Abstract
Purpose
It has been reported that stromal cell features may affect the clinical outcome of breast cancer patients. Cancer associated fibroblasts (CAFs) represent one of the most abundant cell types within the breast cancer stroma. Here, we aimed to explore the influence of CAFs on breast cancer gene expression, as well as on invasion and angiogenesis.
Methods
qRT-PCR was used to evaluate the expression of several cancer progression related genes (S100A4, TGFβ, FGF2, FGF7, PDGFA, PDGFB, VEGFA, IL-6, IL-8, uPA, MMP2, MMP9, MMP11 and TIMP1) in the human breast cancer-derived cell lines MCF-7 and MDA-MB-231, before and after co-culture with CAFs. Stromal mononuclear inflammatory cell (MIC) MMP11 expression was used to stratify primary tumors. In addition, we assessed the in vitro effects of CAFs on both MDA-MB-231 breast cancer cell invasion and endothelial cell (HUVEC) tube formation.
Results
We found that the expression levels of most of the genes tested were significantly increased in both breast cancer-derived cell lines after co-culture with CAFs from either MMP11+ or MMP11- MIC tumors. IL-6 and IL-8 showed an increased expression in both cancer-derived cell lines after co-culture with CAFs from MMP11+ MIC tumors. We also found that the invasive and angiogenic capacities of, respectively, MDA-MB-231 and HUVEC cells were increased after co-culture with CAFs, especially those from MMP11+ MIC tumors.
Conclusions
Our data indicate that tumor-derived CAFs can induce up-regulation of genes involved in breast cancer progression. Our data additionally indicate that CAFs, especially those derived from MMP11+ MIC tumors, can promote breast cancer cell invasion and angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The acquisition of a malignant phenotype does not depend exclusively on intrinsic cancer cell properties, but also on stromal features [1]. Cancer associated fibroblasts (CAFs), one of the most abundant cell types within the breast cancer stroma, contribute to tumor progression trough the recruitment of immune cells [2, 3], the degradation and remodeling of extracellular matrix (ECM) components, the promotion of epithelial-mesenchymal transition (EMT) processes and the induction of stem cell traits [4,5,6,7]. These actions are mediated by growth factors and cytokines secreted by CAFs [8,9,10,11]. Mononuclear inflammatory cells (MICs) represent another stromal cell component that may affect both cancer cell and stromal cell behavior. Previously, we found that, depending on the expression of matrix metalloprotease 11 (MMP11) by MICs, breast cancers may exhibit a highly inflammatory profile [12] and a poor prognosis [13,14,15,16]. Indeed, it has been found that CAFs derived from MMP11+ MIC tumors may over-express factors related to tumor progression, such as TIMP1, VEGFA and S100A4 and, in addition, that their co-culture with breast cancer cells may induce a more aggressive behavior [10]. So, different MIC tumors may differentially modulate CAF phenotypes. As yet, however, little is known about the effects of CAFs on breast cancer cell characteristics.
In order to better understand the dynamic interactions between CAFs and breast cancer cells, the objectives of the present study were (i) to investigate the effects of CAFs on breast cancer cells by analyzing the expression of a panel of genes implicated in tumor progression, (ii) to compare the resulting gene expression profiles after co-culture with CAFs derived from MMP11+ or MMP11- MIC tumors and (iii) to explore its effects on the invasive and tube forming capacities of breast cancer and endothelial cells, respectively.
2 Materials and methods
2.1 Patient selection and characteristics
In this non-randomized prospective study 20 cases with a confirmed diagnosis of invasive breast carcinoma were included, as previously reported [10]. Specifically, we selected consecutive T1 or T2 invasive ductal carcinoma cases yielding enough material for cell culture during the period July 2011 to August 2013. All patients included underwent tumor resection as first therapeutic approach. The exclusion criteria were: prior history of a malignant tumor, excluding non-melanoma skin cancer, uterine cervical carcinoma in situ, ductal carcinoma in situ or lobular carcinoma in situ breast cancer, and having received any type of therapy prior to surgery. The clinicopathological characteristics of the 20 patients included in this study are listed in Table 1. The patients were treated according to the guidelines used in our Institution (Fundación Hospital de Jove). Written informed consent was obtained from all patients. The study adheres to national regulations and has been approved by the Fundación Hospital de Jove Ethics and Research Committee.
2.2 CAF isolation, cell lines and co-culture assays
For the isolation of cancer-associated fibroblasts (CAFs), tumor samples were cut into 1 mm3 pieces and enzymatically dissociated, as previously reported [10]. Fibroblast purities were analyzed by flow cytometry after incubation with an anti-CD90 antibody (clone AS02, Dianova, Hamburg, Germany) at 1:50 for 45 min. The specificity of the anti-CD90 antibody used was confirmed by immunohistochemistry [10].
The estrogen-independent human breast cancer-derived cell line MDA-MB-231, the estrogen-dependent human breast cancer-derived cell line MCF-7 and the primary human umbilical vein endothelial cells (HUVECs) were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). The MCF-7 cells were cultured in DMEM-F12 (Lonza, Visp, Switzerland) and the MDA-MB-231 cells were cultured in high glucose DMEM (Sigma, St. Louis, MO, USA). Both media were supplemented with 10% FBS (PAA, New Jersey, NJ, USA) and 1% penicillin-streptomycin solution (Gibco, Paisley, UK). HUVEC cells were used at passages 4–6, and were cultured in VascuLife basal medium supplemented with LifeFactors VascuLife EnGS (Lifeline Cell Technology, Frederick, MD, USA).
CAFs isolated from the 20 breast tumors included were co-cultured with both MDA-MB-231 and MCF-7 cells. To this end, CAFs (2 × 104) were seeded at the bottom of tissue culture wells, whereas the breast cancer-derived cells (2 × 104) were seeded in 0.24 μm pore size tissue culture inserts. The cells were co-cultured for 72 h in DMEM-F12 supplemented with 10% FBS and 1% penicillin-streptomycin solution, after which RNA was extracted. Co-cultures of fibroblasts derived from MMP11+ or MMP11- MIC tumors with the respective breast cancer-derived cell lines were performed using VascuLife basal medium supplemented with LifeFactors VascuLife EnGS as described above. These media were collected and used as conditioned media (CM) for the HUVEC tube-formation assays (see below).
2.3 Immunohistochemistry
Tumors were classified immunohistochemically according to MMP11 expression by intratumoral mononuclear inflammatory cells (MICs). To this end, 5 μm sections were sliced from formalin-fixed and paraffin-embedded (FFPE) tissue samples using a microtome (Leica Microsystems GmbH, Wetzlar, Germany) and transferred to adhesive-coated slides. The sections were stained using a TechMate TM50 autostainer (Dako, Glostrup, Denmark). Immunohistochemistry (IHC) was performed as previously reported [14] and the antibodies used for IHC are listed in Table 2.
2.4 qRT-PCR and Western blotting
A RNeasy Mini Kit (Qiagen, Hilden, Germany) was used for total RNA extraction following the manufacturer’s instructions. For cDNA synthesis, a Transcriptor First Strand cDNA Synthesis Kit (Roche, Mannheim, Germany) was used. Reverse transcription and quantitative real time-PCR (qRT-PCR) were performed as previously reported [10] using specific primers (listed in Table 3).
Western blotting was performed by subjecting 50 μg total protein extracted from HUVEC cells to SDS–PAGE. After electrophoresis, the proteins were transferred to Immobilon-FL membranes (Merck Millipore, Madrid, Spain), blocked and incubated overnight at 4 °C with primary antibodies and, next, incubated with anti-mouse Dylight™ 680-conjugated or anti-rabbit Dylight™ 800-conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, MA, USA). Quantification was performed using LI-COR Odyssey software (Bad Homburg, Germany). The following antibodies were used: anti-phospho(p)-VEGFR2, anti-p-p38, anti-p-ERK1/2 (Cell Signaling, Danvers, MA, USA) and anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
2.5 Cell invasion and tube-formation assays
Cell invasion assays were performed using BD BioCoatMatrigel invasion chambers (BD Biosciences, Madrid, Spain). Briefly, MCF-7 or MDA-MB-231 cells (5 × 104) were placed into the upper chambers in 0.5 ml serum-free DMEM. In the lower chambers DMEM-F12 (1:1) medium supplemented with 20% FBS (controls) or CAF-conditioned medium (CM) (2 CAF cultures from MMP11- MIC tumors and 2 CAF cultures from MMP11+ MIC tumors for 48 h) were added. After incubation for 22 h, cells that were migrated to the lower surfaces of the filters were fixed in methanol for 2 min at room temperature, stained using crystal violet for 2 min, visualized and counted. Values for cell invasion were expressed as the mean number of cells per microscopic field over four fields per one filter of duplicate experiments.
Tube-formation assays were performed in Matrigel (BD Biosciences). Briefly, 100 μl growth factor-reduced Matrigel matrix was loaded into each well of a 96-well plate and allowed to polymerize for 30 min at 37 °C. Next, HUVEC cells (4 × 105) were added to each well and incubated at 37 °C in 5% CO2 for 5 h with conditioned media as described above. The formation of capillary-like structures was assessed under a fluorescence microscope after CellTracker Red CMTPX Dye (0.5 μM; Thermo Fisher Scientific, Waltham, MA, USA) staining for 15 min. Tube formation was evaluated by quantifying vessel lengths and junction numbers using AngioTool software [17].
2.6 Statistical analysis
All statistical analyses were performed using SPSS 19. The Kolmogorov-Smirnov test was used to determine whether the sample data were normally distributed. Comparisons between groups were performed using Student’s t test. Differences were considered significant when p ≤ 0.05.
3 Results
3.1 mRNA expression alterations in MCF-7 and MDA-MB-231 cells after co-culture with CAFs
To investigate the influence of stromal fibroblast on breast cancer cells, MCF-7 and MDA-MB-231 cells were co-cultured with 20 different CAF samples, after which the expression levels of 14 genes (Table 3) were assessed by qRT-PCR. We found that after co-culture the S100A4, TGFβ, FGF7, PDGFB, uPA, IL-6, IL-8, MMP2, MMP11, TIMP1 and VEGFA mRNA expression levels were upregulated in both MCF-7 and MDA-MB-231 cells (Fig. 1). In contrast, we found that the PDGFA mRNA expression level in MCF-7 cells was decreased after co-culture with the CAFs (p < 0.001). Several mRNAs showed increases of at least 5-fold compared to basal levels after co-culture, including uPA in MCF-7 cells and PDGFB, MMP2 and VEGFA in MDA-MB-231 cells. The IL-6 and IL-8 mRNA levels were found to be increased at least 10-fold in both cell lines after co-culture (Fig. 1a, b).
3.2 mRNA expression alterations in MCF-7 and MDA-MB-231 cells after co-culture with CAFs from MMP11- or MMP11+ MIC tumors
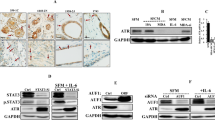
CAFs were classified according to MMP-11 expression by MICs using IHC of FFPE tumor tissues. We found that of the 20 tumors included in the present study, 8 showed MMP11 positive (+) MICs and 12 showed MMP11 negative (−) MICs (Fig. 2a). In the MMP11+ tumors, at least 70% of the MICs showed positive immunostaining in each evaluated field.
a MMP11 immunostaining of primary tumors, showing a negative (left) and a positive (right) example. b qRT-PCR analysis of 14 factors in MCF-7 cells alone, after co-culture with CAFs from MMP11- MIC tumors, or after co-culture with CAFs from MMP11+ MIC tumors, (c) MDA-MB-231 cells alone, after co-culture with CAFs from MMP11- MIC tumors, or after co-culture with CAFs from MMP11+ MIC tumors. CAFs from all 20 patients were included. Data represent mean ± SD (*p ≤ 0.05, ** p ≤ 0.001)
We found that subsequent co-culture of MCF-7 cells with CAFs from MMP11- MIC tumors resulted in uPA mRNA expression upregulation compared to co-culture with CAFs from MMP11+ MIC tumors. Conversely, we found that MCF-7 cells showed increased IL-6 and IL-8 expression levels when co-cultured with CAFs from MMP11+ MIC tumors (Fig. 2b). MDA-MB-231 cells co-cultured with CAFs from MMP11- MIC tumors showed significant (p < 0.001) increases in PDGFB, MMP11 and VEGFA mRNA expression levels. Conversely, we found that MDA-MB-231 cells co-cultured with CAFs from MMP11+ MIC tumors showed significantly higher IL-6 (p < 0.05), IL-8 and TIMP1 (p < 0.001) mRNA expression levels than MDA-MB-231 cells co-cultured with CAFs from MMP11- MIC tumors.
3.3 Protein expression alterations in MDA-MB-231 cells after co-culture with CAFs
Next, an IHC study was performed on the aggressive breast cancer cell line MDA-MB-231 to assess the expression of the IL-6, IL-8, MMP11 and TIMP1 proteins in the presence or absence of CAFs. An expert pathologist blinded to the results (i.e., MDA-MB-231 cells co-cultured or not with CAFs) evaluated the IHC staining intensities using a numerical score ranging from 0 to 3, reflecting the intensities as follows: 0, no staining; 1, weak staining; 2, intense staining. By doing so, we found that the IHC staining intensities, and thus the protein levels, were higher of IL-6, IL-8, MMP11 and TIMP1 when MDA-MB-231 cells were co-cultured with CAFs (Fig. 3a), thereby validating the qRT-PCR data. No cases without IHC staining were found.
a Immunostaining of selected factors (IL6, IL8, MMP11 and TIMP1) differentially expressed in MDA-MB-231 cells alone (left) or after co-culture with CAFs (right). b Invasive capacity of MDA-MB-231 cells in Matrigel after 48 h without or with CAF-conditioned media from MMP11- MIC or MMP11+ MIC tumors as chemoattractants. Ten randomized fields (magnification 20×) were counted. Data represent the mean ± SD. Three independent experiments were performed in each case
3.4 Increased invasion of MDA-MB-231 cells after co-culture with CAFs
Since MCF-7 cells exhibit a poor invasive capacity, they are usually employed as a negative control for invasive studies. Concordantly, we found that the MCF-7 cells showed a poor invasive capacity, even after co-culture with CAFs. Conversely, we found that MDA-MB-231 cells, when co-cultured with CAFs, showed a significant (p = 0.026) increase in invasive capacity (Fig. 3b). Significant differences with regard to CAF populations were also observed, i.e., when MDA-MB-231 cells were co-cultured with CAFs from MMP11+ MIC tumors a significantly higher invasive capacity (p = 0.013) was observed compared to MDA-MB-231 cells co-cultured with CAFs from MMP11- MIC tumors.
3.5 Increased tube formation by HUVECs after co-culture with CAFs
In order to assess the angiogenic capacity induced by CAFs we carried out tube formation assays using HUVEC cells. We found that HUVEC cells showed significant increases in average vessel length when incubated in conditioned media derived from MCF-7 and MDA-MB-231 cells co-cultured with CAFs (p = 0.024 and p = 0.007, respectively), as well as increased numbers of capillary-like junctions (p = 0.006 and p = 0.021, respectively) (Fig. 4a, b). No significant differences were observed in average vessel length when HUVEC cells were incubated in conditioned media derived from MCF-7 cells co-cultured with CAFs from either MMP11- MIC tumors or MMP11+ MIC tumors (Fig. 4a, b), whereas significant (p = 0.014) increases in the numbers of capillary-like junctions were observed after incubation of the HUVEC cells in conditioned medium derived from MCF-7 cells co-cultured with CAFs from MMP11+ MIC tumors compared to conditioned medium derived from MCF-7 cells alone (96.7 ± 20.3) (Fig. 4a, b). On the other hand, we found that both the average vessel lengths and the number of junctions in HUVEC cells significantly increased (p = 0.015 and p = 0.011, respectively) when they were incubated in conditioned medium derived from MDA-MB-231 cells co-cultured with CAFs from MMP11+ MIC tumors compared to conditioned medium derived from MDA-MB-231 cells alone (Fig. 4c, d). Finally, we assessed the expression levels of several angiogenesis related proteins by Western blotting. By doing so, we observed an increased expression of phosphorylated (p)-VEGFR2, p-ERK1/2, and p-p38 in the HUVEC cells after incubation with conditioned media from both MCF-7 and MDA-MB-231 cells co-cultured with CAFs from MMP11+ MIC tumors (lanes 2 and 5), compared the other conditions tested (Fig. 4e).
Human umbilical vein endothelial cell (HUVEC) tube formation assay. a HUVEC cells were cultured for 5 h with conditioned media (CM) from MCF-7 cells (CM-MCF-7), CM-MCF-7/CM-CAFs from MMP11- MIC tumors, or CM-MCF-7/CM-CAFs from MMP11+ MIC tumors, after which average vessel lengths and total numbers of junctions were measured. b Representative images of a. c HUVEC cells were cultured for 5 h with CM from MDA-MB-231 cells (CM-MDA), CM-MDA/CM-CAFs from MMP11- MIC tumors, or CM-MDA/CM-CAFs from MMP11+ MIC tumors, after which average vessel lengths and total numbers of junctions were measured. d Representative images of c. Average vessel lengths and numbers of junctions were quantified using Angiotool software. Data represent mean ± SD from three independent experiments. e Western blot analysis of phosphorylated (p)-ERK1/2, p-p38 and p-VEGFR2 levels in HUVEC cells after treatment for 30 min with CM from MCF-7 and MDA-MB-231 cells alone or after co-culture with CAFs from MMP11+ or MMP11- MIC tumors. GAPDH expression was used as internal control. Data represent mean ± SD from three independent experiments
4 Discussion
First, we assessed the effect of CAFs on the expression of 14 stroma-derived factors, including the calcium-binding protein S100A4, the growth factors TGFβ, FGF2, FGF7, PDGFA, PDGFB and VEGFA, the inflammatory cytokines IL-6 and IL-8, the proteases uPA, MMP2, MMP9 and MMP11 and the metalloprotease inhibitor TIMP1 in MCF-7 and MDA-MB-231 breast cancer-derived cells. Secondly, we assessed the effect of CAFs, stratified by the MMP11 status of stromal MICs of the tumors from which they were isolated, on the expression of the respective factore in MCF-7 and MDA-MB-231 cells. Finally, we assessed the effect of CAFs (stratified or not) on the invasive and angiogenic capacities of MDA-MB-231 and HUVEC cells, respectively.
Previously, we observed different CAF-induced expression patterns depending on the tumor cell line with which they were co-cultured [10]. In addition, we found that both breast cancer-derived cell lines used in this study exhibited TGFβ expression upregulation after co-culture with CAFs [10], which is known to induce EMT in malignant mammary epithelial cells, resulting in the acquisition of highly migratory, invasive and metastatic phenotypes [18]. Additionally, others have reported that TGFβ can induce fibroblasts to secrete a variety of growth factors, cytokines and ECM proteins that can promote tumor development [19, 20]. Here, we show that several of the factors studied (S100A4, TGFβ, FGF7, PDGFB, uPA, IL-6, IL-8, MMP2, MMP11, TIMP1 and VEGFA) were increased in each of the two breast cancer-derived cell lines tested after co-culture with CAFs. Taken together, we conclude that reciprocal influences exist between mammary cancer cells and CAFs.
Of the factors that were found to be induced by CAFs after co-culture with MCF-7 and MDA-MB-231 cells, the upregulation of IL-6 and IL-8 was especially relevant, since both factors are cytokines that are known to promote, through autocrine or paracrine signalling, breast cancer growth, invasion [21, 22] and angiogenesis [23]. Additionally, both cytokines have been found to be associated with advanced breast cancer stages and a poor clinical outcome [24,25,26]. In addition, we noted some differences in gene expression changes between MCF-7 and MDA-MB-231 cells after co-culture with CAFs. Whereas MCF-7 cells were found to overexpress uPA, MDA-MB-231 cells were found to overexpress (more than 5-fold increases) PDFGB, VEGFA, TIMP1 and MMP11, which are key factors involved in invasion and angiogenesis. PDGFB can promote tumor growth and invasion [27] and, through autocrine signalling, metastasis [28]. In addition, PDGFB can induce macrophage recruitment, CAF proliferation [29] and angiogenesis [30]. VEGFA can also stimulate angiogenesis and has been found to be significantly associated with a poor survival [31,32,33]. TIMP1, like others TIMPs, is known to be a multifunctional protein, i.e., in addition to its main role as MMP inhibitor, it can also promote tumor cell growth and angiogenesis, and inhibit apoptosis [34]. Previously, associations between high TIMP1 expression levels and tumor aggressiveness have been reported for breast cancer [35, 36].
MMP11 expression by MICs is considered to serve as a useful biomarker for breast cancer prognosis [12,13,14]. We previously reported that the expression of 22 factors related to inflammation and tumor progression (especially IL-1, -5, -6, -17, IFNβ and NFκB), is associated with MMP11 expression by MICs [12, 37]. In addition, we previously observed an association between MMP11 expression by MICs and a high CD68/(CD3+CD20) ratio in macrophages (CD68+), T-cells (CD3+) and B-cells (CD20+) [38]. Indeed, if there is a high CD68/(CD3+CD20) ratio at the invasive front, most intratumoral MMP11+ MICs are macrophages, suggesting that a high CD68/(CD3+CD20) ratio at the invasive front contributes to the polarization of macrophages in the tumor center to achieve a higher metastatic phenotype. As a result, MMP11 expression by MICs characterizes tumors with a poor prognosis [13, 14], a high inflammatory profile [12, 16, 37], a different stroma composition and a CAF phenotype that contributes to tumor progression [10].
Here, we found that the effects of CAFs from primary MMP11+ or MMP11- MIC tumors on the expression profiles of MCF-7 and MDA-MB-231 cells showed some differences. Interestingly we found that, although conditioned media from both CAF populations increased the invasive phenotype of MDA-MB-231 cells, this effect was more pronounced when the conditioned media were derived from CAFs from MMP11+ MIC tumors. This finding suggests that this CAF population may contribute to a higher invasive capacity of relatively more aggressive breast cancer cells (i.e., MDA-MB-231). In accordance with this notion, we also found that co-culture of MDA-MB-231 cells with CAFs from MMP11+ MIC tumors led to a significantly higher expression of IL-6, IL-8 and TIMP1, which may be related to an increased tumor cell invasiveness. In addition, we found that conditioned media from MDA-MB-231 cells co-cultured with CAFs from MMP11+ MIC tumors significantly increased angiogenesis and p-ERK1/2, p-p38 and p-VEGFR2 expression in HUVEC cells, suggesting that CAFs from MMP11+ MIC tumors act on MDA-MB-231 cells to modify their phenotype and increase their malignancy. Interestingly, previous studies have shown that blocking IL-8 [39] and VEGFA [40] (both up-regulated in breast cancer cells after co-culture with CAFs from MMP11+ MIC tumors) may induce the diminution of both invasive and angiogenic capacities of cancer cells.
In summary, we found that CAFs can induce the expression of several factors related to inflammation and tumor progression, as well as increase invasive and angiogenic capacities in breast cancer and HUVEC cells, respectively. As such, our data support the existence of crosstalk between CAFs and breast cancer cells to promote tumor growth and invasiveness. The various factors that are produced by CAFs and breast cancer cells may include new therapeutic targets. The identification of CAF populations differentially enhancing the malignant phenotype of cancer cells from primary tumors with a high inflammatory profile contributes to our understanding of the clinical heterogeneity of breast cancer.
References
T. Marsh, K. Pietras, S.S. McAllister, Fibroblasts as architects of cancer pathogenesis. Biochim. Biophys. Acta 1832, 1070–1078 (2013)
I. Kogan-Sakin, M. Cohen, N. Paland, S. Madar, H. Solomon, A. Molchadsky, R. Brosh, Y. Buganim, N. Goldfinger, H. Klocker, J.A. Schalken, V. Rotter, Prostate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and IL-8 in response to epithelia-secreted IL-1. Carcinogenesis 30, 698–705 (2009)
M.P. Protti, L. De Monte, Cross-talk within the tumor microenvironment mediates Th2-type inflammation in pancreatic cancer. Oncoimmunology. 1, 89–91 (2012)
S. Koontongkaew, The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 4, 66–83 (2013)
S. Madar, I. Goldstein, V. Rotter, ‘Cancer associated fibroblasts’--more than meets the eye. Trends Mol. Med. 19, 447–453 (2013)
N. Erez, M. Truitt, P. Olson, S.T. Arron, D. Hanahan, Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17, 135–147 (2010)
P.S. Soon, E. Kim, C.K. Pon, A.J. Gill, K. Moore, A.J. Spillane, D.E. Benn, R.C. Baxter, Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocr. Relat. Cancer 20, 1–12 (2013)
R. Kalluri, M. Zeisberg, Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006)
M. Allen, J. Louise Jones, Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J. Pathol. 223, 162–176 (2011)
L. Gonzalez, N. Eiro, B. Fernandez-Garcia, L.O. Gonzalez, F. Dominguez, F.J. Vizoso, Gene expression profile of normal and cancer-associated fibroblasts according to intratumoral inflammatory cells phenotype from breast cancer tissue. Mol. Carcinog. 55, 1489–1502 (2016)
M.M. Koczorowska, C. Friedemann, K. Geiger, M. Follo, M.L. Biniossek, O. Schilling, Differential effect of TGFbeta on the proteome of cancer associated fibroblasts and cancer epithelial cells in a co-culture approach - a short report. Cell. Oncol. 40, 639–650 (2017)
N. Eiro, B. Fernandez-Garcia, L.O. Gonzalez, F.J. Vizoso, Cytokines related to MMP-11 expression by inflammatory cells and breast cancer metastasis. Oncoimmunology. 2, e24010 (2013)
L.O. Gonzalez, I. Pidal, S. Junquera, M.D. Corte, J. Vazquez, J.C. Rodriguez, M.L. Lamelas, A.M. Merino, J.L. Garcia-Muniz, F.J. Vizoso, Overexpression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br. J. Cancer 97, 957–963 (2007)
N. Eiro, B. Fernandez-Garcia, J. Vazquez, J.M. Del Casar, L.O. Gonzalez, F.J. Vizoso, A phenotype from tumor stroma based on the expression of metalloproteases and their inhibitors, associated with prognosis in breast cancer. Oncoimmunology 4, e992222 (2015)
L.O. Gonzalez, S. Gonzalez-Reyes, L. Marin, L. Gonzalez, J.M. Gonzalez, M.L. Lamelas, A.M. Merino, E. Rodriguez, I. Pidal, J.M. del Casar, A. Andicoechea, F. Vizoso, Comparative analysis and clinical value of the expression of metalloproteases and their inhibitors by intratumour stromal mononuclear inflammatory cells and those at the invasive front of breast carcinomas. Histopathology 57, 862–876 (2010)
B. Fernandez-Garcia, N. Eiro, M.A. Miranda, S. Cid, L.O. Gonzalez, F. Dominguez, F.J. Vizoso, Prognostic significance of inflammatory factors expression by stroma from breast carcinomas. Carcinogenesis 37, 768–776 (2016)
E. Zudaire, L. Gambardella, C. Kurcz, S. Vermeren, A computational tool for quantitative analysis of vascular networks. PLoS One 6, e27385 (2011)
M.A. Taylor, Y.H. Lee, W.P. Schiemann, Role of TGF-beta and the tumor microenvironment during mammary tumorigenesis. Gene Expr. 15, 117–132 (2011)
B. Bierie, H.L. Moses, Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 6, 506–520 (2006)
A. Orimo, P.B. Gupta, D.C. Sgroi, F. Arenzana-Seisdedos, T. Delaunay, R. Naeem, V.J. Carey, A.L. Richardson, R.A. Weinberg, Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005)
A.W. Studebaker, G. Storci, J.L. Werbeck, P. Sansone, A.K. Sasser, S. Tavolari, T. Huang, M.W. Chan, F.C. Marini, T.J. Rosol, M. Bonafe, B.M. Hall, Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 68, 9087–9095 (2008)
J.M. Wang, X. Deng, W. Gong, S. Su, Chemokines and their role in tumor growth and metastasis. J. Immunol. Methods 220, 1–17 (1998)
D.E. Hu, Y. Hori, T.P. Fan, Interleukin-8 stimulates angiogenesis in rats. Inflammation 17, 135–143 (1993)
T. Bachelot, I. Ray-Coquard, C. Menetrier-Caux, M. Rastkha, A. Duc, J.Y. Blay, Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br. J. Cancer 88, 1721–1726 (2003)
R. Salgado, S. Junius, I. Benoy, P. Van Dam, P. Vermeulen, E. Van Marck, P. Huget, L.Y. Dirix, Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int. J. Cancer 103, 642–646 (2003)
I.H. Benoy, R. Salgado, P. Van Dam, K. Geboers, E. Van Marck, S. Scharpe, P.B. Vermeulen, L.Y. Dirix, Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin. Cancer Res. 10, 7157–7162 (2004)
Y. Cao, Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol. Med. 19, 460–473 (2013)
M. Jechlinger, A. Sommer, R. Moriggl, P. Seither, N. Kraut, P. Capodiecci, M. Donovan, C. Cordon-Cardo, H. Beug, S. Grunert, Autocrine PDGFR signaling promotes mammary cancer metastasis. J. Clin. Invest. 116, 1561–1570 (2006)
M.M. Mueller, N.E. Fusenig, Friends or foes - bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 4, 839–849 (2004)
D. Engelmann, D. Mayoli-Nussle, C. Mayrhofer, K. Furst, V. Alla, A. Stoll, A. Spitschak, K. Abshagen, B. Vollmar, S. Ran, B.M. Putzer, E2F1 promotes angiogenesis through the VEGF-C/VEGFR-3 axis in a feedback loop for cooperative induction of PDGF-B. J. Mol. Cell Biol. 5, 391–403 (2013)
P. Manders, L.V. Beex, V.C. Tjan-Heijnen, J. Geurts-Moespot, T.H. Van Tienoven, J.A. Foekens, C.G. Sweep, The prognostic value of vascular endothelial growth factor in 574 node-negative breast cancer patients who did not receive adjuvant systemic therapy. Br. J. Cancer 87, 772–778 (2002)
H. Bando, H.A. Weich, M. Brokelmann, S. Horiguchi, N. Funata, T. Ogawa, M. Toi, Association between intratumoral free and total VEGF, soluble VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br. J. Cancer 92, 553–561 (2005)
S. Ghosh, C.A. Sullivan, M.P. Zerkowski, A.M. Molinaro, D.L. Rimm, R.L. Camp, G.G. Chung, High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum. Pathol. 39, 1835–1843 (2008)
S.O. Wurtz, A.S. Schrohl, N.M. Sorensen, U. Lademann, I.J. Christensen, H. Mouridsen, N. Brunner, Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr. Relat. Cancer 12, 215–227 (2005)
A. Neri, T. Megha, F. Bettarini, D. Tacchini, M.G. Mastrogiulio, D. Marrelli, E. Pinto, P. Tosi, Is tissue inhibitor of metalloproteinase-1 a new prognosticator for breast cancer? An analysis of 266 cases. Hum. Pathol. 43, 1184–1191 (2012)
A. Dechaphunkul, M. Phukaoloun, K. Kanjanapradit, K. Graham, S. Ghosh, C. Santos, J.R. Mackey, Prognostic significance of tissue inhibitor of metalloproteinase-1 in breast cancer. Int. J. Breast Cancer 2012, 290854 (2012)
N. Eiro, L. Gonzalez, L.O. Gonzalez, B. Fernandez-Garcia, M.L. Lamelas, L. Marin, S. Gonzalez-Reyes, J.M. del Casar, F.J. Vizoso, Relationship between the inflammatory molecular profile of breast carcinomas and distant metastasis development. PLoS One 7, e49047 (2012)
N. Eiro, I. Pidal, B. Fernandez-Garcia, S. Junquera, M.L. Lamelas, J.M. del Casar, L.O. Gonzalez, A. Lopez-Muniz, F.J. Vizoso, Impact of CD68/(CD3+CD20) ratio at the invasive front of primary tumors on distant metastasis development in breast cancer. PLoS One 7, e52796 (2012)
Y. Lin, R. Huang, L. Chen, S. Li, Q. Shi, C. Jordan, R.P. Huang, Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int. J. Cancer 109, 507–515 (2004)
R.K. Jain, Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003)
Acknowledgments
This work was supported by grants to F. V from the Instituto de Salud Carlos III (PI13/02745) and from the Consejería de Economía y Empleo del Principado de Asturias (GRUPIN14-116), and to R.P-F. from the Ministerio de Economia y Competividad (SAF2015-69221-R). A.M-O. is a predoctoral fellow from the Ministerio de Educación (FPU14/00548). S.C. was recipient of a predoctoral fellowship financed by the Gobierno del Principado de Asturias “Severo Ochoa” PhD Program (BP14-128).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study adheres to national regulations and was approved by our Institution’s Ethics and Investigation Committee.
Conflict of interest
The authors have no conflicts of interests to disclose.
Rights and permissions
About this article
Cite this article
Eiro, N., González, L., Martínez-Ordoñez, A. et al. Cancer-associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell Oncol. 41, 369–378 (2018). https://doi.org/10.1007/s13402-018-0371-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-018-0371-y