Abstract
Purpose
The presence of circulating tumor cells (CTCs) has been found to correlate with colorectal cancer (CRC) prognosis, whereas epithelial-mesenchymal transition (EMT) in CTCs has been found to be associated with CRC metastasis. LGR5 is a known target of Wnt signaling and plays an important role in CRC development. The aim of this study was to assess the clinical relevance of EMT and LGR5 expression in CTCs from CRC patients.
Methods
Sixty-six CRC patients were included in this study. The detection and expression of EMT phenotypes in CTCs from these patients were assessed using CanPatrol™ CTC enrichment and mRNA in situ hybridization (ISH), respectively. LGR5 expression in the CTCs was assessed using mRNA ISH.
Results
CTCs were detected in 86.4% (57/66) of the CRC patients included. Both the numbers of total CTCs and of CTCs displaying a mesenchymal phenotype (M+ CTCs) were found to significantly correlate with advanced disease stages and the occurrence of metastasis (p < 0.05). An adjusted multivariate analysis also indicated that the number of M+ CTCs significantly correlated with the occurrence of metastasis (p = 0.031). Additionally, we found that a high LGR5 expression level significantly correlated with the occurrence of metastasis (p < 0.05). We also found that the presence of ≥ 6 CTCs or ≥ 3 M+ CTCs per 5 ml blood significantly correlated with disease progression (p < 0.05). Patients with ≥ 6 CTCs or ≥ 3 M+ CTCs per 5 ml blood were found to exhibit poorer progression-free survival (PFS) and overall survival (OS) rates (p < 0.05 in all cases). Using Cox regression analyses, we found that only total CTC numbers remained as independent prognostic factors for a worse PFS (p = 0.043).
Conclusions
From our data we conclude that CTC numbers and EMT phenotypes may serve as prognostic markers for disease progression and metastasis in CRC patients. In addition, we conclude that LGR5 expression in CTCs may serve as a marker for CRC metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Each year, about 1.2 million patients are diagnosed with colorectal cancer (CRC), and more than 600 thousand patients die as a direct or indirect result of the disease [1]. The 5-year survival rate among CRC patients with stage I disease is over 90%, but only 10% among those diagnosed with stage IV disease [2]. Although the early prediction and diagnosis of metastasis could have a significant impact on CRC patient management, the diagnostic methods currently in use are usually unable to provide timely information about metastasis, i.e., the likelihood of its occurrence and/or prognosis [3]. However, recent advances in so called liquid biopsies through which circulating tumor cells (CTCs) can be collected at different time points as part of precision cancer treatment programs may be useful for assessing CRC tumor characteristics. To improve the effectiveness of CRC treatment, and therefore patient survival, attempts are being made to employ precision cancer treatment through the selection of treatment regimens based on molecular characteristics of individual patients [4]. The movement of tumor cells from primary tumors to distant sites may be achieved via hematogenous and/or lymphatic systems [5, 6]. In CRC, hematogenous spread is a main route of metastasis [7]. The detection of circulating CTCs may complement current diagnostic techniques used for tumor staging and for monitoring the response to treatment [8].
It has been reported that abnormal activation of epithelial-mesenchymal transition (EMT) may contribute to both tumor cell proliferation and invasion in human cancer cell lines and mouse models [9, 10]. EMT endows epithelial cells with an enhanced invasive potential by eliminating epithelial features and inducing mesenchymal features. CTCs have been detected at all stages of CRC development [11]. Although in stage II CRC cases CTC assessment may help to identify high-risk patients for adjuvant chemotherapy [12], the presence of CTCs has been found to serve as a poor prognostic feature in patients with non-metastatic disease [13, 14]. In addition, it has been reported that molecular characterization and mutation analysis of CTCs could improve biomarker discovery and drug selection [15, 16].
Wnt signaling plays a fundamental role in epithelial stem cell biology, and it has been found that the intestinal crypt stem cell marker leucine-rich repeat containing G protein-coupled receptor 5 (LGR5; also known as G-protein coupled receptor 49, Gpr49) is a key target in canonical Wnt signaling [17]. Cells expressing LGR5 represent the cells-of-origin of intestinal epithelial tumors, and it has been reported that single LGR5-positive crypt stem cells can form multicellular structures in vitro [17, 18]. Increased LGR5 expression has been found to be associated with the development and progression of several types of cancer, including CRC [19,20,21,22,23]. In particular, LGR5 appears to play a key role in colorectal cancer initiation and progression, probably through the Wnt/β-catenin signaling pathway [22, 24,25,26,27], and to be associated with a poor outcome of CRC patients [27,28,29,30]. We therefore suggest that LGR5 expression may serve as a biomarker related to a poor prognosis in CRC.
Here, we aimed at investigating the functional role of LGR5 in CTCs and to assess the potential diagnostic value of CTCs in CRC. We used the CanPatrol™ CTC enrichment technique [31] to characterize CTC phenotypes in peripheral blood samples derived from CRC patients, and mRNA in situ hybridization to detect LGR5 expression in CTCs. The CTC numbers and cell types, as well as the LGR5 expression levels were correlated with clinical stages and the occurrence of metastasis.
2 Materials and methods
2.1 Patients, sample collection and CTC isolation
This study was conducted at the First Affiliated Hospital of Chongqing Medical University from July 2015 to December 2016. Sixty-six patients with CRC (stages I-IV) were included. Peripheral blood samples for CTC analysis were collected at the time of primary surgery. This study was approved by the Institutional Human Ethics Committee, and prior informed and written consent was obtained from all the patients.
From each patient 5 ml peripheral blood was collected in K2-EDTA tubes. Using a tailored connection device, the samples were transferred to sample preservation tubes containing erythrocyte lysis buffer, after which the erythrocytes were lysed for 30 min at room temperature. The resulting lysates were centrifuged for 5 min at 600 g, after which the cell pellets were resuspended in PBS containing 4% formaldehyde for 8 min. Finally, the CTCs were isolated by filtration through calibrated membranes with 8 μm pores.
2.2 Identification and classification of CTCs
A multiplex mRNA in situ hybridization (ISH) assay was used to identify and classify CTCs. The mRNA ISH experiments were performed in 24-well plates at 40 °C for 3 h using a set of EMT markers as probes (Thermo Fisher Scientific, Shanghai, China). The expression of epithelial markers was detected using a multi-marker probe mix encompassing EpCAM, CK8, CK18 and CK19, whereas the expression of mesenchymal markers was detected using a multi-marker probe mix encompassing Twist1, Vimentin, AKT2 and SNAI1. A CD45 probe was used as leukocyte marker [32]. Finally, the cells were counter-stained for 10 min with DAPI after which the ISH results were analyzed by qualified pathologists using an automated imaging fluorescence microscope. If the number of fluorescent dots for the epithelial and mesenchymal markers or for CD45 was ≥ 7 they were considered to be valid fluorescent signals. Red and green fluorescent signals indicated the expression of epithelial and mesenchymal markers, respectively, whereas bright white fluorescent signals indicated CD45 expression.
2.3 LGR5 expression analysis
The expression status of LGR5 in each CTC was assessed using a multiplex mRNA-ISH assay (Thermo Fisher Scientific, Shanghai, China). The results were analyzed by qualified pathologists using an automated imaging fluorescence microscope. A purple fluorescent signal (Alexa Fluor 647, purple dots ≥ 7) from the CTCs indicated LGR5 expression. Any patient positive for CTCs (≥ 1 CTCs in 5 ml blood) was considered eligible for LGR5 expression analysis. The LGR5 expression status in CTC-positive patients was evaluated as follows: (i) patients with no LGR5-expressing CTCs were defined as LGR5-negative; (ii) patients with only one LGR5-positive CTC were considered to have a low or high LGR5 expression based on the detected LGR5 level in the CTC; (iii) patients with 2 or more LGR5-positive CTCs were considered to have a low LGR5 expression when the detected expression was low in more than 50% of the CTCs; otherwise, the LGR5 expression was considered to be high.
2.4 Clinical follow-up
Patients were followed up with regular chart reviews at 3-month intervals for 2 years and at 6-month intervals thereafter. Disease assessment was carried out by the treating physicians, and the patient cohort was divided into two groups based on Response Evaluation Criteria in Solid Tumors (RECIST): (i) non-progressive disease (NPD, including complete response [CR], partial response [PR] and stable disease [SD] categories) and (ii) progressive disease (PD). Progression-free survival (PFS) was defined as the elapsed time from diagnosis to progression, death, or censoring at last follow-up. Overall survival (OS) was defined as the interval between the date of diagnosis to the date of death or the last follow-up.
2.5 Statistical analysis
Statistical analyses were performed using the SPSS 19.0 software package. Correlations between two variables were assessed using Spearman’s rank correlation test. Independent correlations between two variables after adjustment for other variables were assessed using partial correlation analysis. Optimal CTC cutoff values for the stratification of patients into favorable and unfavorable prognostic groups based on CTC counts were determined using receiver operating characteristic (ROC) curve analyses. PFS and OS rates were calculated using the Kaplan-Meier method, and survival rates were compared using the log rank test. Univariate and multivariate Cox regression models with hazard ratios (HRs) and 95% confidence intervals (CIs) were used to evaluate associations between potential prognostic factors and PFS/OS. All statistical analyses were two-tailed, and values p < 0.05 were considered to be significant.
3 Results
3.1 Patients and CTC characteristics
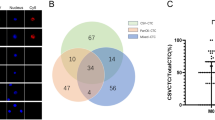
A total of 66 CRC patients (median age, 58 years; range, 26–87 years) was included in this study. The detailed patient characteristics are listed in Table 1. CTCs (≥ 1 per 5 ml blood) were detected in 57 of the 66 patients (86.4%) with a median count of 3.0 CTCs (range 0–36 per 5 ml blood). Based on the EMT mRNA markers, the detected CTCs could be classified into three phenotypes: epithelial, bi-phenotypic and mesenchymal (Fig. 1a). In 49 patients (74.2%) bi-phenotypic and/or mesenchymal CTCs were detected, i.e., CTCs displaying a mesenchymal phenotype (M+ CTCs).
CTCs detected in blood samples from patients with CRC. a CTC phenotypes classified using epithelial and mesenchymal markers. Epithelial CTCs (left) stained with red dots only; bi-phenotypic CTCs (middle) stained with both red and green dots; mesenchymal CTCs (right) stained with green dots only. Bar = 5 μm. b LGR5 expression in CTCs. Representative images based on mRNA-ISH of epithelial (red dots), mesenchymal (green dots), CD45 (bright white dots) and LGR5 (purple dots) markers. Leukocytes expressing CD45 (first line) exhibit bright white dots only; epithelial CTCs expressing LGR5 (second line) exhibiting red and purple dots; bi-phenotypic CTCs expressing LGR5 (third line) exhibiting red, green and purple dots; mesenchymal CTCs expressing LGR5 (fourth line) exhibiting green and purple dots. Bar = 5 μm
3.2 Associations between CTC numbers, phenotypes and patient characteristics
Univariate analyses of associations between CTC phenotypes and patient characteristics are depicted in Table 1 and Fig. 2. We found that there was no significant association between CTC positivity and any of the patient characteristics (p > 0.05 in all cases; Table 1). We did find, however, that the number of CTCs was associated with advanced stage disease and metastasis (p < 0.05; Fig. 2a, b). The median CTC number in patients with advanced stage CRC (stage III or IV) was 5.0, which was higher than that in patients with stage I or II disease (3.0 and 2.0, respectively; Fig. 2a). Similarly, we found that the median CTC number was 2.5 and 5.0 in the non-metastatic and metastatic CRC patients, respectively (Fig. 2b). These data suggest that CTC numbers increase with increasing stages and the development of metastasis.
Although not statistically significant (p = 0.055), we observed a positive trend in the correlation between the presence of M+ CTCs and metastasis, with 67.4% positivity in patients with non-metastatic CRC and 90.0% positivity in patients with metastatic disease (Table 1). Furthermore, we found that the number of M+ CTCs correlated with both advanced disease stages and the occurrence of metastasis (p < 0.01 in both cases; Fig. 2c, d). The median number of M+ CTCs was found to be 5.0 in stage III and IV patients, which was higher than in stage I or II patients (2.0 and 1.0, respectively; Fig. 2c). Likewise, the median M+ CTC count was 1.5 and 5.0 for non-metastatic and metastatic CRC cases, respectively (Fig. 2d). These data suggest that the number of M+ CTCs increases along with increasing disease stages and the development of metastasis.
In a multivariate analysis, the observed association between M+ CTC number and the development of metastasis remained significant after adjustment for age, sex, TNM stage, tumor differentiation and total CTC number (p = 0.031; data not shown). However, the association between M+ CTC number and TNM stage was no longer significant after adjustment for age, sex, tumor differentiation, metastasis and total CTC number (p > 0.05; data not shown). We also found that the association between total CTC number and TNM stage or the development of metastasis lost significance after adjustment for other variables (p > 0.05 in both cases; data not shown).
3.3 Associations between LGR5 expression in CTCs and patient characteristics
Among the 57 patients with ≥1 CTCs per 5 ml blood eligible for LGR5 analysis, 34 (59.6%) were found to be positive for LGR5. LGR5 was expressed in all three CTC phenotypic groups (Fig. 1b). The LGR5 positive fraction was 59.9% (91/152) among all CTCs, 68.8% (44/64) among epithelial CTCs and 53.4% (47/88) among combined bi-phenotypic and mesenchymal CTCs.
The associations between LGR5 expression status and patient characteristics are listed in Table 2. We found that LGR5 expression exhibited no significant association with age, sex, TNM stage or tumor differentiation (p > 0.05 in all cases; Table 2). We did find, however, that high LGR5 expression was associated with the development of metastasis. The fraction of patients exhibiting a high LGR5 expression was 13.2% among non-metastatic patients, but 42.1% among metastatic patients (p = 0.027; Table 2). These data suggest that the level of LGR5 expression increases with the development of metastasis.
3.4 Associations between CTC numbers, LGR5 expression and disease progression
A total of 55 patients were followed-up using RECIST disease assessment. Of these, 46 (83.6%) exhibited non-progressive disease (NPD; 41 had a complete response, CR, and 5 had a stable disease, SD) and 9 (16.4%) exhibited progressive disease (PD). Among the 9 patients with PD, 5 died. We also assessed associations between CTC status and LGR5 expression level and disease status during follow-up. An optimal threshold cutoff of 6 CTCs per 5 ml was calculated using ROC curve analysis (data not shown). As expected, the presence of ≥ 6 CTCs per 5 ml blood was more often found in patients with PD, while the presence of < 6 CTCs per 5 ml blood was more commonly found in patients with NPD (p = 0.023; Table 3). Similarly, the presence of ≥ 3 M+ CTCs per 5 ml blood was more likely to be observed in patients with PD, while the presence of < 3 M+ CTCs per 5 ml blood was more likely to be observed in patients with NPD (p = 0.034; Table 3). No significant association was observed between LGR5 expression and disease status (p > 0.05; Table 3). In a multivariate analysis adjusted for age, sex, TNM stage, tumor differentiation and metastasis, again no significant association was observed between CTC status and disease status (p > 0.05; data not shown).
3.5 Associations between CTC numbers, LGR5 expression and survival
Among the 55 patients with a disease follow-up assessment, the follow-up period ranged from 3 to 49 months (median, 24 months). Next, we analyzed the PFS and OS, taking into consideration CTC status and LGR5 expression. As expected, we found that patients with ≥ 6 CTCs per 5 ml blood exhibited a poorer PFS and OS than those with < 6 CTCs per 5 ml blood (p = 0.012 and 0.015, respectively; Fig. 3a, c). Likewise, patients with ≥ 3 M+ CTCs per 5 ml blood were found to exhibit a poorer PFS and OS than those with < 3 M+ CTCs per 5 ml of blood (p = 0.017 and 0.035, respectively; Fig. 3b, d). Univariate Cox regression analyses revealed significant associations between total CTC and M+ CTC numbers and PFS (p = 0.028 and 0.047, respectively; Table 4), but not OS (p > 0.05 in both cases; Table 4). Multivariate Cox regression analysis further showed that only the total CTC number served an independent prognostic factor for a poor PFS (p = 0.043; Table 4). No significant association was observed between LGR5 expression and survival.
4 Discussion
Metastasis is a multistep process that involves CTCs and disseminated tumor cells that home to distant sites, including the bone marrow, liver and lungs [33,34,35]. Although patient-specific characteristics of CTCs limits their clinical application [36], CTC phenotypes in blood samples have attracted the attention of researchers interested in EMT, which appears to contribute to the process of metastasis [37, 38]. Our findings are consistent with this notion and indicate that EMT is a significant contributor to the metastasis of CRC cells.
In conformity with a previous report [11], we detected CTCs in patients with both late stage (III/IV) and early stage (I/II) tumors. The presence of CTCs in early stage patients may explain why some tumors quickly become more advanced. Furthermore, we found that different CTC subtypes can be discerned using epithelial and mesenchymal mRNA markers. Intriguingly, we found that the presence of M+ CTCs tended to correlate with metastasis, and that the number of CTCs as well as the number of M+ CTCs correlated with advanced disease and metastasis. These results suggest that high CTC numbers, especially those positive for mesenchymal markers, contribute to the development of metastasis, and that EMT is related to this process in CRC. However, in a multivariate analysis adjusting for other factors, we found that only the association between M+ CTC number and metastasis remained significant, indicating that not all patients with high CTC numbers or mesenchymal marker-positive CTCs will develop metastasis. Previous studies have indicated that CTC numbers may serve as risk factors for CRC progression [39, 40]. Consistent with these studies, we found that the presence of ≥ 6 CTCs or ≥ 3 M+ CTCs per 5 ml blood made patients more likely to have PD, while the presence of < 6 CTCs or < 3 M+ CTCs per 5 ml blood was more commonly found in patients with NPD, indicating an association between both total CTC and M+ CTC numbers and disease progression in CRC.
Several additional studies have suggested that the presence of CTCs in the peripheral blood of CRC patients may be associated with a poor prognosis [13, 14, 39, 40]. Concordantly, we found that both the PFS and OS rates were poorer in patients with ≥ 6 CTCs or ≥ 3 M+ CTCs per 5 ml blood than in patients with < 6 CTCs or < 3 M+ CTCs per 5 ml blood. On the other hand, we found through univariate Cox regression analyses that total CTC and M+ CTC numbers were related to PFS but not to OS. It is also noteworthy that after adjusting for age, sex, TNM stage, tumor differentiation and metastasis, only the association between total CTC number and PFS remained significant with a reduced statistical strength. These observations suggest that total CTC numbers overlap with patient characteristics and provide additional independent prognostic value.
LGR5, a member of the G protein-coupled receptor (GPCR) family of proteins, serves as a marker of cancer stem cells that are relevant for the development and progression of CRC [17, 18, 22]. Consistent with previous reports [28,29,30], we found that high LGR5 expression in CTCs was associated with CRC metastasis, which suggests that LGR5 is involved in this process. As such, assessment of LGR5 expression in CTCs could be useful for metastasis evaluation in CRC patients. No significant correlations were observed between LGR5 expression and other clinical parameters such as TNM stage, disease progression and survival. This discrepancy may be attributed to differences in ethnic population background, sample size, technical issues or others. We conclude that additional large sample studies to ascertain the clinical prognostic value of LGR5 are warranted.
In summary, we found that higher total CTC or M+ CTC numbers are associated with advanced disease, metastasis and a poor survival in CRC patients. Multivariate analysis revealed a significant association between M+ CTC number and metastasis and that total the CTC number may serve as an independent prognostic factor for a poor PFS. Our data also indicate that assessment of LGR5 expression in CTCs may be a novel and promising strategy for metastasis evaluation in patients with CRC. Since our study cohort is limited in size, further large-scale studies will be needed to confirm our current data. In addition, it will be important to explore putative correlations between dynamic changes in CTC numbers and phenotypes, and responses to therapy and survival in CRC.
References
F. Grizzi, G. Celesti, G. Basso, L. Laghi, Tumor budding as a potential histopathological biomarker in colorectal cancer: hype or hope? World J Gastroenterol 18, 6532–6536 (2012)
H. Brenner, M. Kloor, C.P. Pox, Colorectal cancer. Lancet 383, 1490–1502 (2014)
I. Zlobec, Novel biomarkers for the prediction of metastasis in colorectal cancer. Expert Opin Med Diagn 7, 137–146 (2013)
F. Andre, E. Mardis, M. Salm, J.C. Soria, L.L. Siu, C. Swanton, Prioritizing targets for precision cancer medicine. Ann Oncol 25, 2295–2303 (2014)
K. Pantel, R.H. Brakenhoff, Dissecting the metastatic cascade. Nat Rev Cancer 4, 448–456 (2004)
R. Paduch, The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol 39, 397–410 (2016)
H. Imamura, Y. Matsuyama, E. Tanaka, T. Ohkubo, K. Hasegawa, S. Miyagawa, Y. Sugawara, M. Minagawa, T. Takayama, S. Kawasaki, M. Makuuchi, Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 38, 200–207 (2003)
N. Bednarz-Knoll, C. Alix-Panabieres, K. Pantel, Clinical relevance and biology of circulating tumor cells. Breast Cancer Res 13, 228 (2011)
R. Kalluri, R.A. Weinberg, The basics of epithelial-mesenchymal transition. J Clin Invest 119, 1420–1428 (2009)
R. Kalluri, EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin Invest 119, 1417–1419 (2009)
J. Sastre, M.L. Maestro, J. Puente, S. Veganzone, R. Alfonso, S. Rafael, J.A. García-Saenz, M. Vidaurreta, M. Martín, M. Arroyo, M.T. Sanz-Casla, E. Díaz-Rubio, Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol 19, 935–938 (2008)
P. Gazzaniga, W. Gianni, C. Raimondi, A. Gradilone, G. Lo Russo, F. Longo, O. Gandini, S. Tomao, L. Frati, Circulating tumor cells in high-risk nonmetastatic colorectal cancer. Tumor Biol 34, 2507–2509 (2013)
N.N. Rahbari, M. Aigner, K. Thorlund, N. Mollberg, E. Motschall, K. Jensen, M.K. Diener, M.W. Büchler, M. Koch, J. Weitz, Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 138, 1714–1726 (2010)
M. Thorsteinsson, P. Jess, The clinical significance of circulating tumor cells in non-metastatic colorectal cancer–a review. EJSO-Eur J Surg Onc 37, 459–465 (2011)
S. Maheswaran, L.V. Sequist, S. Nagrath, L. Ulkus, B. Brannigan, C.V. Collura, E. Inserra, S. Diederichs, A.J. Iafrate, D.W. Bell, S. Digumarthy, A. Muzikansky, D. Irimia, J. Settleman, R.G. Tompkins, T.J. Lynch, M. Toner, D.A. Haber, Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 359, 366–377 (2008)
E. Pailler, J. Adam, A. Barthélémy, M. Oulhen, N. Auger, A. Valent, I. Borget, D. Planchard, M. Taylor, F. André, J.C. Soria, P. Vielh, B. Besse, F. Farace, Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J Clin Oncol 31, 2273–2281 (2013)
N. Barker, H. Clevers, Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138, 1681–1696 (2010)
A. Merlos-Suarez, F.M. Barriga, P. Jung, M. Iglesias, M.V. Céspedes, D. Rossell, M. Sevillano, X. Hernando-Momblona, V. da Silva-Diz, P. Muñoz, H. Clevers, E. Sancho, R. Mangues, E. Batlle, The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 8, 511–524 (2011)
Y. Yamamoto, M. Sakamoto, G. Fujii, H. Tsuiji, K. Kenetaka, M. Asaka, S. Hirohashi, Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with β-catenin mutations. Hepatology 37, 528–533 (2003)
T. McClanahan, S. Koseoglu, K. Smith, J. Grein, E. Gustafson, S. Black, P. Kirschmeier, A.A. Samatar, Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther 5, 419–426 (2006)
K. Tanese, M. Fukuma, T. Yamada, T. Mori, T. Yoshikawa, W. Watanabe, A. Ishiko, M. Amagai, T. Nishikawa, M. Sakamoto, G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am J Pathol 173, 835–843 (2008)
H. Uchida, K. Yamazaki, M. Fukuma, T. Yamada, T. Hayashida, H. Hasegawa, M. Kitajima, Y. Kitagawa, M. Sakamoto, Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in colorectal cancer. Cancer Sci 101, 1731–1737 (2010)
E. Simon, D. Petke, C. Böger, H. Behrens, V. Warneke, M. Ebert, C. Röcken, The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS One 7, e35486 (2012)
A.G. Schepers, H.J. Snippert, D.E. Stange, M. van den Born, J.H. van Es, M. van de Wetering, H. Clevers, Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337, 730–735 (2012)
S. Yui, T. Nakamura, T. Sato, Y. Nemoto, T. Mizutani, X. Zheng, S. Ichinose, T. Nagaishi, R. Okamoto, K. Tsuchiya, H. Clevers, M. Watanabe, Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18, 618–623 (2012)
H.C. Hsu, Y.S. Liu, K.C. Tseng, B.C. Tan, S.J. Chen, H.C. Chen, LGR5 regulates survival through mitochondria-mediated apoptosis and by targeting the Wnt/β-catenin signaling pathway in colorectal cancer cells. Cell Signal 26, 2333–2342 (2014)
S. He, H. Zhou, X. Zhu, S. Hu, M. Fei, D. Wan, W. Gu, X. Yang, D. Shi, J. Zhou, J. Zhou, Z. Zhu, L. Wang, D. Li, Y. Zhang, Expression of Lgr5, a marker of intestinal stem cells, in colorectal cancer and its clinicopathological significance. Biomed Pharmacother 68, 507–513 (2014)
X.S. Wu, H.Q. Xi, L. Chen, Lgr5 is a potential marker of colorectal carcinoma stem cells that correlates with patient survival. World J Surg Onc 10, 244 (2012)
H.C. Hsu, Y.S. Liu, K.C. Tseng, C.L. Hsu, Y. Liang, T.S. Yang, J.S. Chen, R.P. Tang, S.J. Chen, H.C. Chen, Overexpression of Lgr5 correlates with resistance to 5-FU-based chemotherapy in colorectal cancer. Int J Color Dis 28, 1535–1546 (2013)
Z. Liu, W. Dai, L. Jiang, Y. Cheng, Over-expression of LGR5 correlates with poor survival of colon cancer in mice as well as in patients. Neoplasma 61, 177–185 (2014)
S. Wu, S. Liu, Z. Liu, J. Huang, X. Pu, J. Li, D. Yang, H. Deng, N. Yang, J. Xu, Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One 10, e0123976 (2015)
R. Zhao, Z. Cai, S. Li, Y. Cheng, H. Gao, F. Liu, S. Wu, S. Liu, Y. Dong, L. Zheng, W. Zhang, X. Wu, X. Yao, Expression and clinical relevance of epithelial and mesenchymal markers in circulating tumor cells from colorectal cancer. Oncotarget 8, 9293–9302 (2017)
L. Wan, K. Pantel, Y. Kang, Tumor metastasis: moving new biological insights into the clinic. Nat Med 19, 1450–1464 (2013)
A. Chavez-Gonzalez, B. Bakhshinejad, K. Pakravan, M.L. Guzman, S. Babashah, Novel strategies for targeting leukemia stem cells: sounding the death knell for blood cancer. Cell Oncol 40, 1–20 (2017)
F.H. Shek, R. Luo, B.Y.H. Lam, W.K. Sung, T.W. Lam, J.M. Luk, M.S. Leung, K.T. Chan, H.K. Wang, C.M. Chan, R.T. Poon, N.P. Lee, Serine peptidase inhibitor Kazal type 1 (SPINK1) as novel downstream effector of the cadherin-17/β-catenin axis in hepatocellular carcinoma. Cell Oncol 40, 443–456 (2017)
T. Masuda, N. Hayashi, T. Iguchi, S. Ito, H. Eguchi, K. Mimori, Clinical and biological significance of circulating tumor cells in cancer. Mol Oncol 10, 408–417 (2016)
A. Brouwer, B. De Laere, D. Peeters, M. Peeters, R. Salgado, L. Dirix, S. Van Laere, Evaluation and consequences of heterogeneity in the circulating tumor cell compartment. Oncotarget 7, 48625–48643 (2016)
L.M. McInnes, N. Jacobson, A. Redfern, A. Dowling, E.W. Thompson, C.M. Saunders, Clinical implications of circulating tumor cells of breast cancer patients: role of epithelial-mesenchymal plasticity. Front Oncol 5, 42 (2015)
B.G. Koerkamp, N.N. Rahbari, M.W. Büchler, M. Koch, J. Weitz, Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol 20, 2156–2165 (2013)
L.T. Seeberg, A. Waage, C. Brunborg, H. Hugenschmidt, A. Renolen, I. Stav, B.A. Bjørnbeth, K.W. Brudvik, E.F. Borgen, B. Naume, G. Wiedswang, Circulating tumor cells in patients with colorectal liver metastasis predict impaired survival. Ann Surg 261, 164–171 (2015)
Acknowledgments
The authors sincerely thank all the investigators and coordinators who contributed to this study.
Funding
This study was supported by Guangzhou collaborative innovation major projects for “development and industrialization of key technology and product for tumor precision medical real time monitoring” (number 201604010039) and by Guangzhou health care collaborative innovation major projects for “development and industrialization of the clinical application of circulating tumor cell separation, typing and analysis of automatic equipment and reagents” (number 201604020014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Informed consent
Informed consent was obtained from all the patients included in the study.
Rights and permissions
About this article
Cite this article
Wang, W., Wan, L., Wu, S. et al. Mesenchymal marker and LGR5 expression levels in circulating tumor cells correlate with colorectal cancer prognosis. Cell Oncol. 41, 495–504 (2018). https://doi.org/10.1007/s13402-018-0386-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-018-0386-4