Abstract
Biomass treatment and upcycling is attracting considerable critical attention. Upcycling is a converting procedure to alter a valueless or low-value product to another form with higher value, and in this regard, biomass gasification is an upcycling process which converts biomass into a hydrogen-rich syngas. In recent years, researchers have shown an increased interest in biomass gasification; however, there is a lack of a comprehensive and systematic research considering simultaneously various biomass types and gasifying mediums. This study set out to simultaneously consider twenty biomass types and three gasifying mediums and perform a systematic multi-criteria decision analysis to select the best coupling of biomass type/gasifying medium considering nine criteria of syngas compositions and different efficiencies. Biomass types were ranked using the technique for order of preference by similarity to ideal solution method for each gasifying medium, and a sensitivity analysis was performed to select the first and second biomass types with respect to different criteria weights. Performance of gasification processes was multi-objective optimized using response surface methodology. A systematic multi-criteria decision analysis and a sensitivity analysis were conducted to choose the best gasification performance of the best biomass types in their optimum conditions. The findings revealed that gasification with a steam gasifying agent by pine sawdust biomass had the best performance and produced 46.96% of hydrogen and only 4.99% of carbon dioxide and led to energy and exergy efficiencies of 80.91% and 86.03%, respectively. The findings can contribute to a better understanding of the biomass gasification process with different feedstocks and agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biomass has several advantages, such as its independence from climate and location, ease of storing and transport, and availability compared to other renewable sources. Biomass gasification as an efficient upcycling technology is one of the most promising alternatives for the direct utilization of fossil fuels with a bright outlook [1]. A combustible syngas containing hydrogen, methane, and carbon monoxide is resulted from biomass gasification having valuable heating values for applications in power, combined, and multi-generation systems [2]. There are different biomass types and gasifying mediums for this good process and many researchers have studied their performances.
Zhang et al. [3] developed a co-generation system of heat and power based on biomass gasification. They considered municipal solid waste, paddy husk, paper, and wood as the potential feedstocks for their system. Their findings confirmed that municipal solid waste gasification resulted in higher electricity production, higher heat production, and larger exergy efficiencies. Therefore, they introduced municipal solid waste as the best feedstock concerning the mentioned criteria. Municipal solid waste gasification also ranked second concerning energy efficiency and exergy destruction rate criteria. A combined heat and power system was activated using rice straw gasification based on air and steam as gasifying mediums by Wu et al. [4]. Their findings revealed that hydrogen, carbon dioxide, and methane productions were increased with rising of steam to biomass ratio; however, carbon monoxide production was reduced. Safari and Dincer [5] developed a co-generation system using algal biomass gasification for producing green hydrogen and electrical power. They showed that increasing the biomass flow rate decreased the exergy efficiency of the system and increased the total cost rate. Also, their results indicated that the total cost rate significantly decreased when the gasifier worked at higher temperatures. However, the changes in the exergy efficiency versus the gasifier temperature were negligible. An integrated tri-generation system of power, heat, and liquefied natural gas was developed by Ebrahimi and Ziabasharhagh [6] using rice husk gasification. They used oxygen and steam as gasifying mediums and concluded that changing gasifier pressure did not affect syngas compositions. Cao et al. [7] used biomass in a gas turbine unit coupled with an organic Rankine cycle/absorption refrigeration cycle and generated power by waste heat from the biomass process. They compared these two cases from thermo-economic viewpoints and applied a genetic algorithm for multi-objective optimization. The results indicated that the gas turbine unit coupled with inlet cooling had an optimal state with 11.2% higher exergy efficiency than other cases. Lin et al. [8] proposed a co-generation system of electrical power and liquid hydrogen based on biomass gasification. They studied the influence of biomass flow rate on the system performance. Their findings revealed that the exergy efficiency decreased and the total cost rate increased when the biomass mass flow rate enlarged. Also, more power was produced, and the mass flow rate of liquid hydrogen improved when the biomass flow rate increased. AlNouss et al. [9] performed an analysis on gasification of coconut coir pith and its char in the presence of steam as the gasifying medium. Coconut coir pith gasification yielded more hydrogen production at lower gasifier temperatures and steam to biomass ratios. However, hydrogen production was higher in coconut coir pith char gasification at higher gasifier temperatures and steam to biomass ratios than coconut coir pith gasification. Safarian et al. [10] developed a combined heat and power system based on air biomass gasification of garden waste, timber and wood waste, and mixed paper waste. Their results indicated that the gasification of timber and wood waste generated higher electrical efficiencies at all gasifier temperatures and equivalence ratios compared to the other two biomasses. Cao et al. [11] studied a co-generation system producing electrical power and hydrogen fueled by biomass gasification and digestion. Their main purpose was to conduct a comparison investigation from thermodynamic, economic, and electrochemical viewpoints. Their results showed that the system based on biomass digestion had a better performance from a thermodynamic viewpoint; however, the system based on biomass gasification was better concerning economic criteria. A comparative study between biomass gasifications with steam and oxygen mediums in a poly-generation system was conducted by AlNouss et al. [12]. A blend of sludge, food waste, manure, and date pit was considered as biomass and a techno-economic-environmental investigation was performed. Their results revealed that a steam biomass gasification-based system presented economically and environmentally better performance compared to a system based on oxygen gasification. Cao et al. [13] developed a co-generation system of electrical power and heat based on peach stone gasification. Their results showed that increasing the equivalence ratio decreased the net produced electrical power and increased the produced heat. Li et al. [14] triggered a solid oxide fuel cell by a biomass gasifier to generate electrical power and heat. Steam, air, and oxygen were considered as the gasifying agents and this process was simulated in Aspen Plus software. The findings illustrated that their system could reduce the dependence on fossil fuels using agricultural waste, and the energy and exergy efficiencies of the system were achieved by 67.3% and 29.2%, respectively. Ishaq et al. [15] studied the effects of gasifier parameters on a multi-generation system based on rice husk gasification using a steam gasifying agent. Sugarcane bagasse gasification with an air/steam gasifying medium was comprehensively assessed by Jahromi et al. [16]. Their findings reveal that hydrogen production and conversion efficiency were markedly improved by increasing steam to air ratio. Drying sugarcane bagasse also led to gasification with higher hydrogen production and a more efficient conversion rate. Biomass downdraft gasifier reactor was coupled with a desalination unit to run a co-generation (electrical power and freshwater) system by Sorgulu and Dincer [17]. Air and municipal solid waste were selected as gasifying medium and feedstock, respectively. The results showed that energy efficiency of 37.04% and exergy efficiency of 19.78% were obtained for the proposed system.
Biomass gasification as one of the most promising candidates to resolve the issues of fossil fuels can be performed using different biomass types and gasifying mediums. Several studies have been performed on the performance of systems based on biomass gasification with different biomass types and/or gasifying mediums. However, there is a lack of comprehensive and systematic research considering various biomass types and gasifying mediums. The main objective of this paper is to perform a systematic research study on various biomass types and gasifying mediums using multi-criteria decision analysis techniques. Five syngas composition criteria of hydrogen, carbon monoxide, carbon dioxide, methane, and nitrogen productions and four efficiency criteria of energy, chemical, exergy, and hydrogen efficiencies are considered, and the best performance of the gasification process is selected concerning the feedstock and agent. The main contributions of the present study can be summarized as follows:
-
(1)
Considering simultaneously various biomass types and gasifying mediums, including twenty different biomass types and three gasifying mediums of steam, air, and oxygen
-
(2)
Selecting the best biomass type for each gasifying medium based on a systematic multi-criteria decision analysis considering nine different criteria
-
(3)
Performing sensitivity analysis on the best biomass-relevant gasifying medium
-
(4)
Optimizing performance of best biomass-relevant gasifying medium using response surface methodology
-
(5)
Performing a systematic multi-criteria decision analysis to select the best combination of biomass/gasifying agents between sixty possible alternatives (twenty biomass types × three gasifying mediums)
2 System modeling
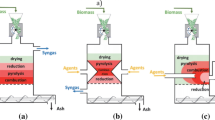
Gasification is a process that takes place in the presence of biomass and gasifying agent. The biomass can be converted to combustible gases using a gasifying agent. The gasifying agent is selected between steam, air, carbon dioxide, and oxygen and also a combination of them, such as steam–air [18]. Each of them has some advantages and disadvantages; therefore, steam, air, and oxygen are fed to the gasifier reactor separately in this study. Twenty types of biomass, apricot stone [19], beech wood [20], cedar wood [21], coffee husk [22], corn cob [23], cotton stem [24], grapevine pruning waste [25], holm oak [26], jute stick [27], legume straw [28], olive refuse [29], peach stone [30], pine sawdust [31], rice straw [31], spruce wood pellet [32], sugarcane bagasse [33], sunflower shell [29], switchgrass [33], wheat straw [34], and wood sawdust [35], are fed to the gasifier. Drying, pyrolysis, oxidation, and reduction are four steps in the gasifier reactor. Biomass crosses through a screw feeder and enters the gasifier and then is dried in the first step. This step needs heat for the heating process obtained from the exothermic reactions happening in the gasifier reactor. The pyrolysis process is done in the second step. The pyrolyzed biomass reacts with a gasifying agent, and the oxidation is done in the third step. Finally, the fourth step occurs, and synthesis gas is produced including carbon monoxide, carbon dioxide, methane, hydrogen, steam, and nitrogen.
The equilibrium reactions that take place through the gasification process are as follows [36]:
In general, a universal gasification reaction is as follows [37]:
The chemical formula of biomasses is \({{CH}}_{\alpha }{{O}}_{\beta }\) and \(\alpha\) and \(\beta\) are hydrogen to carbon and oxygen to carbon ratios achieved from ultimate and proximate analyses presented in Table S.1 (available in the supplementary information file). \(\varphi\) is the moisture content and \(\delta\), \(\varepsilon\), and \(\theta\) are feeding steam, oxygen, and air to the gasifier reactor. The amount of \(\delta\) is zero in oxygen and air gasifying agents, the amount of \(\varepsilon\) is zero in the steam gasifying agent and equal to \(\theta\) in oxygen and air gasifying agents, and the amount of \(\theta\) is zero in steam and oxygen gasifying agents. \({\gamma }_{i}\) is mole of synthesis gas produced per 1 mol of biomass. Obtaining the synthesis gas contents is the main question.
Four molar balance relations are applied to achieve six unknowns \({\gamma }_{i}\) as follows:
As mentioned, Eqs. (1–11) happen through the gasification process which their equilibrium constants can be calculated. As regards the equilibrium constants which characterize the reaction’s extent, some main reactions can be selected because of their higher significant equilibrium constants. Two equilibrium reactions are water–gas shift (Eq. (7)) and methanation (Eq. (9)) reactions.
The equilibrium constants of these two equilibrium reactions are defined as follows [38]:
where \({K}_{WGS}\) and \({K}_{MTN}\) are equilibrium constants of water–gas shift and methanation reactions, respectively. \({P}_{i}\) and \({x}_{i}\) are partial pressure and molar fraction of synthesis gas species, and \({\gamma }_{t}\) is the sum of \({\gamma }_{i}\). Also, the equilibrium constant is a function of temperature and can be expressed as follows [38]:
where \(\Delta {G}_{T}^{o}\), \(\Delta {H}_{T}^{o}\), and \(\Delta {S}_{T}^{o}\) are the Gibbs free energy, enthalpy, and entropy at standard state, respectively. \({R}_{m}\) and \(T\) are universal gas constant and temperature. Equations (21) and (22) are achieved by substituting Eqs. (19) and (20) in Eqs. (17) and (18):
In this way, the six unknown molar contents are found.
The energy balance equation for the gasification process versus three considered gasifying agents is expressed as follows [39]:
where \({Q}_{{in}}\) and \({Q}_{{out}}\) are input and output heat and \({\overline{h} }_{f}^{^\circ }\) is the formation enthalpy. The enthalpy difference between the considered and reference states (\({\Delta \overline{h} }_{T,i}^{^\circ }\)) can be expressed as [40]:
The amounts of A, B, C, and D are presented in Table S.2 (available in the supplementary information file).
The proposed unit can be evaluated from exergy perspective by applying the thermodynamic second law. This law is expressed in Eq. (25) at steady state condition and by neglecting the difference in kinetic and potential exergies [41, 42]:
where \({E}_{W}\) and \({E}_{d}\) are the power and destruction exergies and \(E\) is the total flow exergy as follows:
The standard chemical exergy of species (\({\overline{ex} }_{i}^{ch,0}\)) is presented in the literature [43, 44].
Exergy of input biomass (\({E}_{{Biomass}}\)) can be defined as [45]:
The main efficiencies including energy (\({\eta }_{en}\)), chemical (\({\eta }_{ch}\)), exergy (\({\eta }_{ex}\)), and hydrogen (\({\eta }_{{H}_{2}}\)) efficiencies are considered in this study as follows:
It is noteworthy to mention that the system modeling is conducted in EES (Engineering Equation Solver) software.
3 Multi-criteria decision analysis and optimization methods
In this study, the best biomass type for each gasifying medium is first identified using a systematic multi-criteria decision analysis based on a technique for order preferences by similarity to ideal solution (TOPSIS) method. There are twenty alternatives which are biomass types and nine criteria for each multi-criteria decision problem. Sensitivity analysis is performed by changing the weights of considered criteria. The best alternative, i.e., biomass type, is selected after sensitivity analysis. Afterward, an optimization procedure based on the response surface methodology is implemented to multi-objective optimize the performance of the best biomass type gasification for each gasifying medium. Later, a systematic multi-criteria decision analysis is again conducted to select the best biomass type/gasifying medium combination in its optimum performance state. The theoretical details of TOPSIS and response surface methodology are briefly presented in the following subsections.
3.1 TOPSIS method
The TOPSIS method is one of the most applicable techniques for alternative ranking in multi-criteria decision problems, which is based on distances from the ideal and the non-ideal solutions. The best alternative should have the shortest distance from the ideal solution and the longest distance from the non-ideal solution [46]. This method can be briefly explained as follows [47, 48].
Steps of the TOPSIS method begin with normalization of the decision matrix as follows:
where \({X}_{ij}\) indicates the performance value of the ith alternative versus the jth criterion and \({N}_{ij}\) is the related normalized index. n and m are the numbers of criteria and alternatives contributing to the multi-criteria decision problem, respectively.
The normalized decision matrix is weighted as follows:
where \({\omega }_{j}\) is the weight of the jth criterion and \({W}_{ij}\) is the weighted normalized index.
Ideal solutions are maximum \({W}_{ij}\) in larger-is-better criterion and minimum \({W}_{ij}\) in smaller-is-better criterion. Non-ideal solutions are minimum and maximum \({W}_{ij}\) in larger-is-better and smaller-is-better criteria, respectively.
The best alternative should be near to ideal solutions and far from non-ideal solutions. Distances of each alternative from ideal and non-ideal solutions are as follows, respectively:
where \({D}_{j}^{+}\) and \({D}_{j}^{-}\) are distances from ideal and non-ideal solutions, respectively. \({W}_{i}^{+}\) and \({W}_{i}^{-}\) are ideal and non-ideal solutions, respectively.
The closeness coefficient (\(\xi\)) for each alternative is calculated as follows:
The higher the closeness coefficient is, the better the ranking is. TOPSIS software was employed for performing TOPSIS analysis.
3.2 Response surface methodology
The design of the experiment method has a high potential for optimization and response surface methodology is one of the most applicable techniques, especially in the fields of energy. This technique, due to regulating diversity as the leading cause of poor quality, guarantees a continuous enhancement [49,50,51]. Response surface methodology has several valuable tools and analyses; however, in this study, the optimization approach of this technique is utilized based on the aims defined in the present research work. All the statistical and optimization analyses of response surface methodology are implemented in Minitab software.
4 Results and discussion
4.1 Model validation
The synthesis gas production by this study is compared with an experimental study by Loha et al. [52] and a modeling study by Loha et al. [53] and also compared with an experimental study by Jayah et al. [54] and a modeling study by Jarungthammachote et al. [55] for result validation presented in Fig. 1. These comparisons indicate that the present study has a good agreement with Loha et al. experimental study [52] and Loha et al. modeling study [53] and also with the Jayah et al. experimental study [54] and Jarungthammachote et al. modeling study [55]. It is noteworthy to mention that the root mean square of the modeling is as low as 1.434 compared to the experimental results of Loha et al. [52] and 1.370 compared to the experimental study of Jayah et al. [54].
4.2 Multi-criteria decision problem
Twenty types of biomass were considered as alternatives, including apricot stone, beech wood, cedar wood, coffee husk, corn cob, cotton stem, grapevine pruning waste, holm oak, jute stick, legume straw, olive refuse, peach stone, pine sawdust, rice straw, spruce wood pellet, sugarcane bagasse, sunflower shell, switchgrass, wheat straw, and wood sawdust. Syngas composition and four efficiencies were considered as criteria, including hydrogen, carbon monoxide, carbon dioxide, methane, and nitrogen concentration and energy, chemical, exergy, and hydrogen efficiencies. Decision matrixes for gasification in the presence of air, oxygen, and steam gasifying mediums are presented in Tables 1, 2, and 3, respectively.
Table 1 indicates that the most hydrogen production is in the case of switchgrass gasification in the presence of an air gasifying medium, which is 22.85%. Cedar wood gasification leads to the highest carbon monoxide production (30.00%); however, the most minor carbon dioxide production is in the case of jute stick gasification (5.92%). According to the results, the most methane production is in the case of pine sawdust gasification, which is 1.40%. The lowest nitrogen production belongs to switchgrass gasification, and the highest efficiencies are obtained in wood sawdust gasification. Therefore, choosing the best biomass for gasification in the presence of an air gasifying medium is a challenging issue and needs a multi-criteria decision analysis.
Table 2 reveals that in the case of the oxygen gasifying medium, the highest hydrogen production belongs to pine sawdust gasification, which is 36.51%. Holm oak gasification produces the highest carbon monoxide content, and the highest methane productions are in the case of pine sawdust gasification, which are 52.51% and 3.62%, respectively. Gasification of jute stick leads to the lowest carbon dioxide production, which is 11.35%. The highest efficiencies are obtained in the gasification of wood sawdust in the presence of oxygen as the gasifying medium. Since selecting the best biomass type in the presence of the oxygen gasifying medium is very challenging, there is a need for systematic multi-criteria decision analysis.
The most challenging decision-making problem belongs to gasification in the presence of a steam gasifying medium. Table 3 shows that pine sawdust gasification produces the highest hydrogen content, which is 47.74%. However, the highest carbon monoxide and the lowest carbon dioxide productions belong to the gasification of holm oak and cedar wood, respectively. According to the results, the gasification of pine sawdust leads to the highest methane content which is 6.62%. The results reveal that the highest chemical and exergy efficiencies are in the case of jute stick gasification, which are 88.30% and 91.36%, respectively. However, the gasification of pine sawdust and wood sawdust leads to the highest energy and hydrogen efficiencies, respectively. Similar to the other two cases, biomass gasification in the case of the steam gasifying medium needs a systematic multi-criteria decision analysis to select the best biomass type according to nine considered criteria.
4.3 Multi-criteria decision-making on the best biomass types
The alternative rankings for all three cases of air, oxygen, and steam gasifying mediums were conducted using the TOPSIS method based on considered criteria. In the first state, the alternative ranking was implemented using equal weights for nine criteria, which was almost 0.11. In other states, as a sensitivity analysis, the alternatives were ranked by changing the criteria weights. All states are presented in Table S.3 (available in the supplementary information file).
As mentioned previously, state 1 demonstrates a situation in which all considered criteria have equal weights. State 2 is a situation with a priority on hydrogen production, and state 3 is defined for problems in which pollution issues are essential and emission of carbon dioxide is a significant criterion. State 4 is a situation with a priority of carbon dioxide and nitrogen productions in the case of the air gasifying medium and with a priority of production of gases with heating value in the cases of oxygen and steam gasifying mediums. State 5 is considered for decision-making problems in which efficiencies are more critical compared to syngas composition.
Table 4 shows the alternative ranking results for the first states. The results reveal that wood sawdust is the best biomass type for gasification in the cases of air and oxygen gasifying mediums in the first state. In this state, jute stick is the best alternative for gasification in the presence of steam as the gasifying medium. According to the results, cotton stem has the second rank for air and oxygen gasifying mediums, while pine sawdust stands in this rank for steam gasifying medium. In all three cases, olive refuse has the last rank.
The results of the sensitivity analysis on criteria weights are presented in Table 5. The results reveal that in the case of the air gasifying medium, wood sawdust has the first rank in states 1, 2, and 5, and in the other two cases, pine sawdust is in the first rank. Therefore, wood sawdust and pine sawdust are selected as the biomass types for air gasifying mediums. According to the results, wood sawdust is in the first rank in all states for the oxygen gasifying medium. Cotton stem stands in the second rank of gasification using oxygen gasifying medium in states 1, 2, and 5. Hence, wood sawdust and cotton stem are chosen as the best alternative for gasification in the case of the oxygen gasifying medium. Table 5 indicates that jute sawdust is the biomass type for steam gasification in almost all cases, and pine sawdust gets the second rank. Therefore, jute stick and pine sawdust are selected as the best alternative for the steam gasifying medium.
4.4 Optimization of the performance of the best biomass type
In the previous section, two biomass types were selected as the best alternatives for each gasifying medium. Therefore, there are six conditions to be optimized, including coupled biomass types/gasifying mediums:
-
(i)
Air/wood sawdust
-
(ii)
Air/pine sawdust
-
(iii)
Oxygen/wood sawdust
-
(iv)
Oxygen/cotton stem
-
(v)
Steam/jute stick
-
(vi)
Steam/pine sawdust
Response surface methodology was employed for multi-objective optimization of the performance of each condition based on considered criteria. In this regard, the design of trials was implemented using the central composite design tool of response surface methodology considering gasifier temperature and moisture content of the biomass as processing parameters and is presented in Table S.4 (available in the supplementary information file).
The output results of these thirteen trials for each condition are presented in Table S.5 (available in the supplementary information file).
The multi-objective optimization was implemented using the response optimizer tool of the response surface methodology, and the results are presented in Fig. 2. The results showed that the multi-objective optimum condition for air/wood sawdust state is gasifier temperature of 813.83 °C and moisture content of biomass of 19.85%. These conditions for all cases are as follows:
-
(i)
Air/wood sawdust: gasifier temperature of 813.83 °C and moisture content of biomass of 19.85%
-
(ii)
Air/pine sawdust: gasifier temperature of 825.41 °C and moisture content of biomass of 15.11%
-
(iii)
Oxygen/wood sawdust: gasifier temperature of 818.00 °C and moisture content of biomass of 12.88%
-
(iv)
Oxygen/cotton stem: gasifier temperature of 817.54 °C and moisture content of biomass of 11.76%
-
(v)
Steam/jute stick: gasifier temperature of 760.81 °C and moisture content of biomass of 5.61%
-
(vi)
Steam/pine sawdust: gasifier temperature of 750.88 °C and moisture content of biomass of 6.16%
4.5 Multi-criteria decision-making on the best gasifying medium/biomass type case
In this step, the performance of the six cases is assessed in their optimum condition, and a systematic multi-criteria decision analysis is performed to select the best gasifying medium/biomass type case. Table 6 presents the performance of all six cases at their optimum conditions.
Results of the multi-criteria decision analysis on the best gasifying medium/biomass type case are presented in Fig. 3.
The sensitivity analysis was conducted on the alternative ranking based on the criteria weights considered in Table S.3 (available in the supplementary information file). Figure 3 shows that the combination of steam/pine sawdust as gasifying medium/biomass type is the best choice for gasification in all considered states. The combination of steam/jute stick has the second rank in all states considered as Table S.3 (available in the supplementary information file). Therefore, steam gasification with pine sawdust biomass is the best alternative for gasification based on the criteria considered in this study.
4.6 Performance assessment of the best gasifying medium/biomass type gasification
Figure 4 indicates the effect of temperature and moisture on the synthesis gas composition and energy, chemical, exergy, and hydrogen efficiencies. As Fig. 4a shows, the hydrogen percentage is improved from 43.58 to 46.68% and then is dropped to 46.18% by raising the temperature. Increasing gasification temperature raised carbon monoxide and reduced the carbon dioxide and methane percentages in gas composition. These phenomena appear because the gasification temperature affects the equilibrium reactions. Figure 4b illustrates that raising temperature decreases the energy, chemical, and exergy efficiencies while it enhances the hydrogen efficiency to 44.68% and then drops it. The reason for the hydrogen efficiency trend is referred to the hydrogen content in synthesis gas. The energy efficiency is reduced from 77.74 to 73.27%, the chemical efficiency is decreased from 70.81 to 58.32%, and exergy efficiency is dropped from 73.62 to 64.06%. These are because of an increment in input energy to the system. The effects of moisture on the synthesis gas composition are investigated in Fig. 4c. Hydrogen, carbon monoxide, and methane production are raised by increasing the moisture content. In contrast, the carbon dioxide production is reduced. This phenomenon influenced the efficiencies. As shown in Fig. 4d, all efficiencies (energy, chemical, exergy, and hydrogen) are decreased with raising the moisture content because the combustible gases are produced with lower value at high moisture content. The hydrogen efficiency is almost constant at the moisture content of 5–15%, and then, is reduced with a high trend.
Performance of the best gasifying medium/biomass type gasification versus temperature and moisture; a effect of temperature on synthesis gas composition, b effect of temperature on efficiencies, c effect of moisture content on synthesis gas composition, and d effect of moisture content on efficiencies
5 Conclusions
In this investigation, the aim was to develop a comprehensive and systematic study considering simultaneously various biomass types and gasifying mediums in the biomass gasification process. A systematic multi-criteria decision analysis and a sensitivity study were conducted to choose the best biomass type/gasifying medium combination considering nine different criteria. Firstly, the best biomass types were selected for each gasifying medium using the TOPSIS method. The primary outcomes can be concluded as follows:
-
Wood sawdust and pine sawdust were the best biomass for gasification with an air gasifying medium.
-
Wood sawdust and cotton stem were the best alternatives in the case of the oxygen gasifying medium, and jute stick and pine sawdust were the best biomass for gasification in the presence of steam as the gasifying medium.
-
The findings revealed that the steam/pine sawdust case was the best combination for gasification with the best syngas compositions having desirable energy, chemical, exergy, and hydrogen efficiencies.
-
A gasification temperature of 750.88 °C and a moisture content of 6.16% were the optimal gasification condition for steam/pine sawdust gasification.
-
Steam/pine sawdust gasification had energy and exergy efficiencies of 80.91% and 86.03%, respectively.
-
Chemical and hydrogen efficiencies were 83.11% and 44.61%, respectively, for steam/pine sawdust gasification.
-
More hydrogen and carbon monoxide and less methane were attained at higher temperatures.
-
Increasing moisture content declined hydrogen, carbon monoxide, and methane.
-
Energy and exergy efficiencies were decreased with temperature and moisture content in steam/pine sawdust gasification.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Callén MS, Martínez I, Grasa G, López JM, Murillo R (2022) Principal component analysis and partial least square regression models to understand sorption-enhanced biomass gasification. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02496-z
Mojaver P, Hasanzadeh R, Chitsaz A, Azdast T, Mojaver M (2022) Tri-objective central composite design optimization of co-gasification of eucalyptus biomass and polypropylene waste. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02597-9
Zhang G, Li H, Xiao C, Sobhani B (2022) Multi-aspect analysis and multi-objective optimization of a novel biomass-driven heat and power cogeneration system; utilization of grey wolf optimizer. J Clean Prod 355:131442
Wu Z, Zhu P, Yao J, Zhang S, Ren J, Yang F, Zhang Z (2020) Combined biomass gasification, SOFC, IC engine, and waste heat recovery system for power and heat generation: energy, exergy, exergoeconomic, environmental (4E) evaluations. Appl Energy 279:115794
Safari F, Dincer I (2022) Assessment and multi-objective optimization of a vanadium-chlorine thermochemical cycle integrated with algal biomass gasification for hydrogen and power production. Energy Convers Manag 253:115132
Ebrahimi A, Ziabasharhagh M (2020) Energy and exergy analyses of a novel integrated process configuration for tri-generation heat, power and liquefied natural gas based on biomass gasification. Energy Convers Manag 209:112624
Cao Y, Mihardjo LW, Dahari M, Tlili I (2021) Waste heat from a biomass fueled gas turbine for power generation via an ORC or compressor inlet cooling via an absorption refrigeration cycle: a thermoeconomic comparison. Appl Therm Eng 182:116117
Lin H, Wu X, Ayed H, Mouldi A, Abbas SZ, Ebrahimi-Moghadam A (2022) A new biomass gasification driven hybrid system for power and liquid hydrogen cogeneration: parametric study and multi-objective evolutionary optimization. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2022.01.110
AlNouss A, Parthasarathy P, Shahbaz M, Al-Ansari T, Mackey H, McKay G (2020) Techno-economic and sensitivity analysis of coconut coir pith-biomass gasification using ASPEN PLUS. Appl Energy 261:114350
Safarian S, Unnthorsson R, Richter C (2020) Performance analysis and environmental assessment of small-scale waste biomass gasification integrated CHP in Iceland. Energy 197:117268
Cao Y, Dhahad HA, Hussen HM, Anqi AE, Farouk N, Issakhov A (2022) Development and tri-objective optimization of a novel biomass to power and hydrogen plant: a comparison of fueling with biomass gasification or biomass digestion. Energy 238:122010
AlNouss A, McKay G, Al-Ansari T (2020) A comparison of steam and oxygen fed biomass gasification through a techno-economic-environmental study. Energy Convers Manag 208:112612
Cao Y, Dhahad HA, Rajhi AA, Alamri S, Anqi AE, El-Shafay AS (2022) Combined heat and power system based on a proton conducting SOFC and a supercritical CO2 Brayton cycle triggered by biomass gasification. Int J Hydrogen Energy 47(8):5439–5452
Li H, Liang F, Guo P, He C, Li S, Zhou S, Deng L, Bai C, Zhang X, Zhang G (2020) Study on the biomass-based SOFC and ground source heat pump coupling cogeneration system. Appl Therm Eng 165:114527
Ishaq H, Islam S, Dincer I, Yilbas BS (2020) Development and performance investigation of a biomass gasification based integrated system with thermoelectric generators. J Clean Prod 256:120625
Jahromi R, Rezaei M, Samadi SH, Jahromi H (2021) Biomass gasification in a downdraft fixed-bed gasifier: optimization of operating conditions. Chem Eng Sci 231:116249
Sorgulu F, Dincer I (2021) Development and assessment of a biomass-based cogeneration system with desalination. Appl Therm Eng 185:116432
Hasanzadeh R, Mojaver P, Azdast T, Chitsaz A, Park CB (2022) Low-emission and energetically efficient co-gasification of coal by incorporating plastic waste: a modeling study. Chemosphere 299:134408
Li S, Xu S, Liu S, Yang C, Lu Q (2004) Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process Technol 85(8–10):1201–1211
Radmanesh R, Chaouki J, Guy C (2006) Biomass gasification in a bubbling fluidized bed reactor: experiments and modeling. AIChE J 52(12):4258–4272
Asadullah M, Miyazawa T, Ito SI, Kunimori K, Koyama S, Tomishige K (2004) A comparison of Rh/CeO2/SiO2 catalysts with steam reforming catalysts, dolomite and inert materials as bed materials in low throughput fluidized bed gasification systems. Biomass Bioenerg 26(3):269–279
Vélez JF, Chejne F, Valdés CF, Emery EJ, Londoño CA (2009) Co-gasification of Colombian coal and biomass in fluidized bed: an experimental study. Fuel 88(3):424–430
Lu YJ, Jin H, Guo LJ, Zhang XM, Cao CQ, Guo X (2008) Hydrogen production by biomass gasification in supercritical water with a fluidized bed reactor. Int J Hydrogen Energy 33(21):6066–6075
Guo B, Li D, Cheng C, Lü ZA, Shen Y (2001) Simulation of biomass gasification with a hybrid neural network model. Biores Technol 76(2):77–83
Lapuerta M, Hernández JJ, Pazo A, López J (2008) Gasification and co-gasification of biomass wastes: effect of the biomass origin and the gasifier operating conditions. Fuel Process Technol 89(9):828–837
Franco C, Pinto F, Gulyurtlu I, Cabrita I (2003) The study of reactions influencing the biomass steam gasification process☆. Fuel 82(7):835–842
Asadullah M, Miyazawa T, Ito SI, Kunimori K, Yamada M, Tomishige K (2004) Gasification of different biomasses in a dual-bed gasifier system combined with novel catalysts with high energy efficiency. Appl Catal A 267(1–2):95–102
Wei L, Xu S, Zhang L, Liu C, Zhu H, Liu S (2007) Steam gasification of biomass for hydrogen-rich gas in a free-fall reactor. Int J Hydrogen Energy 32(1):24–31
Haykiri-Acma H, Yaman S, Kucukbayrak S (2006) Gasification of biomass chars in steam–nitrogen mixture. Energy Convers Manag 47(7–8):1004–1013
Arvelakis S, Gehrmann H, Beckmann M, Koukios EG (2005) Preliminary results on the ash behavior of peach stones during fluidized bed gasification: evaluation of fractionation and leaching as pre-treatments. Biomass Bioenerg 28(3):331–338
Li K, Zhang R, Bi J (2010) Experimental study on syngas production by co-gasification of coal and biomass in a fluidized bed. Int J Hydrogen Energy 35(7):2722–2726
Miccio F, Piriou B, Ruoppolo G, Chirone R (2009) Biomass gasification in a catalytic fluidized reactor with beds of different materials. Chem Eng J 154(1–3):369–374
Jin H, Larson ED, Celik FE (2009) Performance and cost analysis of future, commercially mature gasification-based electric power generation from switchgrass. Biofuels Bioprod Biorefin 3(2):142–173
Oesch P, Leppämäki E, Ståhlberg P (1996) Sampling and characterization of high-molecular-weight polyaromatic tar compounds formed in the pressurized fluidized-bed gasification of biomass. Fuel 75(12):1406–1412
Lv PM, Xiong ZH, Chang J, Wu CZ, Chen Y, Zhu JX (2004) An experimental study on biomass air–steam gasification in a fluidized bed. Biores Technol 95(1):95–101
Wojnicka B, Ściążko M, Schmid JC (2021) Modelling of biomass gasification with steam. Biomass Convers Biorefin 11(5):1787–1805
Anniwaer A, Yu T, Chaihad N, Situmorang YA, Wang C, Kasai Y, Abudula A, Guan G (2020) Steam gasification of marine biomass and its biochars for hydrogen-rich gas production. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00868-x
Mojaver P, Jafarmadar S, Khalilarya S, Chitsaz A (2019) Study of synthesis gas composition, exergy assessment, and multi-criteria decision-making analysis of fluidized bed gasifier. Int J Hydrogen Energy 44(51):27726–27740
Hasanzadeh R, Mojaver M, Azdast T, Park CB (2021) Polyethylene waste gasification syngas analysis and multi-objective optimization using central composite design for simultaneous minimization of required heat and maximization of exergy efficiency. Energy Convers Manag 247:114713
Mojaver M, Azdast T, Hasanzadeh R (2021) Assessments of key features and Taguchi analysis on hydrogen rich syngas production via gasification of polyethylene, polypropylene, polycarbonate and polyethylene terephthalate wastes. Int J Hydrogen Energy 46(58):29846–29857
Dincer I, Rosen MA (2012) Exergy: energy, environment and sustainable development. Newnes 31–49
Petković B, Petković D, Kuzman B (2020) Adaptive neuro fuzzy predictive models of agricultural biomass standard entropy and chemical exergy based on principal component analysis. Biomass Convers Biorefin 12:2835–2845
Caglar B, Tavsanci D, Biyik E (2021) Multiparameter-based product, energy and exergy optimizations for biomass gasification. Fuel 303:121208
Styrylska T, Szargut J (1964) Approximate determination of fuel exergy (approximate determination of chemical exergy of fuels with known absolute entropy). Brennstoff-Wärme-Kraft 16:589–596
Heyne S, Thunman H, Harvey S (2013) Exergy-based comparison of indirect and direct biomass gasification technologies within the framework of bio-SNG production. Biomass Convers Biorefin 3(4):337–352
Dhanalakshmi CS, Madhu P, Karthick A, Mathew M, Vignesh Kumar R (2020) A comprehensive MCDM-based approach using TOPSIS and EDAS as an auxiliary tool for pyrolysis material selection and its application. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-01009-0
Hasanzadeh R, Azdast T, Mojaver M, Park CB (2022) High-efficiency and low-pollutant waste polystyrene and waste polystyrene foam gasification: comprehensive comparison analysis, multi-objective optimization and multi-criteria decision analysis. Fuel 316:123362
Nayak PP, Datta AK (2022) An entropy-based TOPSIS approach for selecting best suitable rice husk for potential energy applications: pyrolysis kinetics and characterization of rice husk and rice husk ash. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02824-3
Nandi D, Pulikkalparambil H, Parameswaranpillai J, Siengchin S (2022) Application of a biowaste of fish (Labeo rohita) scale for the removal of methyl orange from aqueous solutions: optimization of sorption conditions by response surface method and analysis of adsorption mechanism. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02614-x
Hasanzadeh R, Azdast T, Doniavi A, Lee RE (2019) Multi-objective optimization of heat transfer mechanisms of microcellular polymeric foams from thermal-insulation point of view. Therm Sci Eng Prog 9:21–29
Hasanzadeh R, Mojaver M, Azdast T, Park CB (2022) A novel systematic multi-objective optimization to achieve high-efficiency and low-emission waste polymeric foam gasification using response surface methodology and TOPSIS method. Chem Eng J 430:132958
Loha C, Chatterjee PK, Chattopadhyay H (2011) Performance of fluidized bed steam gasification of biomass–modeling and experiment. Energy Convers Manag 52(3):1583–1588
Loha C, Chattopadhyay H, Chatterjee PK (2011) Thermodynamic analysis of hydrogen rich synthetic gas generation from fluidized bed gasification of rice husk. Energy 36(7):4063–4071
Jayah TH, Aye L, Fuller RJ, Stewart DF (2003) Computer simulation of a downdraft wood gasifier for tea drying. Biomass Bioenerg 25(4):459–469
Jarungthammachote S, Dutta A (2007) Thermodynamic equilibrium model and second law analysis of a downdraft waste gasifier. Energy 32(9):1660–2166
Author information
Authors and Affiliations
Contributions
Parisa Mojaver: methodology, software, validation, investigation, formal analysis, writing—original draft
Shahram Khalilarya: conceptualization, investigation, writing—review and editing, supervision
Ata Chitsaz: conceptualization, investigation, writing—review and editing, supervision
Samad Jafarmadar: conceptualization, investigation, writing—review and editing, supervision
Corresponding author
Ethics declarations
Ethics approval
This manuscript is the authors’ own original work, which has not been previously published elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mojaver, P., Khalilarya, S., Chitsaz, A. et al. Upcycling of biomass using gasification process based on various biomass types and different gasifying agents: systematic multi-criteria decision and sensitivity analysis. Biomass Conv. Bioref. 14, 13157–13171 (2024). https://doi.org/10.1007/s13399-022-03280-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03280-9