Abstract
Background
In addition to their pivotal roles in coagulation and thrombosis, platelets are crucial in tumor progression, with plenty of clinical and experimental data demonstrating that the interplay of platelets and tumor cells is essential for hematogenous tumor metastasis. After detach from primary sites, tumor cells intravasate into the blood circulation becoming circulating tumor cells and induce platelet activation, aggregation and encasement around tumor cells to form micro tumor thrombi, which create a permissive tumor microenvironment for metastasis. Platelets in micro tumor thrombi protect tumor cells from immune surveillance and anoikis (detachment-triggered apoptosis) through various pathways, which are significant for tumor cell survival in the bloodstream. Moreover, platelets can facilitate tumor metastasis by expediting epithelial-mesenchymal transition (EMT), adhesion to the endothelium, angiogenesis, tumor proliferation processes and platelet-derived microvesicle (PMV) formation.
Conclusions
Here, we provide a synopsis of the current understanding of the formation of micro tumor thrombi and the role of micro tumor thrombi in tumor hematogenous metastasis based on the tumor-platelet interplay. We also highlight potential therapeutic strategies targeting platelets for tumor treatment, including cancer-associated platelet-targeted nanomedicines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The prognosis of tumor patients has substantially improved due to standardized therapy, including surgical excision, chemotherapy and radiotherapy. However, the development of metastasis, which represents advanced disease, has become the major cause of cancer-related death [1]. Therefore, unraveling the mechanism of tumor metastasis is of great importance to improve the prognosis of patients with advanced tumors. Hematogenous tumor metastasis commonly involves the following events: (i) invasion of tumor cells into the extracellular matrix (ECM) of primary sites and intravasation; (ii) escape from immune surveillance and acquisition of anoikis resistance, which are vital for circulating tumor cells (CTCs) survival in blood circulation; and (iii) adhesion to blood vascular endothelium, extravasation and invasion of metastatic sites, in which tumor cells adapt to the new microenvironment and proliferate (Fig. 1).
Overview of the tumor hematogenous metastasis process. Tumor cells migrate from primary sites and undergo intravasation by breaking through the extracellular matrix (ECM) and vascular endothelium. In circulation, circulating tumor cells (CTCs) induce platelet activation and aggregation. Activated platelets encase CTCs to form micro tumor thrombi and protect CTCs from immune surveillance mediated by NK cells. In addition, platelets trigger CTCs to undergo the epithelial-mesenchymal transition (EMT) process, during which tumor cells obtain mesenchymal traits and enhanced invasiveness. Platelets facilitate CTC adhesion to the vascular endothelium and extravasation to distal organs, in which tumor cells adapt to the local microenvironment and proliferate into metastatic foci
Generated from megakaryocytes in bone marrow, platelets are small enucleated cells circulating in the bloodstream. Physiologically, platelets are essential in coagulation and thrombosis processes, maintaining the integrity of vascular walls. Upon vascular injury, platelets adhere to disrupted vascular endothelium through the interaction of (GP)Ibα and von Willebrand factor (vWF). Platelet surface integrins could bind to collagen on disrupted endothelium surface, thus leading to platelet activation and thereafter release of ADP, TXA2 and serotonin that facilitate platelet aggregation. Recently, platelet-derived microvesicles (PMVs) have attracted much attention due to their participation in many physiological and pathological conditions. In fact, PMVs are the major part of microvesicles in blood circulation [2]. PMVs are derived from inversion of the plasma membrane and carry cytosolic components to the outer space. Due to their origin, PMVs contain platelet-derived components encapsulated with a lipid bilayer mirroring the platelet membranes. Thus, PMVs could be deemed mediators of platelet functions and play their roles through both internal bioactive components and external surface ligands [3].
Patients with tumor thrombi have much higher metastatic rates and poorer prognoses than other patients [4]. During intravasation, cells within the tumor mass can secrete microvesicles that induce tumor-derived signatures in platelets [5]. Moreover, those cells could detach and intravasate, becoming CTCs. After contact with platelets, CTCs can be activated and encased by platelets, thus forming a micro tumor thrombi [6, 7]. The storage of a plethora of cytokines and platelets has recently been indicated to participate in the regulation of inflammatory responses, including tumor progression [8]. During tumor-induced inflammation, platelets are the first responding cells because they are small, abundant and store numerous bioactive molecules [9]. Activated platelets can aid in tumor progression and metastasis via various pathways: (i) platelets are able to secrete immunosuppressive cytokines and transfer inhibitive ligands to the CTC surface to protect CTCs from immune surveillance; (ii) platelet derived growth factors can facilitate tumor proliferation and EMT; and (iii) by regulating immune cells, including neutrophils, monocytes and macrophages, platelets create a permissive microenvironment for CTC metastasis.
2 Micro tumor thrombi formation
During hematogenous metastasis, CTCs in the bloodstream have to survive in high shear forces and bypass immune surveillance to form metastatic foci [10]. To escape from shear forces and immune surveillance, tumor cells trigger abnormal platelet activation and aggregation (tumor cell-induced platelet aggregation, TCIPA), which leads to tumor thrombi formation [6, 7]. CTCs achieve this via direct cell interaction and secretion of tumor-derived releasates (Fig. 2). Podoplanin (PDPN), a membrane protein expressed on some tumor cells, including osteosarcoma, squamous cell carcinoma and brain tumors, is the ligand of C-type lectin receptor type 2 (CLEC-2), which is expressed almost exclusively on platelets [11,12,13]. Through such direct binding, tumor cells can stimulate platelets, induce TCIPA, and expedite hematogenous tumor metastasis [11, 14, 15]. Studies have shown that abrogation of either PDPN or CLEC-2 function could significantly impede tumor hematogenous metastasis in mouse models [12, 16, 17]. Disruption of the PDPN and CLEC-2 interaction leads to blockade of platelet and CTC adhesion, which is detrimental for CTC survival in the circulation. After direct contact, stimulated platelets secrete the chemokines CXCL5 and CXCL7, leading to neutrophil recruitment and tumor microenvironment formation [18].
Schematic diagram of micro tumor thrombi formation. Circulating tumor cells (CTCs) induce platelet activation via direct contact and tumor-derived mediators (TF, thrombin and ADP, etc.). Activated platelets are capable of encasing CTCs, leading to the formation of micro tumor thrombi and the tumor microenvironment
Aside from their direct interactions with cells, tumor cells can release numerous mediators (e.g., tissue factor (TF) [19], thrombin [20], ADP [21], TXA2 [22], matrix metalloproteinases (MMPs) [23], high-mobility group box1 (HMGB1) [24], CD97 [25], cancer cell-derived IgG [26], and mucins [27]) to initiate platelet activation and subsequent micro tumor thrombi formation. In fact, patients with tumors are more prone to thrombocytosis and elevated biomarkers of platelet activation, and the risk of venous thromboembolism in tumor patients is much higher than that in the general population (5–15% for tumor patients vs. 1–2 cases per 1000 people per year in the general population) [11, 28, 29]. Tumor patients exhibit overexpression of TF and coagulation factor VII, which are critical in the extrinsic coagulation pathway [30]. TF expressed on tumor cell membranes and secreted as tumor-derived microparticles can initiate the extrinsic coagulation pathway by interacting with coagulation factor VII and coagulation factor X [19, 31]. Thrombin generated from the extrinsic coagulation cascade and some types of tumor cells (e.g., pancreatic and lung cancer cell lines) can activate platelets and accelerate tumor thrombosis [20, 32]. Moreover, a study indicated that platelets could upregulate TF expression in ovarian cancer [33], implicating the interplay between tumor cells and platelets via TF.
ADP and TXA2 are classical platelet agonists that participate in the coagulation cascade and induce thrombosis [34]. ADP expressed by tumor cells can interact with platelet receptors P2Y12 and P2Y1, which contributes to TCIPA and the release of ADP and TXA2, which are stored in platelet granules [28]. Increased ADP and TXA2 expression in the tumor anoxic microenvironment leads to positive feedback in the platelet activation cascade, which is conducive to the generation of metastatic foci [21, 22, 32].
MMPs are proteolytic enzymes that participate in ECM degradation and remodeling, which are crucial in the processes of tumor invasion and metastasis [35]. Interestingly, MMPs derived from tumor cells are indicated to elicit platelet activation and TCIPA [36]. MMP-1 released from breast cancer cells could foster ADP release, triggering TIPCA through a positive feedback pathway [28]. Tumor cell derived MMP-2 can induce TCIPA by binding to platelet integrin αIIbβ3 [37, 38]. In turn, activated platelets can release factors that upregulate MMP expression in melanoma cells [23]. Moreover, MMPs restored in platelet α-granules are released upon platelet activation and can degrade the basement membrane, thus supporting tumor metastasis [39].
Some tumor cells can release high-mobility group box 1 (HMGB1), which binds to platelet toll-like receptor 4 (TLR4), leading to platelet activation and tumor extravasation [24]. Both primary and metastatic tumors have been suggested to express CD97, which can activate platelets and enhance lysophosphatidic acid release, leading to increased tumor invasiveness and transendothelial migration [25, 40]. In contrast to B lymphocytes, tumor cells have been reported to produce immunoglobin G (IgG), which can bind to platelet FcgRIIa, thus initiating platelet activation [26, 41]. Shao et al. demonstrated that cancer mucins bind to both platelets and neutrophils and trigger their mutual activation [27, 42].
Among all the mediators listed above, some could serve as biomarkers for cancer-associated thrombosis and are related to tumor prognosis. Circulating TF-positive microparticles are related to venous thromboembolism in many tumor types (including pancreatic cancer, colorectal cancer and glioma) [43,44,45]. High expression of PDPN is associated with worse prognosis in glioma [46]. Moreover, PDPN expression in glioma patients has a positive correlation with the D-dimer level [47], which is a classical marker reflecting coagulation function.
Overall, tumor cells are capable of inducing platelet activation by various pathways. Activated platelets surround tumor cells and form micro tumor thrombi that are conducive to tumor cell survival in the blood circulation. Releasates from activated platelets recruit leukocytes and form tumor niches facilitating tumor development and metastasis.
3 Role of micro tumor thrombi during hematogenous metastasis
3.1 Immune evasion
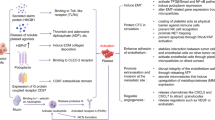
Tumor cells entering the blood circulation as CTCs are prone to be recognized and eliminated by cytotoxic immune cells (e.g., natural killer (NK) and CD8+ T cells) [48]. This process is called immune surveillance. CTCs are capable of inducing platelet activation and aggregation via direct interaction and release of various mediators. Activated platelets can encase CTCs via integrins, fibrin, and P-selectin, shielding them physically and immunologically from immune surveillance and leading to distant metastasis [14, 42, 49, 50]. It has been demonstrated that platelets protect CTCs from both NK and CD8+ T cells. Nieswandt et al. first used thrombocytopenic mice to indicate that platelets impede NK-cell-mediated cytolysis of tumor cells [51]. Palumbo et al. further substantiated this hypothesis using mice lacking Gαq (a G protein vital for platelet activation) and fibrin [52]. Deficiency in any of them resulted in diminished tumor cell survival. Moreover, the diminution of tumor cell survival was obliterated in mice with NK-cell depletion [53]. Platelets impede NK-cell-mediated cytolysis via cell contact and cytokine interactions [54]. Platelets can transfer ligands that are suppressive for NK-cell function to the CTC surface, such as MHC class I [55], glucocorticoid-induced TNF-related protein ligand (GITRL) [56], receptor activator of NF-kB ligand (RANKL) [57], and PD-L1 [58]. Placke et al. revealed that platelets encasing CTCs could transfer MHC class I molecules onto the tumor cell surface, thus disrupting immune recognition and immune surveillance by NK cells [55]. Recently, Zaslavsky et al. reported that PD-L1-negative tumors could escape immune surveillance with the help of platelet-derived PD-L1 [59]. In addition to direct contact with tumor cells, platelets activated by CTCs can secrete transforming growth factor-β (TGF-β), which is capable of downregulating NKG2D on NK cells and suppressing antitumor immunity [60]. In fact, it has been reported that platelets are the major source of TGF-β in both the tumor microenvironment and human body [61,62,63]. Furthermore, platelets express TGF-β-docking receptor glycoprotein A repetitions predominant (GARP), which can activate latent TGF-β in nearby platelets [61]. In addition to inhibiting NK-cell activity, TGF-β can convert CD4+ T cells into regulatory T cells that lead to immunosuppression in the tumor microenvironment [64]. TGF-β and lactate are major platelet-derived mediators that dampen CD4+ and CD8+ T-cell activity [61]. Moreover, TGF-β has been proven to be capable of downregulating NK-cell function by impeding cytokine production and degranulation [60, 65].
As regulators of inflammation, platelets can modulate not only NK cells and CD8+ T cells but also neutrophils, monocytes and macrophages [66]. Neutrophils are recruited to inflammatory sites via L-selectin and PECAM-1 [66, 67]. Activated platelets can trigger neutrophil extracellular trap (NET) formation by binding to neutrophils, which is mediated by the binding of platelet P-selectin and neutrophil P-selectin glycoprotein ligand-1 (PSGL-1) [68]. Platelet ICAM-2, CD40L, GPIb and GPIIb/IIa can facilitate the binding between platelets and neutrophils [66]. GSF released by tumor cells can promote neutrophil production and NET formation [69]. Tumor cell-derived HMGB1 is capable of inducing NET formation through ligation with RAGE (receptor for advanced glycation end products) or TLR4 on neutrophils [70]. In addition, tumor-primed platelets could facilitate tumor cell-induced NET formation [71]. Neutrophil extracellular traps (NETs) consist of nuclear or mitochondrial DNA decorated by histones and proteins secreted from activated neutrophils [72]. Conventionally, the major role of NETs is to assist neutrophils in eliminating pathogens [73]. In recent years, NETs have been indicated to be involved in tumor progression, dissemination and metastasis [74]. CTCs can be entrapped by NETs via β1-integrin-mediated interactions, thus preventing immune surveillance and promoting tumor thrombosis and metastasis [75]. NETs can augment tumor growth through direct alteration of metabolic programming [76]. Recently, NETs were found to be engaged in tumor relapse, during which NETs could reinvigorate dormant tumor cells and lead to metastasis [77]. Monocytes adhered to platelets are activated by direct binding and release procoagulant and TF-positive microvesicles [66]. TF-positive CTCs could activate procoagulant proteins and coat themselves with fibrin, which led to immune evasion and increased adherence to distant sites. Platelet-derived TGF-β suppresses macrophage proinflammatory function and may correlate with the polarization of the M2 phenotype, which exerts immunosuppressive and proangiogenic functions [78, 79]. In general, platelets induce CTC immune evasion by interacting with immunocytes in direct and indirect manners (Fig. 3).
3.2 Anoikis resistance
Anokis is a form of programmed cell death due to the loss of interaction between the cell and ECM [80]. Platelets were indicated to induce CTC anokis resistance by several pathways (Fig. 3). Platelet-derived autotaxin converts lysophosphatidylcholine to lysophosphatidic acid, which can adhere to the CTC LPAR-1 receptor and initiate CTC anokis resistance via the RhoA-Gα12/13-YAP-1 pathway [25, 81, 82]. Through initiating RhoA-MYPT1-PP1-mediated YAP1 dephosphorylation as well as facilitating its nuclear translocation, platelets impede apoptosis in tumor cells and upregulate gene expression with a prosurvival trait [82]. Li et al. reported that plateletderived growth factorBB inhibited anoikis and promoted tumor progression via the Hippo/YAP signaling pathway [83]. PDGF, a growth factor secreted from platelets, has been reported to induce fibroblast apoptosis resistance through the Ras/PI3K/Akt pathway [84]. Cacic et al. found that platelet microparticles could protect acute myelogenous leukemia cells from daunorubicin-induced apoptosis via overexpression of miR-125a and miR-125b, thus leading to chemotherapy resistance [85].
3.3 Promotion of proliferation
Platelets can release various mitogenic proteins and growth factors to boost tumor cell proliferation [14, 28, 86], including TGF-β, PDGF, VEGF, platelet factor 4 (PF4), insulin-like growth factor (IGF)-I, stromal cell-derived factor-1 (SDF-1) and angiopoietin (Fig. 3). Platelet-derived TGF-β can foster tumor growth by binding to the tumor cell receptor TGFβR1 in ovarian cancer [62, 87]. PDGF receptor (PDGFR) is normally expressed on mesenchymal cells, while epithelial tumor cells can express PDGFR via the EMT process induced by TGF-β, thus leading to a proliferation response to PDGF [88]. VEGF released by platelets was indicated to increase tumor cell proliferation in breast cancer via interplay with VEGFR-2 and the integrin signaling pathway [89]. The role of PF4 in tumor proliferation is controversial. Pucci et al. reported that PF4 is a cancer-enhancing endocrine signal and can accelerate tumor growth in lung cancer [90]. However, Wang et al. demonstrated that PF4 can be a potential antitumor target due to its inhibitory role in tumor angiogenesis [91]. Platelet-derived SDF-1 can initiate intracellular signaling through several diverse pathways, leading to upregulated proliferation in ovarian cancer [92].
In addition to releasing of growth factors, platelets can expedite tumor growth by direct contact [39]. The interaction of platelet-expressed CLEC-2 and tumor cell-expressed PDPN can stimulate proliferation in lung cancer [93]. Contact of ADP and the P2Y12 receptor expressed on platelets results in tumor growth via the ASK1-JNK/P38 signaling pathway [94].
3.4 Facilitation of EMT
EMT is a process during which epithelial cells lose their epithelial identity and obtain mesenchymal traits, which is associated with tumor invasiveness and metastasis [95]. A recent study suggested that retarding tumor-platelet crosstalk using activated platelet-targeting nanoparticles could suppress the tumor EMT process and metastasis in breast cancer [96]. In fact, platelets were found promote EMT via several pathways (Fig. 3) [97]. TGF-β is the major cytokine mediating the acceleration of the EMT effect by platelets [97, 98]. In ovarian cancer, patients with higher TGF-β levels were found to have elevated platelet counts [97]. Ovarian cancer cells cocultured with platelets showed increased TGF-β levels and higher expression of mesenchymal markers [97]. The TGF-β type I receptor inhibitor A83-01 inhibited EMT and platelet-mediated tumor progression in vitro and in vivo [97], indicating that platelets induce the EMT process via a TGF-β-dependent pathway. Moreover, direct platelet-tumor cell interactions were demonstrated to synergistically enhance the EMT process and metastasis with platelet-derived TGF-β via the TGF-β/Smad and NF-kB pathways in colorectal cancer [98]. In line with this, PDPN-CLEC-2 and integrin α2β1 interactions could trigger TGF-β release from platelets and accelerate the EMT process [99,100,101]. In addition to TGF-β, TANK-binding kinase 1 (TBK1) mediates the platelet-induced EMT process [102]. TBK1 activation paralleled the platelet-induced EMT process in mammary carcinoma cells. The platelet-induced EMT process and NF-kB activation were impeded via ablation of TBK1 expression, suggesting that TBK1 is involved in platelet-induced EMT and NF-kB signaling.
3.5 Mediation of tumor cell adhesion
Platelet membranes express various adhesion molecules, including integrins (αIIbβIII, α2β1, α5β1, α6β1, αLβ2, αvβ3), glycoprotein (GP) Ib-IX-V, CLEC-2, GPVI and P-selectin [103,104,105]. These molecules mediate the adhesion of platelets, endothelial cells, and CTCs [106]. Platelet αIIbβIII and P-selectin mediate tumor cell rolling, tethering and stable adhesion along the endothelium under dynamic flow conditions [107]. P-selectin has been reported to exert a pivotal role in the interaction of platelets, endothelial cells, and CTCs [42]. In platelets, P-selectin is normally stored in α-granules and can be translocated to the platelet surface upon platelet activation, leading to the contact of platelets, endothelial cells, and CTCs [108]. In addition, P-selectin induces the interaction of platelets, endothelial cells and neutrophils by binding to mucins and PSGL-1 [109].
Integrin α6β1 expressed on platelets can bind to ADAM9 expressed on tumor cells, which induces tumor metastasis [110]. Expressed on tumor cells, αvβ3 can facilitate the interaction among tumor cells, platelets and vasculature [111]. When it colocalizes with αvβ3, nectin-like molecule 5 (NECL5) is capable of inducing tumor cell adhesion of the endothelium via contact with CD226 on the platelet surface [112].
As a key receptor for collagen, platelet GPVI can facilitate tumor cell adhesion to endothelial cells, promoting tumor cell arrest and metastasis [113]. Tumor cell-derived galectin-3 could interact with GPVI on platelets to promote tumor cell extravasation and metastasis [114].
3.6 Induction of angiogenesis and modulation of endothelial cells
During tumor progression and metastasis, tumor cells require angiogenesis to generate new microvessels that provide sufficient nutrients and growth factors to support tumor growth [115]. Platelets have been found to participate in both early and advanced stages of cancer during angiogenesis and are pivotal in stabilizing neovascularization (Fig. 3) [116]. Platelet α-granules contain multiple molecules that can regulate angiogenesis and are released upon platelet activation [104, 117]. Molecules, including VEGF, PDGF, epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), are proangiogenic factors and can induce angiogenesis [118]. Moreover, platelet α-granules also contain anti-angiogenic factors, including angiostatin, PF4, thrombospondin-1 (TSP1) and endostatin [117]. Platelets can release different kinds of angiogenic molecules according to external stimuli [117, 119]. Platelets stimulated by ADP can expedite the release of proangiogenic factors such as VEGF via activation of the PAR1 receptor, while platelets stimulated by TXA2 induce the release of antiangiogenic factors such as endostatin through activation of PAR4 [117]. As an agonist of platelet activation, thrombin also participates in angiogenesis by facilitating the release of proangiogenic factors as well as increasing the permeability of the endothelial cell barrier [120]. Thrombin-, ADP- and TF-stimulated platelets are prone to secrete a plethora of VEGF, which is the most efficient proangiogenic factor [121]. PMVs contain plentiful RNAs, receptors and proteins and were shown to exert proangiogenic effects just as strong as platelets in angiogenesis through the PI3-kinase, Src kinase and ERK signaling pathways [49, 122]. In addition to secreting angiogenic molecules and PMVs, platelets were found to induce angiogenesis via direct contact with endothelial cells [123]. Integrin αIIbβIII and tetraspanin were reported to participate in the direct interaction of platelets and endothelial cells as well as in platelet-induced angiogenesis [124].
In addition to the induction of angiogenesis, platelets also modulate endothelial cells to facilitate the intravasation and extravasation of tumor cells during metastasis. Tumors in primary sites induce local angiogenesis to intravasate into the blood circulation, since new blood vessels have infirm tight junctions and are easy to cross [125]. During this process, platelets secrete factors (e.g., VEGF and TGF-β) to coordinate angiogenesis and disrupt endothelial cell function [126]. After tumor cell intravasation into the bloodstream, platelets further promote the transendothelial migration of tumor cells, which is the process by which tumor cells cross the endothelial barrier. Ward et al. showed that platelet interaction with tumor cell CD97 led to bidirectional signaling that caused platelet release of ATP and tumor cell activation of CD97/LPAR dependent RHO signaling [25]. Platelet-derived ATP resulted in disruption of endothelial tight junction thus increasing vascular permeability [127], while RHO signaling activation made tumor cells more invasive. The bidirectional signaling mentioned above coordinated tumor cell transendothelial migration, which is crucial for tumor metastasis.
Role of micro tumor thrombi in hematogenous tumor metastasis. Platelets in micro tumor thrombi could induce CTC anokis resistance and immune evasion, allowing CTCs to survive in blood circulation. In addition, platelets in micro tumor thrombi are indicated to enhance tumor progression and metastasis via several mechanisms, including EMT, tumor proliferation, angiogenesis and adhesion
3.7 Platelet-derived microvesicle (PMVs)
Upon activation, platelets can release PMVs that contain mRNA, microRNA, DNA, proteins, cytokines, and second messengers [128, 129]. As extracellular membrane vesicles, PMVs have been found to promote metastasis through multiple mechanisms: (i) delivery of membrane receptors to tumor cells and stromal cells; (ii) transfer of mRNA, protein and second messengers that lead to epigenetic alteration in recipient cells; and (iii) direct activation of target cells through PMV surface ligands [130]. Recent studies have shown that PMVs can transfer their membrane receptors, including CXCL4, CD41, CD61 and CD62, to tumor cells to increase tumor migration, metastasis and adhesion [128, 131]. PMVs, which are rich in mRNAs and microRNAs, can regulate the gene expression of recipient cells. With a plethora of genetic material, PMVs can also regulate the gene expression of tumor cells. In an ovarian cancer cell line, PMVs induced the EMT process and increased tumor progression by delivering miR-939 [132]. In breast cancer cells, PMVs delivered TPM3 mRNA to tumor cells, thus augmenting tumor invasion [133]. However, another study pointed out that PMVs infiltrated solid tumors and transferred miRNAs to tumor cells to induce apoptosis and inhibit tumor growth [134]. In the tumor microenvironment, PMV surface phosphatidylserine can bind to the phosphatidylserine receptor on immune cells, thus inhibiting the antitumor immune response and initiating an immunosuppressive environment [135]. In lung cancer, PMVs acted as a chemoattractant for 4 of 5 lung cancer cell lines and promoted proliferation via the MAPK-p42/44 and PI-3 K-AKT signaling pathways [136].
4 Therapeutic strategies targeting platelets for tumor treatment
Current platelet-based antitumor therapeutic strategies can be classified into two major approaches. One method is directly abrogating platelet function via antiplatelet agents. The other method is delivering antitumor or antiplatelet drugs through cancer-associated platelet-targeted nanomedicines [86, 137]. Antiplatelet agents including nonsteroidal anti-inflammatory drugs (NSAIDs) and antagonists targeting integrins, ADP, CLEC-2 and P-selectin have shown therapeutic potential for tumor treatment [138, 139]. For example, the classical antiplatelet agent aspirin was found to have an antitumor effect in colorectal cancer as early as 1988 [140]. Since then, the antitumor effect of aspirin has gradually been extended to other types of tumors, including breast cancer, gastric cancer, liver cancer, and ovarian cancer [141,142,143]. Nevertheless, most studies focusing on these agents were in the early stage and could not reach a unified conclusion. In the ASPREE trial, McNeil et al. enrolled 19,114 community-dwelling persons with 70 years of age or older and randomly divided them into a low-dose aspirin group (100 mg of enteric-coated aspirin) and a placebo group [144,145,146]. The ASPREE trial results showed that use of low-dose aspirin caused higher risk of death from any cause and higher cancer-related death compared to placebo, indicating the clinical application of aspirin for primary prevention of tumor in patients older than 70 years old should be cautious. Moreover, the side effects of antiplatelet agents, including hemorrhage and thrombocytopenia, limit their clinical application [147]. Strategies targeting downstream signaling following platelet activation, such as TGF-β, PDGF, and VEGF signaling, have shown potential therapeutic value with better safety profiles [148,149,150]. Lenvatinib, an inhibitor of VEGF and PDGF receptors, has shown activity with noninferior efficacy to sorafenib (a first-line treatment for unresectable hepatocellular carcinoma) in terms of overall survival in advanced hepatocellular carcinoma [151]. Bintrafusp Alfa, a reprogrammed antibody that can simultaneously inhibit TGF-β and PD-L1, has been proven to exert antitumor effects by overcoming immune evasion both in vitro and in vivo [152, 153].
Another category of platelet-based therapy accomplishes targeted therapy using cancer-associated platelet-targeted nanomedicines that could adhere to platelets via their platelet adhesion molecules [154]. With the design of nanoparticles with antibodies targeting platelet receptors, including P-selectin, GPIb and GPIIb/IIIa, tumors can be imaged and localized in vivo [155]. Similarly, the coating of antitumor agents with platelet membranes has been shown to exert tumoricidal effects and is not affected by tumor heterogeneity [156]. A P-selectin-targeting peptide was applied, and nanoparticles with PSN peptide modification exhibited increased accumulation at both the primary tumor site and metastases due to their ability to capture activated platelets [96]. Therefore, nanoparticles could inhibit tumor metastasis in nearly every crucial stage in a consecutive manner. Coating of drugs with platelet membranes by using mesoporous silica nanoparticles, vascular disruption agents and antiangiogenic drugs achieved significant vascular disruption and antiangiogenic efficacy because the platelet membrane could aggregate at the damaged vessel walls of the tumor through targeted adhesion [157]. Likewise, the TLR agonist R848 coated with platelet membranes initiated a local immune response and caused tumor regression in colorectal and breast tumor models [158]. Platelets conjugated with anti-programmed cell death-ligand 1 (aPDL1) antibody could induce an immune response and inhibit tumor metastasis in breast tumors through the release of aPDL1-decorated PMVs [159]. A type of bioengineered platelet composed of internal-loaded doxorubicin and external-loaded aPDL1 has been reported to decrease tumor recurrence and metastasis in a postsurgical melanoma model [160]. In summary, the major advantages of cancer-associated platelet-targeted nanomedicines are as follows: (i) they protect nanoparticles from elimination in the circulation; (ii) they have relatively high targetability due to the close interplay between platelets and CTCs; and (iii) they exhibit increased circulation period and loading capacity [161]. However, there are still limitations that need to be overcome before these cancer-associated platelet-targeted nanomedicines can be clinically applied. For example, all of these nanomedicines have more or less bleeding risk and some nanomedicines are found not only in tumor tissues but also in the liver, which may lead to liver toxicity [86]. Therefore, the design of more specific and tolerable agents is necessary for the clinical application of nanomedicines.
5 Conclusion
As a critical process during tumor metastasis, tumor cells that undergo intravasation become CTCs with no tumor stroma component and have to cope with high shear force, immune surveillance and anokis to achieve distal metastasis. To survive in the circulation, tumor cells interact with platelets to form micro tumor thrombi, during which process platelets are activated and educated by direct contact as well as the action of tumor cell-derived bioactive molecules. Activated platelets then aggregate and encase tumor cells to form micro tumor thrombi, interact with tumor cells and immune cells to form a permissive tumor microenvironment, and foster tumor progression and metastasis through various pathways. Therefore, therapies targeting platelets and their corresponding signaling pathways have encouraging therapeutic prospects for tumor treatment. Unraveling the interplay between platelets and tumor cells during micro tumor thrombi formation and tumor metastasis will provide a deeper understanding of the mechanism of tumor progression and will be conducive to designing novel therapeutic agents that specifically target tumor lesions without apparent effects on platelet physiological functions.
Data availability
Not applicable.
References
G.P. Gupta, J. Massague, Cancer metastasis: building a framework. Cell 127(4), 679–695 (2006)
D. Varon, E. Shai, Platelets and their microparticles as key players in pathophysiological responses. J. Thromb. Haemost 13(Suppl 1), S40–S46 (2015)
M. Dovizio et al., Platelets and extracellular vesicles in cancer: diagnostic and therapeutic implications. Cancer Metastasis Rev 37(2–3), 455–467 (2018)
P.H. Liu, T.I. Huo, R.A. Miksad, Hepatocellular Carcinoma with Portal Vein Tumor involvement: best management strategies. Semin Liver Dis 38(3), 242–251 (2018)
P. Kanikarla-Marie et al., Platelets, circulating tumor cells, and the circulome. Cancer Metastasis Rev 36(2), 235–248 (2017)
L.J. Gay, B. Felding-Habermann, Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11(2), 123–134 (2011)
X. Zhong et al., Circulating tumor cells in cancer patients: developments and clinical applications for immunotherapy. Mol. Cancer 19(1), 15 (2020)
A.T. Franco, A. Corken, J. Ware, Platelets at the interface of thrombosis, inflammation, and cancer. Blood 126(5), 582–588 (2015)
D.G. Menter et al., Platelet “first responders” in wound response, cancer, and metastasis. Cancer Metastasis Rev 36(2), 199–213 (2017)
K.J. Luzzi et al., Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol 153(3), 865–873 (1998)
K. Suzuki-Inoue, Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood 134(22), 1912–1918 (2019)
A. Takemoto, K. Miyata, N. Fujita, Platelet-activating factor podoplanin: from discovery to drug development. Cancer Metastasis Rev 36(2), 225–234 (2017)
K. Suzuki-Inoue, M. Osada, Y. Ozaki, Physiologic and pathophysiologic roles of interaction between C-type lectin-like receptor 2 and podoplanin: partners from in utero to adulthood. J. Thromb. Haemost 15(2), 219–229 (2017)
L. Yu et al., Bidirectional interaction between cancer cells and platelets provides potential strategies for cancer therapies. Front. Oncol 11, 764119 (2021)
X. Wang et al., Blocking podoplanin inhibits platelet activation and decreases cancer-associated venous thrombosis. Thromb. Res 200, 72–80 (2021)
Y. Kato et al., Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci 99(1), 54–61 (2008)
T. Shirai et al., C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J. Thromb. Haemost 15(3), 513–525 (2017)
J.M. Ponert et al., The mechanisms how heparin affects the tumor cell induced VEGF and chemokine release from platelets to attenuate the early metastatic niche formation. PLoS ONE 13(1), e0191303 (2018)
A. Mitrugno et al., The prothrombotic activity of cancer cells in the circulation. Blood Rev 30(1), 11–19 (2016)
E. Heinmoller et al., Studies on tumor-cell-induced platelet aggregation in human lung cancer cell lines. J. Cancer Res. Clin. Oncol 122(12), 735–744 (1996)
M. Zucchella et al., Human tumor cells cultured “in vitro” activate platelet function by producing ADP or thrombin. Haematologica 74(6), 541–545 (1989)
B.W. Steinert et al., Studies on the role of platelet eicosanoid metabolism and integrin alpha IIb beta 3 in tumor-cell-induced platelet aggregation. Int. J. Cancer 54(1), 92–101 (1993)
V.O. Melnikova et al., Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J. Biol. Chem 281(5), 2911–2922 (2006)
L.X. Yu et al., Platelets promote tumour metastasis via interaction between TLR4 and tumour cell-released high-mobility group box1 protein. Nat. Commun 5, 5256 (2014)
Y. Ward et al., Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell. Rep 23(3), 808–822 (2018)
S. Miao et al., Cancer cell-derived immunoglobulin G activates platelets by binding to platelet FcgammaRIIa. Cell. Death Dis 10(2), 87 (2019)
B. Shao et al., Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood 118(15), 4015–4023 (2011)
S. Mezouar et al., Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb. Res 139, 65–76 (2016)
F. Khan et al., Venous thromboembolism. Lancet 398(10294), 64–77 (2021)
A.K. Kakkar et al., Extrinsic-pathway activation in cancer with high factor VIIa and tissue factor. Lancet 346(8981), 1004–1005 (1995)
A. Mitrugno et al., The role of coagulation and platelets in colon cancer-associated thrombosis. Am. J. Physiol. Cell. Physiol 316(2), C264–C273 (2019)
C.N. Hill et al., Deciphering the role of the coagulation cascade and autophagy in cancer-related thrombosis and metastasis. Front. Oncol 10, 605314 (2020)
R. Orellana et al., Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer 15, 290 (2015)
R. Lordan, A. Tsoupras, I. Zabetakis, Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: potential role of antiplatelet agents. Blood Rev 45, 100694 (2021)
S. Mondal et al., Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. Eur. J. Med. Chem 194, 112260 (2020)
D. Alonso-Escolano et al., Membrane type-1 matrix metalloproteinase stimulates tumour cell-induced platelet aggregation: role of receptor glycoproteins. Br. J. Pharmacol 141(2), 241–252 (2004)
P. Jurasz et al., Matrix metalloproteinase 2 in tumor cell-induced platelet aggregation: regulation by nitric oxide. Cancer Res 61(1), 376–382 (2001)
M. Sebastiano et al., A novel mechanism regulating human platelet activation by MMP-2-mediated PAR1 biased signaling. Blood 129(7), 883–895 (2017)
R. Li et al., Presence of intratumoral platelets is associated with tumor vessel structure and metastasis. BMC Cancer 14, 167 (2014)
M. Steinert et al., Expression and regulation of CD97 in colorectal carcinoma cell lines and tumor tissues. Am. J. Pathol 161(5), 1657–1667 (2002)
G. Lee et al., Molecular and immuno-characteristics of immunoglobulin-like glycoproteins in cancer cell-expressed biomarker, CA215. Immunol. Invest 41(4), 429–446 (2012)
M. Schlesinger, Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol 11(1), 125 (2018)
J.E. Geddings, N. Mackman, Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood 122(11), 1873–1880 (2013)
A.A. Khorana et al., Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J. Thromb. Haemost 6(11), 1983–1985 (2008)
R.S. Kasthuri et al., Effect of chemotherapy and longitudinal analysis of circulating extracellular vesicle tissue factor activity in patients with pancreatic and colorectal cancer. Res. Pract. Thromb. Haemost 4(4), 636–643 (2020)
A. Ernst et al., Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin. Cancer Res 15(21), 6541–6550 (2009)
J. Riedl et al., Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 129(13), 1831–1839 (2017)
H. Gonzalez, C. Hagerling, Z. Werb, Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32(19–20), 1267–1284 (2018)
A. Braun et al., Platelet-cancer interplay: molecular mechanisms and new therapeutic avenues. Front. Oncol 11, 665534 (2021)
L. Schmied, P. Hoglund, S. Meinke, Platelet-mediated protection of cancer cells from immune surveillance - possible implications for cancer immunotherapy. Front. Immunol 12, 640578 (2021)
B. Nieswandt et al., Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59(6), 1295–1300 (1999)
J.S. Palumbo et al., Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105(1), 178–185 (2005)
J.S. Palumbo et al., Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood 110(1), 133–141 (2007)
D.F. Quail, J.A. Joyce, Microenvironmental regulation of tumor progression and metastasis. Nat. Med 19(11), 1423–1437 (2013)
T. Placke et al., Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 72(2), 440–448 (2012)
T. Placke, H.R. Salih, H.G. Kopp, GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J. Immunol 189(1), 154–160 (2012)
K.L. Clar et al., Inhibition of NK Reactivity against solid tumors by platelet-derived RANKL. Cancers (Basel). 11(3), 277 (2019)
S. Pesce et al., PD/1-PD-Ls checkpoint: insight on the potential role of NK cells. Front. Immunol 10, 1242 (2019)
A.B. Zaslavsky et al., Platelet PD-L1 suppresses anti-cancer immune cell activity in PD-L1 negative tumors. Sci. Rep 10(1), 19296 (2020)
H.G. Kopp, T. Placke, H.R. Salih, Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 69(19), 7775–7783 (2009)
S. Rachidi et al., Platelets subvert T cell immunity against cancer via GARP-TGFbeta axis. Sci Immunol. 2(11), eaai7911 (2017)
Q. Hu et al., Role of platelet-derived Tgfbeta1 in the progression of ovarian cancer. Clin. Cancer Res 23(18), 5611–5621 (2017)
R.K. Assoian et al., Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem 258(11), 7155–7160 (1983)
D. Haribhai et al., TGF-beta1 along with other platelet contents augments Treg cells to suppress anti-FVIII immune responses in hemophilia A mice. Blood Adv 1(2), 139–151 (2016)
S. Sadallah et al., Platelet-derived ectosomes reduce NK cell function. J. Immunol 197(5), 1663–1671 (2016)
D. Stoiber, A. Assinger, Platelet-leukocyte interplay in cancer development and progression. Cells. 9(4), 855 (2020)
Y.M. Feng, X.H. Chen, X. Zhang, Roles of PECAM-1 in cell function and disease progression. Eur. Rev. Med. Pharmacol. Sci 20(19), 4082–4088 (2016)
J. Etulain et al., P-selectin promotes neutrophil extracellular trap formation in mice. Blood 126(2), 242–246 (2015)
M. Demers et al., Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. U S A 109(32), 13076–13081 (2012)
J.M. Tadie et al., HMGB1 promotes neutrophil extracellular trap formation through interactions with toll-like receptor 4. Am. J. Physiol. Lung Cell. Mol. Physiol 304(5), L342–L349 (2013)
N. Abdol Razak, O. Elaskalani, P. Metharom, Pancreatic cancer-induced Neutrophil Extracellular Traps: a potential contributor to cancer-associated thrombosis. Int J Mol Sci. 18(3), 487 (2017)
J. Cedervall, A. Hamidi, A.K. Olsson, Platelets, NETs and cancer. Thromb. Res 164(Suppl 1), S148–S152 (2018)
N. Branzk et al., Neutrophils sense microbe size and selectively release Neutrophil Extracellular Traps in response to large pathogens. Nat. Immunol 15(11), 1017–1025 (2014)
M.T. Masucci et al., The emerging role of Neutrophil Extracellular Traps (NETs) in tumor progression and metastasis. Front. Immunol 11, 1749 (2020)
S. Najmeh et al., Neutrophil Extracellular Traps sequester circulating tumor cells via beta1-integrin mediated interactions. Int. J. Cancer 140(10), 2321–2330 (2017)
H.O. Yazdani et al., Neutrophil Extracellular Traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res 79(21), 5626–5639 (2019)
J. Albrengues et al., Neutrophil Extracellular Traps produced during inflammation awaken dormant cancer cells in mice. Science. 361(6409), eaao4227 (2018)
E. Batlle, J. Massague, Transforming growth factor-beta signaling in immunity and Cancer. Immunity 50(4), 924–940 (2019)
V. Thorsson et al., The immune landscape of cancer. Immunity 48(4), 812-830 e14 (2018)
E. Kakavandi et al., Anoikis resistance and oncoviruses. J. Cell. Biochem 119(3), 2484–2491 (2018)
R. Leblanc et al., Interaction of platelet-derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood 124(20), 3141–3150 (2014)
M. Haemmerle et al., Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat. Commun 8(1), 310 (2017)
T. Li et al., Plateletderived growth factorBB mediates pancreatic cancer malignancy via regulation of the Hippo/Yesassociated protein signaling pathway. Oncol. Rep 45(1), 83–94 (2021)
J.A. Romashkova, S.S. Makarov, NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401(6748), 86–90 (1999)
D. Cacic et al., Platelet microparticles protect acute myelogenous leukemia cells against daunorubicin-induced apoptosis. Cancers (Basel). 13(8), 1870 (2021)
M. Geranpayehvaghei et al., Cancer-associated-platelet-inspired nanomedicines for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 13(5), e1702 (2021)
M.S. Cho et al., Platelets increase the proliferation of ovarian cancer cells. Blood 120(24), 4869–4872 (2012)
C.H. Heldin, J. Lennartsson, B. Westermark, Involvement of platelet-derived growth factor ligands and receptors in tumorigenesis. J. Intern. Med 283(1), 16–44 (2018)
L. Jiang et al., Platelet releasate promotes breast cancer growth and angiogenesis via VEGF-integrin cooperative signalling. Br. J. Cancer 117(5), 695–703 (2017)
F. Pucci et al., PF4 promotes platelet production and lung cancer growth. Cell. Rep 17(7), 1764–1772 (2016)
Z. Wang, H. Huang, Platelet factor-4 (CXCL4/PF-4): an angiostatic chemokine for cancer therapy. Cancer Lett 331(2), 147–153 (2013)
B.A. Teicher, S.P. Fricker, CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res 16(11), 2927–2931 (2010)
S. Takagi et al., Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS ONE 8(8), e73609 (2013)
M.S. Cho et al., Role of ADP receptors on platelets in the growth of ovarian cancer. Blood 130(10), 1235–1242 (2017)
B. Bakir et al., EMT, MET, plasticity, and tumor metastasis. Trends Cell. Biol 30(10), 764–776 (2020)
S. Li et al., Targeted inhibition of tumor inflammation and tumor-platelet crosstalk by nanoparticle-mediated drug delivery mitigates cancer metastasis. ACS Nano. 16, 50 (2021)
Y. Guo et al., Platelets promote invasion and induce epithelial to mesenchymal transition in ovarian cancer cells by TGF-beta signaling pathway. Gynecol. Oncol 153(3), 639–650 (2019)
M. Labelle, S. Begum, R.O. Hynes, Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20(5), 576–590 (2011)
N. Fujita, S. Takagi, The impact of Aggrus/podoplanin on platelet aggregation and tumour metastasis. J. Biochem 152(5), 407–413 (2012)
A. Takemoto et al., A critical role of platelet TGF-beta release in podoplanin-mediated tumour invasion and metastasis. Sci. Rep 7, 42186 (2017)
X.X. Zuo et al., Platelets promote breast cancer cell MCF-7 metastasis by direct interaction: surface integrin alpha2beta1-contacting-mediated activation of wnt-beta-catenin pathway. Cell. Commun. Signal 17(1), 142 (2019)
Y. Zhang et al., TANK-binding kinase 1 is a mediator of platelet-induced EMT in mammary carcinoma cells. FASEB J 33(7), 7822–7832 (2019)
X.R. Xu et al., Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb. J 14(Suppl 1), 29 (2016)
N. Li, Platelets in cancer metastasis: to help the “villain” to do evil. Int. J. Cancer 138(9), 2078–2087 (2016)
S. Schwarz et al., Glycosaminoglycans as tools to decipher the platelet tumor cell interaction: a focus on P-selectin. Molecules. 25(5), 1039 (2020)
S. Anvari, E. Osei, N. Maftoon, Interactions of platelets with circulating tumor cells contribute to cancer metastasis. Sci. Rep 11(1), 15477 (2021)
O.J. McCarty et al., Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood 96(5), 1789–1797 (2000)
Y.J. Kim et al., Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium. Am. J. Pathol 155(2), 461–472 (1999)
G.A. Zimmerman, Two by two: the pairings of P-selectin and P-selectin glycoprotein ligand 1. Proc. Natl. Acad. Sci. U S A 98(18), 10023–10024 (2001)
E. Mammadova-Bach et al., Platelet integrin alpha6beta1 controls lung metastasis through direct binding to cancer cell-derived ADAM9. JCI Insight 1(14), e88245 (2016)
M.R. Weber et al., Activated tumor cell integrin alphavbeta3 cooperates with platelets to promote extravasation and metastasis from the blood stream. Thromb. Res 140(Suppl 1), S27–S36 (2016)
K. Morimoto et al., Interaction of cancer cells with platelets mediated by Necl-5/poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene 27(3), 264–273 (2008)
S. Jain, S. Russell, J. Ware, Platelet glycoprotein VI facilitates experimental lung metastasis in syngenic mouse models. J. Thromb. Haemost 7(10), 1713–1717 (2009)
E. Mammadova-Bach et al., Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood 135(14), 1146–1160 (2020)
G. Eelen et al., Basic and therapeutic aspects of angiogenesis updated. Circ. Res 127(2), 310–329 (2020)
M.Z. Wojtukiewicz et al., Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev 36(2), 249–262 (2017)
E.M. Battinelli, B.A. Markens, J.E. Italiano Jr, Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood 118(5), 1359–1369 (2011)
S. Sabrkhany, A.W. Griffioen, M.G. Oude, Egbrink, The role of blood platelets in tumor angiogenesis. Biochim. Biophys. Acta 1815(2), 189–196 (2011)
J.E. Italiano Jr et al., Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 111(3), 1227–1233 (2008)
N.E. Tsopanoglou, M.E. Maragoudakis, Thrombin’s central role in angiogenesis and pathophysiological processes. Eur. Cytokine Netw 20(4), 171–179 (2009)
N.M. Bambace, J.E. Levis, C.E. Holmes, The effect of P2Y-mediated platelet activation on the release of VEGF and endostatin from platelets. Platelets 21(2), 85–93 (2010)
A. Brill et al., Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res 67(1), 30–38 (2005)
E. Pipili-Synetos, E. Papadimitriou, M.E. Maragoudakis, Evidence that platelets promote tube formation by endothelial cells on matrigel. Br. J. Pharmacol 125(6), 1252–1257 (1998)
Z. Huang et al., Tetraspanin CD151 and integrin alpha6beta1 mediate platelet-enhanced endothelial colony forming cell angiogenesis. J. Thromb. Haemost 14(3), 606–618 (2016)
S.M. Weis, D.A. Cheresh, alphaV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med 1(1), a006478 (2011)
N. Reymond, B.B. d’Agua, A.J. Ridley, Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 13(12), 858–870 (2013)
D. Schumacher et al., Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24(1), 130–137 (2013)
S. Lazar, L.E. Goldfinger, Platelets and extracellular vesicles and their cross talk with cancer. Blood 137(23), 3192–3200 (2021)
M. Zmigrodzka, O. Witkowska-Pilaszewicz, A. Winnicka, Platelets extracellular vesicles as regulators of Cancer Progression-An updated perspective. Int J Mol Sci. 21(15), 5195 (2020)
J. Ratajczak et al., Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20(9), 1487–1495 (2006)
M. Baj-Krzyworzeka et al., Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp. Hematol 30(5), 450–459 (2002)
M. Tang et al., Platelet microparticle-mediated transfer of miR-939 to epithelial ovarian cancer cells promotes epithelial to mesenchymal transition. Oncotarget 8(57), 97464–97475 (2017)
B. Yao et al., Delivery of platelet TPM3 mRNA into breast cancer cells via microvesicles enhances metastasis. FEBS Open. Bio 9(12), 2159–2169 (2019)
J.V. Michael et al., Platelet microparticles infiltrating solid tumors transfer miRNAs that suppress tumor growth. Blood 130(5), 567–580 (2017)
M. Park, K.W. Kang, Phosphatidylserine receptor-targeting therapies for the treatment of cancer. Arch. Pharm. Res 42(7), 617–628 (2019)
A. Janowska-Wieczorek et al., Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer 113(5), 752–760 (2005)
A. Lin et al., Shear-regulated uptake of nanoparticles by endothelial cells and development of endothelial-targeting nanoparticles. J. Biomed. Mater. Res. A 93(3), 833–842 (2010)
A. Bruno et al., Antithrombotic agents and cancer. Cancers (Basel). 10(8), 253 (2018)
J. Ma et al., The anti-tumor effect of aspirin: what we know and what we expect. Biomed. Pharmacother 95, 656–661 (2017)
M.J. Thun, M.M. Namboodiri, C.W. Heath Jr, Aspirin use and reduced risk of fatal colon cancer. N Engl. J. Med 325(23), 1593–1596 (1991)
I. Torjesen, Daily aspirin reduces risk of developing and dying from cancer, researchers find. BMJ 349, g5037 (2014)
R.C. van Kruijsdijk et al., Individualised prediction of alternate-day aspirin treatment effects on the combined risk of cancer, cardiovascular disease and gastrointestinal bleeding in healthy women. Heart 101(5), 369–376 (2015)
C. Coyle et al., ADD-ASPIRIN: a phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp. Clin. Trials 51, 56–64 (2016)
J.J. McNeil et al., Effect of aspirin on disability-free survival in the healthy elderly. N Engl. J. Med 379(16), 1499–1508 (2018)
J.J. McNeil et al., Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl. J. Med 379(16), 1509–1518 (2018)
J.J. McNeil et al., Effect of aspirin on all-cause mortality in the healthy elderly. N Engl. J. Med 379(16), 1519–1528 (2018)
P. Gresele et al., Platelet-targeted pharmacologic treatments as anti-cancer therapy. Cancer Metastasis Rev 36(2), 331–355 (2017)
F. Sousa et al., Intratumoral VEGF nanotrapper reduces gliobastoma vascularization and tumor cell mass. J. Control Release 339, 381–390 (2021)
M. Cadamuro et al., Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J. Hepatol 70(4), 700–709 (2019)
S. Li et al., Cancer immunotherapy via targeted TGF-beta signalling blockade in TH cells. Nature 587(7832), 121–125 (2020)
M. Kudo et al., Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126), 1163–1173 (2018)
Y. Lan et al., Simultaneous targeting of TGF-beta/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell 39(10), 1388-1403 e10 (2021)
L. Paz-Ares et al., Bintrafusp Alfa, a Bifunctional Fusion protein targeting TGF-beta and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J. Thorac. Oncol 15(7), 1210–1222 (2020)
M.L. Yap et al., Activated platelets in the tumor microenvironment for targeting of antibody-drug conjugates to tumors and metastases. Theranostics 9(4), 1154–1169 (2019)
M.L. Yap et al., Targeting activated platelets: a unique and potentially universal approach for cancer imaging. Theranostics 7(10), 2565–2574 (2017)
J. Li et al., Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials 76, 52–65 (2016)
B. Li et al., Platelet-membrane-coated nanoparticles enable vascular disrupting agent combining anti-angiogenic drug for improved tumor vessel impairment. Nano Lett 21(6), 2588–2595 (2021)
B. Bahmani et al., Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat Commun. 12(1), 1999 (2021)
H. Li et al., Disrupting tumour vasculature and recruitment of aPDL1-loaded platelets control tumour metastasis. Nat. Commun 12(1), 2773 (2021)
Q. Lu et al., Bioengineered platelets combining chemotherapy and immunotherapy for postsurgical melanoma treatment: internal core-loaded doxorubicin and external surface-anchored anti-PD-L1 antibody backpacks. Nano Lett 22(7), 3141–3150 (2022)
Y. Lu et al., Platelet for drug delivery. Curr. Opin. Biotechnol 58, 81–91 (2019)
Funding
This study received funding from the National Natural Science Foundation of China (81872180).
Author information
Authors and Affiliations
Contributions
Qianyu Shi: Conceptualization, Investigation, Writing, Original Draft, Visualization; Tao Ji: Editing, Resources, Supervision, Funding Acquisition, Investigation; Xiaodong Tang: Resources; Wei Guo: Resources, Project administration.
Corresponding author
Ethics declarations
Ethic approval
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Q., Ji, T., Tang, X. et al. The role of tumor-platelet interplay and micro tumor thrombi during hematogenous tumor metastasis. Cell Oncol. 46, 521–532 (2023). https://doi.org/10.1007/s13402-023-00773-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00773-1