Abstract
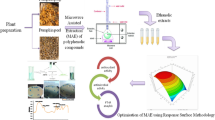
The present study compares three methods viz. microwave assisted extraction (MAE), ultrasonic-assisted extraction (UAE) and conventional solvent extraction (CSE) for extraction of phenolic compounds from black carrot pomace (BCP). BCP is the major by-product generated during processing and poses big disposal problem. Box–Behnken design using response surface methodology was employed to investigate and optimize the MAE of phenolics, antioxidant activity and colour density from BCP. The conditions for maximum recovery of polyphenolics were: microwave power (348.07 W), extraction time (9.8 min), solvent–solid ratio (19.3 mL/g) and ethanol concentration (19.8%). Under these conditions, the extract contained total phenolic content of 264.9 ± 10.02 mg gallic acid equivalents (GAE)/100 mL, antioxidant capacity (AOC) of 13.14 ± 1.05 µmol Trolox equivalents (TE)/mL and colour density of 68.63 ± 5.40 units. The total anthocyanin content at optimized condition was 753.40 ± 31.6 mg/L with low % polymeric colour of 7.40 ± 0.42. At optimized conditions, MAE yielded higher colour density (68.63 ± 5.40), polyphenolic content (264.9 ± 10.025 mg GAE/100 mL) and AOC (13.14 ± 1.05 µmol TE/mL) in a short time as compared to UAE and CSE. Overall results clearly indicate that MAE is the best suited method for extraction in comparison to UAE and CSE. The phenolic rich extract can be used as an effective functional ingredient in foods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Black carrot is an industrial crop mainly processed for the production of anthocyanin-rich concentrate for pigment industry. At present Turkey is the prominent black carrot producing country in the world and the tonnage is increasing each year due to rising demand from pigment and functional food industry. The production yield has increased from 18,000 to 46,000 tons between 2005 and 2010 (Meyed 2011). In 2013, roughly 14,000 tons of black carrot concentrate (ca. 60% dry matter) was produced in Turkey and mostly exported to countries such as Italy, Denmark, France, Japan and China as natural food colorant in foods. However, this large-scale processing generates huge amount of pomace, disposal of which is a major concern as high organic matter and moisture content of pomace makes it highly susceptible to microbial degradation. Thus disposal of BCP is an intractable problem for the black carrot processing industry. Despite the extraction of high amounts of polyphenols (1028.6 mg GAE/100 g reported by Singh et al. 2016b) and anthocyanin in juice, BCP is still left with significant amounts of residual polyphenolic compounds with high bioactivity, which can be put into use in various industries especially the food and nutraceutical industries (Galanakis 2012). These polyphenolic compounds are in huge demand in these industries because of their diverse structures and bioactivities, such as antioxidant, anti-inflammatory and anti-cancerous properties. However, the extraction process of polyphenols is inefficient and usually consume a lot of time and solvent and different processing conditions (temperature, pH, light) has usually been found to cause changes in the colour, stability and functionality of the black carrot anthocyanins/phenolics present in the extract, probably due to copigmentation (Kumar et al. 2018a) and formation of polymeric pigments (Wrolstad et al. 2005). The high temperature used during the extraction process and storage may lead to formation of polymeric pigments. It is also established that anthocyanin degradation and % PC formation is highly correlated (r = 0.989–0.997). This means that low polymeric colour is direct indication of high TAC. Hence, identification of methods for effective and efficient recovery of these bioactives for utilisation in food, pharmaceuticals and cosmetic industry is the need of the hour.
Many innovative technologies are being developed to improve the extraction of beneficial compounds (Barba et al. 2014) from industrial waste. Among various extraction techniques, MAE is a relatively new method used for the extraction of natural products. It is being widely used for the extraction of total phenolics from grape seeds (Hong et al. 2001), peanuts (Tameshia et al. 2010), Radix Astragali (Xiao et al. 2008), Phaseolus vulgaris L. (Sutivisedsak et al. 2010), sour cherry Marasca (Garofulic et al. 2013), potato downstream wastes (Wu et al. 2012) and from rice grains (Setyaningsih et al. 2015). It has been identified as one of the most promising green extraction method (Chemat et al. 2012) because of unequivocal merits viz, higher extraction yield, shorter extraction time and better quality of target extracts, associated with this extraction technique compared with other conventional extraction methods (Tanongkankit et al. 2013). However, still there is a lot of scope for improvement as various functional parameters like time, temperature, microwave power, solvent to solid ratio and ethanol concentration in the solvent are yet to be optimized. Furthermore, these factors might be having their interactive influence on the extraction of the phenolic compounds from the plant sample. During recent times, a statistical tool known as Response Surface Methodology (RSM) has been extensively used for the optimization of various parameters and to study the interactions between these parameters during the extraction process. MAE of total phenolic from C. sinensis (Nayak et al. 2015) and from Pistacia lentiscus L. (Dahmoune et al. 2014), has also been optimised using the RSM. However, there is a lack of literature on the microwave-assisted recovery of polyphenols from BCP. Therefore, it is of interest to develop a cost-effective and eco-friendly protocol using MAE, which will not only provide enhanced extraction yield but would also result in better bioactivity and chemical composition of target extracts. Accordingly, the purpose of present study was to identify optimum MAE parameters using RSM for extraction of residual phenolics from BCP for its valorisation, which would not only help the processing industry to offset the problem of its disposal but would also help them to turn waste into wealth thereby making the whole process economically viable.
Materials and methods
Raw material
Freshly harvested medium size black carrot (Pusa Asita) from the fields of were taken for the study were brought to the Division of Biochemistry, Indian Agricultural Research Institute. Black carrots were washed thoroughly with water to remove adhering soil and dirt. Peeling was done with the stainless steel knife and cut in slices followed by crushing by a waring blender. The activity of inherent polyphenol oxidase (PPO) was inactivated by heating the crushed mass to 90 °C for 1 min and later on rapidly cooled to 40 °C. The mass was partially de-juiced and the 10 g pomace of the black carrot was poured into the flask for further extraction of phenolics in microwave setup at different levels of microwave power, time of extraction, solvent/solid ratio and concentration of acidified ethanol (pH = 4) in the extraction solvent. The extract was filtered through the Whatman no. 1 filter paper. Heidolph rotary evaporator (Schwabach, Germany) was used to concentrate the obtained extract in a water bath at 40 °C so that stability of extracted sample can be increased. The extract was analyzed for phenolics, antioxidant activity and colour density.
Microwave-assisted extraction (MAE)
Phenolics were extracted from BCP using a microwave oven (LG Model). The model was provided with a digital system for extraction time and power (adjustable from 170 to 850 W). The microwave system was modified so that vapours generated during extraction can come into the sample. Acidified ethanol in water was used at different levels of concentration for efficient and non-toxic extraction of phenolics (Li et al. 2012). Ten grams of BCP was stirred into aqueous ethanol for extraction assisted with microwave. RSM was used to evaluate the effect of MAE parameters viz. power (340–680 W), extraction time (5–15 min), solvent to solid (S/S) ratio (10–30 mL/g) and ethanol proportion (10–30%) and to identify optimal conditions for three defined responses as per designed RSM (Table 1). After treatment, the extract was filtered using Whatman no. 1 filter paper and the extract was kept at 4 °C until further use. The extract was analysed for TPC, AOC and colour density.
Conventional solvent extraction (CSE)
Ten grams of BCP was stirred with 30% of ethanol with the solvent to solid ratio of 30:1 with an extraction time of 300 min at acidic pH of 5 in a beaker. After treatment the extract was filtered by using Whatman no. 1 filter paper and extract was kept at 4 °C until further analysis. The extract was analysed for TPC, AOC by Ferric Reducing Antioxidant Assay (FRAP), colour density and % PC and was compared with the best condition for the extraction of anthocyanin by MAE and UAE.
Ultrasound assisted extraction (UAE)
Extraction was done by using probe Ultrasonicator (S-4000; Misonix, USA) with the maximal amplitude of 100 while working at 25 in practical process. For performing UAE 10 g of material was taken in a beaker with S/S ratio of 40:1 for 30 min. 30% ethanol was taken as solvent system for UAE. Insertion of the probe was done inside the solvent to get the maximum ultrasonic waves inside the extraction unit. The temperature was kept constant by using a chilled water bath. The extract was analysed for TPC, AOC by FRAP, colour density and % PC and was compared with MAE and CSE.
Single factor experiments
Single-factor experiments were carried out in triplicate to investigate the influence of each factor (supplementary Fig. 1). The effect of all four parameters (power, time, S/S ratio and ethanol concentration) was determined by using various levels of factors.
Box–Behnken design and statistical analysis
Based on preliminary experiment results, the approximate range for each factor was defined and then a four-factor Box–Behnken design was adopted to design the experiment. As shown in Table 1, the four factors chosen for this study were designated as microwave power (A), extraction time (B), solvent to solid ratio (C) and ethanol concentration (D) and prescribed into three levels, coded + 1, 0, − 1 for high, intermediate and low value, respectively.
There were a total of 29 experimental units/trials, to be carried out in random order. The responses viz., experimental values of TPC, antioxidant potential by FRAP analysis and colour density of the extract is detailed in Table 1. Data obtained from the experiment were used for developing the following second-order response surface model connecting responses and input variables:
where u = 1, 2,…, 29, \( {\text{x}}_{\text{iu}} \) is the level of the ith (i = 1, 2, 3 and 4) factor in the uth treatment combination, \( {\text{f(x}}_{\text{u}} ) \) denotes the response obtained from uth treatment combination and \( {\text{e}}_{\text{u}} \) is the random error associated with the uth observation that is independently and normally distributed with mean zero and common variance \( {{\upsigma }}^{2} \), β0 is a constant, βi is the ith linear regression coefficient, βii is the ith quadratic regression coefficient and \( {{\upbeta }}_{{{\text{ii}}^{\prime}}} \) is the \( ( {\text{i,i}}^{\prime}) \)th interaction coefficient.
The responses and input variables were subjected to regression analysis and performed analysis of variance (ANOVA) to determine the significance of the fitted model and the significance of effects of linear, quadratic, and interaction terms in the model separately for TPC, Ferric Reducing Antioxidant Assay (FRAP) and colour density. The optimum combination of levels of extraction variables was determined by multi-response optimization technique and based on the desirability function using Design Expert software (Version 9.0).
Predictive model for response variables and adequacy of model
The predictive models obtained by fitting the second order polynomial model, three responses were tested for adequacy and fitness by analyses of variance (ANOVA) and the results are presented in Sect. 3. The results of ANOVA were confirmed using good predictive model criteria: R2 (coefficient of determination) ≥ 0.80, a significance level of p < 0.1; lack of fit test p > 0.05 and adequate precision > 4 (Myers and Montgomery 2002).
Validation of optimized conditions and predictive models
The suitability of the developed model equations for predicting the optimum response values was verified using the optimal extraction condition. Experiments in triplicate were conducted on the optimal condition and the mean data obtained from the experiments were analysed with predicted data in order to confirm the efficacy of the models.
Total anthocyanin content, total flavonoids and total phenolics
The pH differential method (Wrolstad et al. 2005) was used for determining total monomeric anthocyanins (mg/L) and total flavonoids content (Quercetin equivalents/L) in BCP was determined by method (Khandare et al. 2011). TPC (mg GAE/100 mL) was estimated by the method described by Singleton et al. (1999).
AOC using ferric reducing antioxidant power (FRAP)
Benzie and Strain (1996) method was performed for the determination of antioxidant properties of the extracts by FRAP assay.
Colour density and polymeric colour
Colour density has been taken as one of the parameters for the optimization, which is a very important parameter depicting the stability of colouring components of polyphenolic compounds (example: Anthocyanins). Colour density of the extract is directly proportional to the anthocyanin content of the extract. % (PC) is also a very important component as it gives an idea of polymeric compounds present in the extract. The % PC is an indication of the degradation of anthocyanins in the polyphenolic extract (Danisman et al. 2015).
Colour density and polymeric colour were analysed according to the Khandare et al. (2011) method.
Result and discussion
Industrial applications often require valorisation of the pomace and byproducts to prevent microbial decomposition of the waste and for environmental reasons. The pomace generated, was hence subjected to solvent assisted MAE for developing phenolics rich extract.
Preliminary experiment
Influence of ethanol concentration
The recovery of TPC from the BCP with increasing concentration of ethanol followed a parabolic curve from 10 to 50% (Supplementary Fig. 1.1). The yield of TPC increased with increasing amounts of ethanol concentration in the extraction medium up to 20% and declined thereafter (Supplementary Fig. 1.1). A percent of 10–30 was further used in the optimization of process parameters for RSM.
Influence of extraction solvent to solid ratio
A significant increase in recovery of phenolic content was observed with increasing ratios of solvent to solid (S/S), maximized up to 20:1 and declined thereafter at higher levels (Supplementary Fig. 1.2). Thus, the optimal solvent–solid ratio of 20:1 was chosen for further process parameters. A ratio of 10–30 (v/w) was further used in the optimization of process parameters for RSM.
Influence of extraction time
An optimum extraction facilitates increased recovery of phenolics, although there is the risk of the degradation at longer exposure times. The recovery of TPC from BCP increased with the increase in extraction time from 2.5 to 10 min, and reached a peak at 10 min, then significantly decreased. Maximum recovery of 159.9 mg GAE/100 mL was obtained after 10 min followed by a substantial decrease to the tune of 2.7 folds (Supplementary Fig. 1.3).
Influence of microwave power
The effects of microwave power on the recovery of TPC from BCP were investigated at the levels ranging from 170 to 850 W with fixed solvent concentration (20% ethanol), extraction time of 10 min and a solvent to solid ratio of 20:1 (mL/g). A similar trend was observed, maximum recovery (171.2 mg GAE/100 mL) at 340 W, followed by a decrease (Supplementary Fig. 1.4). Understandably, higher microwave power level might degrade phenolic compounds. These observations revealed that extraction at higher microwave output power levels at 510 W or more, do not ensure better recovery of phenolic compounds than those extracted at medium power. Taking these factors into account, the optimal power level was found to be 340–680 W for RSM.
Model fitting
The results obtained (in Table 1) ranged as follows: TPC (37.20–276.0 mg GAE/100 mL), FRAP (2.82–14.24 µmol TE/mL) and colour density (6.60–65.26 units). Variations in the content of total phenols, FRAP and colour density of the extracts was observed depending on the experimental conditions. Experimental results suggested regression model fitted is a good fit to the data for all the responses. Model parameter estimates along with their standard errors and model fitness measures were obtained and given in Table 2.
Total phenolic content (TPC)
Black carrot phenolics were extracted according to the experimental design in order to select microwave power, extraction time, solvent to solid ratio and ethanol concentration which are optimal for extraction of phenolic acids.
The quadratic regression model in terms of coded variables showing the effect of operating parameters on the TPC is given in the following equation.
where A, B, C and D were the coded values of microwave power, extraction time, the ratio of solvent to solid and ethanol concentration, respectively.
From the p values of each model term, it could be concluded that effect of A, interactions effect AB and four quadratic terms A2, B2, C2 and D2 are significant model terms (p < 0.1) affecting the TPC. The microwave power and solvent–solid ratio were found to be the most significant linear effect and AB i.e. microwave power and time were the most significant interactive effect, which influenced total phenolic yield. Here, determination coefficient (R2), model F-value, predicted and adjusted R2, adequate precision, lack of fit F-value explain the statistical significance of the model fitted. The values of each term are also presented in Table 2.
According Sun et al. (2010) and Yin and Dang (2008), the interaction effects of the factors on the responses can be evident from the three-dimensional response surface plots (Fig. 1a–f) served very effective as they depicted the sensitiveness of response value towards the variable manipulation. Maran et al. (2013), stressed that such graphs can be drawn by keeping two factors as constant and the other two factors should be varying so as to comprehend their main and interactive effects on the dependent variables.
Response surface analysis of black carrot for the effect of microwave power and time (a), power and solvent to solid ratio (b), power and ethanol concentration in solvent (c), time and solvent to solid ratio (d), time and ethanol concentration in solvent (e), solvent to solid ratio and ethanol concentration in solvent (f) on TPC using microwave assisted extraction (MAE). TPC total phenolic content in mg gallic acid equivalents (GAE)/100 mL
The effect of microwave power (A) and irradiation time (B) on the yield of total phenolics from BCP at a fixed solvent: solid ratio and concentration of ethanol at its central level can be seen in Fig. 1a. At a definite microwave power of 340 W, an increase in the extraction time from 5 to 15 min led to an increase in the extraction efficiency of TPC (190.32–275.49 mg GAE/100 mL); similarly at a microwave power of 680 W, there was a drastic decrease in the extraction efficiency from 110.53 to 27.33 mg GAE/100 mL as the time increased from 5 to 15 min. TPC increased linearly when the power levels are decreased from 680 to 340 W and achieved maximum at around 340 W. Extraction time of 10 min was found to be having maximum TPC further increasing the time level at 340 W decreased the TPC content slightly. This may be attributed to the fact that the extraction power is main factor which plays crucial role in the extraction of TPC and extraction time plays an important role to enhance the extraction yield till a threshold level, after this no increase in the rate of extraction occurs due to saturation (Arvindekar and Laddha 2015). The extending extraction time could result in a higher extraction recovery up to 10 min. However, the recovery decreased with the extension of extraction time above 10 min, which was possibly due to the degradation of total phenolics by microwave wave. Similar results were also obtained by Song et al. (2011). Alara et al. (2018), reported that increase in microwave power and irradiation time beyond the limit leads to decrease in the recovery of TPC. Increasing the microwave power up to 340 W resulted in the highest recovery of the TPC that may be due the ionic conduction and dipole rotation effects of MAE which facilitate leaching out of phenolic compounds from the plant matrix through molecular interaction (Chan et al. 2011; Alara et al. 2018).
Another reason for enhanced recovery of TPC was the use of ethanol–water mixture as an extraction solvent because the mixture has a high dielectric constant and dissipation factor to absorb microwave energy effectively and was able to increase the penetration of the solvent into the sample matrix, and consequently, heating efficiency was increased and as a result of presence of a small amount of water in the solvent extraction, increased the diffusion of water into the cell matrix, delivers better heating, and facilitates high-speed mass transfer compound into the solvent. The results are in line with the findings of the Mun’im et al. (2017), Azlim et al. (2010) and Sochar et al. (2010).
These results also coincide with preliminary single factor analysis experiment in which the maximum yield of TPC was obtained at 340 W and irradiation time of 10 min. The mutual interaction between the microwave power and time was found to be significant at p < 0.05 (Table 2). The mutual interactions between all other interactive terms were found to be non-significant (Table 2 and Fig. 1b–f).
Antioxidant capacity (AOC)
Lachman et al. (2008) and Rumbaoa et al. (2009) have described multifarious health benefits of phenolics, especially antioxidants. The antioxidant activity of the MAE extracts of BCP were evaluated through FRAP as in vitro method. The AOC evaluated using the FRAP method ranged from 2.82 to 14.24 µmol TE/mL (Table 1) for all treatments. The quadratic regression model in terms of coded variables showing the effect of operating parameters on the antioxidant activity by FRAP assay is given in following equation.
The parameter estimates along with standard error and model significance are presented in Table 2.
ANOVA of regression model fitted to experimental results of antioxidant activity by FRAP showed that linear terms power (A) and time (B) are significant (p < 0.01) model terms and AOC increased with decreasing power and time. However, S/S ratio (C) and concentration of ethanol (D) in solvent had no significant (p > 0.10) effect on AOC. Whereas ANOVA of regression model fitted to experimental results of AOC by FRAP showed that all quadratic terms A2, B2, C2 and D2 are significant model terms (p < 0.01) (Table 2). AOC was significantly and inversely dependent on power, time, S/S ratio and ethanolic concentration and its content reduces with increasing effect of quadratic terms as followed in TPC. TPC and AOC were found to be positively correlated in juices of red grapes by Dani et al. (2007) and in jambolan fruit by Singh et al. (2016a). In another study by Xu et al. (2018) showed that AOC of the extract was positively correlated with the TPC and its conjugated polysaccharides in Eucommia ulmoides Oliver leaves and total flavonoid content in black carrot marc (Kumar et al. 2018b). Hence it is clear that enhanced antioxidant capacity at 340 W is the resultant of the high recovery of the TPC at similar conditions.
All the interactive terms are non-significant model terms (p > 0.10). The model F-value of 14.32 implies the model is significant (p < 0.01). A non-significant lack of fit (p > 0.05) and R2 (0.93) indicated fair suitability of model in predicting AOC of the extract by the FRAP (Table 2). The mutual interaction for AOC was found to be non-significant for all the interactive terms (Table 2 and Fig. 2a–f).
Response surface analysis of black carrot for the effect of microwave power and time (a), power and solvent to solid ratio (b), power and ethanol concentration in solvent (c), time and solvent to solid ratio (d), time and ethanol concentration in solvent (e), solvent to solid ratio and ethanol concentration in solvent (f) on Antioxidant capacity (FRAP) using microwave assisted extraction (MAE). FRAP ferric reducing antioxidant potential in µmol TE/mL
Colour density
Colour density as an important index for measuring colouring potential of natural colourants and for tracking deterioration of colour during processing.
The quadratic regression model in terms of coded variables showing the effect of operating parameters on the colour density of the sample is given in following equation.
The results are presented in Table 2. The quadratic polynomial equation with significant (p < 0.001) and non-significant terms (p > 0.10) of colour density of BCP extract were given in Table 2. In this case only one linear term i.e. ‘A’ (Table 2) was found to be statistically significant (p < 0.01). Antioxidant activity by FRAP was significantly dependent on power and increased with decreasing power. Microwave power had an inverse effect on the colour density and causes specific degradation of colour due to heating effect. A study by Sala et al. (1995) and Tchabo et al. (2015) highlighted that cavitation which fastens chemical reactions, enhances diffusion rates, dispersion of aggregates can also be a possible factor that prompts degradation of colour. Whereas ANOVA of regression model fitted to experimental results of colour density showed that quadratic terms A2 (p < 0.05) and B2, C2 and D2 (p < 0.01) are significant model terms (Table 2). Colour density was significantly and inversely dependent on all quadratic terms.
The change in the colour density may be attributed to changes in TPC here which were significantly and negatively dependent on the interaction of power and time; as the power and time increases there is a decrease in the colour density. This may be due to degradation of coloured compounds in the extract due to prolonged heating effect in the microwave resulted in colour degradation (Wang and Xu 2007). The model F-value of 15.99 implies the model is significant (p < 0.05). A non-significant lack of fit (p > 0.05) and R2 (0.936) indicated fair suitability of model in predicting colour density of the extract. The mutual interaction between all the interactive terms for colour density was found to be non-significant at p > 0.1 (Table 2 and Fig. 3a–f).
Response surface analysis of black carrot for the effect of microwave power and time (a), power and solvent to solid ratio (b), power and ethanol concentration in solvent (c), time and solvent to solid ratio (d), time and ethanol concentration in solvent (e), solvent to solid ratio and ethanol concentration in solvent (f) on CD using microwave assisted extraction (MAE). CD colour density (units)
Determination and validation of optimum conditions
Best condition for the MAE of total phenolics, antioxidant activity and colour density from the pomace of black carrot was done by multi-response optimization technique with an objective to obtain higher content of phenolics, antioxidant activity and colour density. To derive at the stated ideal conditions, the three second order polynomial models were used. Results, indicate that a microwave power 348.06 W, extraction time 9.86 min, solvent–solid ratio of 19.3 g/mL and ethanol concentration of 19.8% in the solvent gives 277.07 mg GAE/100 mL of TPC, 14.01 µmol TE/mL of antioxidant activity by FRAP and 69.99 units of colour density with an overall desirability value of 0.993. These optimal conditions could be considered as feasible. The experimental values (TPC = 264.9 ± 10.025, AOC = 13.14 ± 1.05, colour density = 68.63 ± 5.40 units) were in accordance with the values estimated as given in Supplementary Table 1, which is indicative of the appropriateness of the quadratic models developed (Table 2).
Comparison of MAE with UAE and CSE
The optimized MAE conditions, CSE and UAE were set side by side for comparison (Table 3). For performing UAE selected conditions were 30 min, 30% ethanol and S/S ratio 40. Extraction was done by using probe Ultrasonicator (S-4000; Misonix, USA) with maximal amplitude of 100, while working at 25 in practical process. In case of CSE conditions were 300 min, 30% ethanol, S/S ratio 30, 50 at pH 3.0, based on previous preliminary trials. The results show that the three investigated extraction techniques gave statistically different TPC yields (264.9 ± 10.025, 194.5 ± 12.24, 108.7 ± 5.63 mg GAE/100 mL MAE, UAE and CSE respectively). The TFC was greatly variable for all the three studied protocols and found to be maximum with MAE (1662.22 ± 47.3 mg QE/L) than with UAE and CSE (1076.66 ± 36.66 and 683.33 ± 34.80 mg QE/L respectively). Also, the anthocyanin content was superior with MAE (753.40 ± 31.6 mg/L) than with UAE (607 ± 55.69 mg/L) and with CSE (323.4 ± 21.7 mg/L). Similar findings of high anthocyanin content were reported by Karabegovic et al. (2014) from cherry leaves, Sun et al. (2007) from red raspberry and Yang and Zhai (2010) regardless the extraction time and this is generally because of the microwave irradiation mechanism (Sun et al. 2007; Zhang et al. 2008). Localized temperature rise in plant tissues caused by microwaves results in their disruption and subsequent migration of phenolic compounds to the nearby solvent. Forty milligram GAE/g dw of TPC was extracted with 40% aqueous ethanol from blackcurrant leaves by Nour et al. (2014).
The optimized MAE conditions, CSE and UAE were set side by side for comparison. For performing UAE selected conditions were 30 min, 30% ethanol and S/S ratio 40. Extraction was done by using probe Ultrasonicator (S-4000; Misonix, USA) with the maximal amplitude of 100 while working at 25 in practical process. In the case of CSE, conditions were 300 min, 30% ethanol, S/S ratio 30, 50 at pH 3.0, based on previous preliminary trials. The results show that the three investigated extraction techniques gave statistically different TPC yields (264.9 ± 10.025, 194.5 ± 12.24, 108.7 ± 5.63 mg GAE/100 mL MAE, UAE and CSE respectively). The TFC was greatly variable for all the three studied protocols and found to be maximum with MAE (1662.22 ± 47.3 mg QE/L) than with UAE and CSE (1076.66 ± 36.66 and 683.33 ± 34.80 mg QE/L respectively). Also, the anthocyanin content was superior with MAE (753.40 ± 31.6 mg/L) than with UAE (607 ± 55.69 mg/L) and with CSE (323.4 ± 21.7 mg/L). Similar findings of high anthocyanin content were reported by Karabegovic et al. (2014) from cherry leaves, Sun et al. (2007) from red raspberry and Yang and Zhai (2010) regardless the extraction time and this is generally because of the microwave irradiation mechanism (Sun et al. 2007; Zhang et al. 2008). The localized temperature rise in plant tissues caused by microwaves results in their disruption and subsequent migration of phenolic compounds to the nearby solvent. Forty milligram GAE/g dw of TPC was extracted with 40% aqueous ethanol from blackcurrant leaves by Nour et al. (2014).
Conclusion
In the present study, microwave assisted extraction has been identified as an efficient method for the extraction of bioactives such as total phenolics, anthocyanins and other antioxidants; clearly surpassing the performance of other conventional extraction methods including ultrasonication. It can, therefore, undoubtedly prove to be a successful proposition for the cost-effective and eco-friendly extraction of polyphenolic compounds from black carrot pomace and other by-products of food industry for valorisation purposes.
References
Alara OR, Abdurahman NH, Afolabi HK, Olalere OA (2018) Efficient extraction of antioxidants from Vernonia cinerea leaves: comparing response surface methodology and artificial neural network. J Basic Appl Sci 7:276–285
Arvindekar AU, Laddha KS (2015) An efficient microwave-assisted extraction of anthraquinones from Rheum emodi: optimisation using RSM, UV and HPLC analysis and antioxidant studies. Ind Crops Prod 52:6574–6582
Azlim AA, Ahmed JKC, Syed ZI, Mustapha SK, Aisyah M, Kamarul RK (2010) Total phenolic content and primary antioxidant activity of methanolic and ethanolic extracts of aromatic plants leaves. Int Food Res J 17(4):1077–1083
Barba FJ, Esteve MJ, Frígola A (2014) Bioactive components from leaf vegetable products. Stud Nat Prod Chem 41:321–346
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant” power: the FRAP assay. Anal Biochem 239:70–76
Chan CH, Yusoff R, Ngoh GC, Kung FWL (2011) Microwave-assisted extractions of active ingredients from plants. J Chromatogr A 1218:6213–6225
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products: concept and principles. Int J Mol Sci 12:824–829
Dahmoune F, Spigno G, Moussi K, Remini H, Cherbal A, Madani K (2014) Pistacia lentiscus leaves as a source of phenolic compounds: microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind Crops Prod 61:31–40
Dani C, Oliboni LS, Bonatto D, Vanderlinde R, Salvador M, Henriques JA (2007) Phenolic content and antioxidant activities of white and purple juices manufactured with organically-produced grapes. Food Chem Toxicol 45:2574–2580
Danisman G, Arslan E, Toklucu AK (2015) Kinetic analysis of anthocyanin degradation and polymeric colour formation in grape juice during heating Czech. J Food Sci 33(2):103–108
Galanakis CM (2012) Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci Technol 26:68–87
Garofulic IE, Dragović-Uzelac V, Jambrak AR, Jukic M (2013) The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. marasca). J Food Eng 117:437–442
Hong N, Yaylayan VA, Raghavan GSV, Paré JRJ, Belanger JMR (2001) Microwave assisted extraction of phenolic compounds from grape seed. Nat Prod Res 15:197–204
Karabegović IT, Stojičević SS, Veličković DT, Todorović ZB, Nikolić NC, Lazić ML (2014) The effect of different extraction techniques on the composition and antioxidant activity of cherry laurel (Prunus laurocerasus) leaf and fruit extracts. Ind Crops Prod 54:142–148
Khandare V, Walia S, Singh M, Kaur C (2011) Black carrot (Daucus carota ssp. sativus) juice: processing effects on antioxidant composition and color. Food Bioprod Process 89:482–486
Kumar M, Dahuja A, Sachdev A, Kaur C, Varghese E, Saha S, Sairam KVSS (2018a) Black Carrot (Daucus carota ssp.) and black soybean (Glycine max (L.) Merr.) anthocyanin extract: a remedy to enhance stability and functionality of fruit juices by copigmentation. Waste Biomass Valorization. https://doi.org/10.1007/s12649-018-0450-3
Kumar M, Dahuja A, Sachdev A, Kaur C, Varghese E, Saha S, Sairam KVSS (2018b) Valorization of Black carrot marc: antioxidant properties and enzyme assisted extraction of flavonoids. Res J Biotechnol 13(11):12–21
Lachman J, Hamouz K, Šulc M, Orsák M, Dvořák P (2008) Differences in phenolic content and antioxidant activity in yellow and purple-fleshed potatoes grown in the Czech Republic. Plant Soil Environ 54:1–6
Li H, Deng Z, Wu T, Liu R, Loewen S, Tsao R (2012) Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem 130:928–936
Maran J, Manikandan S, Vigna Nivetha C, Dinesh R (2013) Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab J Chem 18:235–239
Meyed (Fruit Juice Industry Corporation) (2011) Turkey fruit juice like products industry report. http://www.meyed.org.tr. Accessed 10 Mar 2016
Mun’im A, Nurpriantia S, Setyaningsih R, Syahdi RR (2017) Optimization of microwave-assisted extraction of active compounds, antioxidant activity and angiotensin converting enzyme (ACE) inhibitory activity from Peperomia pellucida (L.) Kunth. J Young Pharm 9(1):73–78
Myers RH, Montgomery DC (2002) Response surface methodology: process and product optimization using designed experiments, 3rd edn. Wiley, New York
Nayak B, Dahmoune F, Moussi K, Remini H, Dairi S, Aoun O, Khodir M (2015) Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem 187:507–516
Nour V, Trandafir I, Cosmulescu S (2014) Antioxidant capacity, phenolic compounds and minerals content of blackcurrant (Ribes nigrum L.) leaves as influenced by harvesting date and extraction method. Ind Crops Prod 53:133–139
Rumbaoa RGO, Cornago DF, Geronimo IM (2009) Phenolic content and antioxidant capacity of Philippine potato (Solanum tuberosum) tubers. J Food Compos Anal 22:546–550
Sala FJ, Burgos J, Condon S, Lopez P, Raso J (1995) Effect of heat and ultrasound on microorganisms and enzymes. In: Gould GW (ed) New methods of food preservation. Blackie Academic and Professional, London, pp 176–204
Setyaningsih W, Saputro IE, Palma M, Barroso CG (2015) Optimisation and validation of the microwave-assisted extraction of phenolic compounds from rice grains. Food Chem 169:141–149
Singh JP, Kaur A, Shevkani K, Singh N (2016a) Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. https://doi.org/10.1007/s13197-016-2412-8
Singh JP, Kaur A, Singh N, Nim L, Shevkani K, Kaur H, Arora DS (2016b) In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT Food Sci Technol 65:1025–1030
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Methods Enzymol 299:152–178
Sochar J, Ryvolova M, Krystofova O, Salas P, Hubalek J, Adam V (2010) Fully automated spectrometric protocols for determination of antioxidant activity: advantages and disadvantages. Molecules 15(12):8618–8640
Song J, Li D, Liu C, Zhang Y (2011) Optimized microwave-assisted extraction of total phenolics (TP) from Ipomoea batatas leaves and its antioxidant activity. Innov Food Sci Emerg 12:282–287
Sun Y, Liao X, Wang Z, Hu X, Chen F (2007) Optimization of microwave-assisted extraction of anthocyanins in red raspberries and identification of anthocyanin of extracts using high-performance liquid chromatography–mass spectrometry. Eur Food Res Technol 225:511–523
Sun Y, Liu J, Kennedy JF (2010) Extraction optimization of antioxidant polysaccharides from the fruiting bodies of Chroogom phisrutilus (Schaeff. Fr.) O.K. Miller by Box–Behnken statistical design. Carbohydr Polym 82:209–214
Sutivisedsak N, Cheng HN, Willett JL, Lesch WC, Tangsrud RR, Biswas A (2010) Microwave-assisted extraction of phenolics from bean (Phaseolus vulgaris L.). Food Res Int 43:516–519
Tameshia SB, Parameswarakumar M, Kequan Z, Sean O (2010) Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem 120:1185–1192
Tanongkankit Y, Sablani SS, Chiewchan N, Devahastin S (2013) Microwave-assisted extraction of sulforaphane from white cabbages: effects of extraction condition, solvent and sample pretreatment. J Food Eng 117:151–157
Tchabo W, Ma Y, Engmann FN, Zhang H (2015) Ultrasound-assisted enzymatic extraction (UAEE) of phytochemical compounds from mulberry (Morus nigra) must and optimization study using response surface methodology. Ind Crops Prod 63:214–225
Wang WD, Xu SY (2007) Degradation kinetics of anthocyanins in blackberry juice and concentrate. J Food Eng 82:271–275
Wrolstad RE, Durst RW, Lee J (2005) Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol 16:423–428
Wu T, Yan J, Liu R, Marcone MF, Aisa HA, Tsao R (2012) Optimization of microwave-assisted extraction of phenolics from potato and its downstream waste using orthogonal array design. Food Chem 133:1292–1298
Xiao W, Han L, Shi B (2008) Microwave-assisted extraction of flavonoids from Radix astragali. Sep Purif Technol 62:614–618
Xu OLJ, Hou H, Hu J, Liu B (2018) Optimized microwave extraction, characterization and antioxidant capacity of biological polysaccharides from Eucommia ulmoides. Sci Rep 8:6561
Yang Z, Zhai W (2010) Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC–MS. Innov Food Sci Emerg Technol 11:470–476
Yin GH, Dang YL (2008) Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr Polym 74:603–610
Zhang B, Yang R, Liu C (2008) Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep Purif Technol 62:480–483
Acknowledgements
The facilities and financial support provided by the ICAR-Indian Agricultural Research Institute (IARI), Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Confederation of Indian Industry (CII), Government of India and Prathista Industries Limited under Prime Minister Fellowship Scheme to undertake the research work are highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, M., Dahuja, A., Sachdev, A. et al. Valorisation of black carrot pomace: microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box–Behnken design. J Food Sci Technol 56, 995–1007 (2019). https://doi.org/10.1007/s13197-018-03566-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-03566-9