockwood K, Mitnitski A, Song X (2011) Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ 59:814–821
Shi J, Song X, Yu P, Tang Z, Mitnitski A, Fang X, Rockwood K (2011) Analysis of frailty and survival from late middle age in the Beijing longitudinal study of aging. BMC Geriatr 11:17
Singh MP, Tambay JL, Krawchuk S (1994) The National Population Health Survey: design and issues. In: Proceedings of the Survey Research Methods Section, American Statistical Association pp 803–808. Available: www.amstat.org/sections/srms/proceedings/papers/1994_138.pdf. Accessed 15 Mar 2015
Taneja S, Rutenberg A, Mitnitski A, Rockwood K (2014) A dynamical network model for frailty-induced mortality. Bull Am Phys Soc 59:1
Theou O, Brothers TD, Peña FG, Mitnitski A, Rockwood K (2014) Identifying common characteristics of frailty across seven scales. J Am Geriatr Soc 62:901–906
Wang E (2007) MicroRNA, the putative molecular control for mid-life decline. Ageing Res Rev 6:1–11
Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, Howlett SE (2014) A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci 69:621–632
Woo J, Goggins W, Sham A, Ho SC (2006) Public health significance of the frailty index. Disabil Rehabil 28:515–521
Yanai H, Budovsky A, Tacutu R, Fraifeld VE (2011) Is rate of skin wound healing associated with aging or longevity phenotype? Biogerontology 12:591–597
Yanai H, Toren D, Vierlinger V, Hofner M, Nöhammer C, Chilosi M, Budovsky A, Fraifeld VE (2015) Wound healing and longevity: lessons from long-lived αMUPA mice. Aging (Albany NY) 7:167–176
Yashin AI, Arbeev KG, Akushevich I, Kulminski A, Akushevich L, Ukraintseva SV (2007) Stochastic model for analysis of longitudinal data on aging and mortality. Math Biosci 208:538–551
Yashin AI, Arbeev KG, Akushevich I, Kulminski A, Ukraintseva SV, Stallard E, Land KC (2012) The quadratic hazard model for analyzing longitudinal data on aging, health, and the life span. Phys Life Rev 9:177–188
Acknowledgments
This project was sponsored by an operating grant from the Canadian Institutes of Health Research, No. MOP24388. The National Population Health Study was carried out by Statistics Canada. The authors obtained access to the data through an agreement with the Atlantic Research Data Centre of Statistics Canada, which obliged them to operate, for these purposes only, as “deemed employees” of Statistics Canada. Statistics Canada officials reviewed the analyses to ensure that confidentiality had not been breached and that the data were weighted properly to produce uniform estimates across reports. In no other way did any sponsor have influence over the data analyses. The authors also thank Dr. X Song for her for assistance in data preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitnitski, A., Rockwood, K. The rate of aging: the rate of deficit accumulation does not change over the adult life span. Biogerontology 17, 199–204 (2016). https://doi.org/10.1007/s10522-015-9583-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-015-9583-y