Abstract
Co-gasification of biomass and plastic waste has a bright outlook and, in this regard, air and steam co-gasification of eucalyptus and polypropylene was investigated in this study. Various composition ratios of eucalyptus and polypropylene were considered and air and steam co-gasification performances have been analyzed with respect to polypropylene content, moisture content of feedstock, and gasification temperature. Air- and steam-based system performances have been tri-objective optimized using central composite design to attain a clean and efficient gasification performance. The results confirmed that hydrogen production was improved by increasing polypropylene content based on water–gas reaction. The polypropylene concentration significantly affected the efficiencies of steam co-gasification. Increasing polypropylene concentration from 0 to 100 wt% improved energy efficiency of steam co-gasification from 56 to 83%. Moisture content of 26 wt%, temperature of 955 °C, and polypropylene concentration of 54 wt% were optimum conditions of air co-gasification. Steam co-gasification was optimized at moisture content of 30 wt%, temperature of 1000 °C, and polypropylene concentration of 100 wt%. At the optimum conditions, air co-gasification resulted in higher energy efficiency (81% compared with 77%) while steam co-gasification led to higher hydrogen efficiency (50% compared with 42%) and lower CO2 emission (2.2 g/s compared with 9.4 g/s).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Gasification, as a thermochemical conversion, converts a feedstock into a combustible gas mixture [1]. The feedstock can be a worthless carbonaceous material and is converted into the syngas using a gasifying medium which should be an oxygen-carrier [2]. The syngas typically contains hydrogen, carbon monoxide, methane, carbon dioxide, steam, and nitrogen [3]. Gasification process has been well established for biomass and plastic wastes in the literature [4, 5].
Yong and Rasid [6] studied gasification of empty fruit bunch using air and steam gasifying mediums and evaluated the influence of gasification temperature, equivalence, and steam-to-biomass ratios on syngas composition. Their results showed that increasing equivalence ratio declined hydrogen generation and increased carbon monoxide generation. Awais et al. [7] studied a downdraft gasification of coconut shells and sugarcane bagasse and showed that equivalence ratio and biomass type significantly affected the gasification performance. Samimi et al. [8] assessed gasification of three types of biomass consisting of horse manure, pinewood, and sawdust using three types of gasifying mediums of steam, air, and steam/air mixture. Their comparison results demonstrated that steam gasification of pinewood had the best performance. Khalilarya et al. [9] investigated municipal solid waste gasification and showed that at low moisture content, hydrogen generation of steam gasification was larger than that of air and oxygen gasification types; however, oxygen gasification led to higher hydrogen generation than steam and air gasification types at high levels of moisture content. Mojaver et al. [10] investigated gasification of various biomass types and showed that straw was the best biomass. Dang et al. [11] investigated air/steam wood residue gasification and their results revealed that higher temperature, higher steam-to-biomass ratio, lower equivalence ratio, and higher moisture resulted in higher hydrogen generation. Habibollahzade et al. [12] examined biomass gasification and concluded that steam gasification had better performance from efficiency viewpoints compared to other cases.
Recently, gasification process attracted the attentions of the researchers in the field of plastic waste. Bai et al. [13] studied various models of polypropylene gasification and used supercritical water as an agent to enhance gasification efficiency. Mojaver et al. [14] studied steam plastic waste gasification of various plastic types. Their results showed that hydrogen generations of polyethylene and polypropylene waste were improved more than polycarbonate and polyethylene terephthalate waste with temperature. Hasanzadeh et al. [15] examined plastic gasification to study the influences of temperature and steam-to-plastic ratio on hydrogen generation. Their results showed that higher processing conditions resulted in higher hydrogen generation and hydrogen and exergy efficiencies. Han et al. [16] studied air gasification of mixed plastic waste. Their results showed that higher temperature and lower equivalence ratio enhanced hydrogen and CO generations. Janajreh et al. [17] examined gasification performance of plastic waste and their results indicated that gasification efficiencies were 63%, 73%, and 59% for polypropylene, polystyrene, and polyethylene, respectively, and this value was improved to 89% in the case of their mixture. Wang et al. [18] studied polypropylene gasification and compared performances of catalytic and non-catalytic gasification types from syngas composition and efficiency viewpoints. They revealed that catalytic polypropylene gasification enhanced hydrogen generation compared with non-catalytic type.
Nowadays, biomass and plastic waste co-gasification is among the hot topics for scientific purposes. Li et al. [19] investigated polyethylene and pine wood co-gasification assisted by CO2 and evaluated the influence of pretreatment of biomass on co-gasification performance. Supercritical water co-gasification of soda lignin with four plastic waste kinds was analyzed by Cao et al. [20]. Their results showed a synergistic effect of soda lignin with plastic wastes. Zhu et al. [21] evaluated co-gasification of beech wood and polyethylene and studied effect of altering biomass/plastic ratio on co-gasification performance. Basha et al. [22] investigated co-gasification of oil palm kernel shell with polystyrene in an air gasifier and studied influences of gasification temperature and polystyrene content on syngas composition. Burra and Gupta [23] examined a pinewood/plastic waste steam co-gasification and concluded that co-gasification of biomass/plastic resulted in higher syngas yield compared with their mono-gasification processes. Du et al. [24] developed a numerical modeling for co-gasification of coal and polyethylene terephthalate and indicated that increasing inlet gas velocity reduced hydrogen and CO2 generations and enlarged CO generation. Bian et al. [25] investigated supercritical water lignite coal/plastic waste co-gasification and their findings revealed that carbon conversion and gasification efficiencies were improved by increasing plastic content.
Although gasification performances of different biomass and plastic waste types have been well established, co-gasification of biomass/plastic waste needs more studies. The studies on co-gasification of biomass and polypropylene are limited and investigating co-gasification of eucalyptus and polypropylene was not observed in the literature. Eucalyptus had the best performance among thirteen different biomass types in gasification process according to our previous study [10] and polypropylene is one of the most predominant plastic waste types in the world [26], and therefore, their mixture has been considered in this study. The literature review showed that co-gasification of biomass and plastic waste improves their mono-gasification performances and a synergistic effect takes place. Therefore, the co-gasification of eucalyptus and polypropylene waste could be a beneficial process to achieve a clean and efficient gasification. A comparison analysis has been implemented between air and steam co-gasification of eucalyptus and polypropylene waste which endows another novelty aspect to this study. Tri-objective optimization of co-gasification of eucalyptus and polypropylene waste using central composite design to achieve a clean and efficient gasification is also one of the main novelties and contributions of this study.
2 Theoretical procedure
2.1 Gasification modeling
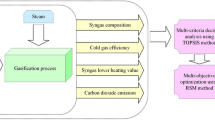
In this study, air and steam co-gasification of eucalyptus and polypropylene waste has been investigated and its schematic is presented in Fig. 1. Eucalyptus and polypropylene waste are mixed at specific weight ratio and fed to the gasifier reactor as feedstock and reacted with gasifying agent which is air or steam.
Air and steam gasification reactions are as [27]:
where \({\mathrm{CH}}_{\mathrm{x}}{\mathrm{O}}_{\mathrm{y}}\) indicates chemical composition of feedstock, x denotes hydrogen-to-carbon ratio, and y indicates oxygen-to-carbon ratio; \(\delta\) and \(\alpha\) are moles of air and steam fed to the gasifier reactor as gasifying agents, \(\beta\) denotes moisture of feedstock, and \({y}_{i}\) is the mole number of syngas component. These mole numbers of components are unknown and should be calculated for evaluating gasification performance. There are different approaches in this regard. This study utilizes a minimization of Gibbs free energy coupled with Lagrange of undetermined multipliers in which its details have been well presented in the literature [14, 15, 28].
G, the total Gibbs free energy, is defined as [14]:
where \({y}_{i}\) is the molar flow, \(\overline{G }\) is the standard G, R is the universal gas constant, and T is the temperature. The following equations conserve the elements [14]:
where \({p}_{e}\) is ith element total atom number.
λ, as the Lagrangian multiplier, is defined as [15]:
For minimization of G [28]:
Therefore, mole numbers of components are found.
Neglecting potential and kinetic energies variations, considering 25 °C of temperature and 101 kPa of pressure for environmental conditions, considering ideal gas and neglecting reactor design, considering equilibrium state and neglecting tar amount, considering dimensionless gasifier reactor, and neglecting heat loss are the assumptions considered in the modeling.
For evaluating co-gasification performance of eucalyptus and polypropylene waste, hydrogen and energy efficiencies are calculated as follows [29]:
where \({\eta }_{h}\) and \({\eta }_{e}\) are hydrogen and energy efficiencies, respectively, \(\beta\) is energy, \({\beta }_{in}\) is input energy, and \({\beta }_{{H}_{2}}\), \({\beta }_{CO}\), and \({\beta }_{{CH}_{4}}\) are energies of hydrogen, carbon monoxide, and methane in syngas. \({\beta }_{i}\) is calculated as follows [29]:
where \({\mu }_{i}\) is HHV (high heating value) and can be calculated based on proximate and ultimate analyses as follows [30]:
Table 1 shows ultimate and proximate analyses for eucalyptus and polypropylene waste considered for co-gasification in this study.
2.2 Central composite design
This is a group of statistical and mathematical procedures valuable for modeling, analysis, and optimization of engineering problems in which response outputs are affected by several input variables [32,33,34]. Consider the response output (y) as a function of input variables (\({x}_{i}\)) as follows:
where \(\varepsilon\) denotes error representing all possible errors in response output including experimental and measurement errors and any kind of deviations which are not considered in f. y is typically considered a first- or second-order model as [35, 36]:
where \({\sigma }_{0}\) is constant term, \({\sigma }_{1}\) and \({\sigma }_{2}\) are linear terms, \({\sigma }_{11}\) and \({\sigma }_{22}\) are square terms, and \({\sigma }_{12}\) is interaction term.
Minitab software version 20 has been utilized in this study for performing the analysis of the central composite design method. For this purpose, moisture content of feedstock, gasifier temperature, and polypropylene waste concentration in the feedstock are considered the variable factors. Moisture content is considered in the range of 0–30 wt%, gasifier temperature is set on 700–1000 °C, and polypropylene waste concentration is changed from 0 to 100 wt%. Hydrogen and energy efficiencies and carbon dioxide emission are considered the response variables. Based on the considered variable factors, 21 runs are conducted to study and optimize the process. The maximization of the hydrogen and energy efficiencies and the minimization of the carbon dioxide emission are considered the goals.
3 Results and discussion
3.1 Modeling validation
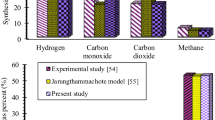
Four comparisons are conducted to validate the gasification performances modeled in this study consisting of air and steam gasification of biomass and air and steam gasification of plastic waste. The results are presented in Fig. 2. Figure 2a shows comparison analysis of syngas composition for air gasification of rubber wood at gasification temperature of 827 °C (as conducted in experiments) between experimental results reported by Jayah et al. [37], modeling results presented by Jarungthammachote and Dutta [38], and modeling results presented in this study. The results proved that the syngas composition of the present model agrees well with both experimental [37] and theoretical [38] results, and therefore, its validity is confirmed. Figure 2b indicates syngas compositions of the present model compared with results of Rapagnà et al. [39] and modeling results presented by Karmakar et al. [40] for steam gasification of olivine particle at 770 °C of temperature and 101 kPa of pressure. The results reveal that the syngas compositions predicted by the present model are in line with both experimental [39] and theoretical [40] results in the same conditions. Hence, the model developed for steam gasification of biomass is verified. Figure 2c shows comparison analysis of syngas composition in air gasification of a mixture plastic waste between the model presented in this study and experimental results reported by Cho et al. [41] at 803 °C of temperature and 101.3 kPa of pressure. The results show an appropriate agreement between the syngas composition obtained by the model presented in this study and those reported in [41]. Hence, the present model for air gasification of plastic waste is verified. Figure 2d indicates syngas composition comparative analysis for steam gasification of polypropylene waste at 800 °C of temperature and 101.3 kPa of pressure. The comparison was conducted between the results of the present model with experimental results of [42] and theoretical findings of [43]. The results demonstrate a good agreement between the present model with experimental [42] and theoretical [43] results.
Therefore, validity of all gasification types consisting of air and steam biomass gasification and plastic waste gasification is confirmed.
In the following, firstly, the influences of moisture content of feedstock, gasification temperature, and polypropylene waste content are studied as the key features on co-gasification performances. In this regard, syngas composition and efficiencies are considered criteria. Afterward, central composite design is utilized for single- and tri-objective optimization of co-gasification of eucalyptus and polypropylene waste with respect to hydrogen and energy efficiencies and CO2 emission criteria. For studying the effect of each key feature on co-gasification performance, other parameters are set on their middle level.
3.2 Co-gasification assessment
Figure 3 indicates the influences of moisture content of feedstock on co-gasification performance of eucalyptus and polypropylene waste with a weight fraction of 50/50. Figure 3a reveals that by increasing moisture content of feedstock in air co-gasification of eucalyptus and polypropylene waste, hydrogen generation is improved, carbon monoxide generation is reduced, and carbon dioxide generation is increased while methane generation is negligible. Hydrogen generation is enhanced from 22.86 to 27.06%, carbon monoxide generation is decreased from 25.65 to 18.9%, and carbon dioxide generation is increased from 2.22 to 7.48% by increasing moisture content from 0 to 30 wt%. These observations are justified using water–gas shift reaction as follows [44]:
Higher H2O content is available for co-gasification process in higher moisture content, and therefore, based on water–gas shift reaction, more CO reacts with H2O and more CO2 and hydrogen are produced.
Comparing these results with those reported in the literature confirms the validity of these trends as Xie et al. [45] informed similar observations for air gasification of sewage sludge and concluded that increasing moisture content of sewage sludge enlarged hydrogen and CO2 generations and decreased CO generation. Jahromi et al. [46] observed similar tendencies in gasification of sugarcane bagasse and reported that hydrogen generation was enhanced, carbon dioxide generation was increased, and carbon monoxide generation was reduced by increasing moisture content from 1 to 20% which are in agreement with the observations of this study.
Figure 3b indicates the influences of moisture content of feedstock on co-gasification performance of eucalyptus and polypropylene waste with a weight fraction of 50/50. The results show that hydrogen generation does not change markedly by altering moisture content and only slightly decreased from 66.25 to 65.49% by increasing moisture content from 0 to 30 wt%. CO generation was augmented from 24.13 to 26.85% and carbon dioxide generation was reduced from 9.59 to 7.62% by increasing moisture content from 0 to 30 wt%. Methane generation in steam co-gasification of eucalyptus and polypropylene waste is insignificant. Similar observations were reported for steam gasification of four types of plastic waste [14].
Figure 4a shows effects of gasification temperature on syngas composition in co-gasification of eucalyptus and polypropylene waste with a weight fraction of 50/50. The results demonstrated that hydrogen efficiency does not change noticeably with gasification temperature while carbon monoxide generation is increased from 20.32 to 23.44% and carbon dioxide generation is reduced from 6.31 to 3.95% by increasing gasification temperature from 700 to 1000 °C. According to the results of Fig. 4b, similar trends are occurred for steam co-gasification of eucalyptus and polypropylene waste with a weight fraction of 50/50. Carbon monoxide generation is increased from 21.52 to 28.17% and carbon dioxide generation is mitigated from 11.62 to 6.64% by increasing gasification temperature from 700 to 1000 °C while hydrogen generation remains constant at about 65%. These trends are verified using Boudouard reaction as follows [47]:
Boudouard reaction is an endothermic reaction and is shifted to the production side in higher temperatures, and therefore, CO2 is converted to CO. Therefore, increasing CO generation and decreasing CO2 generation in higher temperatures are verified.
Jahromi et al. [46] reported similar observations for gasification of sugarcane bagasse and detected that carbon monoxide generation is increased and carbon dioxide generation is decreased with rise of gasification temperature. Mojaver et al. [28] showed that carbon monoxide generation is increased and carbon dioxide generation is reduced for gasification of four different types of plastic waste and six various biomass kinds. Saebea et al. [43] reported decreasing behavior of carbon dioxide generation and increasing trend of carbon monoxide generation for steam gasification of polyethylene waste. Similar trends have been reported for steam gasification of beech chips [48], air/steam gasification of wood residue [11], and air, steam, and oxygen municipal solid waste gasification [9]. Therefore, the tendencies of syngas composition versus temperature in this study are verified compared to the literature.
It is noteworthy to mention that CH4 generation in co-gasification of eucalyptus and polypropylene waste with a weight fraction of 50/50 is negligible; however, its insignificant content tends to zero by increasing gasification temperature.
Figure 5 shows effects of polypropylene waste content on syngas composition of co-gasification of eucalyptus and polypropylene waste from 0 to 100 wt%. It is noteworthy to mention that 0 wt% of polypropylene waste indicates mono-gasification of eucalyptus and 100 wt% of polypropylene waste denotes mono-gasification of polypropylene waste. Figure 5a indicates that air mono-gasification of eucalyptus results in hydrogen generation of 22.34% and air mono-gasification of polypropylene waste produces 26.18% of hydrogen. An improving behavior was observed in air co-gasification of eucalyptus and polypropylene waste and 23.65%, 24.57%, 25.25%, and 25.77% of hydrogen generation are obtained in polypropylene waste content of 20, 40, 60, and 80 wt%, respectively. Adding polypropylene waste content does not change carbon monoxide generation while evidently decreased carbon dioxide generation from 9.13 to 3.03% by adding polypropylene content from 0 to 100 wt%. Improving hydrogen generation by addition of polypropylene waste content can be justified by water–gas reaction as follows [49]:
Polypropylene waste has noticeably more carbon content compared with eucalyptus (85.56% compared with 48.2%), and therefore, carbon content of feedstock is increased by addition of polypropylene waste content. Hence, more hydrogen is produced in water–gas reaction.
Decreasing carbon dioxide generation by increasing polypropylene waste content can be justified using Boudouard reaction because more carbon dioxide reacts with more carbon content of feedstock and its generation is reduced.
Figure 5b demonstrates that increasing polypropylene waste content from 0 to 100 wt% markedly improves hydrogen generation from 61.79 to 66.6%, enhances carbon monoxide generation from 21.3 to 30.35%, and mitigates carbon dioxide generation from 16.9 to 2.52%. These trends are verified using water–gas and Boudouard reactions.
Figure 6 indicates effects of variable parameters on efficiencies of co-gasification of eucalyptus and polypropylene waste. Figure 6a shows effects of moisture content of feedstock on efficiencies of air and steam co-gasification of eucalyptus and polypropylene waste and shows that H2 efficiencies of co-gasification are boosted by increasing moisture content. These improvements are from 34.94 to 44.76% and from 39.34 to 46.08%, respectively. Steam co-gasification results in higher hydrogen efficiency compared with air co-gasification at all moisture contents. Energy efficiency in air co-gasification remains constant because hydrogen content is increased and carbon monoxide content is decreased (see Fig. 3a) and these changes neutralize each other. Energy efficiency of steam co-gasification is improved markedly from 56.03 to 68.13%. It is important to note that air co-gasification leads to higher energy efficiency compared with steam co-gasification. Figure 6b indicates effects of gasification temperature on efficiencies of air and steam co-gasification of eucalyptus and polypropylene waste. Higher temperatures lead to slight improvement of hydrogen efficiency followed by its minor decrement. The hydrogen efficiency of air co-gasification is enhanced from 39.68 to 40.36% and, then, is decreased to 38.25%. This enhancing trend is from 41.85 to 42.79%, and then, the decreasing behavior is to 41.24% in steam co-gasification. According to the results, energy efficiency of air co-gasification is slightly improved from 79.79 to 81.09% while this improvement is from 61.10 to 61.94% in steam co-gasification by increasing gasification temperature. The findings reveal that hydrogen efficiency of steam co-gasification is higher compared with air co-gasification; however, air co-gasification results in higher energy efficiency. Figure 6c shows effects of polypropylene waste content on efficiencies of co-gasification of eucalyptus and polypropylene waste. Increasing polypropylene waste content from 0 to 100 wt% improved hydrogen efficiency from 37.4 to 39.89% in air co-gasification and from 40.08 to 53.71% in steam co-gasification. Energy efficiency of air co-gasification is slightly reduced from 81.7 to 78.82% and that of steam co-gasification is dramatically improved from 56.13 to 82.73%. Steam co-gasification leads to higher hydrogen efficiency while energy efficiency of air co-gasification is higher especially at lower polypropylene waste contents.
3.3 Central composite design analysis
Figure 7 shows single-objective optimization results for efficiencies and carbon dioxide emission in air co-gasification of eucalyptus and polypropylene waste. Figure 7a indicates that the maximum hydrogen efficiency is almost 45% at moisture contents higher than 26 wt% and temperatures lower than 800 °C. Figure 7b demonstrates that the maximum hydrogen efficiency is reached at moisture contents higher than 27 wt% and polypropylene waste contents higher than 20 wt% and this optimum hydrogen efficiency equals 44%. Figure 7c demonstrates that the maximum energy efficiency equals 40% and is gained at temperatures lower than 800 °C and polypropylene waste contents between 30 and 70 wt%. According to Fig. 7d, the maximum energy efficiency is almost 81% and is attained at simultaneous maximum values of moisture content and temperatures. Figure 7e reveals that the ideal energy efficiency is 82% and is obtained at moisture contents higher than 20 wt% and polypropylene waste contents lower than 10 wt%. Figure 7f shows that temperatures higher than 900 °C and polypropylene waste contents lower than 10 wt% result in the maximum energy efficiency of 82%. Figure 7g indicates that the minimum carbon dioxide emission is 4 g/s and is reached at temperatures higher than 800 °C and moisture contents lower than 5 wt%. The optimum carbon dioxide emission is reached at moisture contents lower than 5 wt% and polypropylene waste contents higher than 60 wt% and equals almost 3 g/s, as the results of Fig. 7h show. Figure 7i indicates that the minimum carbon dioxide emission is reached at simultaneous maximum values of polypropylene waste content and temperature and this optimum carbon dioxide emission is almost 5 g/s.
Optimization results in air co-gasification: a hydrogen efficiency versus temperature and moisture, b hydrogen efficiency versus polypropylene content and moisture, c hydrogen efficiency versus temperature and polypropylene content, d energy efficiency versus temperature and moisture, e energy efficiency versus polypropylene content and moisture, f energy efficiency versus polypropylene content and temperature, g CO2 emission versus temperature and moisture, h CO2 emission versus polypropylene content and moisture, and i CO2 emission versus polypropylene content and temperature
Figure 8 depicts the results of single-objective optimization analysis for efficiencies and carbon dioxide emission in steam co-gasification of eucalyptus and polypropylene waste. Figure 8a reveals that the maximum H2 efficiency is reached at moisture contents higher than 25 wt% for all gasification temperatures and equals 45%. According to the results of Fig. 8b, simultaneous maximum levels of polypropylene waste and steam contents result in the maximum H2 efficiency equals to 48%. Figure 8c shows that polypropylene waste contents higher than 80 wt% at all gasification temperatures lead to the maximum hydrogen efficiency about 46%. Figure 8d demonstrates that moisture contents higher than 28% at all gasification temperatures result in the maximum energy efficiency of 67%. The maximum energy efficiency, equals 75%, is reached at simultaneous maximum levels of polypropylene waste and moisture contents, as the results of Fig. 8e indicate. Figure 8f reveals that polypropylene waste contents higher than 85% at all gasification temperatures lead to the maximum energy efficiency of 68%. The minimum carbon dioxide emission is attained at simultaneous maximum values of temperature and moisture equals to 8 g/s, as Fig. 8g shows. Figure 8h demonstrates that moisture content higher than 20 wt% and polypropylene waste content higher than 80 wt% result in the minimum carbon dioxide emission of 5 g/s. According to the results of Fig. 8i, gasification temperatures higher than 850 °C and polypropylene waste content higher than 85% lead to the optimum carbon dioxide emission which is almost 5 g/s.
Optimization results in steam co-gasification: a hydrogen efficiency versus temperature and moisture, b hydrogen efficiency versus polypropylene content and moisture, c hydrogen efficiency versus temperature and polypropylene content, d energy efficiency versus temperature and moisture, e energy efficiency versus polypropylene content and moisture, f energy efficiency versus temperature and polypropylene content, g CO2 emission versus temperature and moisture, h CO2 emission versus polypropylene content and moisture, and i CO2 emission versus polypropylene content and temperature
Figure 9 presents the results of tri-objective optimization of efficiencies and carbon dioxide emission in co-gasification of eucalyptus and polypropylene waste. Figure 9a indicates that moisture content of 25.76 wt%, gasification temperature of 954.55 °C, and polypropylene waste content of 53.81 wt% are multi-objective optimum conditions to achieve an efficient and clean air co-gasification. The optimum outputs are hydrogen efficiency of 71.87%, energy efficiency of 81.14%, and CO2 emission of 9.37 g/s in these conditions. Figure 9b shows that simultaneous highest levels of parameters result in an efficient and clean steam co-gasification, and moisture content of 30 wt%, gasification temperature of 1000 °C, and polypropylene waste content of 100 wt% are the multi-objective optimum conditions. These conditions lead to hydrogen efficiency of 49.86%, energy efficiency of 77.21%, and CO2 emission of 2.21 g/s. The results indicated that air co-gasification of eucalyptus and polypropylene waste leads to higher H2 efficiency and lower carbon dioxide emission compared with steam co-gasification. However, energy efficiency is higher in steam co-gasification than air co-gasification.
4 Conclusions
Air and steam co-gasification processes of eucalyptus and polypropylene waste were studied in detail and tri-objective optimized with respect to efficiencies and carbon dioxide emission using central composite design methodology. The main achievements of this study can be summarized as:
-
Increasing polypropylene waste content mitigated carbon dioxide emission in co-gasification, improved efficiencies in steam co-gasification, and did not change markedly efficiencies of air co-gasification.
-
Increasing moisture content of feedstock increased carbon dioxide emission of air co-gasification, reduced carbon dioxide emission of steam co-gasification, improved noticeably hydrogen efficiencies, and enhanced slightly energy efficiencies of co-gasification.
-
Polypropylene waste fractions of about 40% resulted in optimum conditions for air co-gasification while this value is 100% for steam co-gasification.
-
Air co-gasification of eucalyptus and polypropylene waste led to higher energy efficiency compared with steam co-gasification while higher hydrogen efficiency and lower carbon dioxide emission were attained in steam co-gasification.
-
A clean and efficient air co-gasification of eucalyptus and polypropylene waste was obtained at moisture content of 26 wt%, gasification temperature of 955 °C, and polypropylene concentration of 54 wt%.
-
Moisture content of 30 wt%, gasification temperature of 1000 °C, and polypropylene concentration of 100 wt% resulted in a clean and efficient steam co-gasification of eucalyptus and polypropylene.
Data availability
Not applicable.
References
Bahadar A, Kanthasamy R, Sait HH, Zwawi M, Algarni M, Ayodele BV, Cheng CK, Wei LJ (2022) Elucidating the effect of process parameters on the production of hydrogen-rich syngas by biomass and coal co-gasification techniques: a multi-criteria modeling approach. Chemosphere 287:132052
Yang SS, Ding MQ, He L, Zhang CH, Li QX, Xing DF, Cao GL, Zhao L, Ding J, Ren NQ, Wu WM (2021) Biodegradation of polypropylene by yellow mealworms (Tenebrio molitor) and superworms (Zophobas atratus) via gut-microbe-dependent depolymerization. Sci Total Environ 756:144087
Yang M, Li C, Luo L, Li R, Long Y (2021) Predictive model of convective heat transfer coefficient in bone micro-grinding using nanofluid aerosol cooling. Int Comm Heat Mass Tran 125:105317
Tanigaki N, Ishida Y, Osada M (2015) A case-study of landfill minimization and material recovery via waste co-gasification in a new waste management scheme. Waste Manage 37:137–146
Said Z, Arora S, Farooq S, Sundar LS, Li C, Allouhi A (2022) Recent advances on improved optical, thermal, and radiative characteristics of plasmonic nanofluids: Academic insights and perspectives. Sol Energ Mater Sol Cell 236:111504
Yong YS, Rasid RA (2021) Process simulation of hydrogen production through biomass gasification: introduction of torrefaction pre-treatment. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2021.07.010
Awais M, Li W, Munir A, Omar MM, Ajmal M (2021) Experimental investigation of downdraft biomass gasifier fed by sugarcane bagasse and coconut shells. Biomass Convers Biorefinery 11(2):429–444
Samimi F, Marzoughi T, Rahimpour MR (2020) Energy and exergy analysis and optimization of biomass gasification process for hydrogen production (based on air, steam and air/steam gasifying agents). Int J Hydrogen Energy 45(58):33185–33197
Khalilarya S, Chitsaz A, Mojaver P (2021) Optimization of a combined heat and power system based gasification of municipal solid waste of Urmia University student dormitories via ANOVA and Taguchi approaches. Int J Hydrogen Energy 46(2):1815–1827
Mojaver P, Jafarmadar S, Khalilarya S, Chitsaz A (2019) Study of synthesis gas composition, exergy assessment, and multi-criteria decision-making analysis of fluidized bed gasifier. Int J Hydrogen Energy 44(51):27726–27740
Dang Q, Zhang X, Zhou Y, Jia X (2021) Prediction and optimization of syngas production from a kinetic-based biomass gasification process model. Fuel Process Technol 212:106604
Habibollahzade A, Ahmadi P, Rosen MA (2021) Biomass gasification using various gasification agents: optimum feedstock selection, detailed numerical analyses and tri-objective grey wolf optimization. J Clean Prod 284:124718
Bai B, Wang W, Jin H (2020) Experimental study on gasification performance of polypropylene (PP) plastics in supercritical water. Energy 191:116527
Mojaver M, Azdast T, Hasanzadeh R (2021) Assessments of key features and Taguchi analysis on hydrogen rich syngas production via gasification of polyethylene, polypropylene, polycarbonate and polyethylene terephthalate wastes. Int J Hydrogen Energy 46(58):29846–29857
Hasanzadeh R, Mojaver M, Azdast T, Park CB (2021) Polyethylene waste gasification syngas analysis and multi-objective optimization using central composite design for simultaneous minimization of required heat and maximization of exergy efficiency. Energy Convers Manag 247:114713
Han SW, Lee JJ, Tokmurzin D, Lee SH, Nam JY, Park SJ, Ra HW, Mun TY, Yoon SJ, Yoon SM, Moon JH (2022) Gasification characteristics of waste plastics (SRF) in a bubbling fluidized bed: effects of temperature and equivalence ratio. Energy 238:121944
Janajreh I, Adeyemi I, Elagroudy S (2020) Gasification feasibility of polyethylene, polypropylene, polystyrene waste and their mixture: experimental studies and modeling. Sustain Energy Technol Assess 39:100684
Wang Z, Liu X, Burra KG, Li J, Zhang M, Lei T, Gupta AK (2021) Towards enhanced catalytic reactivity in CO2-assisted gasification of polypropylene. Fuel 284:119076
Li J, Burra KRG, Wang Z, Liu X, Gupta AK (2021) Co-gasification of high-density polyethylene and pretreated pine wood. Appl Energy 285:116472
Cao C, Bian C, Wang G, Bai B, Xie Y, Jin H (2020) Co-gasification of plastic wastes and soda lignin in supercritical water. Chem Eng J 388:124277
Zhu HL, Zhang YS, Materazzi M, Aranda G, Brett DJ, Shearing PR, Manos G (2019) Co-gasification of beech-wood and polyethylene in a fluidized-bed reactor. Fuel Process Technol 190:29–37
Basha MH, Sulaiman SA, Uemura Y (2020) Co-gasification of palm kernel shell and polystyrene plastic: effect of different operating conditions. J Energy Inst 93(3):1045–1052
Burra KG, Gupta AK (2018) Synergistic effects in steam gasification of combined biomass and plastic waste mixtures. Appl Energy 211:230–236
Du S, Yuan S, Zhou Q (2021) Numerical investigation of co-gasification of coal and PET in a fluidized bed reactor. Renew Energy 172:424–439
Bian C, Zhang R, Dong L, Bai B, Li W, Jin H, Cao C (2020) Hydrogen/methane production from supercritical water gasification of lignite coal with plastic waste blends. Energy Fuels 34(9):11165–11174
Europe P (2020) Plastics the facts 2020: an analysis of European plastics production, demand and waste data. Plastic Europe, Brussels
Hasanzadeh R, Mojaver M, Azdast T, Park CB (2021) A novel systematic multi-objective optimization to achieve high-efficiency and low-emission waste polymeric foam gasification using response surface methodology and TOPSIS method. Chem Eng J. https://doi.org/10.1016/j.cej.2021.132958
Mojaver M, Hasanzadeh R, Azdast T, Park CB (2022) Comparative study on air gasification of plastic waste and conventional biomass based on coupling of AHP/TOPSIS multi-criteria decision analysis. Chemosphere 286:131867
Hasanzadeh R, Azdast T, Mojaver M, Park CB (2022) High-efficiency and low-pollutant waste polystyrene and waste polystyrene foam gasification: comprehensive comparison analysis, multi-objective optimization and multi-criteria decision analysis. Fuel 316:123362
Channiwala SA, Parikh PP (2002) A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 81(8):1051–1063
Pio DT, Ruivo LCM, Tarelho LAC, Frade JR, Kantarelis E, Engvall K (2021) Tar formation during eucalyptus gasification in a bubbling fluidized bed reactor: effect of feedstock and reactor bed composition. Energy Convers Manag 229:113749
Perwitasari U, Agustina NT, Pangestu R, Amanah S, Saputra H, Andriani A, Juanssilfero AB, Thontowi A, Widyaningsih TD, Eris DD, Amaniyah M (2021) Cacao pod husk for citric acid production under solid state fermentation using response surface method. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01690-9
Zhang Y, Li C, Jia D, Li B, Wang Y, Yang M, Hou Y, Zhang X (2016) Experimental study on the effect of nanoparticle concentration on the lubricating property of nanofluids for MQL grinding of Ni-based alloy. J Mater Process Technol 232:100–115
Zalazar-García D, Feresin GE, Rodriguez R (2020) Optimal operational variables of phenolic compound extractions from pistachio industry waste (Pistacia vera var. Kerman) using the response surface method. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00862-3
Yang M, Li C, Zhang Y, Jia D, Li R, Hou Y, Cao H, Wang J (2019) Predictive model for minimum chip thickness and size effect in single diamond grain grinding of zirconia ceramics under different lubricating conditions. Ceram Int 45(12):14908–14920
Poy H, Lladosa E, Gabaldón C, Loras S (2021) Optimization of rice straw pretreatment with 1-ethyl-3-methylimidazolium acetate by the response surface method. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02111-7
Jayah TH, Aye L, Fuller RJ, Stewart DF (2003) Computer simulation of a downdraft wood gasifier for tea drying. Biomass Bioenerg 25(4):459–469
Jarungthammachote S, Dutta A (2007) Thermodynamic equilibrium model and second law analysis of a downdraft waste gasifier. Energy 32(9):1660–1669
Rapagnà S, Jand N, Kiennemann A, Foscolo PU (2000) Steam-gasification of biomass in a fluidised-bed of olivine particles. Biomass Bioenerg 19(3):187–197
Karmakar MK, Datta AB (2011) Generation of hydrogen rich gas through fluidized bed gasification of biomass. Biores Technol 102(2):1907–1913
Cho MH, Mun TY, Kim JS (2013) Production of low-tar producer gas from air gasification of mixed plastic waste in a two-stage gasifier using olivine combined with activated carbon. Energy 58:688–694
Wu C, Williams PT (2009) Hydrogen production by steam gasification of polypropylene with various nickel catalysts. Appl Catal B 87(3–4):152–161
Saebea D, Ruengrit P, Arpornwichanop A, Patcharavorachot Y (2020) Gasification of plastic waste for synthesis gas production. Energy Rep 6:202–207
Wojnicka B, Ściążko M, Schmid JC (2019) Modelling of biomass gasification with steam. Biomass Convers Biorefinery 11:1787–1805
Xie LP, Tao LI, Gao JD, Fei XN, Xia WU, Jiang YG (2010) Effect of moisture content in sewage sludge on air gasification. J Fuel Chem Technol 38(5):615–620
Jahromi R, Rezaei M, Samadi SH, Jahromi H (2021) Biomass gasification in a downdraft fixed-bed gasifier: optimization of operating conditions. Chem Eng Sci 231:116249
Liu W, Tian Y, Yan H, Zhou X, Tan Y, Yang Y, Li Z, Yuan L (2021) Gasification of biomass using oxygen-enriched air as gasification agent: a simulation study. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-02035-2
Schuster G, Löffler G, Weigl K, Hofbauer H (2001) Biomass steam gasification–an extensive parametric modeling study. Biores Technol 77(1):71–79
Mauerhofer AM, Müller S, Bartik A, Benedikt F, Fuchs J, Hammerschmid M, Hofbauer H (2021) Conversion of CO2 during the DFB biomass gasification process. Biomass Convers Biorefinery 11(1):15–27
Author information
Authors and Affiliations
Contributions
Dr. Parisa Mojaver: methodology, software, validation, investigation; formal analysis, writing-original draft
Dr. Rezgar Hasanzadeh: methodology, software, validation, investigation; formal analysis, writing-original draft
Prof. Ata Chitsaz: conceptualization, investigation, writing-review and editing, supervision
Prof. Taher Azdast: conceptualization, investigation, writing-review and editing, supervision
Mr. Mehran Mojaver: methodology, software, formal analysis, writing-original draft
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Parisa Mojaver and Rezgar Hasanzadeh contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mojaver, P., Hasanzadeh, R., Chitsaz, A. et al. Tri-objective central composite design optimization of co-gasification of eucalyptus biomass and polypropylene waste. Biomass Conv. Bioref. 14, 4829–4841 (2024). https://doi.org/10.1007/s13399-022-02597-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02597-9