Abstract
Second-generation bioethanol is a promising source of renewable energy. In Brazilian mills, the production of ethanol from sugarcane (first generation, 1G) is a consolidated process performed by Saccharomyces cerevisiae and characterized by high substrate concentrations, high cell density, and cell recycle. The main bacterial contaminants in 1G fermentation tanks are lactic acid bacteria, especially bacteria from the Lactobacillus genus, which is associated with a decrease in ethanol yield and yeast cell viability, among other negative effects. Second-generation (2G) bioethanol production is characterized by the conversion of glucose and xylose into ethanol by genetically modified or non-Saccharomyces yeasts. Spathaspora passalidarum is a promising non-Saccharomyces yeast for 2G ethanol production due to its ability to effectively convert xylose into ethanol. The effect of bacterial contamination on the fermentation of this yeast is unknown; therefore, L. fermentum, a common bacterium found in Brazilian 1G processes, was studied in coculture with S. passalidarum in a fed-batch fermentation process similar to that used in 1G mills. Individually, L. fermentum I2 was able to simultaneously consume glucose and xylose in nutrient-rich broth (Man, Rogosa, and Sharpe (MRS + xylose) but failed to grow in a glucose- and xylose-based synthetic broth. In coculture with S. passalidarum, the bacteria remained at a concentration of 108 UFC/mL throughout cell recycling, but no flocculation was observed, and it did not affect the fermentative parameters or the cellular viability of the yeast. Under both conditions, the maximum ethanol production was 21 g L−1 with volumetric productivity ranging from 0.65 to 0.70 g L−1 h−1. S. passalidarum was thus shown to be resistant to L. fermentum I2 under the conditions studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental damage and declining oil and coal reserves have made it necessary to replace these energy sources with renewable options—for example, by replacing fossil fuels with bioethanol (Jönsson and Martín 2016). One promising biofuel is the second-generation bioethanol (2G) produced from lignocellulosic biomass, the most abundant material on earth and a raw material that does not compete with food production (Macrelli et al. 2012; Paulova et al. 2015; Brandenburg et al. 2018).
In Brazil, sugarcane bagasse is the most advantageous lignocellulosic biomass to produce second-generation bioethanol (de Carvalho et al. 2016). Brazilian mills produce bioethanol from the fermentation of sugarcane juice and/or molasses by Saccharomyces cerevisiae. First-generation (1G) bioethanol production is a consolidated process because this technology has been used commercially for the last 30 years (Macrelli et al. 2012). The process adopted is the Melle-Boinot process, which reaches 92–93% of the theoretical fermentation yield (in terms of the conversion of sugar into ethanol) and requires a very short fermentation time (Basso et al. 2008; Amorim et al. 2011).
However, a common problem in Brazilian first-generation bioethanol plants is contamination by bacteria and wild yeasts (Saccharomyces and non-Saccharomyces species) (Amorim et al. 2011), mainly the genus Lactobacillus (Lucena et al. 2010; Bonatelli et al. 2017). This problem is the result of difficulties in sterilizing large volumes of substrate and the successive recycling of yeast cells (Basso et al. 2008; Amorim et al. 2011). It can cause economic losses, reducing the growth, viability, and fermentation capacity of yeast (Lucena et al. 2010).
Among the bacteria belonging to the genus Lactobacillus, L. fermentum is the dominant species isolated from Brazilian fermentation processes. As with most lactic acid bacteria (LABs), it is tolerant to ethanol, low pH, and high temperature (Lucena et al. 2010). In the production of ethanol with S. cerevisiae, L. fermentum is associated with cell flocculation (Carvalho-Netto et al. 2015), high organic acid production, and decreased ethanol concentrations (Reis et al. 2018). L. fermentum has a wide ecological distribution and can be found in several habitats, which is reflected by flexible metabolism and the ability to use a wide range of carbohydrates, such as xylose (Zhang et al. 2016).
The nature of the feedstock used in the fermentative process is relevant to the severity of bacterial contamination in bioethanol production. Microbial interactions and selection depend on the mineral composition of the substrate and the presence of certain substances that come from the processing of raw material (Bassi et al. 2018).
Sugarcane bagasse is one of the most commonly used raw materials for 2G bioethanol production and is composed of the following (%): cellulose (36.9 – 45.7), hemicellulose (25.6 – 26.9), and lignin (18.9 – 26.1) (Rocha et al. 2015). The conversion of the lignocellulosic biomass into ethanol requires a pretreatment step followed by enzymatic hydrolysis and fermentation of the sugars into ethanol (de Carvalho et al. 2016). The conditions of the pretreatment process (time, temperature, use of a catalyst, and its concentration) define the production of inhibitor compounds that influence fermentation, such as weak acids, furan derivatives, and phenolic compounds (Palmqvist and Hahn-Hägerdal 2000).
Glucose (from cellulose) and xylose (from hemicellulose) are the major sugars in lignocellulosic hydrolysate. A feasible second-generation process depends on the conversion of these sugars into bioethanol (Hou and Yao 2012). S. cerevisiae is the yeast widely used in the production of first-generation bioethanol due to its high ethanol production and robustness, but it cannot convert xylose into bioethanol (Hahn-Hägerdal et al. 2007; Gombert and van Maris 2015; Su et al. 2015). Many yeasts are capable of consuming xylose and arabinose naturally, but only approximately 1% can convert xylose into ethanol (Hahn-Hägerdal et al. 2007). Spathaspora passalidarum is a promising yeast that naturally ferments xylose. It metabolizes xylose with the same efficiency as glucose in a broth composed of either sugar individually and co-ferments glucose, xylose, and cellobiose in aerobic conditions (Long et al. 2012). This yeast has a higher conversion of xylose into ethanol under anaerobic conditions than S. stipitis, the most studied of the yeasts that naturally ferment xylose (Veras et al. 2017).
There are two 2G industrial plants in Brazil: Raízen (http://www.raizen.com.br) and Granbio (http://www.granbio.com.br), both inaugurated in 2014 (Carpio and Simone de Souza 2017). Although 2G ethanol is already commercially produced, the cost remains high due to equipment handling during bagasse pretreatment and the use of enzymes. In addition, there are several initiatives for the selection of the strains for enzyme production and fermentation that lead to the best results in industrial conditions (Lopes et al. 2016). Melle-Boinot fermentation strategies, such as cell recycle (Santos et al. 2016; Nakanishi et al. 2017), high cell concentrations, and fed-batch mode (Nakanishi et al. 2017), have already been studied to improve the performance of S. stipitis and S. passalidarum in fermenting xylose to ethanol.
The 2G ethanol production process still needs improvement, and using the same strategies and structure as 1G bioethanol production may be an option to support the economic viability of this process. Bacterial contamination is the main problem in the 1G process; however, there are few studies on 2G processes. Bacterial contamination may result from the addition of the feedstocks to the ferments as must, inoculum, or aerosol (Muthaiyan et al. 2011). High temperature, applied for sterilization, can degrade the sugar in lignocellulosic hydrolysate, resulting in the formation of inhibitors (Palmqvist and Hahn-Hägerdal 2000). In addition, bacterial contamination can be associated with bacterial biofilms in the tanks and transfer lines (Muthaiyan et al. 2011). Schell et al. (2007) verified that the genus Lactobacillus was predominant in bioethanol production from corn fiber treated with sulfuric acid on a pilot scale; L. brevis, L. collinoides, L. paracasei, L. buchneri, and L. plantarum were the species isolated in this process. The genera Lactobacillus and Acetobacter were isolated from the industrial ethanol production plant Dömsjö Fabriker AB in Sweden, and the predominant species were L. buchneri, L. plantarum, A. tropicalis, and A. syzygii (Albers et al. 2011).
The available literature does not describe the effect of bacterial contamination on the fermentation process of yeasts that naturally ferment xylose, which are promising organisms for the 2G process. Thus, in the present study, we investigated the effect of L. fermentum I2, a bacterial contaminant of the 1G process, in the process of fermentation by S. passalidarum in synthetic broth with glucose and xylose as carbon sources under 1G process conditions (fed-batch fermentation, H2SO4 yeast-cream treatment, high cell density, and cell recycle).
Materials and methods
Strains
S. passalidarum NRRL Y-27907 (Agricultural Research Service Culture Collection, National Center for Agricultural Utilization Research, Illinois, USA) is a yeast that naturally ferments xylose and is considered promising for the production of 2G bioethanol. L. fermentum I2 (Zimotec, “Luiz de Queiroz” College of Agriculture, Piracicaba, Brazil) was the LAB studied in this work; this species is the main bacterial contaminant that has been isolated from the 1G process. The strains were used in fed-batch fermentations with cell recycle and were stored at − 80 °C in 50% (v/v−1) of glycerol.
Yeast cultivation
S. passalidarum NRRL Y-27907 was reactivated in YPX (20%) (10 g L−1 of yeast extract, 20 g L−1 of peptone and 20 g L−1 of xylose) for 24 h at 30 °C and 200 rpm. Preinoculum was transferred to media containing the following (g L−1): yeast extract (3.0), urea (2.3), MgSO4.7H2O (1.0), xylose (12.0), and glucose (1.32). The inoculum was incubated in a shaker (Innova® 44R, New Brunswick) for 15 h at 30 °C and 200 rpm. The propagation was performed in a bioreactor (Bioflo® 115, 3 L, New Brunswick) at 30 °C with the percentage of dissolved oxygen controlled at 40 to 50% saturation in growth media containing the following (g L−1): sugar cane molasses (total reducing sugars [TRS], 30.0), K2PO4 (2.0), and urea (5.0). After 10–11 h, a pulse (g L−1) of K2PO4 (2.0) and urea (5.0) was applied, and a continuous feed of 3gTRS. L−1 h−1 was established with sugarcane molasses. The propagation was finished after 28 h, and the cells were recovered by centrifugation at 15860 × g for 20 min and suspended in a minimal volume of sterile water (Nakanishi et al. 2017). The yeast cream was stored at 4 °C for a maximum of 50 h. Molasses was acquired from the “Melaço de Cana” company (Brazil, São Paulo, Saltinho), which provides the molasses for several 1G bioethanol plants. The molasses was diluted by a factor of 10 with distilled water.
Bacterial cultivation
L. fermentum was precultivated on de Man, Rogosa, and Sharpe (MRS) plate agar. The preinoculum was obtained by adding the colonies to MRS and incubating the mix in a shaker (Innova ® 44R, New Brunswick) for 10 h at 30 °C and 50 rpm. An aliquot of the preinoculum (corresponding to 10% of the total volume of the inoculum) was transferred to the flask with MRS. The flask was incubated for 15 h, at 30 °C and 50 rpm. The cells were centrifuged at 3100 × g for 20 min and suspended in sterile saline solution (0.9%).
Consumption of sugars by L. fermentum I2 in different culture media
The consumption of sugars by L. fermentum I2 was evaluated in MRS + xylose, molasses, and glucose- and xylose-based synthetic broth. The experiments were performed according to Zhang et al. (2016) and Basso et al. (2014) with adaptations. MRS + xylose broth was composed of the following (g L−1): glucose (14.0); xylose (6.0); proteose peptone (7.0); beef extract (7.0); yeast extract (3.5); polysorbate 80 (0.7); and ammonium citrate (1.4), C2H3NaO2 (3.5), MgSO4 (0.07), MnSO4 (0.07), and K2PO4 (1.4). The molasses, raw material for 1G ethanol production, was used with control. The molasses broth was composed of the following (g L−1): TRS (glucose, fructose, and sucrose) (20.0); K2PO4 (3.0), urea (2.4), MgSO4.H2O (0.5), and trace elements (1 mL.L−1) (C6H8O7.H2O (5), ZnSO4.7H2O (1.6), CuSO4.H2O (0.05), MgSO4 (0.05), H3BO3 (0.05), NaMoO4.H2O (0.05), and FeSO4.H2O (1.0)). The sugar concentration was obtained by diluting the molasses (351 g L−1) of TRS with distilled water and adding the supplements. Glucose- and xylose-based synthetic broth was composed of the following (g L−1): glucose (14.0), xylose (6), K2PO4 (3.0), rea (2.4), MgSO4.H2O (0.5), and trace elements (1 mL L−1). L. fermentum I2 was inoculated into the broth at a concentration of 108 CFU (colony-forming units)/mL, for an optical density (OD) of 0.2 at 600 nm as measured in a spectrophotometer (Thermo Scientific®, Evolution 60s). The bacterial cultures for the experiments were incubated in duplicate for 48 h at 30 °C and 50 rpm in a shaker (New Brunswick®, Excella 24).
Fed-batch fermentation with cell recycles
Fed-batch fermentations were performed in a bioreactor (115 Bioflo®, 3.0 L, New Brunswick) as described by Nakanishi et al. (2017) with modifications. Two conditions were evaluated: pure culture (S. passalidarum) and coculture (S. passalidarum and L. fermentum) fermentations. Under both conditions, the yeast cream represented 1/3 (v/v) of the working volume (1.5 L), and the yeast concentration was 60 g L−1 of dry cell weight (DCW). In coculture, 108 CFU/mL of L. fermentum was added to the yeast cream in the first fermentative cycle. The bioreactor was fed at a rate of 0.067 L h−1 with glucose- and xylose-based synthetic broth containing the following (g L−1): glucose (63.0), xylose (27.0), urea (3.6), K2PO4 (4.5), MgSO4.H2O (0.75), and trace elements (1.5 mL L−1). After 15 h, the feeding was interrupted, and the fermentation time was extended to 32 h. No aeration was used. The stirring rate was maintained at 200 rpm and the temperature at 30 °C. The fermentation product was drained aseptically and centrifuged at 15860 × g for 20 min. The cells were resuspended in sterile water, and sulfuric acid 2 M was added until pH 2.5 in laminar flow. The cell suspension was pumped back to the bioreactor, and the acid treatment was performed for 30 min at 30 °C and 200 rpm with the injection of 0.2 vvm of air (Santos et al. 2016). The cells recovered by centrifugation were resuspended in sterile water and used for the subsequent fed-batch fermentation. This procedure was repeated two times for each condition (pure culture and coculture), and the experiments were performed in triplicate each time.
Analytical methodology and calculation of kinetic parameters
DCW was measured by transferring the samples to preweighed tubes and centrifuging them (19,090 × g for 4.5 min). Each pellet was washed with distilled water, dried at 80 °C for 48 h, and weighed. The sugar consumption and the ethanol, organic acid, glycerol, and xylitol production were measured by high-performance liquid chromatography (HPLC). The samples were filtered with a PVDF Millex 22 μm filter and analyzed in the chromatograph (Agilent Infinity, 1260) with a refractive index detector (IR) and a HPX-87H Aminex chromatographic column (Bio-Rad, 300 × 7.8 mm) at 35 °C using a mobile phase of 5 mM H2SO4 degassed with ultrapure water at a flow rate of 0.6 mL min−1.
CFU/mL was determined by serial dilution in saline solution and plating in MRS with 50 μg of cycloheximide (for bacteria) or yeast extract peptone dextrose (YPD) with 200 μg of ampicillin (for yeast). The viability of S. passalidarum was measured using a Neubauer chamber and methylene blue to distinguish dead cells from live cells.
The conversion factor of sugars into ethanol (YP/S) was calculated as the ratio between the ethanol produced (g) and the sugar consumed (g). The ethanol yield (%) was obtained by dividing YP/S by 0.51 and multiplying by 100, according to the theoretical efficiency of Gay-Lussac (0.511 g of ethanol per g of substrate).
The ratio of glycerol to ethanol (KG) was calculated as the ratio between the amount of glycerol produced (g) and the amount of ethanol (g) produced. The ratio of total acid to ethanol (KAC) was calculated as the ratio between the total quantity of lactic, acetic, and succinic acids (g) produced and the quantity of ethanol (g) produced.
The curves of glucose and xylose consumption were calculated as the difference between the provided and the residual sugar (g). The curve of ethanol production was calculated as the difference between the final mass of ethanol (g) and the initial mass of ethanol (g). The specific rates were calculated by adjusting the polynomials with data on glucose and xylose consumption, as well as ethanol production, followed by differentiation of the polynomials with respect to time and division by the cell concentration at the time of analysis. The maximum specific rates were used.
Statistical analysis
The results were expressed as the mean ± standard deviation and were subjected to analysis of variance and Tukey’s test at the 5% probability level (p < 0.05).
Results
Consumption of glucose, molasses, and xylose by L. fermentum in different culture media
In the MRS + xylose broth (Fig. 1a), L. fermentum I2 was able to consume glucose and xylose simultaneously. All sugars were consumed (10.88 ± 0.29 g L−1 of glucose and 7.89 ± 0.33 g L−1 of xylose), producing 15.90 ± 0.69 g L−1 of lactic acid, 2.82 ± 0.15 g L−1 of acetic acid, and 2.83 ± 0.04 g L−1 of ethanol.
When molasses (Fig. 1b) (used as raw material in some 1G Brazilian mills) was used as the substrate, L. fermentum consumed all the glucose and fructose (3.53 ± 0.07 g L−1 and 3.28 ± 0.05 g L−1, respectively), metabolized 2.56 ± 0.23 g L−1 of sucrose (total concentration of 15.83 ± 0.16 g L−1 of sucrose), and produced 3.98 ± 0.02 g L−1 of lactic acid, 0.97 ± 0.02 g L−1 of acetic acid, and 0.47 ± 0.03 g L−1 of ethanol.
The proportion of sugars in the glucose- and xylose-based synthetic broth (Fig. 1c) was based on the alkaline hydrolysate from sugarcane obtained by Nakanishi et al. (2017); in this broth, L. fermentum did not assimilate xylose, and the consumption of glucose was 0.31 ± 0.12 g L−1, producing 0.13 ± 0.00 g L−1 of lactic acid, and 0.07 ± 0.00 g L−1 of acetic acid.
Fed-batch fermentations
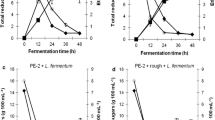
The profiles of glucose, xylose, DCW, and ethanol for pure culture and coculture are shown in Fig. 2. In this figure, it is possible to observe that L. fermentum I2 had a low impact on the fermentation process by S. passalidarum. Ethanol production was approximately the same in both conditions. The initial DCW ranged from 54.19 ± 1.29 g L−1 in fed batch 1 (Fig. 2a) to 45.03 ± 4.37 g L−1 in fed batch 3 (Fig. 2c) for S. passalidarum fermentations and ranged from 55.72 ± 2.87 g L−1 in fed batch 1 (Fig. 2d) to 48.39 ± 4.17 g L−1 in fed batch 3 (Fig. 2f) for fermentations with S. passalidarum and L. fermentum.
Glucose, xylose, dry cell weight (DCW), and ethanol production for fermentation in pure culture (S. passalidarum) and coculture (S. passalidarum + L. fermentum). Pure culture: a cycle 1, b cycle 2, and c cycle 3. Coculture: d cycle 1, e cycle 2, and f cycle 3. Glucose (circle), xylose (square), ethanol (triangle), DCW (inverted triangle)
The conversion factor of sugar into ethanol (Yp/s), ethanol yield (%), glucose consumption (%), xylose consumption (%), ethanol volumetric productivity (g L−1 h−1), and ethanol titer (g L−1) were calculated after 32 h for each fermentative cycle in pure culture and coculture (Table 1). The calculated parameters do not show a significant difference (p < 0.05) between fermentation in pure culture and coculture except for xylose consumption (%) in the second fermentative cycle. The YP/S values ranged from 0.34 to 0.40 with an ethanol yield from 67 to 79%. In pure culture, the conversion of sugars into ethanol increased with successive fermentative cycles (p < 0.05). Under both conditions, xylose consumption (%) decreased from cycle 1 to cycle 3 (p < 0.05). Ethanol production was approximately 21 g L−1 in all conditions, and the productivity ranged from 0.65 to 0.70 g L−1 h−1; these parameters did not present a significant difference (p < 0.05) across fermentative cycles.
Accumulated glucose and xylose consumption, as well as ethanol production (g) as a function of time, are represented in Fig. 3, and the minimum and maximum rates are shown in Table 2 (specific rates vary over time).
Glucose and xylose consumption (g) and ethanol production (g) for fermentations in pure culture (S. passalidarum) and coculture (S. passalidarum + L. fermentum). Pure culture: a cycle 1, b cycle 2, and c cycle 3. Coculture: d cycle 1, e cycle 2, and f cycle 3. Glucose (circle), xylose (square), ethanol (triangle)
In Table 3, the byproducts of the pure culture and coculture fermentations are shown. Lactic acid was produced only by L. fermentum I2 in coculture fermentation, with the maximum production of 0.82 ± 0.02 g L−1 in fed batch 1. There was no significant difference (p < 0.05) in acetic acid, succinic acid, or glycerol production among the conditions. In the presence of the yeast, L. fermentum I2 consumed the sugars and produced lactic acid, acetic acid, and ethanol. The concentrations of lactic acid, glycerol, and xylitol at 32 h were not significantly different (p < 0.05) between the cycles.
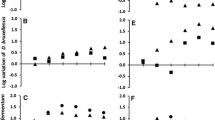
The ratios of total acids (KAG) and glycerol (KG) to ethanol were calculated at 32 h (Fig. 4). KAG was higher in coculture than in pure culture, especially since lactic acid was only produced in coculture. KAG did not show a significant difference (p < 0.05) among the fermentative cycles; for pure culture, the values ranged from 0.05 to 0.06 g g-1, and for coculture, the values ranged from 0.08 to 0.095 g g−1. KG showed no significant difference between the conditions or along the fermentative cycles; the values ranged from 0.01 to 0.02 g g−1.
Ratios of total acids (lactic acid, acetic acid, and succinic acid) (KAG) and glycerol (KG) (g) to ethanol (g) for fermentation in pure culture (S. passalidarum) and coculture (S. passalidarum and L. fermentum). a, b, Statistical test realized comparing the cycles of the same condition. *, **, ***, Statistical test realized comparing the conditions in the same cycle. Means with the same symbol within each column are not significantly different at the 0.05 probability level, according to Tukey’s test (means of triplicate experiments)
Figure 5 illustrates that L. fermentum I2 was able to remain in coculture fermentation with glucose- and xylose-based synthetic broth at a concentration of 108 UFC/mL. The acid treatment applied was not effective in reducing the concentration of the bacteria. The concentration of yeast in the first cycle was approximately 1013 UFC/ mL, and the presence of L. fermentum I2 was not influenced by the concentration of yeast during the fermentative cycles.
Discussion
L. fermentum is a common bacterium in the Brazilian first-generation bioethanol process (Lucena et al. 2010; Carvalho-Netto et al. 2015) and can consume several carbohydrates, as it can be found in different habitats, such as fermented food and the human microflora (Zhang et al. 2016). L. fermentum I2 is able to utilize xylose, which is one of the main sugars in lignocellulosic hydrolysate. L. fermentum 1001 was tested for lactic acid production from corncob hydrolysate broth containing 2.40 g L−1 of glucose, 8.84 g L−1 of xylose, and 1.43 g L−1 of arabinose. Glucose and xylose were assimilated simultaneously, producing 4.84 g L−1 lactic acid and 3.60 g L−1 of acetic acid. Arabinose was not consumed (Zhang et al. 2016). The present work has shown that L. fermentum I2 is able to consume the sugars in sugarcane molasses but not in synthetic broth with glucose and xylose in the absence of yeast. Albers et al. (2011) isolated microbial contaminants from a 2G ethanol plant in Sweden and found that the genus Lactobacillus was the most abundant. They evaluated more than 15 lactobacilli isolated in sulfite liquor and showed that none of the tested strains could grow or maintain its viability in the hydrolysate without the presence of yeast. The authors believe that this characteristic is associated with the supply of nutrients from the yeast to the bacteria and the detoxification of the broths with inhibitors by the yeast. The addition of yeast extract in the hydrolysate had no effect on the loss of bacterial viability (Albers et al. 2011). In the present work, the synthetic broth had no fermentation inhibitors; thus, the inability of L. fermentum to grow in the absence of the yeast was probably caused by a lack of nutrients. Unlike molasses and MRS media, the synthetic broth is poor in amino acids and vitamins, which can affect bacterial growth. LABs compete intensely with yeast, but it is believed that LABs also have a considerable dependence on nutrients that are derived from yeast (Brexó and Sant’Ana 2017). LABs require B vitamins, several amino acids, and purine and pyrimidine bases because they have a complex growth factor (Fitzpatrick and O’Keeffe 2001).
The evaluation of the effect of L. fermentum contamination on the fermentation process by S. passalidarum was carried out in fed-batch fermentation with glucose- and xylose-based synthetic broth by mimicking the fermentation process of Brazilian 1G mills (Santos et al. 2016; Nakanishi et al. 2017) as a proposal for 2G mills. The proportion of 70:30 glucose:xylose was based on the sugarcane bagasse hydrolysate obtained from alkaline pretreatment and enzymatic hydrolysis (Nakanishi et al. 2017). The alkaline pretreatment delignifies the biomass, removes silica (a component of the acid-insoluble ash), and partially removes the hemicellulosic fraction (acetyl and uronic acid groups). These carbohydrates are easily used for ethanol production, additionally, the process has a low generation of inhibitors, and enzymatic hydrolysis results in a hydrolysate free of acetic acid (de Carvalho et al. 2016; Nakanishi et al. 2017). A wide variety of factors influence xylose fermentation by yeast, such as the presence of acetic acid, which is an inhibitor at concentrations greater than 2–5 g L−1 (Nakanishi et al. 2017). In the present study, an inhibitor-free substrate was considered to simulate the alkaline hydrolysate; however, the synthetic broth is less complex than a true broth. In contrast to the procedures of Nakanishi et al. (2017) and Santos et al. (2016), yeast extract was not used to supplement the broth.
S. passalidarum was evaluated in fed-batch fermentation of alkaline hydrolysate of sugarcane bagasse at 30 °C by Nakanishi et al. (2017), who obtained a YP/S of 0.32 g g−1 with maximum bioethanol production of 20.9 g L−1 and volumetric productivity of 0.38 g L−1 h-1. This yeast showed xylitol production of 2.20 g L−1 in the first cycle and 0.67 g L−1 in the next four cycles. This yeast achieved its best performance when the cells were recycled and the temperature was decreased (Nakanishi et al. 2017). The results obtained in the present work were consistent with the results of Nakanishi et al. (2017). Cell recycling is a consolidated process used in Brazilian mills to increase the robustness of the yeast population due to evolutionary adaptation (Basso et al. 2008). Thus, it is expected that the performance of the yeast will improve across successive fermentative cycles. The fermentation in pure culture and coculture showed similar ethanol volumetric productivity and titer in the two cellular recycles, although YP/S and yield (%) in pure culture improved from cycle to cycle. These results may be associated with the reduction in the initial DCW throughout the fermentative cycles. S. passalidarum showed low growth during the fermentation, which was insufficient to replace the cells lost in cell recycling and acid treatment. This behavior can be attributed to the negative Crabtree effect, which means that this yeast requires aeration to maintain cell growth and cell renewal (Nakanishi et al. 2017).
L. fermentum I2 had no effect on the fermentation parameters or the viability of S. passalidarum in glucose- and xylose-based synthetic broth. Basso et al. (2014) studied fermentation in cocultures of Saccharomyces cerevisiae CAT-1 and L. fermentum FT-230 B in sugarcane molasses. In this study, the viability of S. cerevisiae was not affected by the bacteria, but during harvest, under industrial conditions, the bacteria could cause a decrease in ethanol yield and compete with yeast during cell recycling. Costa et al. (2018) studied the effect of acid treatment on the control of the L. fermentum CCT-5852 population and its effect on ethanol production in coculture with S. cerevisiae PE-2. The acid treatment was effective in controlling the bacteria, but they were not completely eliminated. In fermentation with acid treatment, the bacteria had a low effect on ethanol production and sugar consumption. The acid treatment performed in the present study likely contributed to the system stabilization during three subsequent fed-batch fermentations.
In the present work, L. fermentum I2 had low production of metabolites in coculture with S. passalidarum. L. fermentum is a heterofermentative acid lactic bacteria, and according to Brexó and Sant’Ana (2017), this type of bacterium produces lactic acid and other metabolic products. The result was consistent with the result obtained by Basso et al. (2014). After 24 h, the concentration of the metabolites in the substrate with 20% TRS (total reducing sugars) in the coculture of CAT-1 and L. fermentum FT-230 was 0.37 ± 0.03 g L−1 of acetic acid and 0.83 ± 0.14 g L−1 of lactic acid. In experiments in pure culture, lactic acid was not produced, and the concentration of acetic acid was 0.10 ± 0.01 g L−1 (Basso et al. 2014).
The ratio of the total acid (KAC) and glycerol (KG) per ethanol were calculated to measure the efficiency of fermentation. KAC in pure culture fermentation ranged from 0.05 to 0.06 g g-1, and KG ranged from 0.01 to 0.02 g g−1. In the study of Araújo et al. (2018) with PE-2 in batch fermentations with molasses, the values of KAC ranged from 0.016 to 0.022 g g−1 and KG ranged from 0.07 to 0.14 g g−1. The utilization of the fed-batch system rather than the batch system decreases the values of the KG (Alfenore et al. 2002). The values of KG obtained in this work were lower than those obtained by Araújo et al. (2018), but the system utilized was fed-batch fermentations.
The substrate is an important factor for the development and selection of microorganisms. Bassi et al. (2018) and Reis et al. (2018) studied the coculture of S. cerevisiae PE-2 with L. fermentum and Dekkera bruxellensis in sugarcane juice and molasses. The effect of the contamination was more pronounced in sugarcane juice than in the molasses in a single batch; however, in batch fermentation with cell recycle, the substrate had no influence on the effect of the bacteria in the fermentative process. When present as the only bacterial contaminant, L. fermentum had a minor influence on the growth of S. cerevisiae. The severity of the contamination increases with the presence of L. fermentum and D. bruxellensis.
In the present study, L. fermentum I2 was able to consume glucose and xylose simultaneously. Glucose and xylose are the main sugars in lignocellulosic hydrolysates, the substrate for the production of 2G bioethanol. Fed-batch fermentations were performed in glucose- and xylose-based synthetic broth and in 1G process conditions (fed-batch fermentation, acid treatment, and high density and reuse of the cells). L. fermentum I2 had no effect on the viability or the fermentation parameters of S. passalidarum in the conditions under which these fermentations were carried out. L. fermentum I2 produced more organic acids in the presence of the yeast than individually in glucose- and xylose-based synthetic broth, which may be associated with the supply of nutrients from the yeast to the bacteria. When real lignocellulosic hydrolysates are used, the bacteria will probably be influenced by the presence of the inhibitors and nutrients released during the raw material processing.
References
Albers E, Johansson E, Franzén CJ, Larsson C (2011) Selective suppression of bacterial contaminants by process conditions during lignocellulose based yeast fermentations. Biotechnol Biofuels 4:59. https://doi.org/10.1186/1754-6834-4-59
Alfenore S, Molina-Jouve C, Guillouet SE, Uribelarrea JL, Goma G, Benbadis L (2002) Improving ethanol production and viability of Saccharomyces cerevisiae by a vitamin feeding strategy during fed-batch process. Appl Microbiol Biotechnol 60:67–72. https://doi.org/10.1007/s00253-002-1092-7
Amorim HV, Lopes ML, De Castro Oliveira JV, Buckeridge MS, Goldman GH (2011) Scientific challenges of bioethanol production in Brazil. Appl Microbiol Biotechnol 91:1267–1275. https://doi.org/10.1007/s00253-011-3437-6
Araújo TM, Souza MT, Diniz RHS, Yamakawa CK, Soares LB, Lenczak JL, de Castro Oliveira JV, Goldman GH, Barbosa EA, Campos ACS, Castro IM, Brandão RL (2018) Cachaça yeast strains: alternative starters to produce beer and bioethanol. Antonie Van Leeuwenhoek, Int J Gen Mol Microbiol 111:1749–1766. https://doi.org/10.1007/s10482-018-1063-3
Bassi APG, Meneguello L, Paraluppi AL, Sanches BCP, Ceccato-Antonini SR (2018) Interaction of Saccharomyces cerevisiae–Lactobacillus fermentum–Dekkera bruxellensis and feedstock on fuel ethanol fermentation. Antonie Van Leeuwenhoek, Int J Gen Mol Microbiol 111:1661–1672. https://doi.org/10.1007/s10482-018-1056-2
Basso LC, de Amorim HV, de Oliveira AJ, Lopes ML (2008) Yeast selection for fuel ethanol production in Brazil. FEMS Yeast Res 8:1155–1163. https://doi.org/10.1111/j.1567-1364.2008.00428.x
Basso TO, Gomes FS, Lopes ML, De Amorim HV, Eggleston G, Basso LC (2014) Homo- and heterofermentative lactobacilli differently affect sugarcane-based fuel ethanol fermentation. Antonie Van Leeuwenhoek, Int J Gen Mol Microbiol 105:169–177. https://doi.org/10.1007/s10482-013-0063-6
Bonatelli ML, Quecine MC, Silva MS, Labate CA (2017) Characterization of the contaminant bacterial communities in sugarcane first-generation industrial ethanol production. FEMS Microbiol Lett 364:1–8. https://doi.org/10.1093/femsle/fnx159
Brandenburg J, Poppele I, Blomqvist J, Puke M, Pickova J, Sandgren M, Rapoport A, Vedernikovs N, Passoth V (2018) Bioethanol and lipid production from the enzymatic hydrolysate of wheat straw after furfural extraction. Appl Microbiol Biotechnol 102:6269–6277. https://doi.org/10.1007/s00253-018-9081-7
Brexó RP, Sant’Ana AS (2017) Impact and significance of microbial contamination during fermentation for bioethanol production. Renew Sust Energ Rev 73:423–434. https://doi.org/10.1016/j.rser.2017.01.151
Carpio LGT, Simone de Souza F (2017) Optimal allocation of sugarcane bagasse for producing bioelectricity and second generation ethanol in Brazil: scenarios of cost reductions. Renew Energy 111:771–780. https://doi.org/10.1016/j.renene.2017.05.015
Carvalho-Netto OV, Carazzolle MF, Mofatto LS, Teixeira PJPL, Noronha MF, Calderón LAL, Mieczkowski PA, Argueso LL, Pereira GAG (2015) Saccharomyces cerevisiae transcriptional reprograming due to bacterial contamination during industrial scale bioethanol production. Microb Cell Factories 14:1–13. https://doi.org/10.1186/s12934-015-0196-6
Costa MAS, Cerri BC, Ceccato-Antonini SR (2018) Ethanol addition enhances acid treatment to eliminate Lactobacillus fermentum from the fermentation process for fuel ethanol production. Lett Appl Microbiol 66:77–85. https://doi.org/10.1111/lam.12819
de Carvalho DM, de Queiroz JH, Colodette JL (2016) Assessment of alkaline pretreatment for the production of bioethanol from eucalyptus, sugarcane bagasse and sugarcane straw. Ind Crop Prod 94:932–941. https://doi.org/10.1016/j.indcrop.2016.09.069
Fitzpatrick JJ, O’Keeffe U (2001) Influence of whey protein hydrolysate addition to whey permeate batch fermentations for producing lactic acid. Process Biochem 37:183–186. https://doi.org/10.1016/S0032-9592(01)00203-5
Gombert AK, van Maris AJA (2015) Improving conversion yield of fermentable sugars into fuel ethanol in 1st generation yeast-based production processes. Curr Opin Biotechnol 33:81–86. https://doi.org/10.1016/j.copbio.2014.12.012
Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF (2007) Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol 74:937–953. https://doi.org/10.1007/s00253-006-0827-2
Hou X, Yao S (2012) Improved inhibitor tolerance in xylose-fermenting yeast Spathaspora passalidarum by mutagenesis and protoplast fusion. Appl Microbiol Biotechnol 93:2591–2601. https://doi.org/10.1007/s00253-011-3693-5
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Long TM, Su YK, Headman J, Higbee A, Willis LB, Jeffries TW (2012) Cofermentation of glucose, xylose, and cellobiose by the beetle-associated yeast Spathaspora passalidarum. Appl Environ Microbiol 78:5492–5500. https://doi.org/10.1128/AEM.00374-12
Lopes ML, Paulillo SC de L, Godoy A, Cherubin RA, Lorenzi MS, Giometti FHC, Bernardino CD, de Amorim Neto HB, de Amorim HV (2016) Ethanol production in Brazil: a bridge between science and industry. Braz J Microbiol 47:64–76. https://doi.org/10.1016/j.bjm.2016.10.003
Lucena BTL, Santos BM, Moreira JLS, Moreira APB, Nunes AC, Azevedo V, Miyoshi A, Thompson FL, Antonio M, Junior DM (2010) Diversity of lactic acid bacteria of the bioethanol process. BMC Microbiol 10:298. https://doi.org/10.1186/1471-2180-10-298
Macrelli S, Mogensen J, Zacchi G (2012) Techno economic evaluation of 2nd generation bioethanol production from sugar cane bagasse and leaves integrated with the sugar based ethanol process. Biotechnol Biofuels 5:22. https://doi.org/10.1186/1754-6834-5-22
Muthaiyan A, Limayem A, Ricke SC (2011) Antimicrobial strategies for limiting bacterial contaminants in fuel bioethanol fermentations. Prog Energy Combust Sci 37:351–370. https://doi.org/10.1016/j.pecs.2010.06.005
Nakanishi SC, Soares LB, Biazi LE, Nascimento VM, Costa AC, Rocha GJM, Ienczak JL (2017) Fermentation strategy for second generation ethanol production from sugarcane bagasse hydrolyzate by Spathaspora passalidarum and Scheffersomyces stipitis. Biotechnol Bioeng 9999:1–11. https://doi.org/10.1002/bit.26357
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33. https://doi.org/10.1016/S0960-8524(99)00161-3
Paulova L, Patakova P, Branska B, Rychtera M, Melzoch K (2015) Lignocellulosic ethanol: technology design and its impact on process efficiency. Biotechnol Adv 33:1091–1107. https://doi.org/10.1016/j.biotechadv.2014.12.002
Reis VR, Bassi APG, Cerri BC, Almeida AR, Carvalho IGB, Bastos RG, Ceccato-Antonini SR (2018) Effects of feedstock and co-culture of Lactobacillus fermentum and wild Saccharomyces cerevisiae strain during fuel ethanol fermentation by the industrial yeast strain PE-2. AMB Express 8:23. https://doi.org/10.1186/s13568-018-0556-9
Rocha GJ d M, Nascimento VM, Gonçalves AR, Silva VFN, Martín C (2015) Influence of mixed sugarcane bagasse samples evaluated by elemental and physical-chemical composition. Ind Crop Prod 64:52–58. https://doi.org/10.1016/j.indcrop.2014.11.003
Santos SC, de Sousa AS, Dionisio SR, Tramontina R, Ruller R, Squina FM, Vaz Rossell CE, da Costa AC, Ienczak JL (2016) Bioethanol production by recycled Scheffersomyces stipitis in sequential batch fermentations with high cell density using xylose and glucose mixture. Bioresour Technol 219:319–329. https://doi.org/10.1016/j.biortech.2016.07.102
Schell DJ, Dowe N, Ibsen KN, Riley CJ, Ruth MF, Lumpkin RE (2007) Contaminant occurrence , identification and control in a pilot-scale corn fiber to ethanol conversion process. 98:2942–2948. https://doi.org/10.1016/j.biortech.2006.10.002
Su YK, Willis LB, Jeffries TW (2015) Effects of aeration on growth, ethanol and polyol accumulation by Spathaspora passalidarum NRRL Y-27907 and Scheffersomyces stipitis NRRL Y-7124. Biotechnol Bioeng 112:457–469. https://doi.org/10.1002/bit.25445
Veras HCT, Parachin NS, Almeida JRM (2017) Comparative assessment of fermentative capacity of different xylose-consuming yeasts. Microb Cell Factories 16:1–8. https://doi.org/10.1186/s12934-017-0766-x
Zhang C, Guo T, Xin Y, Gao X, Kong J (2016) Catabolite responsive element deficiency of xyl operon resulting in carbon catabolite derepression in Lactobacillus fermentum 1001. J Appl Microbiol 120:126–137. https://doi.org/10.1111/jam.12990
Acknowledgments
We are grateful to the CNPEM and CTBE for assigning the structures for the realization of the experiments and for the help in HPLC analysis.
Funding
The authors acknowledge the financial assistance of the FAPESP- São Paulo Research Foundation (processes no 2017/04997-0 and 2016/06142-0) and the CNPQ - National Council for Scientific and Technological Development (process no 132142/2017-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Collograi, K.C., da Costa, A.C. & Ienczak, J.L. Effect of contamination with Lactobacillus fermentum I2 on ethanol production by Spathaspora passalidarum. Appl Microbiol Biotechnol 103, 5039–5050 (2019). https://doi.org/10.1007/s00253-019-09779-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09779-y