Abstract
Syzygium cumini pomace containing numerous phytochemicals is being neglected and can be used for their extraction for efficient utilization. In the present study, three different methods of phytochemicals extraction, viz., microwave-assisted extraction (MAE), shaking water bath extraction (SWE), and conventional extraction (CE), have been explored for the better extraction of phytochemicals from Indian blackberry pomace using Taguchi orthogonal array design. While comparing the range analysis among these, the extraction efficiencies trend in the decreasing order was CE > MAE > SWE for total phenols, and total flavonoids, MAE > SWE > CE for antioxidant activities, and MAE > CE > SWE for total monomeric anthocyanins. The optimized extraction conditions for MAE were 80% microwave amplitude for 2 min using 1:30 solid to liquid ratio with 0.01% HCl, whereas, for SWE and CE, it was 60 °C for 90 min using 1:30 solid–liquid ratio with 0.05% HCl. The optimized extracts were further analyzed for the qualitative and quantitative characterization using FTIR and HPLC, respectively, which confirmed the presence of these phytochemicals. SEM analysis revealed the better structural opening of pomace in MAE compared to SWE and CE. In a nutshell, it may be concluded that MAE has the potential for better extraction than the SWE and CE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Food processing industries generate a huge amount of waste; the disposal of agri-food processing waste has become a major issue due to its adverse effect on the environment [1]. It is estimated that the waste, i.e., peels, rind, seeds, core, pomace, press cakes, etc., in horticulture crops accounts for up to 40% [2]. These wastes have gained the attention of researchers and industrialists worldwide in the recent era due to the presence of a diverse range of valuable phytochemicals (anthocyanins, carotenoids, phenols, flavonoids) which has the potential for the production of functional/health/supplemented foods [3, 4] and, hence, can be used for the extraction of phytochemicals. Extraction of bioactive compounds from plant materials using low-cost technology to make phytochemical-rich foods can be the best strategy to exploit food processing waste and reduce the volumes of waste generated [5].
In recent years, there is a shift toward the consumption of phytochemical-rich or functional foods with incorporated natural ingredients. Syzygium cumini (jamun) is one of the phytochemical-rich natural sources which has gained the attention of the processors for the development of supplemented/functional foods. The commercial processing of jamun is limited to juice which generates approximately 45% waste in the form of peel, seeds, and residual pulp [6]. Its pomace possesses good amounts of valuable bioactive compounds, minerals, and antioxidant properties; however, after juice extraction, the pomace does not have any value for the processors. This leftover mass being a rich resource of carbon is prone to microbial attack leading to spoilage. In such circumstances, the extraction of phytochemicals is a weapon for its better utilization. However, till date no study has been reported on the utilization of Indian blackberry processing waste and this valuable waste has been neglected. Hence, there is a strong need for the cost-effective utilization of pomace for the extraction of phytochemicals by different methods.
Conventional techniques such as maceration, percolation, decoction, and hot continuous extraction have been used for the extraction of high-value compounds for many decades [7]. The increased interest in the extraction and use of plant-based bioactive compounds has led to the development of new efficient techniques to maximize their extraction with less processing time and cost. Conventional extraction (CE) methods for phytochemicals are often time-consuming, consume large amounts of solvent as well as energy, and can also lead to the degradation of these bioactive compounds [8]. Thus, there is an increasing demand for novel non-conventional extraction methods which shortens extraction time while maximizing the extraction yield of target biomolecules with minimal degradation and reducing environmental impact. Various advanced methods involving the use of microwave, supercritical fluid, high hydrostatic pressure, ultrasound, accelerated solvent, and the enzyme have been explored by various researchers [8,9,10,11,12,13,14,15]. Microwave-assisted extraction (MAE) is one of these promising techniques for the extraction of various biomolecules due to unique benefits such as improved yield and quality of biological compounds, reduced extraction times, and low solvent consumption [13].
The type of solvent used for the extraction of phytochemicals from natural sources is one among the most studied variables. Studies revealed that phytochemicals from natural sources are efficiently extracted using organic solvents [16]; however, health and safety concerns associated with the use of organic solvents have pushed researchers to find alternative green solvents to these. Hence, eco-friendly solvents such as water have been explored for the extraction of bioactive compounds by various researchers [13, 17,18,19]. Apart from this, pH, extraction temperature, time, and solid–liquid ratio affect the extraction from different plant materials [19]. However, generalized extraction conditions cannot be applied to all plant materials. Thus, it is important to optimize these variables for the better extraction of phytochemicals.
Based on above rationale, the present study was aimed at determining the optimal conditions for the extraction of phytochemicals from Syzygium cumini pomace using three different extraction methods namely microwave-assisted, shaking water bath, and conventional extraction.
2 Materials and methods
2.1 Material
Sygyzium cumini pomace was collected from the Food Industry Business Incubation Centre, Punjab Agricultural University, Ludhiana, India, and brought immediately to the laboratory under controlled conditions. The pomace was packed in polyethylene bags (200 gauge; 5-kg capacity) and stored at − 18 °C until further used. HPLC grade standards and solvents were purchased from Sigma-Aldrich Pvt. Ltd. (NSW, Australia). All the chemicals used were of analytical grade and were purchased from Loba Chemie, India.
2.2 Experimental design
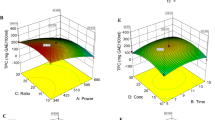
Taguchi orthogonal array design was applied to study the effect of four factors at three levels on the extraction of phytochemicals. Extraction variables namely temperature (40, 50, and 60 °C), extraction time (30, 60, and 90 min), solid–liquid ratio (1:10, 1:20, and 1:30 g/mL), and HCl concentration (0.01, 0.05, and 0.1%) were considered in the present study for the extraction of phytochemicals, i.e., total monomeric anthocyanins, percent polymeric color, total phenolic content, total flavonoids, DPPH free radical scavenging activity, ferric reducing antioxidant power (FRAP), and reducing power using shaking water bath extraction (SWE) and conventional extraction (CE), whereas, for microwave-assisted extraction (MAE), the effect of microwave amplitude (60, 80, and 100%) was studied instead of temperature, and extraction time was 1, 2, and 3 min.
2.2.1 Microwave-assisted extraction (MAE)
Accurately weighed (5 g) pomace was mixed with acidified distilled water and placed in a microwave oven (2450 MHz; Morphy Richards MW025CG, India) having adjustable microwave amplitude and time for the extraction of phytochemicals (Table 1). After microwave treatment, the slurry was centrifuged at 2000 × g for 20 min (ST16R, Thermo Fisher Scientific, Germany) to collect the supernatant (extract). The extracts so obtained were stored at − 18 °C till further analysis.
2.2.2 Shaking water bath extraction (SWE)
Pomace (5 g) was mixed with acidified distilled water. The slurry so prepared was subjected for the extraction of phytochemicals in a shaking water bath (Scientific Industries Inc., Bohemia, NY, USA; 150 rpm) at 40–60 °C for 30–90 min using different experimental combinations as given in Table 2. After extraction of respective combinations, the supernatant was collected and stored as described in MAE.
2.2.3 Conventional extraction (CE)
Five grams of pomace was mixed with acidified distilled water; the prepared slurry was subjected to conventional heating at 40–60 °C for 30–90 min (treatments are given in Table 3). After respective treatments, the extracts were collected and stored as described in MAE.
2.3 Methods
2.3.1 Analysis of phytochemicals of Syzygium cumini pomace
Total monomeric anthocyanin content (TMAC) of the pomace was determined using pH differential method [20]. The extracts were diluted with two buffers, pH 1.0 (0.025 M potassium chloride) and pH 4.5 (0.4 M sodium acetate), and absorbance was read at 520 and 700 nm with UV/Vis spectrophotometer (PG instruments, Leicestershire, UK) after 2-h incubation in dark. TMAC was calculated as mg cyanidin-3-glucoside equivalent (CE)/g (dw).
Percent polymeric color is the ratio of polymerized anthocyanin compounds (polymeric color) to total anthocyanins (color density) and was evaluated using method reported by Wang et al. [21]. 2.8 mL of diluted extract was mixed with 0.2 mL potassium metabisulfite (0.2 g/mL) and distilled water in control. The absorbance was read at 420 nm, λvis-max (500–510 nm), and 700 nm after 30 min incubation at room temperature. Color density and polymeric color were estimated for control and bleached sample, respectively, using the formula:
Total phenolic content (TPC) of the extracts was determined using Folin-Ciocalteu (FC) regent as outlined by Kaur et al. [22]. Aliquot (0.5 mL) was mixed with 0.5 mL FC reagent and 1.5 mL of sodium carbonate (20%, w/v), followed by volume makeup 10 mL with double distilled water. After incubation of 2 h, the absorbance was read at 760 nm and the results were expressed as mg gallic acid equivalent (GAE)/g (dw).
Total flavonoid content (TFC) was determined using method described by Floegel et al. [23]. Extract (0.5 mL) was mixed 0.15 mL sodium nitrite solution (5%, w/v) and 0.15 mL aluminum chloride solution (10%, w/v) and 3.2 mL double distilled water, followed by addition of 1 mL sodium hydroxide solution (1 M). The absorbance was immediately taken at 510 nm and the results were presented as mg quercetin equivalent (QE)/g (dw).
2.3.2 Analysis of in vitro antioxidant activities of Syzygium cumini pomace
Free radical scavenging activities of the extract were measured by DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical scavenging activity (DPPH), ferric reducing antioxidant power (FRAP), and reducing power. Diluted extracts (1 mL) were mixed with 1 mL Tris buffer and 2 mL methanol solubilized DPPH reagent [22], followed by incubation for 30 min in dark at room temperature and the absorbance was then taken at 517 nm. The results were expressed as mg ascorbic acid equivalent (AAE)/g (dw).
The FRAP method is based on reducing Fe3+-TPTZ to a blue-colored Fe2+-TPTZ as described by Jing et al. [24]. FRAP reagent was prepared by mixing stock solutions, 10 mM 2,4,6-tri(2- pyridyl)-1,3,5-triazine (TPTZ) stock solution, 300 mM acetate buffer (pH 3.6), and 20 mM Ferric chloride solution in the ratio 1:10:1, and then incubated at 37 °C for 30 min. Extract (400 μL) was mixed with 3 mL freshly prepared FRAP reagent. The absorbance was read at 593 nm. The results were expressed as mg ascorbic acid equivalent (AAE)/g (dw).
The ability of extracts to reduce ferric ions (Fe+3) was evaluated using method outlined by Philip et al. [25]. One milliliter of extract was mixed with 2.5 mL of each, i.e., phosphate buffer and potassium ferricyanide (1%). After incubation at 50 °C for 20 min, 2.5 mL trichloroacetic acid (10%) was added and mixture was centrifuged at 2000 × g for 20 min. Supernatant (2.5 mL) was mixed with equal amount of double distilled water and 0.5 mL ferric chloride (0.1%), and the absorbance was read at 700 nm. The results were expressed as mg ascorbic acid equivalent (AAE)/g (dw).
2.3.3 Mineral profiling
Mineral composition of the optimized extracts obtained from MAE, SWE, and CE was estimated using inductively coupled plasma atomic emission spectroscopy (X-Series2, Thermo Scientific, USA). Respective extract (0.5 mL) was digested with a mixture of perchloric and nitric acid (1:3) until a clear solution was obtained, followed by volume makeup to 25 mL using deionized water and estimation of minerals. The results were expressed as ppm.
2.3.4 Fourier-transform infrared spectroscopy (FTIR) characterization
The extracts obtained after MAE, SWE, and CE treatments were evaluated qualitatively for the presence of various peaks corresponding to various polyphenolic compounds using FTIR spectrometer (Nicolet 67,000, Thermo Scientific, USA) at ambient temperature (28 ± 2 °C) in 4000–400 cm−1 range and the spectra so obtained were interpreted as per the standard guidelines of Stuart [26].
2.3.5 Scanning electron microscopy (SEM) analysis
To observe the morphology and the effect of respective extraction methods, i.e., MAE, SWE, and CE, the residue was dried at 50 °C, spur coated with gold, and SEM was carried out using scanning electron microscope (XL30, FEI Philips, France; 10 to 300,000 magnification).
2.3.6 HPLC profiling of phenolic compounds
HPLC analysis was performed using a X-Bridge™ C18 column (250 mm × 4.6 mm, 5 μm) (Waters USA) equipped with an X-Bridge™ C18 guard column and a Thermoquest HPLC system with a diode array detector. The mobile phase was comprised of solvent A, 0.1% formic acid in water (v/v), solvent B, 0.1% formic acid in acetonitrile, and solvent C, 0.1% formic acid in water. The flow rate of the mobile phase was 1.0 mL/min, and the injection volumes of the standards and sample extracts were 2.0 µL. Each estimation was run in triplicate and the quantification of phenolic acids was done using external standard method based on the peak area.
2.4 Statistical analysis
All the experiments were performed in triplicate (n = 3). Statistical analysis of the data was performed with ANOVA and a range analysis using SPSS (version 10.0.1.0, Stat-Ease). The differences in means were identified using Tukey’s test (p < 0.05) and the results were expressed as mean ± standard deviation.
Range analysis was carried out to check the effect of individual variable and to determine the optimum level of different extraction variables. The average response (Pij) for each variable (i = 1–3) was calculated at each level (j = 1–3). The difference between highest and lowest values of Pij is represented as Rj and was calculated to determine most contributing variable based on rank (Rj) values of each variable on the yield of phytochemicals.
3 Results and discussion
3.1 Effect of MAE on phytochemicals and antioxidant activities
In the present study, a significant (p < 0.05) effect of various independent variables under study was observed on all phytochemicals (total monomeric anthocyanins, percent polymeric color, total phenols, and flavonoids which ranged from 2.78 to 5.87 mg CE/g, 1.32 to 12.23%, 11.95 to 26.05 mg GAE/g, and 5.46 to 11.17 mg QE/g, respectively), and the antioxidant activities (DPPH, FRAP, and reducing power ranged from 0.30 to 1.5 mg AAE/g, 2.66 to 6.22 mg AAE/g, and 13.44 to 35.96 mg AAE/g, respectively) (Table 1).
To optimize the extraction variables, range analysis was carried out which was found consistent with ANOVA. The solid–liquid ratio was the most significant parameter affecting the yield of total flavonoids, FRAP, and reducing power while HCl concentration was the most significant factor affecting the extraction efficiency of percent polymeric color, total phenols, and DPPH. Total monomeric anthocyanins were most significantly affected by extraction time (Table 1). Moderate microwave power, i.e., 80% amplitude, resulted in the better extraction of phytochemicals and antioxidant activities except for polymeric color irrespective of others (while studying the effect of individual variables independently). This may be attributed to microwave heating which results in the absorption and diffusion of energy in the plant tissues with the help of electromagnetic waves, thus, disrupting the cell walls and releasing the bound phytochemicals into the solvent [13]. However, 100% microwave amplitude resulted in higher temperatures which might have thermally degraded the bioactive compounds [10], whereas, the increase in polymeric color can be attributed to the formation of chalcone: an intermediate formed during anthocyanin degradation at high-temperature processing, leading to polymerization of anthocyanins. The intermediate so formed is unstable and is quickly degraded, resulting in a remarkable increase in polymerized anthocyanins [12]. Microwave exposure of plant material for a longer period leads to an increase in vapor pressure in the cells, which breaks down the cell walls and releases bioactive compounds into the extracting medium, hence results in higher extraction of phytochemicals [27], which was observed in the present study.
The extraction efficiency of phytochemicals and antioxidant activities was positively affected by solid–liquid ratio except for total monomeric anthocyanins and percent polymeric color. The increase in phytochemicals may be due to the dilution of substrate with higher amount of extraction medium (water), which increased the extraction efficiency [13]. However, the decrease of anthocyanin concentration might be due to saturation of extract with the solutes which might have led to steady state. Similar effect of solid–liquid ratio on the extraction of anthocyanins from jamun pulp has been reported by Maran et al. [28]. The addition of HCl into extraction solvent showed increasing trend on the extraction efficiency of anthocyanins, and other phytochemicals up to 0.5%; however, polymeric color was negatively affected. The results of present study were in line with the findings of Ongkowijoyo et al. [16] who reported that the addition of small amounts of acid into solvent increases the extraction and stability of phytochemicals, particularly anthocyanins without degradation, whereas excessive acid may result in partial hydrolysis of glyosidic bond, breaking linkage between anthocyanin and co-pigments. Similar effect of HCl addition was observed on antioxidant activities which can be related to the concentration of heat labile phytochemicals (anthocyanins, flavonoids, and phenolic compounds) [29].
3.2 Effect of SWE on phytochemicals and antioxidant activities
The yield of phytochemicals was significantly (p < 0.05) affected by various independent variables. Total monomeric anthocyanins, polymeric color, total phenols, and flavonoids ranged between 3.23 and 4.99 mg CE/g, 1.36 and 19.29%, 11.10 and 24.23 mg GAE/g, and 6.15 and 10.36 mg QE/g, respectively, and the antioxidant activities in terms of DPPH, FRAP, and reducing power ranged from 0.38 to 1.49, 2.70 to 5.72, and 11.00 to 28.45 mg AAE/g, respectively, due to the effect of various independent variables (Table 2).
While conducting the range analysis, temperature was found to be the most significant factor affecting the yield of total phenols, FRAP, and reducing power while solid–liquid ratio significantly (p < 0.05) affected the extractions of total monomeric anthocyanins and DPPH. Polymeric color and total flavonoids were significantly affected by HCl concentration and extraction time, respectively (Table 2). With the increase in extraction temperature, the extraction efficiency of phytochemicals and antioxidant potential of the extract increased significantly except percent polymeric color which can be explained by the fact that heating the plant tissues before extraction not only inactivates polyphenol peroxidase but also helps in softening of tissue and disrupting the plant cell walls, thus increases the extraction efficiency of phytochemicals and ultimately affects the antioxidant activities [30]. There was a gradual increase in the yield of phytochemicals and antioxidant activities till 60 min of extraction time followed by a decrease in the yield of phytochemicals with further increase in time, whereas percent polymeric color and antioxidant activities were decreased with increase in extraction time. The increase in yield of phytochemicals is due to the dilution of substrate with solvent which takes certain time to reach equilibrium state [31]. Apart from this, longer exposure time facilitates the extraction of higher molecular bioactive compounds which needs more time to diffuse through cellular matrix. Laroze et al. [9] reported similar effect of extraction time on the yield of phytochemicals from raspberry and cranberry. However, extraction time beyond 60 min significantly decreased the yield of monomeric anthocyanins, total phenols, and DPPH free radical scavenging activity which might be due to mass transfer which decreased later with the extension of extraction time.
The solid–liquid ratio significantly enhanced the extraction of phytochemicals as well as antioxidant potential of the extracts except total phenols. This is consistent with mass transfer principles which dictate that the concentration gradient between the solid and bulk of the liquid drives the transfer process. The concentration gradient is greater when there is a higher ratio of solvent to solid [18]. Similar effect of solid–liquid ratio on the extraction of polyphenolic compounds from grape pomace was reported by Pinelo et al. [17]. However, higher solid–liquid ratio exerted steady effect on total flavonoid yield which might be due to saturation of extraction medium with the solutes leading to decrease in its extraction efficiency. Similar effects of solid–liquid ratio were reported by Maran et al. [28]. HCl addition significantly increased extraction of anthocyanins, total phenols, and antioxidant activities in terms of FRAP and reducing power. Addition of small amounts of acids to the extracting solvent improves the stability and better extraction of phytochemicals into the solvent, particularly anthocyanins which are more stable at acidic pH; however, higher acid addition breaks down the linkage between anthocyanin and co-pigments [16]; thus, percent polymeric color content was observed to be decreased.
3.3 Effect of CE on phytochemicals and antioxidant activities
The yield of total monomeric anthocyanins ranged between 3.18 and 5.26 mg CE/g, polymeric color between 1.84 and 20.49%, total phenols between 10.26 and 28.97 mg GAE/g, flavonoids between 5.09 and 15.03 mg QE/g, and the antioxidant activities in terms of DPPH free radical scavenging activity ranged from 0.36 to 1.33 mg/g, FRAP from 2.28 to 5.09 mg/g, and reducing power from 6.96 to 21.11 mg/g due to the effect of various independent variables (Table 3).
Range analysis revealed that temperature was the most significant factor affecting the yield of total phenols, FRAP, and reducing power while solid–liquid ratio significantly (p < 0.05) affected the extractions of total monomeric anthocyanins and DPPH free radical scavenging activity. Polymeric color and total flavonoids were significantly affected by HCl concentration and extraction time, respectively (Table 3). The yield of total flavonoids and antioxidant activities in terms of FRAP and reducing power was found higher when extracted at 60 °C as compared to 40 °C. Increase in temperature results in better softening of pomace, and affecting the extraction positively as discussed earlier [31]. Intermediate formation during thermal degradation of anthocyanins at high temperature of extraction may result in the increase in percent polymeric color [12]. The extraction time had significant positive effect on the extraction of monomeric anthocyanins while it had static or decreasing effect on the other phytochemicals (total phenols and flavonoids), and antioxidant activities. The increase in yield of phytochemicals is due to the dilution of substrate with solvent which takes certain time to reach equilibrium state [31].
With the increase in solid–liquid ratio up to 1:20 (w/v), the extraction efficiency of bioactive compounds and antioxidants significantly improved, which can be explained as higher amount of solvent addition ensures proper hydration and uniform mixing of plant material and development of the driving forces, allowing better penetration of solvent into plant cells [11]. However, further increase in solid–liquid ratio exerted steady effect which might be due to saturation of extraction medium as discussed earlier [17]. The addition of HCl significantly increased the extraction of total phenols, flavonoids, DPPH free radical scavenging activity, FRAP, and reducing power; however, the yield of monomeric anthocyanins decreased with the increase in acid amount in the extracting solvent which might be due to breakdown of linkages between anthocyanins and co-pigments [12].
3.4 Optimization of extraction conditions
The extraction conditions were optimized based on maximum extraction efficiency of majority of phytochemicals as well as antioxidant activities. The optimized conditions for MAE were 80% microwave amplitude for 2 min using 1:30 solid/liquid ratio of distilled water acidified with 0.01% HCl (experimental run 5), while for CE and SWE, the optimized conditions were 60 °C for 90 min using 1:30 solid liquid ratio of distilled water (solvent) acidified with 0.05% HCl (experimental run 6).
3.5 Comparison of extraction methods on phytochemicals recovery
Total phenols and total flavonoid yield followed the order CE > MAE > SWE, while antioxidant activities followed the order MAE > SWE > CE (Tables 1, 2, and 3). Microwave-assisted extraction showed maximum extraction efficiency for total monomeric anthocyanins followed by CE and SWE, respectively (Table 1). A short-time extraction in MAE might have favored the extraction of anthocyanins; however, long-time extraction at comparatively high temperatures (e.g., CE and SWE) degrades the anthocyanins which are heat-labile, thus reduces the yield. Other phenolic compounds such as phenols and flavonoids (thermo-stable) are not affected with long extraction durations [13].
The comparison between the extractability of phytochemicals, antioxidant activities, phenolic acids, and minerals with MAE, SWE, and CE in terms of percentage extractability was carried out, and the results are presented in Table 4. Overall, the application of MAE was found better for the extraction of phytochemicals and antioxidant activities as compared to other methods studied which can be attributed to high-frequency shear, powerful shear, and high-velocity cavitation occurring during the extraction process [18]. The findings were found consistent with the literature [10, 18, 32] which stated increase in phytochemicals and antioxidant activities with reduced extraction time. MAE can improve the bioactive recovery by its heating effect due to dipole rotation of solvent used (water) in the microwave field [32]. Major advantage of MAE was a significant reduction of extraction time, i.e., 3 min in MAE as compared to 90 min in conventional extraction methods. Microwave treatment also enhances the cell breakage by rapid temperature and internal pressure rise inside the plant cells, which improves the exudation of biochemical constituents from the cells into the surrounding solvents as discussed earlier [10].
Thirteen phenolic acids have been profiled by HPLC (Table 4). The quantification of phenolic compounds using HPLC revealed that MAE extracted significantly higher concentration of gallic acid, 4- amino benzoic acid, 3- hydroxybenzoic acid, catechin ( +) hydrate, p- coumeric acid, 2,3- dihydroxybenzoic acid, transcinnamic acid, and sinapic acid (Table 4 and Fig. 1a(A)), whereas the quantities of vanillic acid, caffeic acid, quercetin, and chlorogenic acid were higher in CE (Fig. 1a(B) and (C)). A significant (p < 0.05) increase in mineral elements was observed in MAE extract as compared to SWE and CE except for magnesium, sodium, zinc, and manganese (Table 4). Evaporative losses of dielectric species in MAE might have contributed to the lower mineral content; however, it further needs research to study the exact mechanism involving in the varying amounts of these constituents [33].
3.6 FTIR spectra
FTIR spectra of the extracts obtained from MAE, SWE, and CE revealed the similar absorption bands which depicts the presence of same phytochemical compounds in the extracts confirming the findings of present study; however, their concentration followed the order MAE > SWE > CE based on the peaks in the range of 1500–1000 cm−1 (Fig. 1b). The peaks in the range of 3335–2938 cm−1 indicate the O–H functional group of sugars and phenols corresponding to the occurrence of anthocyanin compounds and the concentration followed the order MAE > CE > SWE [34]. The sharp peak at 1725 cm−1 indicates pyran ring, depicting the flavonoid compounds. The bands in the range of 1347–1345 cm−1 and 1055–1047 cm−1 attribute to the presence of CH3 symmetrical/asymmetrical stretching and CH-CH/C-O stretching, respectively, which indicates the presence of phenolic compounds [22, 34]. The peaks at 1237 cm−1 indicate stretching of phosphorus with oxygen and sulfur, corresponding to phosphorus compounds [35]. The better extraction of phytochemicals in MAE can be attributed to the cell rupturing by rapid temperature and internal pressure rise inside the plant cells as explained earlier.
3.7 Scanning electron microscopy (SEM) analysis
SEM was employed to observe morphological changes occurred on the structure of residue obtained after extraction of phytochemicals with three different methods of extraction. The SEM images clearly showed that different extraction methods deformed the cell structure of pomace to different extents (Fig. 2A–C). The pomace obtained after CE showed comparatively smooth and unbroken cell walls, whereas in SWE the cells were more crumpled (Fig. 2B). In case of MAE treatment, some cavities were observed which might be due to microwave heating of the inner cells leading to increase in pressure, thus, resulting in more cell disruption, thereby increasing the release of bioactive constituents into the extracting solvent in less time as compared to conventional methods which is evident from the SEM images (Fig. 2A).
4 Conclusion
Three methods of extraction namely MAE, SWE, and CE were compared for the extraction of phytochemicals from Indian blackberry pomace. The optimized extraction conditions for MAE were 80% microwave amplitude for 2 min using 1:30 solid–liquid ratio of water acidified with 0.01% HCl, while for SWE and CE, extraction at 60 °C for 90 min using 1:30 solid liquid ratio with 0.05% HCl was optimized. MAE extracted the phytochemicals with significant reduction in extraction time, i.e., 3 min as compared to 90 min. The optimized extracts analyzed for minerals, phenolic acid profiling by HPLC, FTIR, and SEM analysis revealed that MAE was better for the extraction of phytochemicals, some minerals, and phenolic acids; however, the extraction efficiency of SWE and CE was found comparable. The developed conditions have the potential for the better extraction of phytochemicals from Syzygium cumini pomace.
References
Torres-Valenzuela LS, Ballesteros-Gómez A, Rubio S (2020) Green solvents for the extraction of high added-value compounds from agri-food waste. Food Eng Rev 12:83–100. https://doi.org/10.1007/s12393-019-09206-y
FAO (2019) Moving forward on food loss and waste reduction. The State of Food and Agriculture 2019.
Gorinstein S, Martín-Belloso O, Park YS et al (2001) Comparison of some biochemical characteristics of different citrus fruits. Food Chem 74:309–315. https://doi.org/10.1016/S0308-8146(01)00157-1
Soong YY, Barlow PJ (2004) Antioxidant activity and phenolic content of selected fruit seeds. Food Chem 88:411–417. https://doi.org/10.1016/j.foodchem.2004.02.003
Prakash Maran J, Manikandan S (2012) Response surface modeling and optimization of process parameters for aqueous extraction of pigments from prickly pear (Opuntia ficus-indica) fruit. Dye Pigment 95:465–472. https://doi.org/10.1016/j.dyepig.2012.06.007
Suradkar NG, Pawar VS, Shere DM (2017) Physicochemical, proximate and bioactive composition of jamun (Syzygium cuminii L.) fruit. Int J Chem Stud 5:470–472. https://doi.org/10.1007/s13197-011
Dhanani T, Shah S, Gajbhiye NA, Kumar S (2017) Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem 10:S1193–S1199. https://doi.org/10.1016/j.arabjc.2013.02.015
Ghitescu RE, Volf I, Carausu C et al (2015) Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason Sonochem 22:535–541. https://doi.org/10.1016/j.ultsonch.2014.07.013
Laroze LE, Díaz-Reinoso B, Moure A et al (2010) Extraction of antioxidants from several berries pressing wastes using conventional and supercritical solvents. Eur Food Res Technol 231:669–677. https://doi.org/10.1007/s00217-010-1320-9
Li H, Deng Z, Wu T et al (2012) Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem 130:928–936. https://doi.org/10.1016/j.foodchem.2011.08.019
Pradal D, Vauchel P, Decossin S et al (2016) Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: extraction and energy consumption optimization. Ultrason Sonochem 32:137–146. https://doi.org/10.1016/j.ultsonch.2016.03.001
Cao X, Cai C, Wang Y, Zheng X (2019) Effects of ultrasound processing on physicochemical parameters, antioxidants, and color quality of bayberry juice. J Food Qual 2019. https://doi.org/10.1155/2019/7917419
Alara OR, Abdurahman NH (2019) Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: kinetic modelling and process intensification. Ind Crops Prod 137:528–535. https://doi.org/10.1016/j.indcrop.2019.05.053
Baltacıoğlu H, Baltacıoğlu C, Okur I et al (2021) Optimization of microwave-assisted extraction of phenolic compounds from tomato: characterization by FTIR and HPLC and comparison with conventional solvent extraction. Vib Spectrosc 113. https://doi.org/10.1016/j.vibspec.2020.103204
Okur İ, Baltacıoğlu C, Ağçam E et al (2019) Evaluation of the effect of different extraction techniques on sour cherry pomace phenolic content and antioxidant activity and determination of phenolic compounds by FTIR and HPLC. Waste Biomass Valorization 10:3545–3555. https://doi.org/10.1007/s12649-019-00771-1
Ongkowijoyo P, Luna-Vital DA, Gonzalez de Mejia E (2018) Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: an update. Food Chem 250:113–126. https://doi.org/10.1016/j.foodchem.2018.01.055
Pinelo M, Rubilar M, Jerez M et al (2005) Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem 53:2111–2117. https://doi.org/10.1021/jf0488110
Castro-López C, Ventura-Sobrevilla JM, González-Hernández MD et al (2017) Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem 237:1139–1148. https://doi.org/10.1016/j.foodchem.2017.06.032
Ryu D, Koh E (2018) Application of response surface methodology to acidified water extraction of black soybeans for improving anthocyanin content, total phenols content and antioxidant activity. Food Chem 261:260–266. https://doi.org/10.1016/j.foodchem.2018.04.061
Lee J, Durst RW, Wrolstad RE (2005) Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int 88:1269–1278. https://doi.org/10.1093/jaoac/88.5.1269
Wang W, Jung J, Tomasino E, Zhao Y (2016) Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT - Food Sci Technol 72:229–238. https://doi.org/10.1016/j.lwt.2016.04.041
Kaur N, Aggarwal P, Kaur N, Kaur S (2022) Nutritional improvement of Bhujia by incorporating coloured bell peppers. J Food Process Preserv 1–12. https://doi.org/10.1111/jfpp.16569
Floegel A, Kim DO, Chung SJ et al (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal 24:1043–1048. https://doi.org/10.1016/j.jfca.2011.01.008
Jing P, Ye T, Shi H et al (2012) Antioxidant properties and phytochemical composition of China-grown pomegranate seeds. Food Chem 132:1457–1464. https://doi.org/10.1016/j.foodchem.2011.12.002
Philip JP, Madhumitha G, Mary SA (2011) Free radical scavenging and reducing power of Lawsonia inermis L. seeds. Asian Pac J Trop Med 4:457–461. https://doi.org/10.1016/S1995-7645(11)60125-9
Stuart BH (2004) Infrared spectroscopy: fundamentals and applications. John Wiley & Sons.
Rodríguez De Luna SL, Ramírez-Garza RE, Serna Saldívar SO (2020) Environmentally friendly methods for flavonoid extraction from plant material: impact of their operating conditions on yield and antioxidant properties. Sci World J 2020. https://doi.org/10.1155/2020/6792069
Maran JP, Sivakumar V, Thirugnanasambandham K, Sridhar R (2015) Extraction of natural anthocyanin and colors from pulp of jamun fruit. J Food Sci Technol 52:3617–3626. https://doi.org/10.1007/s13197-014-1429-0
Chaves JO, de Souza MC, da Silva LC et al (2020) Extraction of flavonoids from natural sources using modern techniques. Front Chem 8:507887. https://doi.org/10.3389/fchem.2020.507887
Padayachee A, Netzel G, Netzel M et al (2012) Binding of polyphenols to plant cell wall analogues - Part 1: Anthocyanins. Food Chem 134:155–161. https://doi.org/10.1016/j.foodchem.2012.02.082
Tan MC, Tan CP, Ho CW (2013) Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int Food Res J 20:3117–3123
Da Porto C, Natolino A (2018) Extraction kinetic modelling of total polyphenols and total anthocyanins from saffron floral bio-residues: comparison of extraction methods. Food Chem 258:137–143. https://doi.org/10.1016/j.foodchem.2018.03.059
Hayat K, Abbas S, Hussain S et al (2019) Effect of microwave and conventional oven heating on phenolic constituents, fatty acids, minerals and antioxidant potential of fennel seed. Ind Crops Prod 140:111610. https://doi.org/10.1016/j.indcrop.2019.111610
Swer TL, Mukhim C, Bashir K, Chauhan K (2018) Optimization of enzyme aided extraction of anthocyanins from Prunus nepalensis L. LWT - Food Sci Technol 91:382–390. https://doi.org/10.1016/j.lwt.2018.01.043
Coates J (2000) Interpretation of infrared spectra, a practical approach. In: Encyclopedia of Analytical Chemistry, vol 12. pp 10815–37
Acknowledgements
The authors are highly grateful to The Head, Food Science and Technology, Punjab Agricultural University, Ludhiana, Punjab, India, for providing infrastructure for conducting this research.
Funding
This research was financially supported by the Head, Food Science and Technology, Punjab Agricultural University, Ludhiana, Punjab, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, N., Aggarwal, P., Kumar, V. et al. Influence of different extraction techniques on the extraction of phytochemicals and antioxidant activities from Syzygium cumini (jamun) pomace using Taguchi orthogonal array design: a qualitative and quantitative approach. Biomass Conv. Bioref. 13, 14497–14509 (2023). https://doi.org/10.1007/s13399-022-02826-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02826-1