Abstract
Years of investigation have shed light on a theory in which breast tumor epithelial cells are under the effect of the stromal microenvironment. This review aims to discuss recent findings concerning the phenotypic and functional characteristics of cancer associated fibroblasts (CAFs) and their involvement in tumor evolution, as well as their potential implications for anti-cancer therapy. In this manuscript, we reviewed that CAFs play a fundamental role in initiation, growth, invasion, and metastasis of breast cancer, and also serve as biomarkers in the clinical diagnosis, therapy, and prognosis of this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
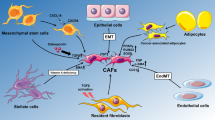
The development of breast cancer does not depend exclusively on the intrinsic characteristics of the tumor epithelial cells, but also on the significant role of the tumor microenvironment [1, 2]. Therefore, it is important to study not only the characteristics of the cancer cells but also the components of the tumor microenvironment in order to find biomarkers of tumor evolution [3,4,5,6,7,8,9,10,11,12,13]. The breast tumor microenvironment is composed of non-neoplastic stromal cells as well as non-cellular components. Cellular components include the endothelial cells, pericytes, tumor-associated macrophages (TAM), dendritic cells and other immune cells, adipocytes, fibroblasts, cancer associated fibroblasts (CAFs), cells derived from bone marrow (BM) and adipose tissue, within which are the mesenchymal stem cells (MSC), mesenchymal precursors and progenitors, hematopoietic stem cells and hematopoietic progenitors, as well as myeloid-derived suppressor cells (MDSC). The non-cellular components involve soluble factors, such as cytokines, chemokines, growth factors, metalloproteinases (MMP), tissue inhibitors of metalloproteinases (TIMP), among others, and extracellular matrix (ECM) components like hyaluronic acid, laminin, fibronectin and collagen type I (Fig. 1) [1, 14, 15].
Crosstalk between normal breast epithelium and components of the microenvironment [Fibroblasts, immune cells, mesenchymal stem cells (MSC), pericytes, endothelial cells, extracellular matrix (ECM)] mediated through direct cell–cell contacts or by secreted molecules, maintain tissue equilibrium. Minor changes in one compartment may cause dramatic alterations in the whole system, thus leading to the development of breast cancer. Consequently, a new crosstalk is established between the breast cancer epithelium and the tumor microenvironment, creating a permissive niche via the formation of cancer associated fibroblasts (CAFs), production and remodeling of ECM, sustained proliferation, stemness, and metabolic reprogramming of cancer cells. In addition, CAFs generate an immunosuppressive microenvironment by promoting monocyte recruitment and inducing their differentiation to M2 macrophages, as well as by increasing the recruitment of regulatory T lymphocytes, endothelial progenitors and precursors, and myeloid-derived suppressor cells (MDSC)
Morsing et al. showed that the normal human mammary gland has two types of stroma. The lobular epithelial cells are surrounded by a loosely connected stroma containing the intralobular fibroblasts, which are surrounded by more fibrous stroma containing the interlobular fibroblasts. Regarding intralobular fibroblasts, they have a different gene expression profile than interlobular fibroblasts. These last ones have low endoglin (CD105) and high CD26 expression and show an immune profile characterized by the expression of Interleukin-1 (IL-1) receptor (R) type 1 (IL-1R1), IL-33, and solute carrier (SLC) 39A8 (SLC39A8) [16]. On the other hand, intralobular fibroblasts present high CD105 and low CD26 expression, have similar characteristics to mesenchymal stem cells and promote epithelial growth and morphogenesis [16]. Moreover, the intralobular fibroblasts exhibit gene expression similar to that of the mammary tumor stroma [16]. This might indicate that these normal fibroblasts are more prone to generating myofibroblasts if cancer arise in the terminal duct lobular unit, which is the predominant site of breast tumor occurrence per se [16].
The fibroblasts are the most predominant cells in solid tumors, such as breast cancer [17]. These are elongated cells with extended cellular processes that present the characteristic spindle-like shape. They synthesize the ECM of the connective tissue, being fundamental in the structural integrity of the tissues [18]. Activated fibroblasts, usually called myofibroblasts, have typical smooth muscle cell characteristics, such as the presence of microfilament bundles, GAP junctions, and the expression of α-smooth-muscle actin (α-SMA). In particular, these activated fibroblasts that are associated with tumor cells are known as CAFs [17, 19,20,21]. Fibroblast activation and consequent CAFs formation involve a loss of CD34 antigen expression, and a gain of α-SMA, platelet-derived growth factor receptor (PDGFR), fibroblast surface protein (FSP) or fibroblast activation protein (FAP), as well as an increase of cell contractility [17, 22, 23].
Although fibroblasts are typically recognized by their morphologic feature, they are still poorly defined by more specific characteristics such as the expression of molecular markers. In fact, the lack of specific markers is a limiting factor when studying fibroblasts in vivo [17]. Furthermore, the role of resting and activated fibroblasts in breast tumor evolution remains a subject of study. Nevertheless, there is increasing evidence that CAFs are important promoters of tumor growth and progression [17]. CAFs are currently known to contribute to tumor initiation, proliferation, invasion, and subsequent metastasis through the production of cytokines, growth factors, chemokines, and matrix-degrading enzymes such as MMP [17, 20, 21]. Also, CAFs induce dysfunctional repairing mechanisms, increasing the production of fibrotic ECM which contains high amounts of collagen fibers and inhibiting MMP effect [21]. This ECM suppresses epithelial cell polarity and stimulates their proliferation, modifications that allow tumor formation and development. Particularly, in the breast tumor, the expansion process of tumor stroma characterized by the activation and proliferation of fibroblasts and consequent production of components of the ECM is called a desmoplastic reaction [24]. In relation with this concept, Giatromanolaki et al. [25] described that this process in which new stroma is formed, called stromatogenesis, is an integral and necessary characteristic for tumor invasion.
Experimental studies showed an active and reciprocal interaction between the tumor cells and the stromal cells, as well as with and other components of the tumor microenvironment, critical events that determine and promote the growth of the primary tumor and its progression towards a future metastasis [1, 26,27,28,29,30,31,32,33,34]. Studies in the last decade have demonstrated that the stromal microenvironment can create a protective niche in which cancer cells are protected from classical treatments, leading to therapeutic failure. However, the microenvironment that surrounds the tumor cells can also potentially impose selective pressure on cancer clones and may hold the key to inhibit or reduce illnesses such as breast cancer [35, 36]. In fact, the breast tumor microenvironment is acidic and hypoxic, the same as an ischemic niche [37,38,39,40,41]. Then, such acidity that induces tumor activation could be a target of breast cancer therapy, regulating the release of drugs and thus reducing cell tolerance [15, 37]. Normal breast epithelial cells and stromal cells undergo a continuous and bilateral molecular crosstalk mediated through direct cell–cell contacts or by secreted molecules (Fig. 1). Therefore, a few changes in one compartment may cause dramatic alterations in the whole system [42]. For example, genetic alterations that lead to a malignant breast epithelial cell during tumor initiation, will consequently change the stromal host compartment to establish a permissive and supportive microenvironment for the tumor cells [43]. Moreover, during early stages of tumor development and invasion, the basement membrane is degraded, and the activated stroma, containing fibroblasts, inflammatory infiltrates and newly formed capillaries, come into direct contact with the tumor cells (Fig. 1). Liotta et al. described that the interaction between the epithelial and mesenchymal compartments create a local heterotypic "invasion field" from which the metastatic cell emerges and disseminates [44]. In relation, animal studies have shown that both wounded and activated stroma provide oncogenic signals to facilitate tumorigenesis [45, 46]. In this context, there is increasing evidence that CAFs affect all the hallmarks of cancer [19, 47]. Therefore, in the present review we will discuss the current knowledge about the origin, the phenotypic and morphological characteristics, as well as the functions of CAFs, mainly in breast cancer. Also, we will focus on their contribution in breast tumor evolution and in the possible implications for cancer therapy.
Morphological and Phenotypic Characteristics of CAFs
In breast carcinomas, most of the stromal cells are fibroblasts, and 80% of them are activated, becoming CAFs [48]. These CAFs have spindle shaped morphology (fusiform) and elongated cytoplasmic projections [49]. Also, these cells have a thin nucleus that is slightly thicker than that of non-activated fibroblasts [49]. Under the microscope, CAFs have abundant basophilic cytoplasm with abundant endoplasmic reticulum, a complex Golgi apparatus, free ribosomes, and myofilaments and fibronexus junctions in the periphery [50]. In addition, Kumar et al. observed that the shape and size of the fibroblast cell line from normal human breast CCD-1126Sk were modified when exposed to conditioned medium of human breast cancer cell lines [51]. They showed that some of these fibroblasts stimulated with conditioned medium from MCF-7 human breast cancer cell line, acquired a spindle shape and increased 30–50% in size, as well as 2–threefold in length. However, with conditioned medium from MDA-MB 321 human breast cancer cell line, the size of CCD-1126Sk fibroblasts increased only 10–15% but the spindle shape was similar to that observed with conditioned medium from MCF-7 [51].
CAFs can be distinguished from normal breast fibroblasts by the differential expression of α-SMA, FAP, FSP1 and PDGFR [19,20,21]. While CAFs are negative for CD34, CD31 and cytokeratin markers; a high percentage of them are positive for α-SMA, vimentin, FSP, FAP, osteonectin, desmin, tenascin, prolyl 4-hydroxylase, PDGFR-α/β, and have low expression of calveolin-1 (CAV1), among other proteins [50, 52, 53]. Moreover, an interesting study showed that the normal human breast fibroblasts also exhibit limited levels of calcium-binding protein (S100A4) and prolyl 4-hydroxylase compared with CAFs [54]. In addition, Purcell et al., when studying their RNA expression profile, found that the messenger RNA of membrane protein leucine rich repeat containing 15 (LRRC15) is highly expressed in CAFs of human breast tumor, among other types of cancer, compared to normal tissue [55]. They also observed that the expression of LRRC15 is induced by the transforming growth factor beta 1 (TGF-β) signal on the CAFs [α-SMA( +)] as well as on the MSC [55].
In a recent study, Sebastian et al. characterized the fibroblast heterogeneity in a mouse allograft model of triple negative breast cancer (TNBC) [56]. Using a single-cell RNA sequencing (scRNA-seq) the authors identified three important CAFs subpopulations: 1)- myofibroblastic CAFs (myoCAFs), enriched with α-SMA and other contractile proteins such as tenascin C, transgelin and myosin light chain 9; 2)- ‘inflammatory’ CAFs (iCAFs), with elevated expression of inflammatory cytokines such as IL-6, IL-33, C-X-C Motif Chemokine Ligand 1 and 12 (CXCL-1, CXCL-12) and chemokine ligand 7 (CCL-7); and 3)- CAFs that express major histocompatibility complex (MHC) class II proteins. The last two subpopulations of fibroblasts were found in higher number in the normal breast stroma while the number of myoCAFs was extremely low. This last observation could suggest that myoCAFs arise during tumorigenesis [56]. Furthermore, this myoCAFs have several growth factor transcripts increased, including TGF-β1/TGF-β2, connective tissue growth factor (CTGF), placental growth factor, vascular endothelial growth factor α (VEGF-α) and Wnt-α [56].
In other reported studies, through the performance of microarrays in human breast tumor tissue samples, it was found that there were different functional subtypes of CAFs based on the membrane antigen that they express, for example: FAPα( +), which are activated or reactivated, and are associated with modulation of the ECM, tumor invasion and immunomodulatory function; FSP-1( +), which are associated with metastatic colonization, macrophage infiltration and protection against carcinogens; and PDGFR-α( +), which have an interrelation with the paracrine signaling that mediates tumor growth and angiogenesis, as well as the recruitment of macrophages [54, 57]. Also, Costa et al. [58] through a gating strategy distinguished four different subpopulations of CAFs in human breast cancer, according to the expression levels of CD29, FAP, α-SMA, PDGFR-β, FSP1, and CAV1. These authors found a significant association between CAFs subsets and breast cancer subtypes. The subpopulations were defined as CAF-S1: CD29 Med, FAP Hi, FSP1 Lo−Hi, α-SMA Hi, PDGFR-β Med−Hi and CAV1 Lo; CAF-S2: CD29 Lo, FAP Neg, FSP1 Neg−Lo, α-SMA Neg, PDGFR-β Neg and CAV1 Neg; CAF-S3: CD29 Med, FAP Neg, FSP1 Med−Hi, α-SMA Neg−Lo, PDGFR-β Med and CAV1 Neg−Lo; CAF-S4: CD29 Hi, FAP Neg, FSP1 Lo−Med, α-SMA Hi, PDGFR-β Lo−Med and CAV1 Neg−Lo. The CAF-S1 population was predominant in TNBC while the CAF-S4 population was predominant in HER2 breast cancers. However, they did not find that the CAFs subsets were indicative of breast cancer patient survival by themselves. Additionally, the phenotypic differences between the four subpopulations reflected different functionalities, in particular those related to immunosuppression. Particularly, in TNBC samples they found that CAF-S1 subpopulation plays a fundamental role in immunosuppression, different from CAF-S4. CAF-S1 favor the attraction of T lymphocytes, increase the survival of CD4( +)/CD25( +) T lymphocytes and promote their differentiation into regulatory CD25( +)/FOXP3( +) cells, which finally inhibit T lymphocytes. We will delve into this immunosuppression capacity of CAFs in the following sections. However, there is no profile of specific markers that recognize all the CAFs present in each type of breast cancer [53, 59]. It is known that the phenotypic characteristics of CAFs can be modified depending on the cancer subtype. In relation with this, Park et al. [54] performed a tissue microarray consisting of 642 human breast cancer samples. In this study, they found high expression of podoplanin, prolyl 4-hydroxylase, FAP, S100A4, PDGFR-α, PDGFR-β, and chondroitin sulfate proteoglycan (CSPG) in CAFs from Her2/neu breast tumors, whereas the expression of these proteins was lower in luminal A (ER+ and / or PR+, Her2−, low Ki-67) and triple negative (ER−, PR− and Her2−) breast tumors. In addition, high expression of these proteins was found in CAFs from breast tumors with desmoplastic stromal type in comparison with the sclerotic and inflammatory types [54].
In summary, CAFs are spindle shape stromal cells and the most have with α-SMA Pos, vimentin Pos, FSP Pos, FAP Pos, osteonectin Pos, desmin Pos, tenascin Pos, prolyl 4-hydroxylase Pos, PDGFR-α/β Pos, CD34 Neg, CD31 Neg, cytokeratin Neg, and CAV1 Lo. The precise classification by cellular markers of the diverse subpopulations of CAFs in the different stages of breast tumor progression is still a subject of study [20]. Finally, it is important to evaluate not only the phenotypical characteristics of CAFs in human primary breast tumor samples but also the morphological changes of these cells during tumor progression. Both analysis could be used in the future to improve breast cancer diagnosis, as well as to develop novel therapies.
Different Origins of CAFs
Currently, the origin of CAFs in breast cancer remains not entirely clear and is a continuous topic of study. Perhaps, the heterogeneity of the CAFs in terms of characteristics and molecular markers, is due to their diverse cellular origins. They could have originated from fibroblasts of mammary tissue, breast tumor epithelial cells [due to their epithelial-mesenchymal transdifferentiation (EMT)], endothelial cells (due to their endothelial-mesenchymal transdifferentiation), pericytes, MSC from BM and adipose tissue (Fig. 2) [7, 23, 59,60,61,62,63,64,65]. Interestingly, Nair et al. recently suggested that cancer stem cells (CSC) could be a new source of CAFs in the stem cell niche of the breast tumor [66]. In a murine model, they generated CSC by treating induced pluripotent stem cells with conditioned media from T47D and BT549 human breast cancer cell lines. Then, these CSC were able to differentiate towards CAFs which supported the maintenance and survival of breast cancer cells [66].
Breast CAFs may Originate from Resident Fibroblast
Breast tumor cells secrete different factors, both soluble and internalized in exosomes that induce the activation of normal resident breast fibroblasts and the subsequent differentiation to CAFs. Some examples are stromal-derived-factor-1 (SDF-1) [50, 59], TGF-β [50, 59], PDGF [50, 67, 68], basic fibroblast growth factor (FGF-β) [50], IL-6 [50, 69], leukemia inhibitory factor [2], osteopontin [70], reactive oxygen species (ROS) [71] survivin [72], miRNAs [73, 74], among others. Albrengues et al. proved in vitro and , in mouse models of breast carcinomas, that leukemia inhibitory factor induces the activation of normal fibroblast through signaling of the JAK / STAT pathway [75]. Recently, Vu et al. [73] established an important role for miR-125b in CAFs differentiation both in human and in in vivo mouse models of breast cancer. They demonstrated that breast tumor cells secrete extracellular vesicles that deliver miR-125b, which are spontaneously captured by normal fibroblasts at a high rate [73]. Then, miR-125b promotes fibroblasts differentiation to CAFs through increased expression of α-SMA, MMP and some cytokines [73]. Furthermore, Chatterjee et al. observed that miR-222 and laminin B receptor are important for the differentiation and maintenance of CAFs, thus influencing the tumorigenesis of human breast cancer cells [74]. In relation, another study showed that this miR-222 confers chemoresistance of human breast cancer cells to adriamycin by targeting PTEN/Akt [76]. Besides, Kojima et al. showed that human breast fibroblasts are progressively converted to CAFs during breast tumor progression using a xenograft murine model. In this work, they used a mix of a primary culture of normal human breast GFP+- fibroblasts with MCF-7 cells, which was injected into immunodeficient nude mice [59]. This group suggested that human breast tumor cells could release TGF-β, which can elicit enhanced endogenous TGF-β and SDF-1 production and induce C-X-C motif chemokine receptor 4 (CXCR4) expression in stromal breast fibroblasts. Consequently, two autocrine signaling loops, mediated by TGF-β and SDF-1, are generated, and they act in a positive feedback way, which maintain the CAFs´ phenotype [59]. On the other hand, other authors showed that ROS promote normal breast fibroblast conversion into CAFs through the accumulation of hypoxia inducible factor-1α (HIF‐1α) and CXCL-12 [77, 78]. Moreover, other studies showed that the release of survivin and osteopontin, by mammary tumor cells upregulate SOD1 expression in normal fibroblasts and then converts them into CAFs, thus inducing breast cancer progression both in vitro and in vivo [70, 72].
Breast CAFs may Originate from Epithelial Cells, Endothelial Cells, Pericytes and Adipocytes
Through the scRNA-seq in a genetic-engineered murine breast cancer model, Bartoschek et al. demonstrated the existence of three spatially and functionally distinct subsets of breast CAFs. This group showed that CAFs can originate from the breast tumor epithelial cell through the EMT process, from resident fibroblasts, as mentioned previously, and from pericytes of perivascular niche [60]. They found that a subpopulation of CAFs shared many marker genes with pericytes, including CSPG-4, RGS5, PDGFR-β, and DES, albeit at comparably low levels. In addition, they observed that endosialin is highly expressed by this CAFs subpopulation, which was previously reported to be a marker for activated MSC, including tumor pericytes. On the other hand, this group recognizes a second subpopulation of CAFs that can originate from resident fibroblasts of the breast, which specifically expressed transcripts of a large variety of ECM-related genes, such as glycoproteins (DCN, LUM, and VCAN), structural proteins. (COL14A1), matricellular proteins (FBlN1, FBlN2, and SMOC), and matrix-modifying enzymes [lysyl oxidase (LOX) and LOXL1] [60]. Finally, they described another subset of CAFs that had a transcriptional signature (SCRG1, SOX9, and SOX10, among others) and histological localization that suggests a possible breast epithelial origin [60]. In relation, fibroblasts that come from breast cancer cells contain mutations in oncogenes and tumor suppressors that make them distinct from CAFs of different origins [79]. However, most studies did not find mutations in CAFs populations, which could suggest that the contribution of CAFs derived from EMT is minimal [79, 80].
Besides, during tumor progression, peritumoral adipocytes could become CAFs present in the desmoplastic reaction of human breast cancers [81, 82]. With this idea in mind, Bochet et al. injected GFP-3T3-F442A mouse pre-adipocytes cell line into the flank of athymic nude mice. Fat pad formation was allowed to proceed for 5 weeks, after which fat pads were injected with 4T1 mouse breast cancer cell line. They found that adipose tissue was composed of adipocytes-GFP Pos and fibroblast-like cells-GFP Pos, containing many small lipid droplets [65]. Thus, they conclude that breast cancer cells are able to force mature adipocytes towards fibroblast-like cells that express some CAFs markers like FSP-1 / S100A4, but not α-SMA [65]. Moreover, Ghiabi et al. demonstrated that breast tumor cells were able to stimulate endothelial-mesenchymal transition in endothelial cells (α-SMA+, FSP-1+, CD31+ and VE-Cadherin+). This result was reached through an in vitro model of cell co-culture between human umbilical vein endothelial cells (HUVECs) and MDA-231/MCF-7 cell lines, as well as by an in vivo model based on co-injection of MDA-231 to the mammary fat pad of NOD/SCID mice with or without E4-ECs (Akt low). The authors proposed that this process is regulated by Smad signaling through the synergistic stimulation of TGFβ and notch pathways [62]. Additionally, Buchsbaum et al. [53] suggested the possibility of an endothelial origin of CAFs in the breast tumor microenvironment based on previous works of Kalluri et al. [19, 83, 84] in relation to the mesenchymal-endothelial transition during cancer development. This is a complex process in which endothelial cells lose their molecular markers, such as vascular endothelial cadherin, and acquire others like α-SMA, type I collagen, and vimentin, typical markers of myofibroblastic cells. Moreover, they suggest that these fibroblastic cells also gain motility and migration capacities [21, 84, 85].
Breast CAFs may Originate from Mesenchymal Stem Cells of Bone Marrow
Regarding the mesenchymal origin, Raz et al. performed adoptive BM transplantations from donor mice that expressed GFP ubiquitously (β-actin-GFP) into MMTV-PyMT female mices. They showed that while GFP + cells were found in both normal and tumoral breast tissues (probably due to infiltration of immune cells derived from BM), BM-derived CAFs (GFP +, α-SMA+) were detected in breast tumors and not in normal breast tissue. Through this lineage tracing experiment, this group demonstrated that BM-MSC (PDGFR-α−, CD45−, CD34−) are recruited into primary breast tumors and they differentiate into CAFs (α-SMA+, PDGFR-α−, CD45−, CD34−) [61]. They also observed that the recruitment of MSC depends on a gradient of SDF-1 and CXCL-16, and on the hypoxia state generated by the tumor cells [86,87,88]. In addition, ECM stiffness could also contribute to MSC recruitment to the tumor microenvironment, where they differentiate to CAFs and promote breast tumor progression. One study showed that when culturing human MSC on a rigid and non-flexible ECM, they developed a CAFs phenotype and contributed to breast tumor progression [89]. It is believed that the cytokines secreted by breast cancer cells have a significant role in the MSC-CAF transformation. In fact, it is well known that breast tumor cells, like other cells, produce epidermal growth factor (EGF), SDF-1, PDGF, VEGF, FGF-β, IL-6, ROS, TGF-β1, among others, that favor the differentiation process from MSC to CAFs, and its subsequent activation [50, 52, 90]. MSC progressively acquire the ability to promote breast tumor growth and become tumor associated-MSC, some of which eventually lose their self-renewal capacity and express high levels of α-SMA, vimentin and FSP1, typical markers of CAFs [88]. Interestingly, Weber et al., through in vitro and in vivo assays with human breast cancer cells, suggested that osteopontin induces MSC-CAF transformation by activating the transcription factor myeloid zinc finger 1, inducing the production of TGF-β [63]. Moreover, other authors found that the lysophosphatidic acid released by breast tumor cells favor its epithelial-mesenchymal transition, invasion and the possibly fibroblastic differentiation of MSC, driving these events to breast cancer progression [91].
Besides, Paunescu and collaborators [92] observed that CAFs isolated from primary tumors of infiltrating ductal breast cancer patients have phenotypic characteristics (CD44 Pos, CD73 Pos, CD90 Pos, CD106 Pos and CD117 Pos, as well as CD14 Neg, CD29 Neg, CD31 Neg, CD34 Neg, CD45 Neg and HLA-DR Neg), similar to BM-MSC from healthy donors. In addition, both cell types expressed vimentin, α-SMA and nestin, while not expressing positivity for E-cadherin and cytokeratin. Moreover, these authors found that the expression of vimentin was not modified in the CAFs when subcultivated, which suggests that the CAFs retain their primitive MSC-like characteristics. Furthermore, the CAFs of the primary tumors of these patients showed a capacity for chondrogenic and osteogenic differentiation similar to the BM-MSC from healthy donors and lower adipogenic differentiation. Also, they showed that CAFs released higher levels of growth factors, as well as immunosuppression and pro-angiogenic factors than normal BM-MSC. However, Del Valle et al., found important differences in gene expression between BM-MSC and CAFs from primary breast tumors and lymph nodes of breast cancer patients. This difference may reflect the adaptation that MSC must do in order to differentiate to CAFs [93]. Furthermore, other authors showed that human BM-MSC exposed to conditioned medium of the human breast cancer cell line MDA-MB-231 over a long period of time, acquire a CAFs phenotype with a high expression of α-SMA, vimentin, FSP and SDF-1 [94]. In addition, Raz et al. [61] found that PDGFR-α expression could define two functionally different populations of CAFs in human breast tumors [61]. They observed that human BM-derived CAFs, unlike the resident CAFs, are PDGFR-α−. Interestingly, a decrease in PDGFR-α in CAFs from human breast tumors was associated with a worse prognosis in patients. Also, it has been observed that 90–95% of the BM-MSC are characterized by presenting CD105+. Interestingly, in breast cancer patients, 50% of CAFs express CD105 in the tumor stroma [95]. Furthermore, CD105 antigen is the co-receptor for TGF-β, which is the modulator of the response. Also, TGF-β favors the differentiation of MSC into CAFs, stimulates the proliferation and activation of CAFs, as well as increases its tumor activity by inducing the release of ECM components [96]. In a previous work, we found that epithelial cells present in the primary tumors of breast cancer patients (invasive ductal carcinoma, clinical-pathological stages I and II, without treatment) produce chemotactic substances for BM-MSC as IL-6, SDF-1 and CCL-2. Furthermore, we observed a significant association between the expression of these ligands in breast tumor cells and the expression of IL-6R, CXCR-4 and C–C chemokine receptor type 2 (CCR-2), present in intratumoral fusiform stromal cells, that are not associated with the vasculature, like MSC or CAFs [97].
While the origin of breast CAFs is controversial, here we present a variety of evidences supporting the origins of CAFs, in particular from resident normal breast fibroblasts, BM-MSC, pericytes of the mammary vascular niche, and breast tumor cells. However, a unique and clear definition of breast CAFs in the context of specific markers, genetic profile and origins is currently lacking. This may be due, not only to the great heterogeneity of CAFs, but also to the lack of lineage tracing experiments and studies with large sample sizes.
Role of CAFs in Breast Tumor Evolution
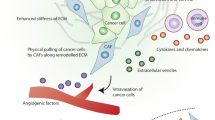
The role of CAFs in breast cancer progression includes a wide range of functions including ECM remodeling, secretion of soluble factors, regulation of motility and stemness, tumor metabolism remodeling and conditioning of a pre-metastatic niche. This role is dual, being able to inhibit or promote malignant growth. They can possibly act as repressors at the beginning of breast tumor progression, facilitating the formation of gap junctions between CAFs and tumor cells, and so establishing an inhibition by contact between tumor cells [98]. At more advanced stages, a greater number of local breast fibroblasts are activated and new fibroblasts that will differentiate into activated CAFs are recruited in through the action of soluble factors secreted by the tumor [98]. In this context, the cytokine TGF‐β plays a fundamental role, since it is a multifunctional cytokine, released by CAFs and breast tumor cells, among other cells, and a potent driver of cancer progression. TGF‐β acts on a variety of cells within the breast tumor niche. During the early stages of breast cancer development this factor could acts as a promoter of apoptosis in breast tumor cells, and is also a powerful stimulator of the conversion of normal fibroblasts into CAFs [15, 55, 99, 100]. However, as the tumor progresses this cytokine could exerts an opposite effect through the mediation of epithelial-mesenchymal transition and the induction of angiogenesis and immunosuppression [99,100,101,102].
Role of CAFs in Breast Tumor Initiation
CAFs may be involved in the breast tumor initiation. Several studies showed that breast CAFs promote the proliferation and malignant transformation of breast epithelial cells. Using a co-transplanting mouse model, a study showed that irradiated normal breast fibroblasts have a high tumor-promoting capacity compared to non-irradiated ones [103]. In relation with this, Kuperwasser et al. [104] found that irradiated normal breast fibroblasts overexpress TGF-β and hepatocyte growth factor (HGF), both factors that favor breast tumor development. Moreover, in a 3D cell–cell interaction model, Shekhar et al. demonstrated that CAFs, through estrogen synthesis, induce the malignant transformation of MCF-10A, a human normal breast epithelial cell line, and its preneoplastic derivative, the MCF10AT1-EIII8 (referred to as EIII8), as well [105]. Also Wang et al., demonstrated that CAFs and normal breast fibroblasts from tumor and healthy breast tissues of the same patient respectively, promoted the self-renewal capacity of breast cancer stem cells and induced the phenotypic transformation of non-stem cells to cancer stem cells. These last results indicated that both CAFs and normal fibroblasts would be involved in the initiation and progression of the breast tumor [106]. Thus, all these studies could suggest that the differentiation of CAFs from normal fibroblasts could occur at the beginning of breast tumor development, preceding the genetic alterations experienced by the mammary epithelial cells, thus promoting the malignant transformation of the neighboring mammary epithelial cells.
Role of CAFs in Breast Tumor Progression
At more advanced stages, CAFs contribute to breast tumor progression through the secretion of growth factors such as EGF, FGF-β, PDGF, VEGF-α, insulin growth factor (IGF-1), HGF, tumor necrosis factor (TNF) [107], SDF-1 [108] and CTGF, as well as a set of cytokines and chemokines including IL-4 [109], IL-6 [109], IL-17A, CXCL-14 [110, 111], CXCL-16 [112] and CCL-5 [19, 20, 113, 114]. These factors stimulate proliferation and inhibit apoptosis of breast tumor cells, as well as vascular and lymphatic endothelial cells. In this way, tumor growth occurs simultaneously with the development of new blood and lymphatic vessels. In particular, the effect of VEGF could depend on the contractile properties of CAFs [89]. In a recent study, Suh et al. demonstrated that CAFs through the release of FGF-β promote the growth, migration and invasion of MDA-MB-231 cells in the mammary tumor microenvironment [115]. Additionally, Wu et al. [116] have demonstrated that crosstalk between 82 T human breast CAFs cell line and MDA-MB-231 human triple negative breast cancer cell line induce reciprocal activation of tyrosine kinase receptors. In particular, EGF and IGFR-1 are activated in CAFs, whereas FGFR-1 and Axl are activated in breast tumor cells [116]. Interestingly, there is consensus about the stability of CAFs’ phenotype, that is a consequence of the conservation of epigenetic changes [80, 117, 118]. The malignant phenotype of CAFs may persist even in the absence of the paracrine signals produced by the human breast tumor epithelium [108]. Besides, non-structural ECM proteins, such as tenascin C and periostin, are also express by human breast CAFs [119]. Wang et al. used an orthotopic mouse tumor model to show that periostin favors breast tumor pulmonary metastasis by stimulating the accumulation of MDSC at the metastatic site [120]. In addition, Huang et al. demonstrated in vitro that tenascin C affects proliferation, migration and metastasis of MDA-MB435 human breast cancer cell line [121]. In a recent work, we found that intra-tumor stromal cells with fusiform shaped morphology that are not associated with the vasculature produce molecules such as TNF-related apoptosis-inducing ligand (TRAIL), a receptor activator of nuclear factor-kappaβ (NF-κB) ligand (RANKL) and CCL-2, which modify the survival, proliferation, migration and the acquisition of a mesenchymal phenotype of tumor cells from primary tumors of breast cancer patients. Furthermore, we observed that there is a significant association between the expression of these ligands in these stromal cells and the expression of TRAIL-R1, TRAIL-R4, RANK and CCR-2 in the breast tumor cells, respectively [97, 122]. Based on a co-culture of normal human fibroblasts and MCF-7 breast cancer cells, Martinez-Outschoorn et al. proposed that the oxidative stress of CAFs, in particular ROS, favors its pro-tumoral potential by increasing the genomic instability of the breast tumor cells [123]. After their activation, CAFs undergo metabolic and transcriptomic changes in order to mimic the "Warburg Effect". This phenotype is further exaggerated in the hypoxic tumor microenvironment of a mouse tumor model. Also, evidence suggests that this metabolic switch can serve to coordinate glucose and lactate metabolism in the tumor microenvironment [124]. In accordance with this, a report showed that well-oxygenated human breast cancer cells support the high glycolysis rate of cells in hypoxia by increasing lactate uptake. [125, 126]. This phenomenon is known as the “Reverse Warburg Effect” when talking about cancer cells reacting to metabolic reprogramming by CAFs [127]. In relation with this, CD36 and CAV1 protein expressions are downregulated in CAFs. Both CD36 and CAV1 decrease stabilize HIF-1α, which promotes a metabolic shift from mitochondrial oxidative phosphorylation to aerobic glycolysis, increasing lactate and glutamate [21]. A recent study also showed that the hypoxia level induces epigenetic reprogramming of human normal breast fibroblasts, resulting in a pro-glycolytic, CAF-like transcriptome [128]. Becker et al. found that human breast CAFs have an activated metabolism with increased glycolytic activity, which is stabilized by epigenetic changes in key genes, such as HIF-1α. This metabolic change of CAFs allows breast tumor cells nutrition and consequently promote tumor growth [128]. In summary, all of these studies suggest that breast CAFs are an established source of growth factors and cytokines known to have critical roles in breast tumor progression.
Role of CAFs in the Development of Metastatic Process
CAFs could facilitate the invasion of human breast tumor cells in several ways. Firstly, as we previously mentioned CAFs stimulate the growth, survival and invasion of tumor cells, as well as stimulates angiogenesis through the release of growth factors such as TGF-β, HGF, PDGF, IGF-2, VEGF, collagen, fibronectin, MMP, cytokines and non-soluble factors (i.e. exosomes), among others [129,130,131,132,133,134,135,136]. Also, certain interleukins, like IL-6, released by CAFs in the mammary tumor microenvironment promote the transition from pre-invasive to an invasive phenotype [134, 137]. In addition, IL-6 signaling between ductal carcinoma in situ cells and human breast CAFs mediates breast tumor cell growth and migration [134, 138]. On the other hand, CAFs increase the aggressiveness of human breast tumor cells by transporting molecules through extracellular vesicles [80, 139, 140]. A recent study using miRNA array showed that exosomes released by CAFs isolated from breast cancer patients had high levels of miR-3613-3p. Furthermore, after exposing breast cancer cell lines with exosomes from CAFs, they found that miR-3613-3p plays a pro-tumoral role, increasing the survival of breast tumor cells and consequently the process of metastasis [139]. Moreover, CAFs contribute to the formation of the metastatic niche, allowing the survival and extravasation of breast tumor cells [80]. Then, through the release of MMP, CAFs create pathways that allow breast tumor cells to spread to other tissues [141−145]
Emerging evidence supports the idea that multicellular breast tumor clusters invade and seed metastasis. Matsumura et al., demonstrated that CAFs induce the formation of breast tumor cell clusters that are composed of two types of cancer cell populations [145]; one of them in a highly epithelial state (E-cadherin Hi ZEB1 Lo/Neg) and the other in a hybrid epithelial/mesenchymal state (E-cadherin Lo ZEB1 Hi). The highly epithelial cells express antigen-related cell adhesion molecule 5 (CEACAM5) and CEACAM6 that associate with E-cadherin, resulting in increased breast tumor cell cluster formation and metastatic seeding. The hybrid epithelial/mesenchymal cells also remain associated with highly epithelial cells leading to collective invasion. SDF-1 and TGF-β produced by CAFs favor both states as well as invasive and metastatic traits via Src activation in human breast tumor cells [145]. In addition, Orimo et al. demonstrated in a human tumor xenograft model that SDF-1 released by CAFs increases proliferation of breast cancer cells and angiogenesis [108]. While breast tumor cells are in circulation, human CAFs could go together with them providing survival signals and an early growth advantage at the metastatic site [146]. It is well known that a pre-metastatic niche can be formed by the secreted soluble factors and extracellular vesicles released by the primary breast cancer cells before their arrival to the target tissue [147,148,149,150]. Once in the target organs, activated breast CAFs are also important for the development of the pre-malignant niche [80, 151]. In reference to it, an interesting study using an orthotopic murine model of breast cancer showed that CAFs also promote metastatic cells seeding by regulating stiffness of the ECM through LOX-dependent collagen crosslinking [152]. Malanchi et al. found that CAFs could contribute to pre-metastatic niche through the production of periostin in a murine breast cancer model [153]. This molecule increases Wnt signaling in the infiltrating breast cancer cells to promote the maintenance of their stem cell-like properties. Also, other work demonstrated that periostin can enhance the accumulation of MDSC in the lungs, which promotes immunosuppression in human ER(-) breast cancers [119]. In summary, CAFs could play a fundamental role in the development of the pre-metastatic niche favoring the evolution of the metastatic cascade.
Immunosuppressive Activity of Breast CAFs
There are still several immunomodulatory mechanisms exerted by CAFs that have not been studied in breast cancer yet. However, suppression of cytotoxic T cells and inflammatory macrophage function by breast CAFs frequently occurs [154]. As we said previously, Costa et al. found four subpopulations of CAFs with different immunosuppressive activities in human breast tumor. Particularly, in TNBC samples it was observed that CAF-S1 attracts T lymphocytes, increase CD4( +)/CD25( +) T lymphocytes survival and promote their differentiation into regulatory CD25( +) FOXP3( +) cells, which finally inhibit T lymphocytes [58]. Also, other authors found that the expression of FAP and SDF-1 in CAFs is related to their immunosuppressive activity, in particular over the cytotoxic T lymphocytes function in breast tumor [155,156,157,158,159]. So, if FAP is removed or SDF-1 release is inhibited, cytotoxic T lymphocytes accumulate, increasing breast tumor response to checkpoint inhibitors such as programmed death-ligand 1 (PD-L1) [155,156,157]. In accordance with this, Liao et al. observed that depletion of FAP( +) CAFs induces an increase of IL-2 and IL-7 expression and a reduction of IL-6/4, VEGF and macrophage-colony stimulating factor (M-CSF) expression in a 4T1 murine breast cancer model [109]. This leads to reduced recruitment of anti-inflammatory M2-macrophages and regulatory T lymphocytes and increased recruitment of mature dendritic cells and cytotoxic T lymphocytes in breast tumor [109]. Furthermore, SDF-1 suppresses antitumor immunity by increasing the number of T regulatory lymphocytes within the human breast tumor microenvironment [58]. Chemokines such as CCL-2 and CCL-5, which attract monocytes/macrophages are also released by CAFs, promoting indirectly more invasion of breast tumor cells and metastasis [160,161,162,163]. Moreover, Yavuz et al. found that CAFs obtained from human invasive breast cancer recruits monocytes by SDF-1/CXCR4 axis, and can also differentiate monocytes to M2-like macrophages, in terms of phenotypic features (increase of CD163 and CD206 expression) and immunosuppressive function through the increase of programmed cell death protein 1 (PD-1) [159]. Besides of immunosuppression, these M2 macrophages promote angiogenesis by secretion of VEGF, as well as epithelial mesenchymal transition of breast tumor cells and CAFs formation by the secretion of TGF-β [109]. Therefore, CAFs and M2 immunosuppressive macrophages are connected by a positive feedback loop [80]. Through studies in murine model, Ouyang et al. suggested that human CAFs stimulate development of both 4T1 and EMT6 murine breast cell lines, in part through the release of SDF-1 [164]. This molecule interacts with its receptor on tumor cells and on MDSC, as well, promoting immune escape [164]. In breast cancer MDSC not only attenuate the anti-tumor immunity to promote the growth and metastasis, but also reduce the effect of other immune therapies [165]. In addition, TGF-β released by CAFs could suppresses the adaptive and innate immune response against breast cancer cells [166, 167]. In particular, TGF-β prevents normal differentiation of dendritic cells and T cells, thus inducing the generation of MDSC, tumor-promoting M2 macrophages and immunosuppressive regulatory T cells [167]. Inhibition of IL-6 also reduces the secretion de CCL-2 and subsequent recruitment of monocytes in vitro [168]. Finally, Cohen et al. observed that chitinase 3-like 1 (Chi3L1), a secretion glycoprotein that was observed to be increased in cancer diseases, is upregulated in CAFs from murine and human primary breast tumors. Although the biological role of Chi3L1 is not yet fully known, the authors found that depletion of Chi3L1 decreased breast tumor burden in vivo, enhanced T cell infiltrate, promoted a T helper 1 (Th1) response and reduced the M2 macrophages [169].
As described, CAFs have extensive functions in breast cancer due to their vast heterogeneity and versatility. Their main functions are depicted in Fig. 3. As previously discussed, CAFs have a dual function. While they could act as repressors at the early stages of breast tumor progression, they promote malignant growth at more advanced stages [15, 55, 98, 101, 115]. The mechanisms by which they promote tumor growth are diverse. CAFs can particularly promote growth, survival and invasion of breast tumor cells, as well as stimulate angiogenesis through the release of growth factors, cytokines, and chemokines and modify the ECM through synthesis and degradation of its components [19,20,21, 80, 107, 109,110,111,112,113,114]. This ECM stimulates the proliferation and migration of breast tumor epithelial cells and could act as a barrier for immune system cells as well as for delivery drugs access [170]. Therefore, CAFs may reduce the effectiveness of therapy delivery. Moreover, CAFs, through the release of MMP, create pathways that are exploited by breast tumor cells in order to spread to other tissues, thus favoring the metastasis process [141, 142, 171]. In addition, human CAFs together with breast tumor cells go in circulation, providing survival signals and an early growth advantage at the metastatic site [146]. Finally, it is known that some subpopulations of CAFs could have immunosuppressive functions [58]. However, current knowledge regarding the precise roles of CAFs in breast cancer is not sufficient and more research is needed.
CAFs as Prognosis Factor
The importance of identifying some markers of CAFs for the clinical diagnosis and prognosis of breast cancer was and continues to be extensively studied. In this regard, Yamashita et al. identified that the expression of α-SMA in myofibroblasts is an independent predictor of metastasis and worse prognosis in patients with invasive breast cancer [172]. In addition, PDGFR-β and FAP are significantly associated with recurrence of disease, disease-free and survival-free time [13, 173, 174]. A recent study, through a microarray from 132 patients with metastatic breast cancer, determined that CAFs differentially express proteins according to the metastatic site and the stromal histologic phenotype [175]. In this study, Kim et al. found that PDGFR-α, S100A4 and podoplanin were increased in bone metastasis and reduced in liver metastasis. On the other hand, stromal PDGFR-β was also upregulated in lung metastasis [175]. Ao et al. developed a pilot study in patients with advanced breast cancer, in which they detected circulating CAFs in peripheral blood. Results showed that circulating CAFs were present in 88% of the patients with metastasis versus 33% of the patients who had a localized tumor [176]. Therefore, CAFs have a great potential in clinical diagnosis since they may provide information for the individualized treatment of breast cancer patients if their molecular markers and derived bio-products are evaluated [50]. Different works have shown that podoplanin expression in CAFs was a suitable poor prognosis factor for invasive breast cancer patients. This target may be used as a strategy for breast cancer treatment of in the future [177, 178]. Also the levels of α-SMA and tumor cytoplasmic high mobility group box 1 protein (HMGB1) could serve as independent prognostic markers for metastatic relapse in breast cancer patients. The α-SMA-high/HMGB1-low profile provided the most reliable metastasis relapse predictors [179]. Interestingly, through the development of a metastatic murine model of ER( +) breast disease, a recent study found that CD146(-) CAFs promote increased metastasis compared to CD146( +) CAFs [180]. In this study, Brechbuhl et al. also showed that CD146 (-) CAFs promote breast cancer cell invasion and metastatic phenotype through tenascin C expression and epidermal growth factor receptor (EGFR) pathway activation [180].
In a previous work, we found that the high expression of CD105 in CD34(-) fusiform stromal cells, not associated to the vasculature, is a novel marker for the identification of early breast cancer patients with invasive ductal carcinoma in clinical- pathological stages I-II which are at high risk of developing metastasis [181]. Furthermore, we demonstrated that the expression of CD105 in these cells is an independent prognostic factor of metastasis-free time and overall survival [181]. In addition, IL-6R expression in these stromal cells was significantly associated with a higher risk of metastatic occurrence in early breast cancer patients. Furthermore, high expression of IL-6R was associated with shorter disease-free survival, metastasis-free survival, and overall survival. Interestingly, we also demonstrated that IL-6R expression was an independent prognostic factor for disease-free survival and metastasis-free survival [182].
Another interesting fact is that each breast tumor subtype can co-evolve with different stromal subtypes of CAFs, which have different genetic changes in the tumor suppressor genes TP53 and PTEN, as well as epigenetic changes [183]. Interestingly, the status of the ER and Her2 of breast tumor cells also modifies and correlates with the gene expression of the intra-tumor CAFs and their later functions [172, 184, 185]. For example, CAFs from breast tumors overexpressing Her2 exhibit an increase in the expression of genes related to integrin and cytoskeletal signaling pathways, compared to CAFs isolated from TNBC and ER( +) breast tumors. These differences favored that the CAFs of Her2( +) breast tumors had a greater capacity to induce the migration of T47D human breast tumor cell line (tumor subtype ER+ and PR+, with low migratory capacity) compared to CAFs from TNBC and ER( +) human breast tumors [184]. Furthermore, these Her2( +) breast tumors may be more aggressive if they co-evolve with CAFs with loss of PTEN [183]. Consequently, it is important to study the phenotypic, molecular and functional characteristics of the CAFs that predominate in the human breast tumor, so as to develop alternative therapies. However, CAFs present heterogeneous subtypes with diverse origins, markers, and functional characteristics in the mammary tumor microenvironment. Therefore, before developing a therapeutic approach using anti-CAF drugs, it is important to accurately identify tumor-promoting subtypes in human breast cancer in order to properly focus the targeting strategy.
CAFs as a Therapeutic Strategy
It is well known that not all the breast cancer patients respond to treatments because of drug resistance, which is a consequence of the genomic instability of cancer cells [186]. Then, it is very important to choose a genetically stable tumor stromal cell, such as CAFs, for targeted treatment [187]. In order to develop a specific therapy it should be taken into account that treatment could be focused not only on their membrane markers but also on the ECM components and factors produced by CAFs. Also, the molecules secreted by CAFs could be targeted by small compound inhibitors, antibodies, peptides, because removing a cell type like CAFs is a more difficult task. Because CAFs are a more genetically stable cell population than tumor cells, developing vaccines against FAP antigen carried by CAFs may be a potential therapeutic strategy [188]. For example, Fang et al. employed a FAP-targeting immunotoxin (αFAP-PE38) that is specific for depletion of FAP-expressing CAFs in a murine model of metastatic breast cancer, and observed a potent tumor suppression [189]. In addition, another study showed that the administration of an oral mouse DNA vaccine directed to FAP induced the death of CAFs mediated by CD8( +) T lymphocytes, increasing intratumoral uptake of chemotherapeutic drugs in multi-drug resistant breast cancer model [188].
Recently, Su et al. found that CAFs expressing CD10 and GPR77 constitute a subgroup that provide a breast tumor CSC survival avoiding chemotherapy effect [190]. The identification of these novel specific CAFs markers can help define a human CAFs subpopulation with pro-tumorigenic functions, thus facilitating the development of therapeutic strategies that target directly to the CD10( +)/GPR77( +) CAFs. In line with this idea, Su et al. demonstrated that when GPR77 was blocked with neutralizing monoclonal antibodies, infiltration of CD10( +)/GPR77( +) CAFs was reduced. They observed that by the employment of that strategy, tumorigenesis decreased while chemotherapy sensitivity increased in a patient-derived xenograft model of breast cancer [190]. Moreover, another subtype of human CAFs expressing IL-7, has been targeted pre-clinically, resulting in impaired tumor stemness and growth, as well as restored chemosensitivity in an orthotopic murine breast cancer model [191].
Angiotensin II (AngII) /AngII type I receptor (AT1R) axis plays pivotal roles in promoting tumor growth and progression. The treatment of CAFs with losartan, which is a selective AT1R blocker, reported an attenuated activation of fibroblasts in a orthotopic murine breast cancer model [192]. This study demonstrated that losartan reduce collagen and hyaluronic production by CAFs in breast cancer, thereby improving vascular perfusion and drug delivery in this malignancy [192]. In addition, Hu et al. highlighted the importance of a molecular design in the preparation of injectable hydrogels and demonstrated that the losartan-loaded peptide hydrogel could improve the effect of chemotherapy in the inhibition of growth and lung metastasis of TNBC through regulation of CAFs and collagen synthesis using a murine model [193].
Moreover, Ryan et al. [194] observed that acetylated HMGB1 through binding to receptor for advanced glycation end products (RAGE) activated naïve MSC in a orthotopic geminin-overexpressing cells (GemOE) breast tumor model. [194]. These MSC activated by acetylated HMGB1 secrete the S100A4, a known promoter of breast cancer proliferation, invasion, and metastasis [195, 196]. Moreover, within the breast tumor, MSC can differentiate into S100A4-secreting CAFs. Then S100A4 activates GemOE to secrete CCL-2 that recruits macrophages from the stroma into the tumor, polarizing them to an M2 macrophage profile [194]. On the other hand, Axl is overexpressed in breast cancers [197,198,199]. These authors [194] found that activation of Axl and RAGE in GemOE tumor cells by protein growth arrest-specific gene 6 protein (Gas6) and acetylated HMGB1, converts them into metastatic precursors capable of dissemination from primary tumors, especially through exacerbating the stemness and epithelial-mesenchymal transition phenotypes [200]. Furthermore, in TNBC, expression of a nuclear/cytoplasmic S100A4 is associated with high histological tumor grade and inferior metastasis-free and overall survival. All these observations suggest that RAGE and Axl could be an additional therapeutic target to prevent GemOE metastatic precursors dissemination from TNBC, not only by affecting tumor cells but also MSC and/or CAFs [194]. In a preclinical approach, treatment with monoclonal antibodies that block Gas6, Axl ligand, decreased tumor growth, inhibited the activity of tumor-associated macrophages, and impaired metastasis in a xenograft murine model of breast cancer [201]. Also, other study showed that treatment with anti-Axl monoclonal antibody 20G7-D9, in a model of TNBC xenograft, prevents EMT, reduces tumor growth, decreases migration, invasion, extravasation and metastasis [197]. On the other hand, targeting RAGE was shown to affect the tumor progression and metastasis, as assayed in vitro and in an animal model [202]. As discussed above, the pivotal role of RAGE in breast cancer progression caused by the induction of several cellular pathways is related to proliferation, migration, invasion, or metastasis of cancer cells. The goal of some studies has been to discover new drugs that are able to alleviate or block the breast cancer progression. Still, these problems require further investigations. The first treatments that used blocking RAGE signaling were performed in cell lines of fibrosarcoma, pheochromocytoma, and glioma, among other tumors [203].Within RAGE inhibitors are papaverine (significant inhibition of RAGE-dependent NF-κB driven by HMGB1 on HT1080 human fibrosarcoma cell line, in vitro), Heparin (attenuated the HMGB1-induced NF-κB activation through RAGE on HT1080 human fibrosarcoma cell line, in vitro), Hispidin (attenuated RAGE expression, and NF-κB pathway activation through antioxidant activities on PC12 rat pheochromocytoma cell line, in vitro), Ethyl Pyruvate (induced reduction in RAGE expression and NF-κB activation on MM human malignant mesothelioma cells, in vitro) and Duloxetine (inhibited S100B-production on GL261 mouse glioma cells line, in vitro, and inhibited the growth of intracranial GL261, in vivo) [203]. It is essential the screening of these anti-RAGE drugs and new ones in order to control breast cancer progression.
Another type of treatment could be based in the targeting of tumor stiffness via the inhibition of LOX enzymatic activity. LOXL2 plays a role in invasion of various tumor, such as breast cancer [204]. Barker et al. [205] showed that blocking LOXL2 significantly inhibits breast tumor invasion and metastasis in transgenic and orthotopic mouse models. Moreover, it was determined that LOXL2 induced the expression of α-SMA in fibroblasts [206]. All this suggest that inhibition of LOXL2 in human breast tumors could reduce not only tumor cell invasion but also attenuates the activation of host cells such as CAFs in the tumor microenvironment [206]. Drugs that target CAFs signals and effectors have become an important complement for therapies directed against tumor cells for multiple solid tumors [36]. For example, co-administration of pirfenidone, an anti-fibrotic agent, together with chemotherapy inhibits tumor growth and metastasis of 4T1 mouse breast cancer cells, presumably due to the attenuation of the TGF-β signal pathway, fibroblasts activation and ECM production by CAFs [207].
Reprogramming CAFs back into their dormant state is another possible strategy for impairing tumorigenesis. miRNAs have attracted interest in this field. For example, Al-Harbi et al., demonstrated that CAFs within human breast tumors had decreased levels of the tumor suppressor miRNA Let-7b compared to their normal fibroblast counterparts. In addition, they found that the inhibition of Let-7b in these normal fibroblasts increased their activation and capacity to induce epithelial mesenchymal transition in breast cancer cells in vitro, and enhanced tumor growth in a murine breast tumor model [208]. In the future, a better understanding of the different mediators involved in fibroblast activation, such as TGF-β or extracellular matrix metalloproteinase inducer (EMMPRIN), could lead to the development of new therapies in breast cancer. As said before, TGF-β is considered to be main inducer of fibroblast activation in breast primary tumor initiation and metastasis [59, 99, 209, 210]. After binding to its receptors, TGF-β induces signaling pathways leading to the upregulation of targeted genes such as α-SMA in human normal fibroblasts [210,211,212]. One study has shown that the expression of α-SMA is controlled by EMMPRIN [213]. Co-culture of 1068SK human normal breast fibroblasts with human breast cancer cell lines (MDA-157, SKBR-3, MCF-7, BT-20, and HS578T), expressing high levels of EMMPRIN, induced the expression of α-SMA in these fibroblasts. Moreover, α-SMA expression was induced after the treatment of 1068SK human normal fibroblasts with conditioned culture medium from these breast cancer cell lines [213]. Therefore, EMMPRIN and TGF-β could be interesting therapeutic targets in breast cancer evolution. Finally, it would be interesting to consider in the future synergistic combinations of therapies against CAFs and other effective treatments such as immunotherapy to combat breast tumor progression.
Conclusion
CAFs are one of the cell populations that most favor breast tumor progression. They are activated at a very early stage, as well as at late stage, and contribute to tumor initiation, growth, metastasis and resistance to treatment through mechanical pressure, paracrine activation by growth factors, cytokines, estrogens, enzymes and proteins of the ECM. Moreover, there is not only a crosstalk between CAFs and breast tumor cells, but also between these two types of cells and other components of the breast tumor microenvironment such as ECM and immune cells. However, there are still some issues that need further study in relation to the role of CAFs in breast cancer. Currently, there is no clear and accurate molecular classification of CAFs in human breast cancer, which makes their use for clinical diagnosis difficult. Therefore, it is important to find specific and effective molecular markers as well as to determine the subpopulations of CAFs present in the breast cancer subtypes. The present review provides accumulated evidence that CAFs are promising as a future target therapy against breast tumor progression. The development of stromal ‘normalization’ therapies in combination with standard drugs could modify the tumor response and patient survival.
Abbreviations
- AT1R:
-
Angiotensin II (AngII) /AngII type I receptor
- BM:
-
Bone marrow
- CAF(s):
-
Cancer associated fibroblast(s)
- CAV1:
-
Calveolin-1
- CCL:
-
Chemokine ligand
- CCR-2 C–C:
-
Chemokine receptor type 2
- CD105:
-
Endoglin
- CEACAM5:
-
Cells express antigen-related cell adhesion molecule 5
- Chi3Li:
-
Chitinase 3-like 1
- CSC:
-
Cancer stem cells
- CTGF:
-
Connective tissue growth factor
- CXCL C-X-C:
-
Motif chemokine ligand
- CXCR4 C-X-C:
-
Motif chemokine receptor 4
- CSPG:
-
Chondroitin sulfate proteoglycan
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- EMMPRIN:
-
Extracellular matrix metalloproteinase inducer
- EMT:
-
Epithelial-mesenchymal transdifferentiation
- ER:
-
Estrogen receptor
- FAP:
-
Fibroblast activation protein
- FGFR-1:
-
Fibroblast growth factor 1 receptor
- FGF-β:
-
Basic fibroblast growth factor
- FSP:
-
Fibroblast surface protein
- Gas6:
-
Protein growth arrest-specific gene 6 protein
- GemOE:
-
Geminin-overexpressing
- HER2/neu:
-
Human epidermal growth factor receptor 2
- HGF:
-
Hepatocyte growth factor
- HIF-1α:
-
Hypoxia inducible factor-1α
- HMGB1:
-
High mobility group box 1 protein
- iCAFs:
-
Inflammatory’ CAFs
- IGF:
-
Insulin growth factor
- IGFR:
-
Insulin growth factor receptor
- IL:
-
Interleukin
- IL-R:
-
Interleukin receptor (R)
- LOX:
-
Lysyl oxidase
- LRRC15:
-
Leucine rich repeat containing 15
- M-CSF:
-
Macrophage-colony stimulating factor
- MDSC:
-
Myeloid-derived suppressor cells
- MHC:
-
Major histocompatibility complex
- MMP:
-
Metalloproteinases
- MSC:
-
Mesenchymal stem cells
- myoCAFs:
-
Myofibroblastic’ CAFs
- NF-κB:
-
Nuclear factor-kappaβ
- PD-1:
-
Programmed cell death protein 1
- PDGF:
-
Platelet-derived growth factor
- PDGFR:
-
Platelet-derived growth factor receptor
- PDL-1:
-
Programmed death-ligand 1
- PR:
-
Progesterone receptor
- RAGE:
-
Receptor for advanced glycation end products
- RANKL:
-
Receptor activator of nuclear factor-kappaβ ligand
- ROS:
-
Reactive oxygen species
- S100A4:
-
Calcium-binding protein
- scRNA-seq:
-
Single-cell RNA sequencing
- SDF-1:
-
Stromal-derived-factor-1
- SLC39A8:
-
Solute carrier (SLC) 39A8
- TAM:
-
Tumor-associated macrophages
- TGF-β:
-
Transforming growth factor beta 1
- TH1 T:
-
Lymphocyte helper 1
- TIMP:
-
Tissue inhibitors of metalloproteinases
- TNBC:
-
Triple negative breast cancer
- TNF:
-
Tumor necrosis factor
- TRAIL TNF:
-
Related apoptosis-inducing ligand
- VEGF:
-
Vascular endothelial growth factor
- α-SMA:
-
Alpha-smooth muscle actin
- αFAP-PE38 FAP:
-
Targeting immunotoxin
References
Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol. 2008;130:1091–103. https://doi.org/10.1007/s00418-008-0530-8.
Bissell MJ, Radisky DC, Rizki A, et al. The organizing principle: Microenvironmental influences in the normal and malignant breast. Differ. 2002;70:537–46. https://doi.org/10.1046/j.1432-0436.2002.700907.x.
Arendt LM, Rudnick JA, Keller PJ, et al. Stroma in breast development and disease. Semin Cell Dev Biol. 2010;21:11–8. https://doi.org/10.1016/j.semcdb.2009.10.003.
Chantrain CF, Feron O, Marbaix E, et al. Bone marrow microenvironment and tumor progression. Cancer Microenviron. 2008;1:23–35. https://doi.org/10.1007/s12307-008-0010-7.
Gao D, Mittal V. The role of bone-marrow-derived cells in tumor growth, metastasis initiation and progression. Trends Mol Med. 2009;15:333–43. https://doi.org/10.1016/j.molmed.2009.06.006.
Gonda TA, Varro A, Wang TC, et al. Molecular biology of cancer-associated fibroblasts: Can these cells be targeted in anti-cancer therapy? Semin Cell Dev Biol. 2010;21:2–10. https://doi.org/10.1016/j.semcdb.2009.10.001.
Spaeth EL, Dembinski JL, Sasser AK, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE. 2009;4:e4992.
Martin FT, Dwyer RM, Kelly J, et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: Stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat. 2010;124:317–26. https://doi.org/10.1007/s10549-010-0734-1.
Rhodes LV, Muir SE, Elliott S, et al. Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat. 2010;121:293–300. https://doi.org/10.1007/s10549-009-0458-2.
El-Haibi CP, Karnoub AE. Mesenchymal stem cells in the pathogenesis and therapy of breast cancer. J Mammary Gland Biol Neoplas. 2010;15:399–409. https://doi.org/10.1007/s10911-010-9196-7.
Klopp AH, Gupta A, Spaeth E, et al. Concise review: Dissecting a discrepancy in the literature: Do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–9. https://doi.org/10.1002/stem.559.
Luo H, Tu G, Liu Z, et al. Cancer-associated fibroblasts: A multifaceted driver of breast cancer progression. Cancer Lett. 2015;361:155–63. https://doi.org/10.1016/j.canlet.2015.02.018.
Paulsson J, Micke P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin Cancer Biol. 2014;25:61–8. https://doi.org/10.1016/j.semcancer.2014.02.006.
Kaushik N, Kim S, Suh Y, et al. Proinvasive extracellular matrix remodeling for tumor progression. Arch Pharmacal Res. 2019;42:40–7. https://doi.org/10.1007/s12272-018-1097-0.
Najafi M, Goradel NH, Farhood B, et al. Tumor microenvironment: Interactions and therapy. J Cell Physiol. 2019;234:5700–21. https://doi.org/10.1002/jcp.27425.
Morsing M, Klitgaard MC, Jafari A, et al. Evidence of two distinct functionally specialized fibroblast lineages in breast stroma. Breast Cancer Res. 2016;18:1–11. https://doi.org/10.1186/s13058-016-0769-2.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. https://doi.org/10.1038/nrc1877.
Yoshida GJ. Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways. J Exp & Clin Cancer Res. 2020;39:112. https://doi.org/10.1186/s13046-020-01611-0.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98. https://doi.org/10.1038/nrc.2016.73.
LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Disease models & mechanisms. 2018;11:dmm029447. https://doi.org/10.1242/dmm.029447.
Yoshida GJ, Azuma A, Miura Y, et al. Activated fibroblast program orchestrates tumor initiation and progression; molecular mechanisms and the associated therapeutic strategies. Int J Mol Sci. 2019;20:2256. https://doi.org/10.3390/ijms20092256.
Barbazán J, Matic VD. Cancer associated fibroblasts: is the force the path to the dark side? Curr Opin Cell Biol. 2019;56:71–9. https://doi.org/10.1016/j.ceb.2018.09.002.
Salimifard S, Masjedi A, Hojjat-Farsangi M, et al. Cancer associated fibroblasts as novel promising therapeutic targets in breast cancer. Pathol Res Pract. 2020;216:152915. https://doi.org/10.1016/j.prp.2020.152915.
Shekhar MPV, Pauley R, Heppner G. Extracellular matrix-stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res. 2003;5:130–5. https://doi.org/10.1186/bcr580.
Giatromanolaki A, Sivridis E, Koukourakis MI. The pathology of tumor stromatogenesis. Cancer Biol Ther. 2007;6:639–45. https://doi.org/10.4161/cbt.6.5.4198.
Bitoux M-A, Stamenkovic I. Tumor-host interactions: the role of inflammation. Histochem Cell Biol. 2008;130:1079–90. https://doi.org/10.1007/s00418-008-0527-3.
Garamszegi N, Garamszegi SP, Shehadeh LA, et al. Extracellular Matrix-Induced Gene Expression in Human Breast Cancer Cells. Mol Cancer Res. 2009;7:319–29. https://doi.org/10.1158/1541-7786.MCR-08-0227.
Ali S, Lazennec G. Chemokines: Novel targets for breast cancer metastasis. Cancer Metastasis Rev. 2007;26:401–20. https://doi.org/10.1007/s10555-007-9073-z.
Ahn S, Cho J, Sung J, et al. The prognostic significance of tumor-associated stroma in invasive breast carcinoma. Tumor Biol. 2012;33:1573–80. https://doi.org/10.1007/s13277-012-0411-6.
Hugo HJ, Lebret S, Tomaskovic-Crook E, et al. Contribution of fibroblast and mast cell (afferent) and tumor (efferent) IL-6 effects within the tumor microenvironment. Cancer Microenviron. 2012;5:83–93. https://doi.org/10.1007/s12307-012-0098-7.
Horimoto Y, Polanska UM, Takahashi Y, et al. Emerging roles of the tumor-associated stroma in promoting tumor metastasis. Cell Adhes Migr. 2012;6:193–202. https://doi.org/10.4161/cam.20631.
Conklin MW, Eickhoff JC, Riching KM, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178:1221–32. https://doi.org/10.1016/j.ajpath.2010.11.076.
Conklin MW, Keely PJ. Why the stroma matters in breast cancer: Insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell Adhes Migr. 2012;6:249–60. https://doi.org/10.4161/cam.20567.
Hill BS, Sarnella A, D’Avino G, et al. Recruitment of stromal cells into tumour microenvironment promote the metastatic spread of breast cancer. Semin Cancer Biol. 2019;60:202–13. https://doi.org/10.1016/j.semcancer.2019.07.028.
Yeldag G, Rice A, del Río HA. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers. 2018;10:471. https://doi.org/10.3390/cancers10120471.
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discovery. 2019;18:99–115. https://doi.org/10.1038/s41573-018-0004-1.
Gillies RJ, Raghunand N, Karczmar GS, et al. MRI of the tumor microenvironment. J Magn Reson Imaging. 2002;16:430–50. https://doi.org/10.1002/jmri.10181.
Wouters BG, Weppler SA, Koritzinsky M, et al. Hypoxia as a target for combined modality treatments. 2002;38:1–9.
Runkel S, Wischnik A, Teubner J, Kaven E, Gaa J MF. Oxygenation of Mammary Tumors as Evaluated by Ultrasound-Guided Computerized-PO2-Histography. In Oxygen Transport to Tissue XV. Springer, Boston, MA. 1994. p. 451–8. https://doi.org/10.1007/978-1-4615-2468-7_60.
Knoop C, Hockel M. Oxygenation of Human Tumors: Evaluation Of Tissue Oxygen Distribution In Breast Cancers By Computerized O2 Tension Measurements. Can Res. 1991;51:3316–22.
Mccarty MF, Whitaker J. Manipulating Tumor Acidification As Cancer Treament Strategys. 2010;15:264–72.
De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–47. https://doi.org/10.1002/path.1398.
Bishop J. Molecular themes in oncogenesis. Cell. 1991;64:235–48. https://doi.org/10.1016/0092-8674(91)90636-d.
Liotta LA, Kohn EC. The microenvironment of the tumour–host interface. Nat. 2001;411:375–9. https://doi.org/10.1038/35077241.
Dolberg DS, Hollingsworth R, Hertle M, et al. Wounding and its role in RSV-mediated tumor formation. Sci. 1985;230:676–8. https://doi.org/10.1126/science.2996144.
Sieweke MH, Thompson NL, Sporn MB, et al. Mediation of wound-related rous sarcoma virus tumorigenesis by TGF-β. Sci. 1990;248:1656–60. https://doi.org/10.1126/science.2163544.
Pietras K, Östman A. Hallmarks of cancer: Interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–31. https://doi.org/10.1016/j.yexcr.2010.02.045.
Sappino A ‐P, Skalli O, Jackson B, et al. Smooth‐muscle differentiation in stromal cells of malignant and non‐malignant breast tissues. Int J Cancer. 1988;41:707–12. https://doi.org/10.1002/ijc.2910410512.
Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–96. https://doi.org/10.1016/j.addr.2015.07.007.
Qiao A, Gu F, Guo X, et al. Breast cancer-associated fibroblasts: their roles in tumor initiation, progression and clinical applications. Front Med. 2016;10:33–40. https://doi.org/10.1007/s11684-016-0431-5.
Kumar S, Shabi TS, Goormaghtigh E. A FTIR imaging characterization of fibroblasts stimulated by various breast cancer cell lines. PLoS ONE. 2014;9. https://doi.org/10.1371/journal.pone.0111137.
Shiga K, Hara M, Nagasaki T, et al. Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers. 2015;7:2443–58. https://doi.org/10.3390/cancers7040902.
Buchsbaum RJ, Oh SY. Breast cancer-associated fibroblasts: Where we are and where we need to go. Cancers. 2016;8:1–19. https://doi.org/10.3390/cancers8020019.
Park SY, Kim HM, Koo JS. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat. 2015;149:727–41. https://doi.org/10.1007/s10549-015-3291-9.
Purcell JW, Tanlimco SG, Hickson JA, et al. LRRC15 is a novel mesenchymal protein and stromal target for antibody-drug conjugates. Can Res. 2018;78:1457–70. https://doi.org/10.1158/0008-5472.CAN-18-0327.
Sebastian A, Hum NR, Martin KA, et al. Single-Cell Transcriptomic Analysis of Heterogeneity in Breast Cancer. Cancers. 2020;12:E1307. https://doi.org/10.3390/cancers12051307.
Cortez E, Roswall P, Pietras K. Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin Cancer Biol. 2014;25:3–9. https://doi.org/10.1016/j.semcancer.2013.12.010.
Costa A, Kieffer Y, Scholer-Dahirel A, et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell. 2018;33:1–17. https://doi.org/10.1016/j.ccell.2018.01.011.
Kojima Y, Acar A, Eaton EN, et al. Autocrine TGF-β and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA. 2010;107:20009–14. https://doi.org/10.1073/pnas.1013805107.
Bartoschek M, Oskolkov N, Bocci M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun. 2018;9. https://doi.org/10.1038/s41467-018-07582-3.
Raz Y, Cohen N, Shani O, et al. Bone marrow–derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J Exp Med. 2018;215:3075–93. https://doi.org/10.1084/jem.20180818.
Ghiabi P, Jiang J, Pasquier J, et al. Breast cancer cells promote a notch-dependent mesenchymal phenotype in endothelial cells participating to a pro-tumoral niche. J Transl Med. 2015;13:1–19. https://doi.org/10.1186/s12967-015-0386-3.
Weber, et al. Osteopontin Mediates an MZF1-TGF-β1-Dependent Transformation of Mesenchymal Stem Cells into Cancer Associated Fibroblasts in Breast Cancer. Oncog. 2015;34:4821–33. https://doi.org/10.1038/onc.2014.410.
Rønnov-Jessen L, Petersen OW, Koteliansky VE, et al. The origin of the myofibroblasts in breast cancer: Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Investig. 1995;95:859–73. https://doi.org/10.1172/JCI117736.
Bochet L, Lehuédé C, Dauvillier S, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Can Res. 2013;73:5657–68. https://doi.org/10.1158/0008-5472.CAN-13-0530.
Nair N, Calle AS, Zahra MH, et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci Rep. 2017;7:6838. https://doi.org/10.1038/s41598-017-07144-5.
Bronzert DA, Pantazis P, Antoniades HN, et al. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc Natl Acad Sci USA. 1987;84:5763–7. https://doi.org/10.1073/pnas.84.16.5763.
Shao ZM, Nguyen M, Barsky SH. Human breast carcinoma desmoplasia is PDGF initiated. Oncog. 2000;19:4337–45. https://doi.org/10.1038/sj.onc.1203785.
Hendrayani SF, Al-Khalaf HH, Aboussekhra A. The cytokine il-6 reactivates breast stromal fibroblasts through transcription factor STAT3-dependent up-regulation of the RNA-binding protein AUF1. J Biol Chem. 2014;289:30962–76. https://doi.org/10.1074/jbc.M114.594044.
Sharon Y, Raz Y, Cohen N, et al. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Can Res. 2015;75:963–73. https://doi.org/10.1158/0008-5472.CAN-14-1990.
Arcucci A, Ruocco MR, Granato G, et al. Cancer: An Oxidative Crosstalk between Solid Tumor Cells and Cancer Associated Fibroblasts. BioMed Res Int. 2016. https://doi.org/10.1155/2016/4502846.
Li K, Liu T, Chen J, et al. Survivin in breast cancer-derived exosomes activates fibroblasts by upregulating SOD1, whose feedback promotes cancer proliferation and metastasis. J Biol Chem. 2020. jbc.RA120.013805. https://doi.org/10.1074/jbc.ra120.013805.
Vu LT, Peng B, Zhang DX, et al. Tumor-secreted extracellular vesicles promote the activation of cancer-associated fibroblasts via the transfer of microRNA-125b. J Extracell Vesicles. 2019;8:1599680. https://doi.org/10.1080/20013078.2019.1599680.
Chatterjee A, Jana S, Chatterjee S, et al. MicroRNA-222 reprogrammed cancer-associated fi broblasts enhance growth and metastasis of breast cancer. Br J Cancer. 2019. https://doi.org/10.1038/s41416-019-0566-7.
Albrengues J, Bertero T, Grasset E, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat Commun. 2015;6:10204. https://doi.org/10.1038/ncomms10204.
Wang D dan, Li J, Sha H huan, et al. miR-222 confers the resistance of breast cancer cells to Adriamycin through suppression of p27kip1 expression. Gene. 2016;590:44–50. https://doi.org/10.1016/j.gene.2016.06.013.
Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32. https://doi.org/10.1016/j.semcancer.2013.12.007.
Manuscript A, Cells D, Cancer B. Role of Oxidative Stress and the Microenvironment in Breast Cancer Development and Progression. 2014;19:1–11. https://doi.org/10.1097/PPO.0000000000000007.Dendritic.
Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Frontiers in bioscience (Landmark edition). 2010;15:166–79. https://doi.org/10.2741/3613.
Kwa MQ, Herum KM, Brakebusch C. Cancer-associated fibroblasts: how do they contribute to metastasis? Clin Exp Metas. 2019;36:71–86. https://doi.org/10.1007/s10585-019-09959-0.
Motrescu ER, Rio MC. Cancer cells, adipocytes and matrix metalloproteinase 11: A vicious tumor progression cycle. Biol Chem. 2008;389:1037–41. https://doi.org/10.1515/BC.2008.110.
Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev. 2011;32:550–70. https://doi.org/10.1210/er.2010-0030.
Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–9. https://doi.org/10.1038/sj.bjc.6604662.
Zeisberg EM, Potenta S, Xie L, et al. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Can Res. 2007;67:10123–8. https://doi.org/10.1158/0008-5472.CAN-07-3127.
Yeon JH, Jeong HE, Seo H, et al. Cancer-derived exosomes trigger endothelial to mesenchymal transition followed by the induction of cancer-associated fibroblasts. Acta Biomater. 2018;76:146–53. https://doi.org/10.1016/j.actbio.2018.07.001.
Chaturvedi P, Gilkes DM, Wong CCL, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Investig. 2013;123:189–205. https://doi.org/10.1172/JCI64993.
Spaeth E, Klopp A, Dembinski J, et al. Inflammation and tumor microenvironments: Defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–8. https://doi.org/10.1038/gt.2008.39.
Shi Y, Du L, Lin L, et al. Tumour-associated mesenchymal stem/stromal cells: Emerging therapeutic targets. Nat Rev Drug Discovery. 2016;16:35–52. https://doi.org/10.1038/nrd.2016.193.
Sewell-Loftin MK, Bayer SVH, Crist E, et al. Cancer-associated fibroblasts support vascular growth through mechanical force. Sci Rep. 2017;7:1–12. https://doi.org/10.1038/s41598-017-13006-x.
Wobus M, List C, Dittrich T, et al. Breast carcinoma cells modulate the chemoattractive activity of human bone marrow-derived mesenchymal stromal cells by interfering with CXCL12. Int J Cancer. 2015;136:44–54. https://doi.org/10.1002/ijc.28960.
Jahn SC, Law ME, Corsino PE, et al. An in vivo model of epithelial to mesenchymal transition reveals a mitogenic switch. Cancer Lett. 2012;326:183–90. https://doi.org/10.1016/j.canlet.2012.08.013.
Paunescu V, Bojin FM, Tatu CA, et al. Tumour-associated fibroblasts and mesenchymal stem cells: More similarities than differences. J Cell Mol Med. 2011;15:635–46. https://doi.org/10.1111/j.1582-4934.2010.01044.x.
Del Valle PR, Milani C, Brentani MM, et al. Transcriptional profile of fibroblasts obtained from the primary site, lymph node and bone marrow of breast cancer patients. Genet Mol Biol. 2014;37:480–9. https://doi.org/10.1590/S1415-47572014000400002.
Mishra PJ, Mishra PJ, Humeniuk R, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Can Res. 2008;68:4331–9. https://doi.org/10.1158/0008-5472.CAN-08-0943.
Pasanen I, Lehtonen S, Sormunen R, et al. Breast cancer carcinoma-associated fibroblasts differ from breast fibroblasts in immunological and extracellular matrix regulating pathways. Exp Cell Res. 2016;344:53–66. https://doi.org/10.1016/j.yexcr.2016.04.016.
Heneberg P. Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Crit Rev Oncol/Hematol. 2016;97:303–11. https://doi.org/10.1016/j.critrevonc.2015.09.008.
Labovsky V, Martinez LM, Davies KM, et al. Association Between Ligands and Receptors Related to the Progression of Early Breast Cancer in Tumor Epithelial and Stromal Cells. Clin Breast Cancer. 2015;15:e13–21. https://doi.org/10.1016/j.clbc.2014.05.006.
Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: A diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31:195–208. https://doi.org/10.1007/s10555-011-9340-x.
Neel J-C, Humbert L, Lebrun J-J. The Dual Role of TGFβ in Human Cancer: From Tumor Suppression to Cancer Metastasis. ISRN Mol Biol. 2012;2012:1–28. https://doi.org/10.5402/2012/381428.
Principe DR, Doll JA, Bauer J, et al. TGF-β: Duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 2014;106:1–16. https://doi.org/10.1093/jnci/djt369.
Knudson KM, Hicks KC, Luo X, et al. M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. OncoImmunology. 2018;7:1–14. https://doi.org/10.1080/2162402X.2018.1426519.
Boyd N, Berman H, Zhu J, et al. The origins of breast cancer associated with mammographic density: A testable biological hypothesis. Breast Cancer Res. 2018;20:1–13. https://doi.org/10.1186/s13058-018-0941-y.
Nguyen DH, Oketch-Rabah HA, Illa-Bochaca I, et al. Radiation Acts on the Microenvironment to Affect Breast Carcinogenesis by Distinct Mechanisms that Decrease Cancer Latency and Affect Tumor Type. Cancer Cell. 2011;19:640–51. https://doi.org/10.1016/j.ccr.2011.03.011.
Kuperwasser C, Chavarria T, Wu M, et al. From The Cover: Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci. 2004;101:4966–71. https://doi.org/10.1073/pnas.0401064101.
Shekhar MPV, Werdell J, Santner SJ, et al. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: Implications for tumor development and progression. Can Res. 2001;61:1320–6.
Wang B, Xi C, Liu M, et al. Breast fibroblasts in both cancer and normal tissues induce phenotypic transformation of breast cancer stem cells : a preliminary study. 2018:1–19. https://doi.org/10.7717/peerj.4805.
Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. cell. 2012. https://doi.org/10.1016/j.cell.2012.04.042.
Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. https://doi.org/10.1016/j.cell.2005.02.034.
Liao D, Luo Y, Markowitz D, et al. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS ONE. 2009;4:e7965. https://doi.org/10.1371/journal.pone.0007965.
Sjöberg E, Augsten M, Bergh J, et al. Expression of the chemokine CXCL14 in the tumour stroma is an independent marker of survival in breast cancer. Br J Cancer. 2016;114:1117–24. https://doi.org/10.1038/bjc.2016.104.
Sjöberg E, Meyrath M, Milde L, et al. A novel ACKR2-Dependent role of fibroblast-derived CXCL14 in epithelial-to-mesenchymal transition and metastasis of breast cancer. Clin Cancer Res. 2019;25:3702–17. https://doi.org/10.1158/1078-0432.CCR-18-1294.
Allaoui R, Bergenfelz C, Mohlin S, et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat Commun. 2016;7:13050. https://doi.org/10.1038/ncomms13050.
Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–23. https://doi.org/10.1084/jem.20140692.
Karnoub AE1, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R WR. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nat. 2007.
Suh J, Kim DH, Lee YH, et al. Fibroblast growth factor-2, derived from cancer-associated fibroblasts, stimulates growth and progression of human breast cancer cells via FGFR1 signaling. Mol carcinog. 2020:1–13. https://doi.org/10.1002/mc.23233.
Wu X, Zahari MS, Renuse S, et al. Quantitative phosphoproteomic analysis reveals reciprocal activation of receptor tyrosine kinases between cancer epithelial cells and stromal fibroblasts. Clin Proteomics. 2018;15:21. https://doi.org/10.1186/s12014-018-9197-x.
DeClerck YA, Pienta KJ, Woodhouse EC, et al. The tumor microenvironment at a turning point knowledge gained over the last decade, and challenges and opportunities ahead: A white paper from the NCI TME network. Can Res. 2017;77:1051–9. https://doi.org/10.1158/0008-5472.CAN-16-1336.
Qiu W, Hu M, Sridhar A, et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40:650–5. https://doi.org/10.1038/ng.117.
Jang I, Beningo KA. Integrins, CAFs and Mechanical Forces in the Progression of Cancer. Cancers. 2019;11:721. https://doi.org/10.3390/cancers11050721.
Wang Z, Xiong S, Mao Y, et al. Periostin promotes immunosuppressive pre-metastatic niche formation to facilitate breast tumor metastasis. J Pathol. 2016;239:484–95.
Huang W, Chiquet-Ehrismann R, Orend G, et al. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Can Res. 2001;61:8586–94.
Labovsky V, Martinez LM, Davies KM, et al. Prognostic significance of TRAIL-R3 and CCR-2 expression in tumor epithelial cells of patients with early breast cancer. BMC Cancer. 2017;17:280. https://doi.org/10.1186/s12885-017-3259-8.
Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–76. https://doi.org/10.4161/cc.9.16.12553.
Sanford-Crane H, Abrego J, Sherman MH. Fibroblasts as Modulators of Local and Systemic Cancer Metabolism. Cancers. 2019;11:619. https://doi.org/10.3390/cancers11050619.
Pavlides S, Whitaker-Menezes D, Castello-Cros R, et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. https://doi.org/10.4161/cc.8.23.10238.
Sotgia F, Martinez-Outschoorn UE, Pavlides S, et al. Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor microenvironment. Breast Cancer Res. 2011;13:1–13. https://doi.org/10.1186/bcr2892.
Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8:57813–25. https://doi.org/10.18632/oncotarget.18175.
Becker LM, O’Connell JT, Vo AP, et al. Epigenetic Reprogramming of Cancer-Associated Fibroblasts Deregulates Glucose Metabolism and Facilitates Progression of Breast Cancer. Cell Rep. 2020;31:107701. https://doi.org/10.1016/j.celrep.2020.107701.
Eiro N, González L, Martínez-Ordoñez A, et al. Cancer-associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell Oncol. 2018:1–10. https://doi.org/10.1007/s13402-018-0371-y.
Lappano R, Rigiracciolo DC, Belfiore A, et al. Cancer associated fibroblasts: role in breast cancer and potential as therapeutic targets. Expert Opinion on Therapeutic Targets. 2020;24:559–72. https://doi.org/10.1080/14728222.2020.1751819.
Yu Y, Xiao CH, Tan LD, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110:724–32. https://doi.org/10.1038/bjc.2013.768.
Matà R, Palladino C, Nicolosi ML, et al. IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget. 2016;7:7683–700. https://doi.org/10.18632/oncotarget.6524.
Dumont N, Liu B, Defilippis RA, et al. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia (United States). 2013;15:249–62. https://doi.org/10.1593/neo.121950.
Wang JM, Deng X, Gong W, et al. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1–17. https://doi.org/10.1016/S0022-1759(98)00128-8.
Primac I, Maquoi E, Blacher S, et al. Stromal integrin α11 regulates PDGFRβ signaling and promotes breast cancer progression. J Clin Investig. 2019;129:4609–28. https://doi.org/10.1172/JCI125890.
Tyan SW, Kuo WH, Huang CK, et al. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS ONE. 2011;6:1–9. https://doi.org/10.1371/journal.pone.0015313.
Osuala KO, Sameni M, Shah S, et al. Il-6 signaling between ductal carcinoma in situ cells and carcinoma-associated fibroblasts mediates tumor cell growth and migration. BMC Cancer. 2015;15. https://doi.org/10.1186/s12885-015-1576-3.
Studebaker AW, Storci G, Werbeck JL, et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Can Res. 2008;68:9087–95. https://doi.org/10.1158/0008-5472.CAN-08-0400.
Liu Y, Yang Y, Du J, et al. MiR-3613–3p from carcinoma-associated fibroblasts exosomes promoted breast cancer cell proliferation and metastasis by regulating SOCS2 expression. IUBMB Life. 2020:1–10. https://doi.org/10.1002/iub.2292.
Donnarumma E, Fiore D, Nappa M, et al. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget. 2017;8:19592–608. https://doi.org/10.18632/oncotarget.14752.
Gaggioli C, Hooper S, Hidalgo-Carcedo C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–400. https://doi.org/10.1038/ncb1658.
Attieh Y, Clark AG, Grass C, et al. Cancer-associated fibroblasts lead tumor invasion through integrin-β3-dependent fibronectin asse. J Cell Biol. 2017;216:3509–20. https://doi.org/10.1083/jcb.201702033.
Nabeshima K, Inoue T, Shimao Y, et al. Matrix metalloproteinases in tumor invasion: Role for cell migration. Pathol Int. 2002;52:255–64. https://doi.org/10.1046/j.1440-1827.2002.01343.x.
Nelson AR, Fingleton B, Rothenberg ML, et al. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2017;18:1135–49.
Matsumura Y, Ito Y, Mezawa Y, et al. Stromal fibroblasts induce metastatic tumor cell clusters via epithelial–mesenchymal plasticity. Life Sci Alliance. 2019;2:1–24. https://doi.org/10.26508/lsa.201900425.
Duda DG, Duyverman AMMJ, Kohno M, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA. 2010;107:21677–82. https://doi.org/10.1073/pnas.1016234107.
Psailaa B, Kaplana RN, Port ER, et al. Priming the “soil” for breast cancer metastasis: The Pre-Metastatic Niche. Breast Dis. 2006;26:65–74. https://doi.org/10.3233/bd-2007-26106.
Ursini-Siegel J, Siegel PM. The influence of the pre-metastatic niche on breast cancer metastasis. Cancer Lett. 2016;380:281–8. https://doi.org/10.1016/j.canlet.2015.11.009.
Feng T, Zhang P, Sun Y, et al. High throughput sequencing identifies breast cancer-secreted exosomal LncRNAs initiating pulmonary pre-metastatic niche formation. Gene. 2019;710:258–64. https://doi.org/10.1016/j.gene.2019.06.004.
Taverna S, Giusti I, D’ascenzo S, et al. Breast cancer derived extracellular vesicles in bone metastasis induction and their clinical implications as biomarkers. Int J Mol Sci. 2020;21:1–21. https://doi.org/10.3390/ijms21103573.
Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nat. 2005;438:820–7. https://doi.org/10.1038/nature04186.
Cox TR, Bird D, Baker A, et al. LOX-Mediated Collagen Crosslinking Is Responsible for Fibrosis-Enhanced Metastasis. Can Res. 2013;73:1721–32. https://doi.org/10.1158/0008-5472.CAN-12-2233.
Malanchi I, Santamaria-Martínez A, Susanto E, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nat. 2012;481:85–91. https://doi.org/10.1038/nature10694.
Houthuijzen JM, Jonkers J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. 2018;37:577–97. https://doi.org/10.1007/s10555-018-9768-3.
Zhang Y and Ertl HC. Depletion of FAP+ cells reduces immunosuppressive cells and improves metabolism and functions CD8+T cells within tumors. Oncotarget. 2016;7:23282–99. https://doi.org/10.18632/oncotarget.7818.
Zhang J, Pang Y, Xie T, et al. CXCR4 antagonism in combination with IDO1 inhibition weakens immune suppression and inhibits tumor growth in mouse breast cancer bone metastases. OncoTargets Ther. 2019;12:4985–92. https://doi.org/10.2147/OTT.S200643.
Xia Q, Zhang FF, Geng F, et al. Improvement of anti-tumor immunity of fibroblast activation protein α based vaccines by combination with cyclophosphamide in a murine model of breast cancer. Cell Immunol. 2016;310:89–98. https://doi.org/10.1016/j.cellimm.2016.08.006.
Kieffer Y, Hocine HR, Gentric G, et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 2020;33:CD-19–1384. https://doi.org/10.1158/2159-8290.cd-19-1384.
Gok Yavuz B, Gunaydin G, Gedik ME, et al. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1 + TAMs. Sci Rep. 2019;9:1–15. https://doi.org/10.1038/s41598-019-39553-z.
Roca H, Varcos ZS, Sud S, et al. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–54. https://doi.org/10.1074/jbc.M109.042671.
Mulholland BS, Forwood MR, Morrison NA. Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) Drives Activation of Bone Remodelling and Skeletal Metastasis. Curr Osteoporos Rep. 2019;17:538–47. https://doi.org/10.1007/s11914-019-00545-7.
Khalid A, Wolfram J, Ferrari I, et al. Recent Advances in Discovering the Role of CCL5 in Metastatic Breast Cancer. Mini-Reviews Med Chem. 2015;15:1063–72. https://doi.org/10.2174/138955751513150923094709.
Swamydas M, Ricci K, Rego SL, et al. Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adhes Migr. 2013;7:315–24. https://doi.org/10.4161/cam.25138.
Ouyang L, Chang W, Fang B, et al. Estrogen-induced SDF-1α production promotes the progression of ER-negative breast cancer via the accumulation of MDSCs in the tumor microenvironment. Sci Rep. 2016;6:39541. https://doi.org/10.1038/srep39541.
Shou D, Wen L, Song Z, et al. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget. 2016;7:64505–11. https://doi.org/10.18632/oncotarget.11352.
Stüber T, Monjezi R, Wallstabe L, et al. Inhibition of TGF- β- Receptor signaling augments the antitumor function of ROR1-specific CAR T-cells against triple-negative breast cancer. J ImmunoTher Cancer. 2020;8:1–7. https://doi.org/10.1136/jitc-2020-000676.
Hargadon K. Dysregulation of TGFβ1 Activity in Cancer and Its Influence on the Quality of Anti-Tumor Immunity. J Clin Med. 2016;5:76. https://doi.org/10.3390/jcm5090076.
Silzle T, Kreutz M, Dobler MA, et al. Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur J Immunol. 2003;33:1311–20. https://doi.org/10.1002/eji.200323057.
Cohen N, Shani O, Raz Y, et al. Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncog. 2017;36:4457–68. https://doi.org/10.1038/onc.2017.65.
Piersma B, Hayward MK and Weaver VM. Fibrosis and cancer: A strained relationship. Biochimica et Biophysica Acta - Rev Cancer. 2020;1873. https://doi.org/10.1016/j.bbcan.2020.188356.
Glentis A, Oertle P, Mariani P, et al. Correction: Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat Commun. 2018;9:75005. https://doi.org/10.1038/s41467-018-03304-x.
Yamashita M, Ogawa T, Zhang X, et al. Role of stromal myofibroblasts in invasive breast cancer: Stromal expression of alpha-smooth muscle actin correlates with worse clinical outcome. Breast Cancer. 2012;19:170–6. https://doi.org/10.1007/s12282-010-0234-5.
Paulsson J, Sjöblom T, Micke P, et al. Prognostic significance of stromal platelet-derived growth factor β-receptor expression in human breast cancer. Am J Pathol. 2009;175:334–41. https://doi.org/10.2353/ajpath.2009.081030.
Ariga N, Sato E, Ohuchi N, et al. Stromal expression of fibroblast activation protein / seprase, a cell membrane serine proteinase and gelatinase, is associated with longer survival in patients with invasive ductal. Int J Cancer. 2001;95:67–72. https://doi.org/10.1002/1097-0215(20010120)95:1%3c67::aid-ijc1012%3e3.0.co;2-u.
Kim HM, Jung WH, Koo JS. Expression of cancer-associated fibroblast related proteins in metastatic breast cancer: an immunohistochemical analysis. J Transl Med. 2015;13:222. https://doi.org/10.1186/s12967-015-0587-9.
Ao Z, Shah SH, Machlin LM, et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Can Res. 2015;75:4681–7. https://doi.org/10.1158/0008-5472.CAN-15-1633.
Schoppmann SF, Berghoff A, Dinhof C, et al. Podoplanin-expressing cancer-associated fibroblasts are associated with poor prognosis in invasive breast cancer. Breast Cancer Res Treat. 2012;134:237–44. https://doi.org/10.1007/s10549-012-1984-x.
Cai D, Wu X, Hong T, et al. CD61+ and CAF+ were found to be good prognosis factors for invasive breast cancer patients. Pathol Res Pract. 2017;213:1296–301. https://doi.org/10.1016/j.prp.2017.06.016.
Amornsupak K, Jamjuntra P, Warnnissorn M, et al. High ASMA+Fibroblasts and Low Cytoplasmic HMGB1+Breast Cancer Cells Predict Poor Prognosis. Clin Breast Cancer. 2017;17:441–52. https://doi.org/10.1016/j.clbc.2017.04.007.
Brechbuhl HM, Barrett AS, Kopin E, et al. Fibroblast subtypes define a metastatic matrisome in breast cancer. JCI Insight. 2020;5:1–16. https://doi.org/10.1172/jci.insight.130751.
Martinez LM, Labovsky V, De Lujan CM, et al. CD105 expression on CD34-negative spindle-shaped stromal cells of primary tumor is an unfavorable prognostic marker in early breast cancer patients. PLoS ONE. 2015;10:1993–6. https://doi.org/10.1371/journal.pone.0121421.
Labovsky V, Martinez LM, Calcagno M de L, et al. Interleukin-6 receptor in spindle-shaped stromal cells, a prognostic determinant of early breast cancer. Tumor Biol. 2016;37:13377–84. https://doi.org/10.1007/s13277-016-5268-7.
Wallace JA, Li F, Leone G, et al. Pten in the breast tumor microenvironment: Modeling tumor-stroma coevolution. Can Res. 2011;71:1203–7. https://doi.org/10.1158/0008-5472.CAN-10-3263.
Tchou J, Kossenkov AV, Chang L, et al. Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles. BMC Med Genomics. 2012;5:39. https://doi.org/10.1186/1755-8794-5-39.
Busch S, Andersson D, Bom E, et al. Cellular organization and molecular differentiation model of breast cancer-associated fibroblasts. Mol Cancer. 2017;16:1–12. https://doi.org/10.1186/s12943-017-0642-7.
Konieczkowski DJ, Johannessen CM, Garraway LA, et al. A convergence-based framework for cancer drug resistance. Cancer Cell. 2019;33:801–15. https://doi.org/10.1016/j.ccell.2018.03.025.A.
Valkenburg KC, De Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018. https://doi.org/10.1038/s41571-018-0007-1.
Loeffler M, Krüger JA, Niethammer AG, et al. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Investig. 2009;119:421–421. https://doi.org/10.1172/jci26532c1.
Fang J, Xiao L, Joo K Il, et al. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. Int J Cancer. 2016;138:1013–23. https://doi.org/10.1002/ijc.29831.
Su S, Chen J, Yao H, et al. CD10+GPR77+ Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell. 2018;172(841–856):e16. https://doi.org/10.1016/j.cell.2018.01.009.
Boesch M, Onder L, Cheng HW, et al. Interleukin 7-expressing fibroblasts promote breast cancer growth through sustenance of tumor cell stemness. OncoImmunology. 2018;7:e1414129. https://doi.org/10.1080/2162402X.2017.1414129.
Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. https://doi.org/10.1038/ncomms3516.
Hu C, Liu X, Ran W, et al. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials. 2017;144:60–72. https://doi.org/10.1016/j.biomaterials.2017.08.009.
Ryan D, Koziol J, ElShamy WM. Targeting AXL and RAGE to prevent geminin overexpression-induced triple-negative breast cancer metastasis. Sci Rep. 2019;9:1–19. https://doi.org/10.1038/s41598-019-55702-w.
Egeland EV, Boye K, Park D, et al. Prognostic significance of S100A4-expression and subcellular localization in early-stage breast cancer. Breast Cancer Res Treat. 2017;162:127–37. https://doi.org/10.1007/s10549-016-4096-1.
Boye K, Mælandsmo GM. S100A4 and metastasis: A small actor playing many roles. Am J Pathol. 2010;176:528–35. https://doi.org/10.2353/ajpath.2010.090526.
Leconet W, Chentouf M, Du Manoir S, et al. Therapeutic activity of anti-AXL antibody against triple-negative breast cancer patient-derived xenografts and metastasis. Clin Cancer Res. 2017;23:2806–16. https://doi.org/10.1158/1078-0432.CCR-16-1316.
Wu X, Liu X, Koul S, et al. AXL kinase as a novel target for cancer therapy. Oncotarget. 2014;5:9546–63. https://doi.org/10.18632/oncotarget.2542.
Park JS, Lee CH, Kim HK, et al. Suppression of the metastatic spread of breast cancer by DN10764 (AZD7762)-mediated inhibition of AXL signaling. Oncotarget. 2016;7:83308–18. https://doi.org/10.18632/oncotarget.13088.
Wang C, Jin H, Wang N, et al. Gas6/Axl axis contributes to chemoresistance and metastasis in breast cancer through Akt/GSK-3β/β- catenin signaling. Theranostics. 2016;6:1205–19. https://doi.org/10.7150/thno.15083.
Ye X, Li Y, Stawicki S, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncog. 2010;29:5254–64. https://doi.org/10.1038/onc.2010.268.
Hudson BI, Lippman ME. Targeting RAGE Signaling in Inflammatory Disease. Annu Rev Med. 2018;69:349–64. https://doi.org/10.1146/annurev-med-041316-085215.
El-Far AH, Sroga G, Al Jaouni SK, et al. Role and mechanisms of rage-ligand complexes and rage-inhibitors in cancer progression. Int J Mol Sci. 2020;21:1–21. https://doi.org/10.3390/ijms21103613.
Hollosi P, Yakushiji JK, Fong KSK, et al. Lysyl oxidase-like 2 promotes migration in noninvasive breast cancer cells but not in normal breast epithelial cells. Int J Cancer. 2009;125:318–27. https://doi.org/10.1002/ijc.24308.
Barker HE, Chang J, Cox TR, et al. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Can Res. 2011;71:1561–72. https://doi.org/10.1158/0008-5472.CAN-10-2868.
Barker HE, Bird D, Lang G, et al. Tumor-secreted LOXL2 activates fibroblasts through fak signaling. Mol Cancer Res. 2013;11:1425–36. https://doi.org/10.1158/1541-7786.MCR-13-0033-T.
Takai K, Le A, Weaver VM, et al. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. 2016;7:82889–901. https://doi.org/10.18632/oncotarget.12658.
Al-Harbi B, Hendrayani SF, Silva G, et al. Let-7b inhibits cancer-promoting effects of breast cancerassociated fibroblasts through IL-8 repression. Oncotarget. 2018;9:17825–38. https://doi.org/10.18632/oncotarget.24895.
Casey TM, Eneman J, Crocker A, et al. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-β1) increase invasion rate of tumor cells: A population study. Breast Cancer Res Treat. 2008;110:39–49. https://doi.org/10.1007/s10549-007-9684-7.
Rønnov-Jessen L PO. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707.
Desmouliere A, Geinoz A, Gabbiani F, et al. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. https://doi.org/10.1083/jcb.122.1.103.
Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. https://doi.org/10.1016/S0092-8674(03)00432-X.
Xu J, Lu Y, Qiu S, et al. A novel role of EMMPRIN/CD147 in transformation of quiescent fibroblasts to cancer-associated fibroblasts by breast cancer cells. Cancer Lett. 2013;335:380–6. https://doi.org/10.1016/j.canlet.2013.02.054.
Acknowledgments
The authors thanks the National Agency of Scientific and Technological Promotion, Argentina; the National Council of Scientific and Technical Research, Argentina; Alberto Roemmers Foundation, Argentina; René Barón Foundation, Argentina and Williams Foundation, Argentina.
Author information
Authors and Affiliations
Contributions
María Belén Giorello, performed the literature search, organize and wrote the review, designed the figures. Francisco Raúl Borzone, critical revised the review. Vivian Labovsky, Critical revised the review. Flavia Valeria Piccioni, designed the figures and critical revised the review. Norma Alejandra Chasseing, had the idea. Performed the literature search, organize and wrote the review, designed the figures together with María Belén Giorello, both corresponding authors.
Corresponding authors
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Ethical Approval
This is a review article and does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giorello, M.B., Borzone, F.R., Labovsky, V. et al. Cancer-Associated Fibroblasts in the Breast Tumor Microenvironment. J Mammary Gland Biol Neoplasia 26, 135–155 (2021). https://doi.org/10.1007/s10911-020-09475-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-020-09475-y