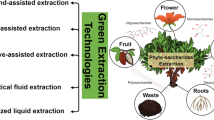
Abstract
Large amounts of agri-food by-products, non-edible food, and waste are produced throughout the supply chain from the initial production to the final consumption stages. The valorization of this biomass to obtain high value-added compounds has been the focus of extensive research in the last decade. For this purpose, the use of green techniques is essential to reduce the negative impact on the health and the environment. In this review, we discuss the use of green solvents for the valorization of agri-food waste and by-products, and we consider their potential to replace conventional organic solvents in order to provide more environmentally friendly and sustainable processes. The use of supercritical fluids, neoteric (ionic liquids and deep eutectic solvents), bio-based, and supramolecular solvents is critically dicussed. Parameters affecting extraction efficiency are detailed for each type of solvent along with advantages and limitations for application at the industrial scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agri-food waste is estimated at 5 billion tons of biomass residues per year globally [90]. Only in EU, the total annual biowaste is estimated at 76.5–102 million tonnes [61]. Nowadays, the final disposal of agri-food waste has become a major challenge for food processing industries due its potential negative impact on the environment [45]. Thus, agri-food by-products account for 3.3 billion tonnes of carbon dioxide emissions each year, globally.
The Food and Agricultural Organization (FAO) estimates that one-third of the edible food is annually wasted [49]. The valorization of non-edible crop residues is also relevant (peels, seed, leaves, pits, pulp, press cakes). Over the last years, the evaluation of these by-products as sources of biologically active compounds has attracted great interest [22] both to decrease the volume of residues and to obtain high added-value compounds [126]. Natural bioactive compounds from agri-food waste constitute a wide variety of molecules with different structures and functionalities for the production of nutraceuticals, functional foods, and cosmetics, such as polyphenols, lycopene, anthocyanins, lipids, sugars, alkaloids, proteins, dietary fibers, and flavors ([70]; see Table 1). Articles reviewing the valorization of certain industrial food waste, such as tomato [126], wine [62, 129], fruit juice [62], and olive oil [8, 111], have been reported in the last years. Other valorization activities include the production of animal feed, compost, fuel, wood-based panels, bio-fertilizers, and biofibers.
Many efforts have been devoted to find simple and inexpensive strategies for the exploitation of agri-food by-products. A variety of solvents and extraction methods, such as high pressure and temperature extraction, supercritical fluids, ultrasound-, and microwave-assisted extractions, and enzymatic treatment have been proposed in an attempt to enhance process efficiency for recovery of high added-value compounds. Organic solvents, such as diethyl ether, N,N-dimethylformamide, ethanol, hexane, toluene, and their aqueous solutions have been the main extractant phases [17]. However, many of the solvent-based extraction processes are nowadays considered inefficient because of the extended times needed to extract/purify the target compounds, the requirement of large solvent volumes per sample so that a high amount of toxic waste is generated. This waste possesses a negative impact on health, safety, and the environment [134] and consequently, the search for solvent reduction consumption and greener solvents has been strongly fostered [35, 102, 130].
Green Solvents: Potential and Limitations in the Extraction and Valorization of Agri-food Waste
Green solvents are non-toxic, non-volatile, recyclable, biodegradable, and may not involve a high energy cost of synthesis [38]. A number of alternative solvents that fulfill, to a greater or lesser extent, this definition are included in Fig. 1. They are grouped in four categories, namely, supercritical fluids, neoteric, bio-based, and supramolecular solvents. Replacement of a harmful solvent by a greener alternative in a separation process is not trivial and, in some cases, novel challenges and limitations can arise due to the different physicochemical properties of the solvents considered. In this review, we discuss briefly the extraction potential and limitations of green solvents for the valorization of agri-food waste.
Supercritical Fluids
Supercritical fluids (SCFs) are substances for which both pressure and temperature are above their critical values [19, 67]. The SCFs are characterized by gas-liquid properties, i.e., gas-like viscosity and diffusivity and liquid-like density and solvating properties. This makes them excellent solvents for extraction processes in the so-called supercritical fluid extraction, SFC [67, 105]. Thus, the fluid diffuses easily through solids and provides faster extraction yields [36]. Additionally, the SCF density can be modified by changing its pressure and/or temperature and since density is related to solubility, the solvent strength of the fluid can be modified [53]. Furthermore, the fluid solubility strength can be tuned by the addition of modifiers. This versatility makes SFCs very interesting for different applications [146].
SCFs have been extensively used in the industry and scientific literature for fractionation of products, dyeing of fibers, treatment of contaminated soils, production of powders in micro/nanometer sizes and novel chemical reactions to replace organic solvents (e.g., catalytic hydrogenation reactions typical for petrochemical industry), energy industry applications, and biofuel production [67]. The most used SCFs are water, carbon dioxide, helium, refrigerants, and hydrocarbon fuels, but health and safety benefits are especially evident in the use of supercritical CO2 and supercritical water.
Water
Water is considered as the cleanest solvent. Supercritical water exists at temperatures above 374 °C and pressures above 22.1 MPa. Supercritical water behaves as a nonpolar solvent because hydrogen bonding is lost under these extreme conditions [40]. Its use has increased during the last two decades and industrial applications have been developed looking for environment-friendly and energy-saving technologies [46, 142]. However, despite extensive research efforts, corrosion problems have not been satisfactorily solved for application at industrial scale up to now [106]. An alternative is the use of pressurized hot water extraction (PHWE) or subcritical water extraction that uses water at temperatures above its boiling point (100 °C) but below the critical point of water (374 °C, 22.1 MPa) [96, 97, 106]. A variety of applications to the extraction of bioactives have been made, such as flavonoids from onion waste [89], pectin from jackfruit peel waste [76], phenolic compounds from grape skin [63], or reducing sugars from wheat straw [1]. However, the risk of hydrolysis and other degradation reactions during extraction are major drawbacks of this technique [106].
Carbon Dioxide
Supercritical fluid (SCF) extraction with carbon dioxide has widely contributed to the development of green extraction processes for bioactive compounds [36]. CO2 is the most used because of its moderate critical temperature (31.3 °C) and pressure (7.38 MPa). CO2 is non-carcinogenic, non-toxic, non-mutagenic, non-flammable, and thermodynamically stable [67] and generally recognized as safe [53].
The applicability of SFE to high added-value compounds from vegetable matrices (agri-waste, algae, etc.) has been reviewed by several authors [19, 36, 42, 67, 94, 121]. The bioactive compounds extracted by SFE include a wide variety, such as phenolic compounds from passion fruit seeds [95], grape seeds [103], and papaya seeds [24], phytochemical compounds from soy bean expeller [6], essential oil from orange peel [141], phenols from olive oil mill waste [72], phytosterol from roselle seeds [93], limonoid glucosides from grapefruit molasses [147], solanesol from tobacco waste [138], and saponins from Agave salmiana bagasse [117] (see Table 2). Most of these studies investigate the influence of pressure and temperature in the extraction yield. Extractions are usually carried out at temperatures and pressures in the ranges 35–80 °C and 10–70 MPa, respectively. The flux ranges from 1.5 to 5000 mL CO2/min and the extraction times from 25 to 150 min. The use of experimental design is common for understanding linear and complex interactions among variables. However, as [121] pointed out, the successful application of an experimental design in SFE relies on the in-depth understanding of both SFE and experimental design techniques [121].
When compared with other extraction techniques, CO2-SFE was superior to ultrasound-assisted extraction for isolation of essential oils from orange peel extracts [141], while for more polar compounds, such as phenolics from olive oil mill waste, CO2-SFE was acceptable but less efficient than extraction with polar solvents (e.g., ethanol). In this sense, many authors propose the use of co-solvents, such as ethanol, for improving recoveries of polar and medium polar compounds [16, 21, 141]. Since CO2 is a gas with low polarity, the addition of a polar solvent (4.7–10%) improves its solubility for compounds with polar functional groups (such as vitamin E, γ-oryzanols, and xanthophylls). Another advantage of SFE processes is the fact that this technology can be easily transferred at industrial scale to extract large quantities of matrix and obtaining great amount of extract in a single step [21].
However, despite the excellent extraction properties and great versatility, the high processing costs and the complex industrial equipment are limiting factors. For example, the economical assessment of SFE into a sugarcane-microalgae biorefinery by Albarelli et al. [3] led to the conclusion that the process was not economically attractive, as it increased the total investment by 71% (respect to traditional biorefinery) and presented a very high energy demand that would lead to high operational costs [3].
Neoteric Solvents
Neoteric solvents is a term that refers to solvents structurally novel or unconventional and usually characterized by physical and chemical properties that can be finely tuned for a range of applications by varying the chemical constituents [50]. Among neoteric solvents, fluorous solvents, ionic liquids, and eutectic solvents have received the highest attention.
Fluorous solvents are made from highly fluorinated compounds, such as perfluorooctane, perfluorohexane, perfluoro (methyl cyclohexane), perfluorodecaline, perfluorotributylamnine, and perfluoropolyether [84]. They are so-called the “third liquid phase,” because of their immiscibility with both water and organic phases, which make their reuse and application easier in separation processes. Furthermore, perfluorocarbons have advantages as solvents because they are chemically unreactive, and non-flammable and have low toxicity [64]. Main drawbacks are their high cost, limited applicability to very non-polar solutes, and the concern about their sustainability due to their high environmental persistence and global warming potential (greenhouse gases) [29]. Fluorous solvents have been employed for extraction of metals and organic compounds. However, to the best of our knowledge, their applicability to the extraction of bioactive compounds for agri-waste has not been explored yet. So, in this review, we focus our discussion on ionic liquids and eutectic solvents.
Ionic Liquids
Ionic liquids (ILs) have been widely applied to the extraction of bioactive compounds [101, 133]. They are a class of salts composed of discrete cations and anions with melting points below 100 °C [52], unique physicochemical properties and pre-organized and tunable solvent structures [127]. Some of their special properties are negligible vapor pressure, excellent thermal and chemical stability, wide electrochemical potential window, and outstanding solubility for organic, inorganic, and organometallic substances. These properties, along with the extraordinary degree of tunability for both cations and anions, make ionic liquids interesting materials for extraction processes [52].
Although the use of ILs in food processes is not regulated by the Federal Drug Administration (FDA) [82], the extraction of alkaloids, terpenoids, flavonoids, phenolic compounds, saponins, etc. from natural sources (mainly plants) has been widely investigated [133]. However, their applicability to agri-food waste is somehow more limited (see Table 3). Among ILs, 1-alkyl-3-methylimidazolium-based ILs are by far the most studied and are usually combined with [BF4], Cl–, and Br– counterions. The application of greener ILs, e.g., ammonium-based cations, such as cholinium, is still scarce [133].
Regarding agri-food waste, ILs have been applied to the extraction of reducing sugars from corn stalk [74] and soybean hulls [56], levulinic acid from rice husk [65], lactic acid from deoiled cottonseed cake, wheat straw and sugarcane bagasse [47], oleanolic acid from olive tree leaves [30], cellulose from coconut husk [148], tyrosol from olive mill wastewater [73], and lignin from sugarcane bagasse [113]. The use of high temperature for extraction is usual (up to 140 °C) as well as long extraction times (2 h); additionally, ultrasonic extraction has been frequently reported. The viscosity of ILs is high and can be lowered by temperature, which is an important factor in the mass tranfer process and fluid flow [65]. Additionally, the high temperature promotes the biomass dissolution [54] and ILs are mostly thermally stable above 200 °C [65]. IL concentration and composition are the other most investigated parameters for extraction processes based on these solvents.
A special advantage of ILs for the extraction of bioactives is their ability to permeate and modify biomass cell walls and tissues and facilitate the release of compounds. Protic ILs may facilitate the hydrolysis of polysaccharides and other components for cell lysis via strong hydrogen bonding. This has been exploited for the extraction of asthaxanthin for algae and levulinic acid from lignocellulosic biomass [65, 120]. The extraction of levunilic acid also involved a catalytic process favored by acidic ILs [65]. Acidic ionic liquids have been proposed for further favoring the hydrolysis of lignocellulosic materials [74].
The versatility of ILs and the wide range of experimental conditions for its use make them very attractive for extraction processes. However, their further practical use has been limited so far, mainly due to their inherent high costs and potential toxicity. The development of more environmentally benign ILs for extraction purposes is still in its infancy [43, 101]. To reduce costs, the utilization of co-solvents, such as methanol, and solvent reuse based on the different solubility of ILs and bioactives in organic solvents and water, are available options [31]. Thus, Khan et al. [65] proposed the recycling of the IL by re-extraction of levulinic acid with ethyl acetate (in which the IL was not soluble) and solubilization of the IL in water (in which levunilic acid was not soluble). The IL was then recovered by evaporation using vacuum rotary and could be reused four times with reasonable yield. The yield of levulinic acid was between 47 and 48%. Saha et al. [113] proposed to recycle the IL and to recover lignin from soybean hulls by adding a mixture of acetone: water (1:1 v/v) to the bagasse:ionic liquid solution 10:1 (v/v). This caused the precipitation of the cellulosic material and left a filtrate solution containing lignin and the IL. Ligning was recovered after evaporation of acetone and the IL was obtained after the further evaporation of water under vacuum. The yield of lignin for the whole process was 90.1% and the efficient recovery of the IL was proved by thermogravimetric analysis.
Deep Eutectic Solvents
Deep eutectic solvents (DESs) were developed to overcome the environmental issues of ILs [43]. They have physical and chemical properties comparable with ionic liquids, but they are easier to synthesize and more stable and cost-competitive and, typically, most of them are environmentally friendly [119, 150]. DESs have shown a great potential in emerging green extraction technologies and they are expected to be widely transferred to industry in coming years [4].
DESs are eutectic mixtures of Lewis or Brønsted acids and bases which can contain a variety of anionic and/or cationic species [123]. They are usually produced by the complexation of a quaternary ammonium salt with a metal salt or hydrogen bond donor. The charge delocalization trough the hydrogen bonding results in a decrease of the melting point of the mixture. This is due to the fact that DESs consist of large, non-symmetric ions with low lattice energy and hence, low melting points [123].
DESs are prepared by simply mixing the components and are classified depending on the nature of the complexing agent into four categories (see Fig. 2). They can be composed of a quaternary ammonium salt and a metal chloride (type I), a metal chloride hydrate (type II) or a hydrogen bond donor (type III) and of a hydrogen bond donor and a metal chloride (type IV). A range of hydrogen bond donors have been studied such as amides, carboxylic acids, and alcohols [123].
DES clasification. Adapted from Smith et al. [123]
One of the attractive features of DES is their tunability. Thus, a huge number of eutectic mixtures with varying viscosity, density, miscibility, and polarity can be obtained by simply changing one or both components in the mixture. In this way, DESs can be easily tailored for specific applications including extraction processes [33, 59, 130].
Regarding the applicability of DESs in the valorization of agri-waste, type III DESs have been the most studied and have the greater potential in biomass processing due to their quick and easy preparation, non-reactivity with water, biodegradable nature, and cost effectiveness [80, 123]. The most used DES has been made up of choline chloride (ChCl) mixed with different chemical functional groups such as amine, alcohol, acid, and sugar, which act as hydrogen bond donors. Choline is non-toxic, have low cost, and is classified as a provitamin in Europe [123].
DESs have been reported for the extraction of tocols from crude palm oil [51], anthocyanins from wine [15, 109], genistin, genistein and apigenin from Pigeon pea roots [32], and lignin from rice straw [55, 69] and anthocyanins from grape pomace [100]. Polyphenols have been extracted from lemon peels, olive leaves, onion solid wastes, red grape pomace and wheat bran [87, 98], grape skins [109], Cajanus cajan leaves [140], Morus alba L. leaves [152], olive pomace [26], and spent coffee grounds [145]. The extraction time and yield for the bioactives varied according to the type of DES, the structure of the bio-compound, the extraction temperature applied and the use of auxiliary energy (such as microwave or ultrasound). Extraction times varied from 11 min to 24 h with temperatures in the range 40–90 °C and frequent dilution with water (5–30 % w/w). Table 4 lists valorization processes of agri-waste with DESs.
The physicochemical properties of DESs greatly influence extraction rates [149]. Polarity and viscosity are two very influencial factors when optimizing the extraction of bioactive compounds with DESs. The high viscosity of DES is a major disadvantage since it reduces the mass transfer of bioactive compounds. Viscosity can be lowered by increasing the temperature at which extraction occurs and by mixing DES with water. For instance, in the case of DESs made up of ChCl:glycerol (1:1), the viscosity decreased by 1/5 at 5% of water and to 1/80 at 20% of water [149]. Additionally, the polarity of DES increased along with the water content [57]. Different hydrogen bond donors (i.e., sugars, polyhydric alcohols, and organic acids) were tested by Cui et al. [32] to lower viscosity and increase polarity of choline-based DESs in the extraction of genistin, genistein, and apigenin from pigeon pea root [32]. The viscosity of DESs with sugars was the greatest while the polarity was higher for sugars and polyhydric alcohols compared with organic acids. Finally, DESs made up of 30% water in 1,6-hexanediol/ChCl (7:1, mol/mol) were selected as optimal. Microwave-assisted extraction and 80 °C were applied to enhance the extraction yield.
Procedures for the recovery of DES and bioactives with solvent back-extraction, such as a washing step with water:ethanol for ChCl:glycerol enriched with glucose and xylose and further drying at 38 °C, have been proposed [108]. In this way, the yield of glucose and xylose were in the ranges 91.5–92.3% and 59.5–95.5 %, respectively. Hadi et al. [51] investigated the reuse of other chloine-based DES after extraction of tocols from crude palm oil. A mixture of water–hexane (4:1 v/v) was employed for liquid-liquid separation. The hexane layer contained the tocols that were later recovered by evaporation at 60 °C. The DES-rich layer, which contained a mixture of methanol, water, and traces of hexane, was dried to remove methanol and water (15 h). The yield of the recycled DES decreased from 18,525 ± 882 to 11,741 ± 566 mg/kg (total tocols concentration). Other procedures have been described for the recovery of DES after extraction. Ruesgas-Ramón et al. [112] reviewed the use of DES for the extraction of phenolic compounds from plants. Authors reported that the use of solid-phase extraction was also a common strategy for the recovery of DES by using different types of resins (e.g., ME-2 polystyrene matrix, XAD-16 styrene–divinylbenzene). Once the extract was loaded, DES was recovered with water while a second elution step with ethanol or methanol was employed to recover the phenolic compounds. Finally, the addition of an anti-solvent for the bioactive compounds, usually water, was used to strongly dilute the DES and break the supramolecular interactions between components (losing of DES’ solvation properties) which led to the precipitation of the extracted compounds.
Bio-based Solvents
Bio-based solvents are defined as solvents produced from renewable biomass sources such as energy crops, forest products, aquatic biomass, and waste materials [90]. They are produced in a biorefinery [137] which aims for the maximum recovery and production of high added-value products [23]. Some bio-based solvents are alcohols (ethanol), esters (ethyl lactate), glycerols, terpenes, furfurals (furfural, furfural alcohol, levulinic acid), and furan [75]. Viscosities are low, which make them easy to handle in extraction processes. Despite their great potential, the scale of biorefineries is still mainly limited to lab-scale or pilot plants [137]. However, some of them are already commercially available.
Alcohols
The first generation of bio-based ethanol was derived from sources like starch, sugar, animal fats, and vegetable oil. The main problem was the food-versus-fuel debate [99]. The second generation was produced from a non-food biomass, such as lignocellulosic materials. The third generation was derived from microalgae [99]. Methanol can also be produced from biomass, but it has toxicity issues [137]. Other bio-alcohols with low toxicity are bio-butanol, bio-2-octanol, bio-1,3-propanediol, and bio-1,3-butanediol [20]. On the other hand, glycerol has been widely obtained as by-product in biodiesel production [137].
Esters
Ethyl acetate is an industrially relevant ester, non-toxic, and fully biodegradable [25]. This bio-solvent is mainly produced by esterification of acetic acid and ethanol in liquid or vapor phase, acetylation of ethylene, and ethanol dehydrogenation [114]. Yeasts, such as Saccharomyces cerevisiae, Wickerhamomyces anomalus, and Kluyveromyces marxianus can also convert sugar into ethyl acetate [68]. Ethyl lactate is widely used as a green solvent to replace chlorinated hydrocarbons [104]. It is very suitable and environmental benign for food applications. It is also allowed as pharmaceutical and food additive by the FDA [14].
Terpenes
α-Pinene is a bicyclic monoterpene hydrocarbon and is one of the most abundant components in the essential oils of various plant species [66]. It has potential for the pharmaceutical, bioenergy, fine chemistry, and flavor industries [91]. D-Limonene is a colorless liquid cyclic terpene extracted from orange peels in orange juice industry. It is widely accepted for cosmetics and food [28]. Finally, p-cymene is another bio-based molecule. It is used for the synthesis of p-cresol and fine chemicals for perfumes, fungicides, and pesticides and as a solvent of dyes and varnishes [81]. It can be obtained for conversion of limonene into p-cymene, also is present in pine trees [143].
Extraction of Compounds from Agroindustrial By-products Using Bio-based Solvents
The extraction of bioactive compounds from agri-food waste with bio-based solvents have been applied in a lesser extent than with SCFs. Studies are mainly focused on extraction from algae or natural resources (not residues) [13, 14, 39, 135]. Table 5 shows research studies concerning the use bio-based solvents to extract bioactive compounds from agri-waste.
Bio-based solvents have been used to extract rosmarinic and caffeic acids from basil wastewater [96, 97], carotenoids and phenols from tomato waste [44, 122, 125, 127], polyphenols, flavonoids, anthocyanins and ellagic acid from pomegranate peel [83], phenolic compounds, flavonoids and sinapine from seeds of rapeseed, mustard crambe and sunflower [85], phenolic compounds from lotus by-products [58], oil from rice bran [77], and volatile compounds from Cooperage woods in winemaking [2]. Ethyl lactate and ethyl acetate, sometimes in mixtures with water, have been by far the most used bio-based solvents. It is usual to employ high temperatures (usually 30–80 °C) and repetitive extractions to reach adequate recovery of bioactives, which is highly dependent on extraction time and the presence (or not) of auxiliary energy such as microwave or ultrasound.
Bio-based solvents have been reported to extract bioactive compounds as efficiently (or with higher efficiency) than conventional organic solvents. In the extraction of rice bran oil, the use of D-limonene showed superior extraction yield (24.6%) than hexane (18.6%). Similarly, in olive oil extraction, the use of D-limonene increase the lipid yield in 8.3% more than hexane [136]. Yara-Varón et al. also reported that cis-pinane and d-limonene extracted more carotenoids from carrot than n-hexane (95.4, 94.8 and 78.1% respectively) [144]. Commonly, energy-assisted extraction techniques are used for enhancing recoveries. Thus, ultrasound extraction increased in 9.4% the lycopene yield in tomato pomace with ethyl lactate–ethyl acetate mixtures [122]. Also, pressurized liquid extraction was suitable for the extraction of phenolic compounds from basil waste using mixtures of water (75% v/v) and ethanol or ethyl lactate at 150 °C, with extraction rates up to 93.9 an 99.2% respectively [96, 97].
Supramolecular Solvents
Supramolecular solvents (SUPRASs) are nanostructured liquids produced in colloidal suspensions of amphiphiles by spontaneous, sequential phenomena of self-assembly, and coacervation [18]. Coacervation is defined as “the separation into two liquid phases in colloidal systems. The phase more concentrated in colloid component is the coacervate, and the other phase is the equilibrium solution” [60].
These nanostructured liquids have been used for extraction since Watanabe and Tanaka in 1978 developed a method to extract zinc using “a micellar solution of a non-ionic surfactant that separates in two phases” also known as the cloud point technique [139]. The name SUPRAS was introduced later, to highlight the differences between these liquid phases and molecular and ionic solvents, to underline the nanostructures formed by non-covalent interactions and to emphasize the synthesis process, which is based on amphiphile self-assembly [12].
The SUPRAS synthesis is made in two steps. First, “an aqueous or organic colloidal suspension of the amphiphile is prepared above its critical aggregation concentration.” This suspension contains supramolecular aggregates, typically aqueous or reverse micelles or vesicles [12]. The formation of these architectures primarily depends on the packing parameter, which in turn depends of the volume and the length of the hydrophobic segment and the cross-sectional area of the head group [79].
In the second step, the generated nanostructures self-assembly in larger aggregates by the action of an external stimulus (coacervating agent) that diminishes the repulsion among the aggregates [118] and separate from the bulk solution as an immiscible liquid via coacervation ([11], p.; [110]). The most used stimulus for the coacervation are pH, temperature, inorganic, and organic salts and poor solvents for the amphiphile [12] (see Fig. 3).
Supramolecular solvents have a unique array of physicochemical properties that render them very attractive to replace conventional organic solvents in extractions [11]. Thus, SUPRAS offer mixed-mechanisms for solute solubilization and produce high extractions rates for solutes covering a wide polarity range. Multiple binding interactions are available which depends on the nature of the amphiphile [11], and due to its internal structure, different polarity regions are generated [12]. Another important characteristic is that they can be tailored to offer programmed characteristics such as molecular-restricted access behavior [10].
SUPRASs have proved high efficiency for the separation, preconcentration, or purification of organic compounds such as such as polycyclic aromatic hydrocarbons, pesticides, surfactants, bioactive compounds and dyes [12, 18]. In terms of green chemistry, they are good alternatives to the conventional extraction systems because of their high performance, low toxicity, and low cost [12, 18, 78, 115]. Furthermore, they are non-volatile and non-flammable and many amphiphiles are bio-compatible and renewable, such as carboxylic acids and rhamnolipids. In summary, sustainable and economical SUPRAS-based extraction processes can be implemented taking into account that the synthesis can be developed with green natural amphiphiles at low cost and thought energyless processes [12].
Despite their great potential, only a few studies have been related to the extraction of bioactives from agroindustrial by-products (Table 6). These studies have focused on the extraction of polyphenols from wine sludge [27], betaine from beet molasses [86], saponins from sisal (Agave sisalana) waste [41], and anthraquinones from aloe peel [128].
The most used amphiphiles were non-ionic surfactants from the Triton X series and the most employed coacervating agent was the temperature. High recoveries have been reported with these solvents. Good recoveries have been also obtained with other non-ionic surfactants, such as those reported by Chatzilazarou et al. [27] [27]. Thus, recoveries found for phenol from wine sludge were 98.5% using PEG 8000 as amphiphile (at pH 2.5, 55 °C) in a fast process that took 30 min. On the other hand, Ribeiro et al. [41] found that SUPRASs were superior for extraction of saponins from sisal waste (98.4%) compared with an ethanolic solution 30% v/v (38.6%) under the same conditions of time (4 h), temperature (50 °C) and sample mass/volume ratio (0.17 g/mL) [41].
Recently, SUPRAS made up of inverse aggregates of 1-hexanol in mixtures ethanol:water have been proposed for the recovery of alkaloids and polyphenols from spent coffee grounds [131]. In this case, the coacervating agent was water (poor solvent for the amphiphile) and the extraction was rapid (1 min) and made at room temperature. SUPRAS components (1-hexanol, ethanol, and water) are authorized for food processing or as food additives so that further industrial implementation is facilitated. Furthermore, extracts showed good antioxidant and antimicrobial properties.
The recovery of bioactives from the surfactant-rich phase has been investigated by some authors. Thus, Mohammadzadeh et al. [86] proposed the back-extraction of betaine (nearly 100%) from beet molasses from the surfactant-rich phase with an aqueous phase at pH 2.5. The recovery of bioactives from the surfactant-rich phase by a change of pH in aqueous solution was also proposed by Tan et al. [128] for the recovery of anthraquinones from aloe peel with an efficiency of 70%.
Future Perspectives
This review aimed to provide an overview of the application of green solvents for the extraction of different classes of bioactive compounds from agri-food waste, mainly small organic extractable compounds (phenolic compounds, carotenoids, tocols, among others) and other high added-value compounds (fermentable sugars, lignin, oils, etc.). Research in this area is increasing in the last years and constitutes an urgent demand since disposal of agri-waste represents both cost and potential negative impact on the environment. In general, it can be concluded that if properly selected, green solvents are able to afford high extraction yields in different agri-food wastes. The sustainable character and costs associated with the extraction depend on the selected solvent, the source of bioactive compound, the temperature and processing time and the presence—or not—of assisted extraction modes, such as the use of microwave, ultrasound, or the use of re-flux.
Despite the efforts made by different authors to develop alternative green solvents and to evaluate different extraction approaches and conditions, many studies are still based on ionic liquids and SFCs. However, the use of SCFs is too expensive and the toxicity of ILs is controversial. Bio-based solvents, natural deep eutectic solvents (NADES) and supramolecular solvents appear to be a more promising and greener option due to their bio-compatibility and low toxicity. The term NADES refers to deep eutectic solvents synthetized from natural compounds, i.e., choline chloride, mixed with natural acids, amines, and alcohols [37]. For these non-volatile (or hardly volatile) green solvents, strategies for the recovery or back-extraction and concentration of bioactives are key for their implementation at industrial scale. However, only few studies investigate possible procedures. Kumar et al. [71] evaluated a biorefinery process for ethanol production from cellulose coming from rice straw. NADES was used as a pretreatment step for delignification, recovery of high purity lignin and xylan, enzymatic hydrolysis, and production of cellulosic ethanol. The study concluded that the proposed biorefinery was effective and economically viable mainly based on the possibility of solvent recovery and reuse, the cheap and energyless synthesis of NADES (lactic acid + choline chloride + water) and the coextraction of value-added products.
The evaluation of the economic viability and implementation at industrial scale are necessary to broaden the applicability for green solvents. Furthermore, studies covering the comparison of different types of green solvents for the same application or of a green solvent with conventional ones would be desirable to further understand the advantages and disadvantages of the different strategies. The development of cost-effective and more sustainable extraction and separation processes is the critical step toward the recovery and commercialization of new and low-cost bioactive products for the nutraceutical, cosmetic, and pharmaceutical sectors. Research in extraction processes with green solvents needs to take into account in the near future: (i) the life cycle analysis of their processes and products, (ii) processes able to be scaled-up, and (iii) economic analyses of the extraction process, solvent, and material costs.
References
Abdelmoez W, Nage SA, Bastawess A et al (2014) Subcritical water technology for wheat straw hydrolysis to produce value added products. J Clean Prod 70:68–77. https://doi.org/10.1016/j.jclepro.2014.02.011
Alañón ME, Alarcón M, Marchante L et al (2017) Extraction of natural flavorings with antioxidant capacity from cooperage by-products by green extraction procedure with subcritical fluids. Ind Crop Prod 103:222–232. https://doi.org/10.1016/j.indcrop.2017.03.050
Albarelli JQ, Santos DT, Ensinas AV et al (2018) Comparison of extraction techniques for product diversification in a supercritical water gasification-based sugarcane-wet microalgae biorefinery: thermoeconomic and environmental analysis. J Clean Prod 201:697–705. https://doi.org/10.1016/j.jclepro.2018.08.137
Alonso DA, Baeza A, Chinchilla R et al (2016) Deep eutectic solvents: the organic reaction medium of the century. Eur J Org Chem 2016:612–632. https://doi.org/10.1002/ejoc.201501197
Alvarez VH, Cahyadi J, Xu D et al (2014) Optimization of phytochemicals production from potato peel using subcritical water: experimental and dynamic modeling. J Supercrit Fluids 90:8–17. https://doi.org/10.1016/j.supflu.2014.02.013
Alvarez MV, Cabred S, Ramirez CL, Fanovich MA (2019) Valorization of an agroindustrial soybean residue by supercritical fluid extraction of phytochemical compounds. J Supercrit Fluids 143:90–96. https://doi.org/10.1016/j.supflu.2018.07.012
Apostolakis A, Grigorakis S, Makris DP (2014) Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Sep Purif Technol 128:89–95. https://doi.org/10.1016/j.seppur.2014.03.010
Araújo M, Pimentel FB, Alves RC, Oliveira MBPP (2015) Phenolic compounds from olive mill wastes: health effects, analytical approach and application as food antioxidants. Trends Food Sci Technol 45:200–211. https://doi.org/10.1016/j.tifs.2015.06.010
Arun KB, Chandran J, Dhanya R et al (2015) A comparative evaluation of antioxidant and antidiabetic potential of peel from young and matured potato. Food Biosci 9:36–46. https://doi.org/10.1016/j.fbio.2014.10.003
Ballesteros-Gómez A, Rubio S (2012) Environment-responsive alkanol-based supramolecular solvents: characterization and potential as restricted access property and mixed-mode extractants. Anal Chem 84:342–349. https://doi.org/10.1021/ac2026207
Ballesteros-Gómez A, Sicilia MD, Rubio S (2010) Supramolecular solvents in the extraction of organic compounds. A review. Anal Chim Acta 677:108–130. https://doi.org/10.1016/j.aca.2010.07.027
Ballesteros-Gómez A, Lunar L, Sicilia MD, Rubio S (2018) Hyphenating supramolecular solvents and liquid chromatography: tips for efficient extraction and reliable determination of organics. Chromatographia 82:1–14. https://doi.org/10.1007/s10337-018-3614-1
Ben-Youssef S, Fakhfakh J, Breil C et al (2017) Green extraction procedures of lipids from Tunisian date palm seeds. Ind Crop Prod 108:520–525. https://doi.org/10.1016/j.indcrop.2017.07.010
Bermejo DV, Luna P, Manic MS et al (2013) Extraction of caffeine from natural matter using a bio-renewable agrochemical solvent. Food Bioprod Process 91:303–309. https://doi.org/10.1016/j.fbp.2012.11.007
Bosiljkov T, Dujmić F, Cvjetko Bubalo M et al (2017) Natural deep eutectic solvents and ultrasound-assisted extraction: green approaches for extraction of wine lees anthocyanins. Food Bioprod Process 102:195–203. https://doi.org/10.1016/j.fbp.2016.12.005
Braga MEM, Santos RMS, Seabra IJ et al (2008) Fractioned SFE of antioxidants from maritime pine bark. J Supercrit Fluids 47:37–48. https://doi.org/10.1016/j.supflu.2008.05.005
Byrne FP, Jin S, Paggiola G, Petchey THM, Clark JH, Farmer TJ, Hunt AJ, Robert McElroy C, Sherwood J (2016) Tools and techniques for solvent selection: green solvent selection guides. Sustain Chem Process 4:7–24. https://doi.org/10.1186/s40508-016-0051-z
Caballo C, Sicilia MD, Rubio S (2017) Chapter 5 - supramolecular solvents for green chemistry. In: Pena-Pereira F, Tobiszewski M (eds) the application of green solvents in separation processes, 1st edn. Elsevier, pp 111–137
Cabeza LF, de Gracia A, Fernández AI, Farid MM (2017) Supercritical CO2 as heat transfer fluid: a review. Appl Therm Eng 125:799–810. https://doi.org/10.1016/j.applthermaleng.2017.07.049
Calvo-Flores FG, Monteagudo-Arrebola MJ, Dobado JA, Isac-García J (2018) Green and bio-based solvents. Top Curr Chem 376:18. https://doi.org/10.1007/s41061-018-0191-6
Campone L, Celano R, Lisa Piccinelli A, Pagano I, Carabetta S, Sanzo RD, Russo M, Ibañez E, Cifuentes A, Rastrelli L (2018) Response surface methodology to optimize supercritical carbon dioxide/co-solvent extraction of brown onion skin by-product as source of nutraceutical compounds. Food Chem 269:495–502. https://doi.org/10.1016/j.foodchem.2018.07.042
Carciochi RA, D’Alessandro LG, Vauchel P, et al. (2017) Chapter 4 - valorization of agrifood by-products by extracting valuable bioactive compounds using green processes. In: Grumezescu AM, Holban AM (eds) Ingredients extraction by physicochemical methods in food. Academic Press, pp 191–228. https://doi.org/10.1016/B978-0-12-811521-3.00004-1
Carmona-Cabello M, Garcia IL, Leiva-Candia D, Dorado MP (2018) Valorization of food waste based on its composition through the concept of biorefinery. Curr Opin Green Sustain Chem 14:67–79. https://doi.org/10.1016/j.cogsc.2018.06.011
Castro-Vargas HI, Baumann W, Ferreira SRS, Parada-Alfonso F (2019) Valorization of papaya (Carica papaya L.) agroindustrial waste through the recovery of phenolic antioxidants by supercritical fluid extraction. J Food Sci Technol-Mysore 56:3055–3066. https://doi.org/10.1007/s13197-019-03795-6
Chan W-C, Su M-Q (2008) Biofiltration of ethyl acetate and amyl acetate using a composite bead biofilter. Bioresour Technol 99:8016–8021. https://doi.org/10.1016/j.biortech.2008.03.045
Chanioti S, Tzia C (2018) Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innovative Food Sci Emerg Technol 48:228–239. https://doi.org/10.1016/j.ifset.2018.07.001
Chatzilazarou A, Katsoyannos E, Gortzi O, Lalas S, Paraskevopoulos Y, Dourtoglou E, Tsaknis J (2010) Removal of polyphenols from wine sludge using cloud point extraction. J Air Waste Manage Assoc 60:454–459. https://doi.org/10.3155/1047-3289.60.4.454
Chemat S, Tomao V, Chemat F (2012) Limonene as green solvent for extraction of natural products. In: Mohammad A (ed) Green solvents I: properties and applications in chemistry. Springer Netherlands, Dordrecht, pp 175–186
Clark JH, Tavener SJ (2007) Alternative solvents: shades of green. Org Process Res Dev 11:149–155. https://doi.org/10.1021/op060160g
Cláudio AFM, Cognigni A, de Faria ELP, Silvestre AJD, Zirbs R, Freire MG, Bica K (2018) Valorization of olive tree leaves: extraction of oleanolic acid using aqueous solutions of surface-active ionic liquids. Sep Purif Technol 204:30–37. https://doi.org/10.1016/j.seppur.2018.04.042
Cooney MJ, Benjamin K (2016) Ionic Liquids in Lipid Extraction and Recovery. In: Xu X, Guo Z, Cheong L-Z (eds) Ionic Liquids in Lipid Processing and Analysis. AOCS Press, pp 279–316. https://doi.org/10.1016/B978-1-63067-047-4.00009-X
Cui Q, Peng X, Yao X-H et al (2015) Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep Purif Technol 150:63–72. https://doi.org/10.1016/j.seppur.2015.06.026
Cunha SC, Fernandes JO (2018) Extraction techniques with deep eutectic solvents. TrAC Trends Anal Chem 105:225–239. https://doi.org/10.1016/j.trac.2018.05.001
Cvjetko Bubalo M, Ćurko N, Tomašević M, Kovačević Ganić K, Radojčić Redovniković I (2016) Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem 200:159–166. https://doi.org/10.1016/j.foodchem.2016.01.040
Cvjetko Bubalo M, Vidović S, Radojčić Redovniković I, Jokić S (2018) New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod Process 109:52–73. https://doi.org/10.1016/j.fbp.2018.03.001
da Silva RPFF, Rocha-Santos TAP, Duarte AC (2016) Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal Chem 76:40–51. https://doi.org/10.1016/j.trac.2015.11.013
Dai Y, van Spronsen J, Witkamp GJ, Verpoorte R, Choi YH (2013) Natural deep eutectic solvents as new potential media for green technology. Anal Chim Acta 766:61–68. https://doi.org/10.1016/j.aca.2012.12.019
Das S, Mondal A, Balasubramanian S (2017) Recent advances in modeling green solvents. Curr Opin Green Sustain Chem 5:37–43. https://doi.org/10.1016/j.cogsc.2017.03.006
de Jesus SS, Ferreira GF, Fregolente LV, Maciel Filho R (2018) Laboratory extraction of microalgal lipids using sugarcane bagasse derived green solvents. Algal Res 35:292–300. https://doi.org/10.1016/j.algal.2018.09.001
DeSimone JM (2002) Practical Approaches to Green Solvents. Science 297:799–803. https://doi.org/10.1126/science.1069622
Dias Ribeiro B, Weingart Barreto D, Zarur Coelho MA (2015) Use of micellar extraction and cloud point preconcentration for valorization of saponins from sisal (Agave sisalana) waste. Food Bioprod Process 94:601–609. https://doi.org/10.1016/j.fbp.2014.07.004
Djas M, Henczka M (2018) Reactive extraction of carboxylic acids using organic solvents and supercritical fluids: a review. Sep Purif Technol 201:106–119. https://doi.org/10.1016/j.seppur.2018.02.010
Dominguez de Maria P (2017) Ionic liquids, switchable solvents and eutectic mixtures. In: Green solvents. Elsevier, Amsterdam, p 533
El-Malah MH, Hassanein MM, Helmy Areif M, Al-Amrousi EF (2015) Utilization of Egyptian tomato waste as a potential source of natural antioxidants using solvents, microwave and ultrasound extraction methods. Am J Food Technol 10:14–25. https://doi.org/10.3923/ajft.2015.14.25
Galanakis CM (2015) Food waste recovery. processing technologies and industrial techniques. Elsevier
Gorbaty Y, Bondarenko GV (2017) Transition of liquid water to the supercritical state. J Mol Liq 239:5–9. https://doi.org/10.1016/j.molliq.2016.06.040
Grewal J, Khare SK (2018) One-pot bioprocess for lactic acid production from lignocellulosic agro-wastes by using ionic liquid stable Lactobacillus brevis. Bioresour Technol 251:268–273. https://doi.org/10.1016/j.biortech.2017.12.056
Grigoras CG, Destandau E, Fougère L, Elfakir C (2013) Evaluation of apple pomace extracts as a source of bioactive compounds. Ind Crop Prod 49:794–804. https://doi.org/10.1016/j.indcrop.2013.06.026
Gustavsson J, Cederberg C, Sonesson U et al (2011) Global food losses and food waste. Food and Agriculture Organization of the United Nations, Rome
Gutiérrez-Arnillas E, Álvarez MS, Deive FJ et al (2016) New horizons in the enzymatic production of biodiesel using neoteric solvents. Renew Energy 98:92–100. https://doi.org/10.1016/j.renene.2016.02.058
Hadi NA, Ng MH, Choo YM et al (2015) Performance of choline-based deep eutectic solvents in the extraction of tocols from crude palm oil. J Am Oil Chem Soc 92:1709–1716. https://doi.org/10.1007/s11746-015-2720-6
Henderson RK, Jiménez-González C, Constable DJC et al (2011) Expanding GSK’s solvent selection guide – embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem 13:854–862. https://doi.org/10.1039/C0GC00918K
Herrero M, Cifuentes A, Ibañez E (2006) Sub- and supercritical fluid extraction of functional ingredients from different natural sources: plants, food-by-products, algae and microalgae: a review. Food Chem 98:136–148. https://doi.org/10.1016/j.foodchem.2005.05.058
Hou Q, Li W, Ju M, Liu L, Chen Y, Yang Q, Wang J (2015) Separation of polysaccharides from rice husk and wheat bran using solvent system consisting of BMIMOAc and DMI. Carbohydr Polym 133:517–523. https://doi.org/10.1016/j.carbpol.2015.07.059
Hou X-D, Lin K-P, Li A-L et al (2018) Effect of constituents molar ratios of deep eutectic solvents on rice straw fractionation efficiency and the micro-mechanism investigation. Ind Crop Prod 120:322–329. https://doi.org/10.1016/j.indcrop.2018.04.076
Hu XM, Zhang BX, Dong SJ et al (2014) Hydrolisis of soybean by-products to prepare reducing sugar in ionic liquids. Asian J Chem 26:8475–8478
Huang Y, Feng F, Jiang J, Qiao Y, Wu T, Voglmeir J, Chen ZG (2017) Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem 221:1400–1405. https://doi.org/10.1016/j.foodchem.2016.11.013
Huang H, Belwal T, Jiang L et al (2019) Valorization of lotus byproduct (Receptaculum Nelumbinis) under green extraction condition. Food Bioprod Process 115:110–117. https://doi.org/10.1016/j.fbp.2019.03.006
Huddleston JG, Willauer HD, Swatloski RP et al (1998) Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction. Chem Commun:1765–1766. https://doi.org/10.1039/A803999B
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. https://doi.org/10.1351/goldbook
Jablonský M, Škulcová A, Malvis A, Šima J (2018) Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J Biotechnol 282:46–66. https://doi.org/10.1016/j.jbiotec.2018.06.349
Kammerer DR, Kammerer J, Valet R, Carle R (2014) Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res Int 65:2–12. https://doi.org/10.1016/j.foodres.2014.06.012
Kashtiban AE, Esmaiili M (2019) Extraction of phenolic compounds from Siah-Sardasht grape skin using subcritical water and ultrasound pretreatment. J Food Process Preserv 43:e14071. https://doi.org/10.1111/jfpp.14071
Kerton F (2009) Alternative solvents for green chemistry. Royal Society of Chemistry, Cambridge
Khan AS, Man Z, Bustam MA, Nasrullah A, Ullah Z, Sarwono A, Shah FU, Muhammad N (2018) Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr Polym 181:208–214. https://doi.org/10.1016/j.carbpol.2017.10.064
Kim M, Sowndhararajan K, Park SJ, Kim S (2018) Effect of inhalation of isomers, (+)-α-pinene and (+)-β-pinene on human electroencephalographic activity according to gender difference. Euro J Integr Med 17:33–39. https://doi.org/10.1016/j.eujim.2017.11.005
Knez Ž, Markočič E, Leitgeb M et al (2014) Industrial applications of supercritical fluids: a review. Energy 77:235–243. https://doi.org/10.1016/j.energy.2014.07.044
Kruis AJ, Levisson M, Mars AE, van der Ploeg M, Garcés Daza F, Ellena V, Kengen SWM, van der Oost J, Weusthuis RA (2017) Ethyl acetate production by the elusive alcohol acetyltransferase from yeast. Metab Eng 41:92–101. https://doi.org/10.1016/j.ymben.2017.03.004
Kumar AK, Parikh BS, Pravakar M (2016) Natural deep eutectic solvent mediated pretreatment of rice straw: bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ Sci Pollut Res 23:9265–9275. https://doi.org/10.1007/s11356-015-4780-4
Kumar K, Yadav AN, Kumar V et al (2017) Food waste: a potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour Bioprocess 4:18–14. https://doi.org/10.1186/s40643-017-0148-6
Kumar AK, Sharma S, Shah E, Patel A (2018) Technical assessment of natural deep eutectic solvent (NADES) mediated biorefinery process: a case study. J Mol Liq 260:313–322. https://doi.org/10.1016/j.molliq.2018.03.107
Lafka T-I, Lazou AE, Sinanoglou VJ, Lazos ES (2011) Phenolic and antioxidant potential of olive oil mill wastes. Food Chem 125:92–98. https://doi.org/10.1016/j.foodchem.2010.08.041
Larriba M, Omar S, Navarro P et al (2016) Recovery of tyrosol from aqueous streams using hydrophobic ionic liquids: a first step towards developing sustainable processes for olive mill wastewater (OMW) management. RSC Adv 6:18751–18762. https://doi.org/10.1039/C5RA26510J
Li C, Wang Q, Zhao ZK (2008) Acid in ionic liquid: an efficient system for hydrolysis of lignocellulose. Green Chem 10:177–182. https://doi.org/10.1039/B711512A
Li Z, Smith KH, Stevens GW (2016) The use of environmentally sustainable bio-derived solvents in solvent extraction applications—a review. Chin J Chem Eng 24:215–220. https://doi.org/10.1016/j.cjche.2015.07.021
Li WJ, Fan ZG, Wu YY, Jiang ZG, Shi RC (2019) Eco-friendly extraction and physicochemical properties of pectin from jackfruit peel waste with subcritical water. J Sci Food Agric 99:5283–5292. https://doi.org/10.1002/jsfa.9729
Liu SX, Mamidipally PK (2005) Quality comparison of rice bran oil extracted with d-limonene and hexane. Cereal Chem 82:209–215. https://doi.org/10.1094/CC-82-0209
Liu W, Zhao W, Chen J, Yang M (2007) A cloud point extraction approach using Triton X-100 for the separation and preconcentration of Sudan dyes in chilli powder. Anal Chim Acta 605:41–45. https://doi.org/10.1016/j.aca.2007.10.034
Liu Y, Liu B, Nie Z (2015) Concurrent self-assembly of amphiphiles into nanoarchitectures with increasing complexity. Nano Today 10:278–300. https://doi.org/10.1016/j.nantod.2015.04.001
Loow Y-L, New EK, Yang GH et al (2017) Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion. Cellulose 24:3591–3618. https://doi.org/10.1007/s10570-017-1358-y
Lycourghiotis S, Makarouni D, Kordouli E et al (2018) Activation of natural mordenite by various acids: characterization and evaluation in the transformation of limonene into p-cymene. Molec Catal 450:95–103. https://doi.org/10.1016/j.mcat.2018.03.013
Martins PLG, Braga AR, de Rosso VV (2017) Can ionic liquid solvents be applied in the food industry? Trends Food Sci Technol 66:117–124. https://doi.org/10.1016/j.tifs.2017.06.002
Masci A, Coccia A, Lendaro E, Mosca L, Paolicelli P, Cesa S (2016) Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem 202:59–69. https://doi.org/10.1016/j.foodchem.2016.01.106
Matsuda H, Hirota Y, Kurihara K et al (2013) Liquid–liquid equilibria containing fluorous solvents as environmentally benign solvent. Fluid Phase Equilib 357:71–75. https://doi.org/10.1016/j.fluid.2012.12.037
Matthäus B (2002) Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem 50:3444–3452. https://doi.org/10.1021/jf011440s
Mohammadzadeh M, Honarvar M, Zarei AR, Mashhadi Akbar Boojar M, Bakhoda H (2018) A new approach for separation and recovery of betaine from beet molasses based on cloud point extraction technique. J Food Sci Technol 55:1215–1223. https://doi.org/10.1007/s13197-017-2999-4
Mouratoglou E, Malliou V, Makris DP (2016) Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valor 7:1377–1387. https://doi.org/10.1007/s12649-016-9539-8
Mourtzinos I, Anastasopoulou E, Petrou A, Grigorakis S, Makris D, Biliaderis CG (2016) Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J Food Sci Technol 53:3939–3947. https://doi.org/10.1007/s13197-016-2381-y
Munir MT, Kheirkhah H, Baroutian S et al (2018) Subcritical water extraction of bioactive compounds from waste onion skin. J Clean Prod 183:487–494. https://doi.org/10.1016/j.jclepro.2018.02.166
Naidu DS, Hlangothi SP, John MJ (2018) Bio-based products from xylan: a review. Carbohydr Polym 179:28–41. https://doi.org/10.1016/j.carbpol.2017.09.064
Ndongou Moutombi FJ, Selka A, Fabiano-Tixier A-S et al (2018) Highly selective solvent-free hydrogenation of pinenes to added-value cis-pinane. Comptes Rendus Chimie. https://doi.org/10.1016/j.crci.2018.09.002
Nyam KL, Tan CP, Karim R et al (2010a) Extraction of tocopherol-enriched oils from Kalahari melon and roselle seeds by supercritical fluid extraction (SFE-CO2). Food Chem 119:1278–1283. https://doi.org/10.1016/j.foodchem.2009.08.007
Nyam KL, Tan CP, Lai OM et al (2010b) Optimization of supercritical fluid extraction of phytosterol from roselle seeds with a central composite design model. Food Bioprod Process 88:239–246. https://doi.org/10.1016/j.fbp.2009.11.002
Oliveira ELG, Silvestre AJD, Silva CM (2011) Review of kinetic models for supercritical fluid extraction. Chem Eng Res Des 89:1104–1117. https://doi.org/10.1016/j.cherd.2010.10.025
Oliveira DA, Mezzomo N, Gomes C, Ferreira SRS (2017) Encapsulation of passion fruit seed oil by means of supercritical antisolvent process. J Supercrit Fluids 129:96–105. https://doi.org/10.1016/j.supflu.2017.02.011
Pagano I, Piccinelli AL, Celano R et al (2018a) Pressurized hot water extraction of bioactive compounds from artichoke by-products. Electrophoresis 39:1899–1907. https://doi.org/10.1002/elps.201800063
Pagano I, Sánchez-Camargo ADP, Mendiola JA et al (2018b) Selective extraction of high-value phenolic compounds from distillation wastewater of basil (Ocimum basilicum L.) by pressurized liquid extraction. Electrophoresis. 39:1884–1891. https://doi.org/10.1002/elps.201700442
Pal CBT, Jadeja GC (2019) Microwave-assisted deep eutectic solvent extraction of phenolic antioxidants from onion (Allium cepa L.) peel: a Box-Behnken design approach for optimization. J Food Sci Technol-Mysore 56:4211–4223. https://doi.org/10.1007/s13197-019-03891-7
Pandiyan K, Singh A, Singh S et al (2019) Technological interventions for utilization of crop residues and weedy biomass for second generation bio-ethanol production. Renew Energy 132:723–741. https://doi.org/10.1016/j.renene.2018.08.049
Panic M, Gunjevic V, Cravotto G et al (2019) Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem 300:125185. https://doi.org/10.1016/j.foodchem.2019.125185
Passos H, Freire MG, Coutinho JAP (2014) Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem 16:4786–4815. https://doi.org/10.1039/C4GC00236A
Pena-Pereira F, Tobiszewski M (2017) The application of green solvents in separation processes|ScienceDirect. Elsevier, Amsterdam, Netherlands
Pérez C, Ruiz del Castillo ML, Gil C, Blanch GP, Flores G (2015) Supercritical fluid extraction of grape seeds: extract chemical composition, antioxidant activity and inhibition of nitrite production in LPS-stimulated Raw 264.7 cells. Food Funct 6:2607–2613. https://doi.org/10.1039/c5fo00325c
Pighin E, Díez VK, Di Cosimo JI (2017) Kinetic study of the ethyl lactate synthesis from triose sugars on Sn/Al2O3 catalysts. Catal Today 289:29–37. https://doi.org/10.1016/j.cattod.2016.10.002
Pitchaiah KC, Sujatha K, Deepitha J et al (2018) Recovery of uranium and plutonium from pyrochemical salt matrix using supercritical fluid extraction. J Supercrit Fluids. https://doi.org/10.1016/j.supflu.2018.10.015
Plaza M, Turner C (2015) Pressurized hot water extraction of bioactives. TrAC Trends Anal Chem 71:39–54. https://doi.org/10.1016/j.trac.2015.02.022
Plaza M, Abrahamsson V, Turner C (2013) Extraction and neoformation of antioxidant compounds by pressurized hot water extraction from apple byproducts. J Agric Food Chem 61:5500–5510
Procentese A, Johnson E, Orr V, Garruto Campanile A, Wood JA, Marzocchella A, Rehmann L (2015) Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour Technol 192:31–36. https://doi.org/10.1016/j.biortech.2015.05.053
Radošević K, Ćurko N, Gaurina Srček V et al (2016) Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT-Food Sci Technol 73:45–51. https://doi.org/10.1016/j.lwt.2016.05.037
Rezaei F, Yamini Y, Asiabi H et al (2016) Supercritical fluid extraction followed by nanostructured supramolecular solvent extraction for extraction of levonorgestrel and megestrol from whole blood samples. J Supercrit Fluids 107:392–399. https://doi.org/10.1016/j.supflu.2015.10.005
Roselló-Soto E, Barba FJ, Parniakov O, Galanakis CM, Lebovka N, Grimi N, Vorobiev E (2015) High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food Bioprocess Technol 8:885–894. https://doi.org/10.1007/s11947-014-1456-x
Ruesgas-Ramón M, Figueroa-Espinoza MC, Durand E (2017) Application of deep eutectic solvents (DES) for phenolic compounds extraction: overview, challenges, and opportunities. J Agric Food Chem 65:3591–3601. https://doi.org/10.1021/acs.jafc.7b01054
Saha K, Dasgupta J, Chakraborty S, Antunes FAF, Sikder J, Curcio S, Santos JC, Arafat HA, Silva SS (2017) Optimization of lignin recovery from sugarcane bagasse using ionic liquid aided pretreatment. Cellulose 24:3191–3207. https://doi.org/10.1007/s10570-017-1330-x
Santaella MA, Orjuela A, Narváez PC (2015) Comparison of different reactive distillation schemes for ethyl acetate production using sustainability indicators. Chem Eng Process Process Intensif 96:1–13. https://doi.org/10.1016/j.cep.2015.07.027
Santalad A, Srijaranai S, Burakham R, Glennon JD, Deming RL (2009) Cloud-point extraction and reversed-phase high-performance liquid chromatography for the determination of carbamate insecticide residues in fruits. Anal Bioanal Chem 394:1307–1317. https://doi.org/10.1007/s00216-009-2663-6
Santos DNE, de Souza LL, Ferreira NJ, de Oliveira AL (2015) Study of supercritical extraction from Brazilian cherry seeds (Eugenia uniflora L.) with bioactive compounds. Food Bioprod Process 94:365–374. https://doi.org/10.1016/j.fbp.2014.04.005
Santos-Zea L, Gutiérrez-Uribe JA, Benedito J (2019) Effect of ultrasound intensification on the supercritical fluid extraction of phytochemicals from Agave salmiana bagasse. J Supercrit Fluids 144:98–107. https://doi.org/10.1016/j.supflu.2018.10.013
Sarkar D, Choudhury P, Dinda S, Das PK (2018) Vesicle formation by cholesterol based hydrazone tethered amphiphiles: stimuli responsive dissipation of self-assembly. J Colloid Interface Sci 530:67–77. https://doi.org/10.1016/j.jcis.2018.06.064
Satlewal A, Agrawal R, Bhagia S et al (2018) Natural deep eutectic solvents for lignocellulosic biomass pretreatment: recent developments, challenges and novel opportunities. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2018.08.009
Shankar M, Chhotaray PK, Agrawal A et al (2017) Protic ionic liquid-assisted cell disruption and lipid extraction from fresh water Chlorella and Chlorococcum microalgae. Algal Res 25:228–236. https://doi.org/10.1016/j.algal.2017.05.009
Sharif KM, Rahman MM, Azmir J et al (2014) Experimental design of supercritical fluid extraction – a review. J Food Eng 124:105–116. https://doi.org/10.1016/j.jfoodeng.2013.10.003
Silva YPA, Ferreira TAPC, Celli GB, Brooks MS (2019) Optimization of lycopene extraction from tomato processing waste using an eco-friendly ethyl lactate–ethyl acetate solvent: a green valorization approach. Waste Biomass Valor 10:2851–2861. https://doi.org/10.1007/s12649-018-0317-7
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/cr300162p
Sookwong P, Suttiarporn P, Boontakham P, Seekhow P, Wangtueai S, Mahatheeranont S (2016) Simultaneous quantification of vitamin E, γ-oryzanols and xanthophylls from rice bran essences extracted by supercritical CO2. Food Chem 211:140–147. https://doi.org/10.1016/j.foodchem.2016.05.001
Strati IF, Oreopoulou V (2011) Effect of extraction parameters on the carotenoid recovery from tomato waste. Int J Food Sci Technol 46:23–29. https://doi.org/10.1111/j.1365-2621.2010.02496.x
Strati IF, Oreopoulou V (2014) Recovery of carotenoids from tomato processing by-products – a review. Food Res Int 65:311–321. https://doi.org/10.1016/j.foodres.2014.09.032
Strati IF, Oreopoulou V (2016) Recovery and isomerization of carotenoids from tomato processing by-products. Waste Biomass Valor 7:843–850. https://doi.org/10.1007/s12649-016-9535-z
Tan Z-J, Li F-F, Xing J-M (2012) Cloud point extraction of aloe anthraquinones based on non-ionic surfactant aqueous two-phase system. Nat Prod Res 26:1423–1432. https://doi.org/10.1080/14786419.2011.601415
Teixeira A, Baenas N, Dominguez-Perles R et al (2014) Natural bioactive compounds from winery by-products as health promoters: a review. Int J Mol Sci. https://doi.org/10.3390/ijms150915638
Tomé LIN, Baião V, da Silva W, Brett CMA (2018) Deep eutectic solvents for the production and application of new materials. Appl Mater Today 10:30–50. https://doi.org/10.1016/j.apmt.2017.11.005
Torres-Valenzuela LS, Ballesteros-Gómez A, Sanin A et al (2019) Valorization of spent coffee grounds by supramolecular solvent extraction. Sep Purif Technol 228:115759. https://doi.org/10.1016/j.seppur.2019.115759
Ueno H, Tanaka M, Machmudah S et al (2008) Supercritical carbon dioxide extraction of valuable compounds from Citrus junos seed. Food Bioprocess Technol 1:357–363. https://doi.org/10.1007/s11947-007-0015-0
Ventura SPM, e Silva FA, Quental MV et al (2017) Ionic-liquid-mediated extraction and separation processes for bioactive compounds: past, present, and future trends. Chem Rev 117:6984–7052. https://doi.org/10.1021/acs.chemrev.6b00550
Vian M, Breil C, Vernes L et al (2017) Green solvents for sample preparation in analytical chemistry. Curr Opin Green Sustain Chem 5:44–48. https://doi.org/10.1016/j.cogsc.2017.03.010
Villanueva-Bermejo D, Reglero G, Fornari T (2017) Recent advances in the processing of green tea biomolecules using ethyl lactate. A review. Trends Food Sci Technol 62:1–12. https://doi.org/10.1016/j.tifs.2016.12.009
Virot M, Tomao V, Ginies C, Chemat F (2008) Total lipid extraction of food using D-limonene as an alternative to n-hexane. Chroma 68:311–313. https://doi.org/10.1365/s10337-008-0696-1
Vovers J, Smith KH, Stevens GW (2017) Chapter 4 - bio-based molecular solvents. In: Pena-Pereira F, Tobiszewski M (eds) The application of green solvents in separation processes, 1st edn. Elsevier, pp 91–110
Wang Y, Gu W (2018) Study on supercritical fluid extraction of solanesol from industrial tobacco waste. J Supercrit Fluids 138:228–237. https://doi.org/10.1016/j.supflu.2018.05.001
Watanabe H, Tanaka H (1978) A non-ionic surfactant as a new solvent for liquid–liquid extraction of zinc(II) with 1-(2-pyridylazo)-2-naphthol. Talanta 25:585–589. https://doi.org/10.1016/0039-9140(78)80151-9
Wei Z, Qi X, Li T et al (2015) Application of natural deep eutectic solvents for extraction and determination of phenolics in Cajanus cajan leaves by ultra performance liquid chromatography. Sep Purif Technol 149:237–244. https://doi.org/10.1016/j.seppur.2015.05.015
Xhaxhiu K, Wenclawiak B (2015) Comparison of supercritical CO2 and ultrasonic extraction of orange peel essential oil from Albanian Moro cultivars. J Essential Oil Bearing Plants 18:289–299. https://doi.org/10.1080/0972060X.2015.1010603
Yang X, Cheng K, Jia G (2019) The molecular dynamics simulation of hydrogen bonding in supercritical water. Phys A: Stat Mech Appl 516:365–375. https://doi.org/10.1016/j.physa.2018.10.022
Yao G, Wang L, Chen X et al (2019) Measurement and correlation of vapor–liquid equilibrium data for binary and ternary systems composed of (−)-β-caryophyllene, p-cymene and 3-carene at 101.33 kPa. J Chem Thermodyn 128:215–224. https://doi.org/10.1016/j.jct.2018.08.015
Yara-Varón E, Selka A, Fabiano-Tixier AS, Balcells M, Canela-Garayoa R, Bily A, Touaibia M, Chemat F (2016) Solvent from forestry biomass. Pinane a stable terpene derived from pine tree byproducts to substitute n-hexane for the extraction of bioactive compounds. Green Chemistry 18 (24):6596–6608
Yoo DE, Jeong KM, Han SY, Kim EM, Jin Y, Lee J (2018) Deep eutectic solvent-based valorization of spent coffee grounds. Food Chem 255:357–364. https://doi.org/10.1016/j.foodchem.2018.02.096
Yoon TJ, Lee Y-W (2018) Current theoretical opinions and perspectives on the fundamental description of supercritical fluids. J Supercrit Fluids 134:21–27. https://doi.org/10.1016/j.supflu.2017.11.022
Yu J, Dandekar DV, Toledo RT, Singh RK, Patil BS (2006) Supercritical fluid extraction of limonoid glucosides from grapefruit molasses. J Agric Food Chem 54:6041–6045. https://doi.org/10.1021/jf060382d
Zahari SMSNS, Amin ATM, Halim NM et al (2018) Deconstruction of Malaysian agro-wastes with inexpensive and bifunctional triethylammonium hydrogen sulfate ionic liquid. AIP Conference Proceedings 1972:030024. https://doi.org/10.1063/1.5041245
Zainal-Abidin MH, Hayyan M, Hayyan A, Jayakumar NS (2017) New horizons in the extraction of bioactive compounds using deep eutectic solvents: a review. Anal Chim Acta 979:1–23. https://doi.org/10.1016/j.aca.2017.05.012
Zhang Q, Vigier KDO, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108–7146. https://doi.org/10.1039/C2CS35178A
Zhang C-W, Xia S-Q, Ma P-S (2016) Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour Technol 219:1–5. https://doi.org/10.1016/j.biortech.2016.07.026
Zhou P, Wang X, Liu P et al (2018) Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind Crop Prod 120:147–154. https://doi.org/10.1016/j.indcrop.2018.04.071
Funding
Authors gratefully acknowledge financial support from Spanish MINECO (Project CTQ2017-83823-R). A. Ballesteros-Gómez acknowledges the funding from Spanish Ministry of Science, Innovation and Universities for a Ramón y Cajal contract (RYC-2015-18482). L.S. Torres-Valenzuela thanks AUIP for her doctoral fellowship.
GAE, gallic acid equivalents (total polyphenolic content); QE, quercetin equivalent (total flavonoids contents)
GAE, gallic acid equivalents
Optimal amphiphile in bold; 5-CGA, 5-chlorogenic acid; 5-CGAE, 5-chlorogenic acid equivalents; TPC, total phenolic compounds
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Torres-Valenzuela, L.S., Ballesteros-Gómez, A. & Rubio, S. Green Solvents for the Extraction of High Added-Value Compounds from Agri-food Waste. Food Eng Rev 12, 83–100 (2020). https://doi.org/10.1007/s12393-019-09206-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-019-09206-y