Abstract
The increasing production of plastics has raised concerns over the depletion of fossil fuels. In addition, the environmental issues associated with the improper management of plastic waste are increasingly alarming. Therefore, there is a sharp rise of researches conducted on one of the most promising biopolymers for the production of biodegradable plastics, which is known as polylactic acid (PLA). Subsequently, this has led to an increased interest in the production of its monomer, lactic acid (LA). However, the high production cost of LA has been limiting its large-scale manufacturing. The utilization of expensive raw materials and complicated downstream processes have led to the high overall production cost of LA. This review explores the potential of 3G feedstock, specifically macroalgae biomass, as a substrate for LA production. Then, the recent technological advancements for LA production and the challenges currently faced in the LA industry are addressed. Lastly, the sustainability aspect of macroalgae biomass is evaluated economically and environmentally by utilizing engineering tools such as life cycle assessment and exergy analysis, which represent the highlights of this review paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the years, the production and consumption of plastic have increased exponentially due to its favorable properties, such as light-weighted, waterproof, economical, and versatile [1]. Currently, the reported annual production capacity of plastics worldwide has approached 350 million tons [2]. According to Garside [3], the worldwide production of plastics would triple to 1100 million tons per year by 2050 [3]. The consumption of plastics follows a “take-make-dispose” pattern, where the single-used plastics were manufactured, used, and discarded. The accumulation of plastic waste worldwide creates a huge burden toward the environment. Meanwhile, the depletion of non-renewable resources driven by the consumption of petroleum-based plastics is also increasingly alarming [1].
The urgent situation poses a threat for humanity, drawing attention to alternative materials such as biodegradable plastics from bio-based polymers to replace petroleum-based plastics. Serle [4] had mentioned that the decomposition of biodegradable plastics could be achieved within three to six months under specific conditions [4]. Minimal carbon is emitted during the decomposition process since plant-based materials are used during the manufacturing of biodegradable plastics where the carbon is absorbed from the atmosphere during the photosynthesis [5]. Hence, biodegradable plastics offer a reduced carbon footprint when compared to traditional plastics made from fossil fuels, mitigating the risk of climate change (CC). Biodegradable plastic from polylactic acid (PLA) is one of the most widely used substitutes as it has low processing temperature, high flexibility, and high mechanical properties [6]. Other than that, PLA is also biocompatible and friendly toward the environment [6]. Due to the increasing demand of PLA, the market demand of l-lactic acid has also expanded as it is an essential building block for the biopolymer. However, PLA is more costly compared to synthetic plastics due to several challenges faced in the LA industry [7, 8].
At present, large-scale production of LA through the fermentation route is not economically feasible due to the expensive carbon sources [9]. Therefore, this challenge requires immediate action from researchers to explore a cheaper alternative renewable carbon source. Initially, researchers had focused their studies on edible food resources which are rich in readily fermentable sugars [10]. However, edible feedstock received opposition due to food security issues. Consequently, residual waste from various sources such as forestry and agriculture has been implemented to overcome the food conflict and has been proven to be feasible by producing a high yield of LA [11,12,13,14]. Even though the food security issue was resolved, the commercialization of LA using lignocellulosic biomass is still hindered due to its high lignin content. Delignification is relatively energy-consuming as a sequence of complex pre-treatment methods are involved, leading to high production cost [15, 16]. Thus, the macroalgae biomass is explored as another viable alternative carbon source. Apart from the limitation in the fermentation phase, technological breakthrough in the downstream processes of LA is essential as the final market price and optical purity of LA are highly dependent on the efficient separation and purification methods.
This review paper highlights the latest information on the emergence of macroalgae biomass as a promising feedstock in LA biorefinery, and the current challenges faced by the industrial production of LA. In order to analyze the sustainability of macroalgae biomass as a feedstock for LA production, effective engineering tools such as life cycle assessment and exergy analysis were further discussed in this context.
1.1 Introduction of lactic acid
Lactic acid (LA) is an organic acid denoted as 2-hydroxy propanoic acid. Typically, the chiral molecule is available as two optical isomers, l-lactic acid and d-lactic acid [6]. An optically inactive form known as a racemic mixture with an equimolar (50:50) mixture of l-lactic acid and d-lactic acid can be obtained through chemical synthesis [17]. It is suggested that the physical properties of products differ in accordance with the proportion of each optical isomer [8]. An inappropriate proportion of l-lactic acid and d-lactic acid would lead to unpredictable properties of final products, whereas a racemic mixture of LA produces an inactive polymer, which is less desired for the PLA production [6]. Hence, pure enantiomers of LA have a higher market value when compared to the optically inactive form [8]. l-lactic acid tends to have the highest demand in the PLA production and food-related industries. This is due to its rapid metabolic conversion, indicating the ease of assimilation inside the human body [17]. On the other hand, d-lactic acid is less desirable because it often causes disruptions on the metabolic system in the human body, impacting human health with adverse conditions such as acidosis and decalcification [8].
The versatility of LA provides a broad range of applications in the food, cosmetic, pharmaceutical, and chemical industries. It acts as an essential platform chemical for producing value-added products. Generally, l-lactic acid, the isomer with higher compatibility with the human body, is preferable for the food, pharmaceutical, cosmetic-related industries [17]. However, the pure isomers or a racemic mixture can sometimes still be needed by the chemical industry, depending on the application. The potential applications of LA are illustrated in Fig. 1.
In the food industry, LA is utilized extensively in various forms as food additives due to its classification as GRAS (generally recognized as safe). It works effectively as a pH regulator, acidulant, emulsifier, preservative, and mineral fortification. The addition of these food additives in processed meat industries allows an increased shelf life and a regulated microflora activity. Meat products are prone toward Salmonella infection, and LA is usually used to reduce the contamination risks with its antimicrobial properties [18]. LA is also functional as an acidulant and preservative to add acidic taste and stabilize the microbial activity in salad dressings, pickled vegetables, and beverages. LA can also be found in confectionery as an acidulant to achieve a sourness effect on sugar candies. Other than that, it allows the production of clear high boiled sweets, which avoiding the occurrence of sugar inversion [18]. As a pH regulator, it adjusts the pH level of beer and wine during the mashing process while enhancing their flavor [18]. At the same time, LA has the potential as a mineral fortification in food and beverages, where it enriches the food products with micronutrients [18].
According to Vijayakumar et al. [18], LA is well known as the mildest form of alpha hydroxy acid (AHA), which is a promising alternative to glycolic acid [18]. It is a potent skincare ingredient for over-the-counter skincare products. LA works as a chemical exfoliant which helps to remove dead cells from the surface of the skin and stimulating skin rejuvenation [20]. This also renders it as a favorable active ingredient in treating acne by reducing the growth of bacteria [20]. Next, LA is also regularly used as a natural pH adjuster in formulations [20]. It maintains the pH of products on the skin at a low pH value to inhibit microbial and fungal infections. Furthermore, LA at low concentration is a natural humectant where it helps to attract moisture from the inner levels of skin to the outer skin surface for hydration. It also helps to restore the natural moisture factor of the skin and keeps the skin moisturized throughout the day [20]. However, it increases the sensitivity of skin toward the sunlight upon application and renders new cells to be more vulnerable to UV damage.
LA, as a normal constituent of blood, is also commonly used in the pharmaceutical industry as intravenous (IV) fluids because it has a lower implication of side effects on human health. IV fluids allow the replenishment of the body fluids for patients with kidney failure during the dialysis session. Several examples of IV fluids are dialysis solutions for conventional kidney machines, Ringer’s Lactate for resuscitation, and continuous ambulant peritoneal dialysis (CAPD). Currently, LA polymer has been given considerable attention in biomedical applications as surgical sutures and orthopedic implants due to its biodegradability and compatibility with the metabolic system of the human body [8]. The antimicrobial property of LA has been applied as a sanitizer and anti-acne topical cream [8].
As a chemical product, LA can be made as a descaling and cleaning agent for decalcification of coffee machines and cleaning of washrooms and washing machines [18]. It also works well as a neutralizer in the production of detergents and personal care products [18]. Furthermore, it has the potential as a metal sequestering agent to bind with heavy metal ions and prevent the metals from oxidizing onto the surfaces of hard water [18]. LA is also used as an acidulant in the deliming process of leather to remove alkali traces and adjust the pH prior to the tanning process [18].
LA also emerges as a good potential monomer for chemical conversion [7]. It possesses the flexibility of undergoing a variety of chemical conversions because of the presence of a hydroxyl group and a carboxylic group. Numerous chemicals such as acetaldehyde, acrylic acid, ethyl lactate, propanoic acid, and polylactic acid (PLA) can be produced through polymerization, dehydration, hydrogenation, and dehydrogenation of LA. Apart from that, PLA is a promising biopolymer for biodegradable plastics with its latest application in additive manufacturing (AM) technology, namely 3D printing [8, 21]. PLA is regarded as one of the best choices for 3D printing owing to its feasibility to recycle and reuse, causing no environmental pollution [21]. It is commonly used to manufacture biomedical components, particularly implants. As reported, metal implants that are coated with PLA tend to have better resistance toward corrosion [21].
In summary, the development of PLA is highlighted due to the alarming environmental issue particularly related to the accumulation of non-biodegradable plastic waste. It has the potential to serve as biodegradable commodity plastics. The developed biopolymer has favorable properties such as durability, heat-resistance, and elasticity. It provides a wide range of applications in food packaging, trash bags, textiles, plates, and trays. Despite the recognition of the society for the advantages of biodegradable plastics, the high production cost of LA is still hampering its commercial viability. The high expenses are resulted from the fermentation and the downstream processes of l-lactic production. This had prevented the wide-spread adaption of bio-based plastics to replace synthetic plastics made from petroleum due to the latter cheaper cost [7, 8].
1.2 Production of l-lactic acid
Recently, l-lactic acid has received tremendous attention as a monomer to produce polylactic acid (PLA), an environmental-friendly biopolymer for biodegradable plastics. The demand for PLA had subsequently driven the worldwide demand for l-lactic acid. Currently, the annual production capacity of LA is around 270,000 tons [13]. Further increment in the production capacity of LA was expected as Parker [14] stated that the annual growth of the LA market was 11.5% until 2026 [14].
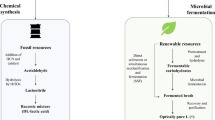
LA can be manufactured industrially by using both routes, chemical process and microbial fermentation, as summarized in Fig. 2 [7]. For the chemical synthesis of LA, the process is based on the hydrolysis of lactronitrile, a product formed when hydrogen cyanide (HCN) reacts with acetaldehyde (CH3CHO) in the presence of a base agent. Meanwhile, ammonium chloride is produced from the process as a by-product. Despite the fact that the production of LA takes a shorter time through the synthesis route, this method only produces a racemic mixture of LA. Furthermore, this method is not ideal for the industrial production of LA because it involves the utilization of petrochemical resources. Increasing reliance on non-renewable resources is not friendly toward the environment and the resources will also suffer from depletion [23]. The experts had mentioned that the production of PLA relied on pure LA enantiomers rather than the racemic mixture of LA due to their potential to produce high crystalline PLA through polymerization, which is great for commercial usage. The high optical purity of l-lactic acid and d-lactic acid enantiomers can be achieved through microbial fermentation with the appropriate microbial strains. Therefore, the microbial production of LA has received a significant amount of attention recently. Industrially, the production of LA derived from microbial fermentation of starch and other polysaccharides accounts for over 90% of the total worldwide production [8]. The advantages of fermentation over chemical synthesis are significantly improved environmental sustainability and a relatively cheaper raw material is needed [24, 25]. It provides opportunities to utilize raw materials such as organic waste and renewable sources, as shown in Table 1. Additionally, microbial fermentation is more favorable because it consumes less energy [25, 42].
Overview of two commercial lactic acid production methods [22]
Currently, the industrial-scale LA is manufactured through fermentation on pure sugars, which are easily available from food crops such as potato, sugar cane, sugar beet, corn, and cassava [16]. The utilization of pure sugar as substrates is not economically feasible as it contributes to a significant proportion of LA production costs. In addition to the financial challenge, the usage of food crops for biochemicals production is sparking debates due to the food security issue. Thus, the discovery of alternative renewable substrates for LA production is urgently prioritized by researchers. Thereafter, it was discovered that non-food crops or cellulosic materials can also be promising raw materials for LA production. These non-food crops and cellulosic biomass have attracted attention because they are easily accessible, renewable, and cheap that does not fall into the food versus fuel debate. However, the effective utilization of cellulosic biomass was still faced with many bottlenecks. For instance, some of the substrates cannot be utilized directly without pre-treatment process due to the presence of lignin or the absence of hydrolytic enzymes in microbial LA producers. The additional pre-treatment is an energy-intensive process which may contribute to the overall processing cost of LA. The expensive downstream processing for recovery and purification of LA is an additional challenge to microbial fermentation.
2 Lactic acid producers
In nature, several microbes have been used for feedstock consumption in microbial fermentation of LA. They can be classified into bacteria, fungi, and metabolically engineered strains [43]. Each microbe has one or more merits over the others, such as low nutritional requirements, enhanced yield, and productivity, or increased optical purity of LA. The properties of microbes used for LA production are described in Table 2.
2.1 Fungi
Among the fungal species, Rhizopus spp. is widely studied in LA production. It involves the utilization of Rhizopus spp. in various renewable resources, including xylo-oligosaccharides [26], rice straw [44], cassava starch [45], and Sophora flavescens residues [37]. This species, Rhizopus spp. consists only l-ldh gene encoding L-LDH. Therefore, it has the ability to produce only optically pure l-Lactic acid via fungal fermentation [22, 46]. According to Thongchul [46], the LA production by Rhizopus spp. allows direct fermentation of various starch-rich raw materials without prior saccharification due to its secretion of hydrolytic enzymes [22, 46, 47]. The next advantage associated with fungal fermentation is its low nutritional requirements [22]. However, the fermentation generally resulted in a low productivity rate, which was below 3 g/L h, possibly because of the requirement of oxygen during fermentation and low reaction rate due to the limitation in mass transfer [19, 22]. The fermentation also produces undesirable by-products such as fumaric acid and ethanol [19, 22, 46].
2.2 Yeast
Yeast has also received attention for fungal fermentation due to its cost-effective nutrient requirements and high tolerance to low pH levels [22, 47]. As such, the regeneration of precipitated lactate can be avoided under low pH levels and reduce the demand for neutralizing agents. However, most wild-type yeast naturally produces low concentrations of LA. Therefore, genetically engineered yeasts were developed to enhance LA production. For instance, Ilmén et al. [48] found out that the application of Candida sonorensis strain could produce 92 g/L of l-lactic acid at a yield of 0.94 g/g glucose. Kong et al. [42] utilized K. marxianus strain YKX071 for fermentation on corncob residue in fed-batch simultaneous saccharification and co-fermentation (SSCF) process to produce 103 g/L of high optical purity of l-lactic acid (99.5%) [42].
2.3 Bacteria
The production of LA by many genera of bacteria includes the four main microorganisms: lactic acid bacteria (LAB), Bacillus strains, Escherichia coli, and Corynebacterium glutamicum. Bacterial fermentation has been extensively used for the commercial production of LA as a primary product of the fermentation process [49].
2.3.1 Bacillus strains
Bacillus spp., especially Bacillus coagulans, has been widely studied in microbial production of LA due to their abilities in producing high optical purity and high yield at thermophilic temperatures [22, 47]. Ma et al. [50] employed Bacillus coagulans to produce l-lactic acid with high optical purity (> 99.5%), with a concentration of 92 g/L, yield of 0.91 g/g, and productivity of 13.8 g/L h from corn stover hydrolysate [50]. Bacillus spp. are Gram-positive bacteria, and the optimal temperature for growth is 50 °C [51]. In contrast to the conventional LAB, Bacillus spp. have several merits, such as the reduction of fermentation cost by growing and producing LA in a simple mineral salt medium with minimal nitrogen sources [51]. The microbe can be fermented at 50 °C, which reduces the risk of contamination since the sterilization of fermentation medium using coolant water is not necessary [47, 51]. Thus, this reduces the cost associated with medium sterilization. Other than that, the usage of Bacillus spp. enables the simultaneous saccharification and fermentation (SSF) on both pentose and hexose sugars for lignocellulosic biomass at optimum temperature [22, 47, 51]. However, the oxygen requirement by Bacillus spp. is a disadvantage for economical LA fermentation as it prefers aerobic growth and anaerobic fermentation [22].
2.3.2 Lactic acid bacteria
Although the LA production through fungal fermentation and Bacillus spp. has been attempted persistently, lactic acid bacteria (LAB) is still the most predominantly used LA producer. Bacteria, in the form of LAB, is Gram-positive, anaerobic, acid-tolerant, and aero-tolerant. The morphology can vary from short cocci or long rod-shaped to excrete LA as the major anaerobic product from the fermentation of reducing sugars, with high yield and high productivity [43, 47]. They are highly adaptable in various optimal growth environments, where some of the producers can grow in the pH range between 3.2 and 10.0 and temperature within 5 to 45 °C [8, 47, 51]. A few strains of LAB can even survive in a harsh environment and produce LA under higher temperature (> 45 °C) [29, 52]. A novel thermophilic LAB strain known as Enterococcus faecium QU 50 facilitated the fermentation of LA at optimal conditions (pH 6.5 and 50 °C), achieving optically pure l-lactic acid (≥ 99.2%) [52]. Similarly, at the same temperature of 50 °C, a thermo-alkaliphilic strain known as Enterococcus durans BP130 was able to produce a high LA titer of 28.8 g/L when demonstrated under the alkaline environment with the pH-controlled at 9.0 [31].
Several of the major naturally found genera are Lactococcus, Lactobacillus, Streptococcus, Leuconostoc, Pediococcus, Aerococcus, Carnobacterium, Enterococcus, Oenococcus, Tetragenococcus, Vagococcus, and Weisella [22, 43, 46]. Among them, Lactobacilli is available abundantly and comprises of approximately 80 species [43]. This species has a good reputation in commercial-scale LA production without affecting the health of both the consumers and the production workers [47]. The Lactobacillus strain, which is a commercially viable LAB strain, has been particularly effective due to their tolerance toward high acidity conditions and their potential to be engineered genetically for the selective production of d- or l-lactic acid.
The conversion of fermentable sugars into LA by LAB via different fermentation patterns can be divided into two groups: homofermentative or heterofermentative. Homofermentative LAB contains an aldolase enzyme where it generates LA as the major product. This group of LAB ferments more yield of LA because one mol of glucose can be converted into 2 mol of LA, without generation of any by-products through Embden-Meyerhof-Parnas (EMP) glycolytic pathway, as illustrated in Fig. 3a [17]. Thus, homofermentative LAB is favored industrially due to its high yield of LA formation and carbon-neutral properties [8]. Several of the genera categorized under homofermentative LAB are Lactococcus, Streptococcus, Pediococcus, Enterococcus, and some species of Lactobacillus [47]. In contrast to homofermentative LAB, the maximum LA yield produced by heterofermentative LAB only reached 0.5 g/g and 0.6 g/g with the utilization of hexoses and pentoses, respectively [46, 47]. The production of LA is accompanied with by-products such as ethanol, acetic acid, and carbon dioxide (CO2), through the phosphoketolase pathway as illustrated in Fig. 3b. Several reported genera of heterofermentative LAB are Leuconostoc, Oenococcus, and species of Lactobacillus [47].
Metabolic pathways of a homofermentative and b heterofermentative LAB in glucose [53]
In general, bacterial fermentation faces a limitation in biosynthetic capabilities where they cannot produce sufficient B-vitamins and amino acids [43, 51, 54]. Therefore, they only show rapid growth when adequate vitamin B, peptides, yeast extract, and fatty acids are supplied to the growth medium [43, 51, 54]. Furthermore, LAB tends to perform efficiently only at lower temperatures. Thus, the sterilization of culture media should be carried out before starting the fermentation. These two factors had contributed to a substantial rise in the downstream production cost of LA. Meanwhile, the requirement of low temperature for cell growth hampers the performance of LAB in the simultaneous saccharification and fermentation (SSF) process since hydrolytic enzymes tend to develop at higher optimal temperatures for saccharification when compared to LAB [47].
According to Abdel-Rahman et al. [55], most of the LAB strains favored the neutral or slightly acidic pH ranged from 5.5 to 6.5 which increased the contamination risks [55]. They further emphasized that the usage of the alkaline solution for fermentation when paired with alkaliphilic LAB strains was a promising solution to overcome this limitation. Most contaminant strains do not favor this alkali growth condition, whereas the usage of alkaliphilic LAB strains can tolerate pH levels at 9.0 and above which resolves the contamination problems during fermentation. Calabia et al. [56] found out that the isolation of alkaliphilic Halolactibacillus halophilus could produce 65.8 g/L of l-lactic acid in batch fermentation at pH 9.0 with a yield of 0.76 g/g [56]. Abdel-Rahman et al. [55] had isolated alkaliphilic Psychrobacter maritimus BoMAir 5 in a batch fermentation [55]. It showed high pH adaptation at 9.0 and produced a high LA production titer of 140.8 g/L with a yield of 0.93–0.99 g/g glucose consumed [55]. The isolated thermo-alkaliphilic Enterococcus faecium FW26 is capable of producing LA yield of 0.84 g/g consumed sugars by direct utilization of mixed lignocellulosic and food waste at pH 9.0 and 50 °C [29]. Aside from this, Hassan et al. [31] also effectively obtained LA concentration of 28.8 g/L from untreated mixed waste through direct utilization, facilitated by Enterococcus durans BP130 at pH 9.0 [31].
2.3.3 Escherichia coli
Escherichia coli has shown good potential in the rapid production of LA from the metabolism of both pentose and hexose sugars [47, 51]. Overall, this lactic acid producer requires a simpler nutritional requirement for growth when compared to the conventional LAB. Other than that, it is easy for gene replacement into engineered E. coli strains to achieve a higher yield of LA production [47, 51]. The major drawback of this LA producer is the generation of undesirable organic acids (d-lactic acid, acetic acid, succinic acid, formic acid), and ethanol due to its carbon flux distribution [47, 51]. There are a few reported studies on the application of engineered Escherichia coli strains for LA production. Parra-Ramírez, Martinez, and Cardona [57] had evaluated the performance of a metabolically engineered Escherichia coli strain JU15 for fermentation using Aspen Plus software to produce d-lactic acid from xylose and glucose. It was reported that the strain achieved a maximum concentration of 40 g/L with a yield of 0.6 g/g sugars consumed [57]. Wang et al. [58] reengineered a d-lactic acid-producing strain, Escherichia coli HBUT-D, to be fitted for l-Lactic acid fermentation through gene replacement. The consumption of this strain on combined wastes of molasses and corn steep liquor achieved 97 g/L of l-lactic acid with high optical purity of 99%, productivity of 3.17 g/L h, and a conversion rate of 90% [57]. Both experiments demonstrated that engineered E. coli strains can be a great approach in waste conversion into the high concentration of LA.
2.3.4 Corynebacterium glutamicum
Corynebacterium glutamicum is a Gram-positive soil bacterium that is extensively used for the industrial production of amino acid [51]. It has several advantages, such as simple fermentation medium, high growth rate, and predominantly producing l-lactic acid as the main product under oxygen-deprived conditions [22, 47, 51]. It produces combined organic acids such as l-lactic acid, succinic acid, and acetic acid with the consumption of various sugars [59]. However, the by-products formed during LA fermentation are disadvantageous since this led to low LA yield [22].
Tsuge et al. [59] reported the use of metabolically engineered Corynebacterium glutamicum strains to consume a mixture of glucose and xylose for LA fermentation [59]. The study conducted the fermentation anaerobically and managed to achieve a high titer of d-lactic acid up to 119.3 g/L with a yield of 0.79 g/g of mixed sugar consumed after 80 h. At the same time, they learned that the gene encoded Corynebacterium glutamicum strains decreased the yield of by-products, DHA, and glycerol by 74% and 24%, respectively. Tsuge et al. [60] conducted another experiment on two different metabolically engineered Corynebacterium glutamicum, namely LPglc429 and LPglc402, respectively for glucose fermentation into l- and d-lactic acid [60]. They found out that each strain had the ability to produce high productivity of LA without the requirement of the nutrient-rich medium for growth. Corynebacterium glutamicum, LPglc429 produced 212 g/L l-lactic acid, and Corynebacterium glutamicum, LPglc402 produced 264 g/L d-lactic acid, where each of the yields was above 95%. They successfully produced high optical purity of l- and d-lactic acid with the usage of inexpensive mineral salts medium. Further study of metabolically engineered Corynebacterium glutamicum strains in minimizing or eliminating the production of by-products is highly encouraged since it has a high potential for industrial LA production.
2.4 Evolution of lactic acid biorefineries
As of today, various feedstocks have been published in the literature for the production of LA [11, 14, 41, 61, 62]. These feedstocks can be categorized into three primary groups based on their sources of biomass, advantages, limitations, and technological progress, as presented in Table 3.
Biorefineries can be represented as either first-generation (1G), second-generation (2G), or third-generation (3G), depending on the sources of the substrate. Currently, commercialized LA in the market is mostly derived from food crops, which are known as 1G feedstock [16]. The sources of 1G feedstock mainly consist of food crops rich in sugar, starch, vegetable oils, and animal fats. The carbon sources are readily extractable for the fermentation of LA without further pre-treatment process accompanied with maturity in conversion technology at commercial scale. However, the use of 1G feedstock as LA feedstock suffers from the food versus energy debate since food prices will increase along with the expansion of the LA industry. The biomass supply for LA production has also increased the demand for more agricultural land for food supply [65]. This also comes with a higher overall production cost for LA as pure carbon sources are economically unfeasible. Several studies of LA production from sugarcane [66], wheat bran [67], rice [68], sweet sorghum juice [62], and cassava [45] had been published in the literature.
The drawbacks in 1G feedstock have subsequently led to the development of 2G feedstock. In contrast to easily extractable carbon sources, 2G focuses on lignocellulosic biomass such as forest and agricultural waste. Lignocellulose is primarily composed of cellulose (35–45%), hemicellulose (20–25%), and lignin (15–20%) [69]. It is rich in hexose and pentose sugars, which are fermentable [70]. The usage of 2G feedstock is effective in overcoming the food security issue encountered in the 1G feedstock. However, it is challenging to release fermentable sugars from lignocellulosic biomass due to its high lignin content. Owing to the complex structure, complicated and cost-intensive pre-treatment methods are required to remove the lignin from the lignocellulosic biomass [15, 16]. The market of LA from lignocellulosic biomass is not as established as 1G feedstock due to the high cost of processing technologies. Even though LA from 2G feedstock is available at a commercial scale, there is still a potential for further cost reduction following the advancement of technologies. A few studies on LA production using lignocellulose biomass had been reported, such as bagasse of cassava [71], orange peel waste [14, 72], corn cob residue [11], waste cooking oil glycerol [73], bagasse from sugar cane [74], municipal solid waste [75], coffee mucilage [12], and coffee pulp [13].
As a result of criticism received from 1G feedstock and technological challenges associated with 2G feedstock, researchers have focused their attention to the macroalgae biomass known as 3G feedstock.
3 Macroalgae: sustainable feedstock for LA
3.1 Diversity of algae species
In this context, algae biomass has recently received much attention as a 3G feedstock because of supply abundance and sustainability. According to Abdul Latif et al. [76], a quarter of 400,000 algae species have been discovered worldwide [76]. Algae biomass can be categorized into macroalgae and microalgae in accordance with its morphological features, size, carbohydrates, and lipids content [77, 78]. In general, microalgae produce a relatively higher lipid content, which serves as raw material mainly for biodiesel production [79, 80]. On the other hand, macroalgae possess a high content of carbohydrates, which are useful for the production of alcohol-based biofuels and biochemicals [77, 78, 81, 82]. The biochemical composition of macroalgae and microalgae is illustrated in Table 4. It shows that most macroalgae generally have a low content of protein (5–13% dry wt.) and lipids (7–12% dry wt.), whereas most microalgae are observed to have a relatively high protein content (30–60% dry wt.) and high composition of lipid (10–20% dry wt.).
Macroalgae can be broadly grouped into three categories: green algae (Chlorophyceae), brown algae (Phaeophyceae), and red algae (Rhodophyceae). The general carbohydrate contents of macroalgae is highly dependent on the species of macroalgae strain such as green, red, and brown algae, which ranges between 30–60%, 30–50%, and 20–30% dry weight, respectively [77]. Since macroalgae have a variety of species, biochemical characterization is performed using proximate analysis after the harvesting of macroalgae [78]. There are four main components which are necessary to be studied on macroalgae strains, including carbohydrate, protein, lipid, and ash content [78]. This information is required so that the macroalgae species can be effectively utilized as carbon sources for bioenergy and bioproducts. The carbohydrate composition of several of the macroalgae species is presented in Table 5.
Based on a review paper published by Gomez-Zavaglia et al. and Renganathan and Takriff [96, 97], the following macroalgae species made up most of the current total cultivated macroalgae: the brown algae (Laminaria japonica, Saccharina japonica, and Undaria pinnatifida), the red algae (Porphyra sp., Eucheuma striatum, Kappaphycus alvarezii, and Gracilaria sp.), and green algae (Monostroma and Enteromorpha). The population of the two species for brown macroalgae (Laminaria japonica and Undaria pinnatifida) and three species of red algae (Eucheuma, Kappaphycus, and Gracilaria spp.) are highly cultivated and they occupied approximately 40% of the total production capacity [97, 98]. When compared to the other two species, the production number of green algae is considered negligible [97, 98].
3.2 Comparisons between macroalgae and lignocellulosic plants
The structural polysaccharides of macroalgae are significantly different from those of terrestrial plants, which are composed of cellulose, hemicellulose, and lignin. The type of principle carbohydrates presents in each species of macroalgae, microalgae, and lignocellulosic biomass is tabulated in Table 6. The unique compositions in macroalgae such as ulvan, carrageenan, agar, mannitol, fucoidan, and alginate are generally differed according to the three macroalgae groups and cannot be found in other microalgae and lignocellulosic biomass [99]. The existence of alginate, fucoidan, and mannitol can be found in brown macroalgae (Phaeophyta); while carrageenan and algae can be found in red macroalgae (Rhodophyta) [100, 101]. The green macroalgae (Chlorophyta) are known to contain cellulose, starch, and mannose [101]. In contrast to the terrestrial plants, the other two categories of macroalgae have a lower starch content and lipid, except for green macroalgae (Chlorophyta) [15].
In terrestrial plants, lignin is an essential constituent for structural support to provide rigidity of the plant cell wall. Macroalgae are cultivated in marine environments and are held up by the buoyant force of water. Hence, they do not require the rigidity provided by lignin [15, 102]. The characteristic of macroalgae having negligible lignin is an added advantage over 2G feedstock as the complexity of the pre-treatment process can be reduced and lower the production cost [51, 77, 78, 103, 104]. Additionally, macroalgae does not compete with food crops for agricultural land since macroalgae are resistant to extreme growth conditions and can be cultivated in a variety of water environments such as wastewater and saline water [64, 105]. In the case where the cultivation of macroalgae is conducted in wastewater, the wastewater can be treated effectively along with the growth of macroalgae biomass [104].
Meanwhile, an added merit of macroalgae biomass would be a shorter harvesting cycle compared to terrestrial plants because of a higher photosynthetic efficiency [104]. A large quantity of macroalgae biomass per unit area can be produced in one harvesting cycle, ensuring an abundance of supply [103, 104, 106]. On average, green algae has a productivity of 7100 g/m2 year compared to brown algae and red algae, which recorded a maximum of 11,300 g/m2 year [77]. The cultivation of macroalgae biomass can also be easily sustained through photosynthesis with light, CO2, and nutrients in the water environments [77]. At the same time, macroalgae can fix CO2 from the atmosphere while releasing oxygen in the process.
3.3 Opportunities for macroalgae-based biorefinery
As mentioned previously, the production of LA in the current market is mostly derived from expensive carbohydrates categorized under 1G feedstock [16]. To resolve this concern, researchers had taken the initiative to explore the sustainability of macroalgae for the production of LA as it showed high potential in biochemical conversion without compromising the food versus fuel debate and agricultural issues. According to Jung et al. [98], the most viable macroalgae species at the moment for biorefinery feedstock were Laminaria japonica, Eucheuma spp., Kappaphycus alvarezii, Undaria pinnatifida, and Gracilaria verrucose [98]. The development in macroalgae-based refinery technology should be based on the exploitation of brown and red algae rather than green algae after considering the rising market demand of PLA and the current technology for macroalgae cultivation.
Macroalgae can be cultivated easily and potentially reach a higher rate of production compared to other terrestrial plants due to their higher photosynthetic rate [104]. The macroalgae-based carbohydrates comprise mostly of cellulose and starch with negligible lignin, which acts as a readily accessible carbon source for microbial fermentation of LA in the biorefinery. Hence, due to the fast growth rate and immense carbohydrates content, macroalgae had received the focus as the feedstock for renewable fuels and chemicals production [92, 107]. From previous studies, the exploitation of macroalgae biomass was focused primarily on generating single products, while the residual solid or liquid waste remained unused and discarded [108]. In addition, most of the researches related to macroalgae was predominantly focused on biofuel production [82, 106, 109,110,111,112]. To the best of the author’s current knowledge, there is no available literature in the production of LA from macroalgae integrated biorefineries. However, it was reported that microalgae had been utilized in LA production at a laboratory scale and proven to be feasible. As of now, there were only three published articles proposing the conversion of LA from microalgae.
By using green microalga, Hydrodictyon reticulatum as substrate, Nguyen et al. [113] achieved 37.11 g/L of l-lactic acid at a yield of 0.46 g/g, and producing 1.03 g/L h with consumption of Lactobacillus paracasei LA104 in a simultaneous saccharification and co-fermentation (SSCF) process. The optical purity of l-lactic acid obtained was between 95.7 and 98%. Similarly, Nguyen et al. [114] also achieved 36.6 g/L of d-lactic acid in approximately 36 hours, from the same substrate, Hydrodictyon reticulatum consumed by Lactobacillus coryniformis subsp. torquens in a SSCF process at a yield of 0.46 g/g and maximum productivity of 1.02 g/L h [114]. Hydrodictyon reticulatum is a promising feedstock for high optical purity d-lactic acid production since this research shows encouraging results ranging from 95.8 to 99.6% [114]. In addition, another microalga, Nannochlorum sp. 26A4, with 40% dry weight of starch content, was successfully converted into d-Lactic acid with a conversion rate of 70%. This fermentation process occurred through an anaerobic reaction when isolated in a dark environment [115].
3.4 Pre-treatment of macroalgae biomass
In the bioprocessing of macroalgae, an effective pre-treatment is essential to achieve high carbohydrates hydrolysis and thereafter a high yield of LA. During pre-treatment, the outer cell wall of macroalgae will be broken down to release complex polysaccharides and further hydrolyzed into simple reducing sugars (majorly glucose and galactose) while avoiding the degradation of carbohydrates. Different from lignocellulosic biomass, macroalgae has negligible lignin, which simplifies the pre-treatment process to become more economical [15]. Currently, the most practiced pre-treatments of macroalgae are physical (e.g., milling and microwave-assisted extraction), chemical (e.g., dilute acid treatment), and biological process (e.g., enzymatic hydrolysis).
3.4.1 Physical pre-treatment
Physical pre-treatment is one of the most effective methods because it involves size reduction of macroalgae biomass [43, 116]. This process increases the surface area and liberates complex sugars for better accessibility to enzymes, thereby optimizing LA production. The physical methods can be categorized into mechanical pre-treatment and microwave-assisted extraction.
In general, mechanical pre-treatment comprises of three steps, which are washing, drying, and milling. Initially, the freshly collected macroalgae biomass undergoes a washing process using fresh water to remove salts, sand, and debris on the surface. Next, the removal of water content is carried out through drying to prolong the shelf life of macroalgae biomass for the subsequent procedure [78]. The biomass can be dried in a hot air oven or exposed to the sun. At the last step, milling is performed to reduce the size and subjected to sieving. Together with other pre-treatment methods, this approach can be utilized to reduce energy expenses and enhance the overall performance of the process for substrate extraction [117]. Although mechanical processes do not require temperature as a driving force or any usage of chemicals, energy consumption is perceived to be high [117, 118].
Microwave-assisted extraction has been used to extract multiple compounds of interest from lignocellulose, macroalgae, and microalgae biomass [119, 120]. This approach is based on the assistance of a short electromagnetic spectrum with high frequencies ranging from 0.3 to 300 GHz, where it raises the temperature to a boiling state rapidly and causes disruption in the cells [43, 120, 121]. This method induces the exposure of the intracellular contents available for fermentation. The main drawback of this method is that the generated heat might also damage the compounds which are sensitive toward heat and not commercially viable because it is energy-intensive [43, 120].
3.4.2 Acid and alkali hydrolysis
The chemical route is widely accepted for pre-treatment of macroalgae biomass because it is efficient in depolymerizing cellulose and solubilizing hemicellulose using dilute alkali or acid treatment. Sodium hydroxide (NaOH) is mostly used in alkali treatment, where it facilitates the simultaneous solvation and saponification on biomass [122]. This causes the fibres of macroalgae to swell and enlarge in pore sizes. Substantially, this enables the release of reducing sugars for efficient enzymatic hydrolysis or fermentation. The major drawback of alkali treatment is the requirement of large quantities of water for desalting, where it increases the overall production cost and wastewater generation [78].
For acidic pre-treatment, dilute sulfuric acid (H2SO4) is applicable to all the classes of macroalgae [78, 123]. In comparison to other pre-treatment methods, the energy consumption in acid pre-treatment is relatively low, and it delivers a higher sugar yield [77, 124]. However, the use of dilute acid pretreatment is restricted due to the generation of inhibitory compounds such as furfural, 5-hydroxymethylfurfural (HMF), levulinic acid, furfural, and caffeic acid [77, 98, 125]. The former two toxins can inhibit the activity of enzymes, thereby mitigating the glycolysis pathway. Levulinic acid, which is a type of weak acid, can hinder the growth of cells. These inhibitors can be mitigated with the addition of sodium hydroxide for neutralization to pH 7 prior to fermentation or using alternative methods such as enzymatic hydrolysis [77]. As discussed above, a host of factors such as acid strength, treatment duration, amount of biomass loading, temperature, and pH can affect the yield of acid hydrolysis [78, 117].
The above statement can be justified by the following studies conducted. Sudhakar et al. [126] investigated the effect of different concentrations of H2SO4 (0.1%, 0.5%, and 1%) for hydrolysis of spent seaweed [126]. They reported that a higher amount of total sugar can be obtained from the biomass by increasing to 1% diluted H2SO4. There was an increment of 30% from 62.62 mg/g of total sugar obtained when 1% of diluted H2SO4 was used compared to 0.1%. They also found that the biomass loading and volume of substrate loading ratio at 4:50 displayed the maximum amount of reducing sugar yield, which was 13.07 mg/g. The concentration greater than this value showed a declined trend in the reducing sugar yield. Hong et al. [127] pretreated red macroalgae, Gelidium amansii with 1% H2SO4 for 15 min at 111 °C. The concentration of reducing sugar in hydrolysate was found to improve by 80.95% when the treatment temperature was raised to 131 °C [127].
3.4.3 Enzymatic hydrolysis
As aforementioned, enzymatic hydrolysis is a more effective practice for fermentation processes. Although it has a longer incubation period for the release of sugar as compared to acid hydrolysis, this method is highly practiced due to the low production of toxic inhibitory compounds as compared to acid hydrolysis [15, 104, 128]. Meanwhile, it yields more reducing sugars from cellulosic biomass due to the high conversion rate. Jmel et al. and Trivedi et al. [84, 129] carried out an in-depth study concerning enzymatic hydrolysis on macroalgae biomass (Ulva fasciata Delile and Ulva lactuca) where cellulase preparations were used to yield a high concentration of reducing sugars, 97.5 mg/g and 206.82 mg/g, respectively [84, 129]. However, the enzymes utilized are highly specific in converting certain types of polysaccharides, while macroalgae are often composed of several types of polysaccharides [15, 117]. In addition, the application of expensive enzymes causes this method to be not economical for industrial-scale biomass pretreatment [117].
According to Kim et al. [130], the efficiency of saccharification tends to improve when accompanied with mechanical pretreatment prior to acid and enzymatic hydrolysis [130]. These methods are sometimes co-utilized on macroalgae biomass for enhanced yield in fermentable sugars. From several recent studies, macroalgae biomass was typically pretreated using dilute acid and then followed by enzymatic hydrolysis. Wu et al. [131] pre-treated the macroalgae biomass, 15% (w/w) of Ulva lactuca with 4% H2SO4 for 120 min at 80 °C before proceeding with LA production in ensilage at acidic condition (pH < 4) [131]. They observed a maximum reducing sugar yield of 155 mg/g as a result of acid hydrolysis. The high reducing sugar yield reflected the feasibility of ensilage and presented encouraging pretreatment effects of acid hydrolysis. The importance of enzymatic hydrolysis was further demonstrated where it resulted in a further spike in reducing sugar yield from 155 to 198 mg/g [131]. The reducing sugar was later consumed by Lactobacillus plantarum during fermentation for 24 h and achieved a LA yield of 0.58 g/g. Different combinations of pre-treatment methods and macroalgae-extracted reducing sugar yield are described in Table 7.
3.5 Lactic acid conversion technologies
Prior to the conversion of LA, macroalgae is subjected to mechanical processing, which involves drying and reduction in size before followed by pretreatment of biomass. Later, saccharification is incorporated to degrade the polysaccharides into reducing sugars, and finally, these substrates are subjected to the fermentation process of LA. The saccharification process can be combined with fermentation in the following sequence: (1) separate hydrolysis and fermentation (SHF), (2) simultaneous saccharification and fermentation (SSF), and (3) simultaneous saccharification and co-fermentation (SSCF).
SHF is a process where hydrolysis of polysaccharides from biomass and fermentation of reducing sugars are performed separately in two different units along with different reaction conditions [15]. This allows both hydrolysis and fermentation to be performed at optimal conditions to convert reducing sugars into LA and lowering the intake of enzymes [139]. However, this configuration could lead to increased contamination risk and unwanted inhibitory effects [139]. An increased inhibitory effect indirectly resulted in rate-limiting hydrolysis due to inhibition of cellulase activity [139]. Since this technique requires two vessels for operation, the operating cost may increase [43].
On the other hand, SSF aims to produce LA through polysaccharides’ hydrolysis and fermentation of hexoses in one-step [139]. This step is carried out in a single reactor, where the same reaction conditions are applied to both processes [43, 139]. SSF configuration effectively overcomes the drawbacks in SHF by reducing the risk of contamination, inhibitory effects and decreasing the operating cost by utilizing only one reactor [43, 139]. The main limitation of this configuration is the failure to maintain the performance of hydrolysis and fermentation under optimal conditions [43, 139]. It is relatively difficult to maintain the optimal pH below 5 and a temperature of about 50 °C for hydrolysis, and optimal pH between 5 and 7 at 37 to 43 °C for fermentation [43].
Even though both configurations were reported to deliver encouraging results in LA production, SSF was shown superior to SHF from previous studies. Zhang et al. [26] reported a l-lactic acid conversion yield of 0.34 g/g with SHF and a higher yield of 0.6 g/g with SSF from xylo-oligosaccharides [26]. In addition, Zaini et al. [40] studied the production of d-lactic acid from dried distiller’s grains and also discovered a higher d-lactic acid yield of 0.423 g/g with SSF when compared to SHF [40].
Contrarily to lignocellulosic biomass, macroalgae is composed of cellulose and galactan, which have the potential to be converted into reducing sugars after the hydrolysis process. The enzymatic hydrolysis can be performed simultaneously with the co-fermentation of hexoses and pentoses in a SSCF configuration [139]. This approach allows the LA producers to commence a continuous fermentation process without separating the sugars and the involvement of sterilization [140]. This configuration poses less contamination risk and shortens the time duration of the process while producing a higher yield of LA [43]. SSCF is currently a highly researched area that has yet to achieve commercialization status [140]. Similar to SSF, the hydrolysis and fermentation are conducted simultaneously in a single reactor, thus the difficulty in providing optimal conditions to satisfy both processes [43].
Currently, the studies of LA production from macroalgae biomass through SHF, SSF, and SSCF are still scarce when compared to lignocellulosic biomass. A study by Talukder et al. [141] had investigated the production of l-lactic acid from lipid depleted microalgae, Nannochloropsis salina using the SSF method [141]. The microalgae biomass was pretreated using 5% of H2SO4 at 120 °C for 1 h. The SSF configuration was studied in a batch fermentation with the addition of Lactobacillus pentosus to produce LA at optimal conditions. The maximum yield of l-lactic acid reached 0.93 g/g with a productivity of 0.45 g/L h. Talukder et al. [141] discussed that low productivity might be the result of inhibitor compounds such as furfural and HMF [141]. Nguyen et al. [113] investigated the feasibility of l-lactic acid production from microalgae, Hydrodictyon reticulum through the SSCF process [113]. Lactobacillus paracasei LA104 was isolated from traditional Korean dishes and utilized for fermentation because it is highly tolerant toward heat and low pH [113]. Other than that, it is also tolerant toward high concentrations of glucose and can eliminate unfavorable inhibitory effects. The final concentration of l-lactic acid attained at optimal conditions was 37.11 g/L, with a yield of 0.46 g/g, and a productivity of 1.03 g/L h [113].
3.6 Separation and purification of LA
Regardless of the conversion route, the separation and purification of LA from fermentation broth is crucial. The downstream processes play a significant part in the purity of LA since the expenses of downstream processes represent half of the overall production cost [61]. In order to achieve commercially feasible LA, an efficient upstream process must be matched with an economically and environmentally feasible downstream separation method to remove impurities such as cells, proteins, residual sugar, and cell metabolites present inside the fermentation broth. Many studies had been extensively reported on LA downstream processes, including precipitation, solvent extraction [142,143,144,145], various distillation systems [74], ion exchange [12], and membrane separation processes [146,147,148,149,150,151]. A summary of the main advantages and disadvantages of each method is tabulated in Table 8.
Industrially, the purification of LA is mainly based on precipitation [61]. During the purification, an excess base, either calcium carbonate or calcium hydroxide is added into the fermentation broth to neutralize the LA produced. This step maintains the pH of the growth media around 5 to 6 so that the LA producers can adapt and work efficiently at pH near neutrality. The precipitated calcium lactates can then be easily recovered by distillation and re-acidified using sulfuric acid for the release of LA accompanied by the generation of gypsum. The generated gypsum from this process will later be filtered and evaporated to obtain the technical grade (22–44%) LA [152]. However, the high optical purity of LA enantiomers is essential for the manufacturing of PLA to define the physical properties of the end products [8]. For high-purity products, the technical-grade LA will go through esterification with methanol or ethanol to form either methyl or ethyl lactate. The lactate salt can be recovered sequentially using distillation, hydrolysis, evaporation, and crystallization. This method is a mature technology but the requirement for large amounts of sulfuric acid leads to higher cost. Other than that, the purification of LA from this method is accompanied with gypsum as a solid waste, which poses a threat from an environmental standpoint. Thereby, to date, researchers had been studying the recovery of LA through methods such as solvent extraction [142,143,144,145], membrane separation [146,147,148,149,150,151], and emulsion liquid membrane [153].
Another popular method of LA purification is solvent extraction. The LA is removed from the aqueous phase by the addition of an organic solvent extractant. Afterward, the phase loaded with LA known as the organic phase is back-extracted. This method seems to be promising because the LA is removed directly from the fermentation broth without the need of chemicals to regulate the pH. The merit of solvent extraction is that the process of extracting LA with the extractant and diluent can be repeated through proper recycling. An innovative hybrid process accompanied with reactive extraction has justified the importance of repeated extraction. Lan et al. [145] studied the efficiency of a two-step extraction incorporating salting-out and reactive extraction for LA recovery from corn stover hemicellulose derived-liquor [145]. This two-step hybrid system achieved an extraction efficiency of 89.4% after five successive extraction cycles with back-extraction. This recorded a slight improvement as compared to 83% obtained from one-step extraction at optimized conditions [145]. At the same time, this process consumes less energy to produce a high yield of LA. Unlike purification, this method avoids the generation of gypsum waste since the process does not involve any calcium salts. However, solvent extraction requires expensive equipment to achieve an efficient separation at high mass transfer rate. The use of a large amount of toxic extractants increases the additional cost to the process and might be limiting the performance of the LA producers to produce LA.
Membrane-based separation technologies such as electrodialysis, ultrafiltration, and nanofiltration have also been extensively studied [146,147,148,149,150,151]. López-Gómez et al. [28] reported the recovery of l-lactic acid from organic fraction municipal solid waste through a series of filtration followed by electrodialysis [28]. They managed to reduce the content of impurities to around 0.3 g/L, a value lower than the previously reported studies by Olszewska-Widdrat et al. and Neu et al. [12, 62]. Thus, a recovery percentage of 51.5% with a high purity of l-lactic acid at 98.7% was accomplished. Lee et al. [150] reported an integrated membrane separation process which is inclusive of ultrafiltration and nanofiltration for the recovery of LA from fermentation broth [150]. Both separation processes successfully separated most of the organic and inorganic components inside the fermentation broth. The recovery process was later combined with ion exchange and vacuum-assisted evaporation to remove residual salt ions further to achieve LA with high purity (> 99.5%). Pal and Dey [151] managed to obtain 95% pure l-lactic acid with a yield of 0.96 g/g and productivity of 12.4 g/L h using a three-stage membrane-integrated hybrid reactor system [151]. The hybrid system, which combined two processes, namely microfiltration, and nanofiltration, had permitted the selective production of l-lactic acid with repeated recycling of cells and unconverted reducing sugars. While separation through membrane provides high purity of LA, the advancement in this technology was limited due to the expensive membrane cost, membrane fouling, and polarization issues [150].
Recently, emulsion liquid membrane (ELM) technology was considered a new breakthrough for the recovery of LA. Emulsion liquid membrane is a technique used to separate solute molecules or ions using a liquid film between two miscible liquid phases [25, 153]. The transport mechanism of ELM is driven by kinetic instead of affinity in the solvent extraction process [153]. LA will first be extracted into the liquid membrane before being back-extracted from the membrane. This technology has been confirmed as a promising LA separation method due to its high extraction efficiency, the requirement of a small volume ratio between organic and aqueous phases, and large mass transfer area [25, 153]. Kumar et al. [154] managed to accomplish the extraction efficiency of LA at 96.59% at optimal conditions using ELM. The main concern of this method is due to the usage of non-renewable resources as organic solvent [25, 153]. In view of the importance of sustainable development, researchers are working progressively to search for a replacement for petroleum-based organic solvents in ELM. With respect to this constraint, the application of vegetable oils as the green solvent has been nominated as the most important development of ELM in recent years, proven to be feasible by several researchers [155,156,157].
4 Lactic acid sustainability assessment methods
Several useful methodologies have been extensively practiced to date to measure the efficiency and environmental sustainability of a system or process, namely life cycle assessment, techno-economic analysis, energy, and exergy analysis.
The approach of life cycle assessment can effectively evaluate the energy efficiency and the environmental hotspots in a system or process [158]. It provides data-driven insights on environmental impacts such as climate change, ecotoxicities in terms of marine, freshwater, or terrestrial and depletion of water or fossil resources. Thereby, future optimizations can be made to the whole system to reduce the environmental effects identified in a LA biorefinery.
When evaluating an energy system, energy analysis is the most commonly used engineering tool. It considers all the input and output energy in a boundary system to provide insights into the efficiency of the system. However, the reliance on energy analysis can be confusing since it cannot identify how the energy in a system is transferred or converted into one another. The sustainability of a system cannot be justified by energy analysis solely. On the other hand, an exergy analysis that quantifies both the quantity and quality of energy streams is a better approach than energy analysis. Exergy analysis can effectively discover the locations and the causes of thermodynamic irreversibility in a system by providing a quantitative analysis. After identification of the locations of thermodynamic irreversibility, potential enhancements can be made to the system to improve the overall system efficiency. As the exergy efficiencies are enhanced, the sustainability of the system can also be improved with the reduction in environmental impacts initiated by these existing thermodynamic losses. In general, exergy analysis provides more meaningful insights as a method which closely resembles ideal scenarios.
4.1 Exergy analysis
4.1.1 Energy versus exergy
The first law of thermodynamics states that energy is neither created nor destroyed but changed from one form into another. Based on the second law of thermodynamics, the exergy does not obey the conservation laws and tends to be destroyed when a process is irreversible. When exergy is destroyed, this phenomenon indicates that the quality or usefulness of the energy is lost and is identified as exergy destruction [74]. Exergy is conserved during real processes where they are reversible. Thus, energy differs from exergy such that energy is a measure of only the quantity, whereas exergy may be viewed as a measure of quality energy in a system. In other words, exergy is a property to measure the capacity of an energy form to do useful work. In fundamental theory, exergy is defined as the maximum amount of work that can be extracted from a system as it is brought to equilibrium relative to a reference environment [159,160,161]. The exergy will be equaled to zero when the system properties correspond to the reference environment that is called the dead state.
Energy analysis is mostly used to determine the thermodynamic efficiency in processes. It is important to note that, while the use of energy allows one to construct an energy balance around a given process, a focus on energy analysis alone can be misleading because it does not distinguish between heat and work. It does not provide any resourceful information with regards to how energy is transferred or whether the energy can be completely converted to another. Conversely, the exergy analysis can overcome these drawbacks from energy analysis in various energy processes. Table 9 presents the comparison between the key properties of energy and exergy.
4.1.2 Exergy reference environment
The evaluation of exergy concepts is with respect to a reference environment which acts as an infinite system. The specification of the concise characteristics for the reference environment is of utmost importance. This is commonly done by defining the temperature (To) and pressure (Po) at 25 °C and 101.325 kPa, respectively, and the chemical composition of the reference environment [160, 163]. The outcome of exergy analysis is usually relative to the specified reference environment mentioned, which is modeled after the actual local environment in most applications. When the system is in equilibrium with the reference environment, the exergy of a system is denoted as zero [161]. The exergy consumption is also zero in a reversible process [161].
4.1.3 Exergy efficiency and exergy destruction
The aforementioned energy analysis is incompetent at providing a measure of the performance of a system approaches ideal condition [164]. Furthermore, it is insufficient in pinpointing the factors and locations of energy losses, which causes the performance of the system to deviate from ideal conditions [164]. In contrast, exergy analysis tends to provide useful insights into improving the efficiency of a process under ideal conditions, practically overcoming the drawbacks associated with energy analysis.
Exergy analysis is an efficient methodology based primarily on the concept of irreversible production of entropy principle from the second law of thermodynamics and the conservation of energy principle from the first law of thermodynamics [160]. According to the exergy analysis, energy can neither be created nor destroyed, but the energy quality can be degraded during a process [160, 161]. The degraded energy eventually reaches the equilibrium state with the surroundings and cannot be used to perform tasks efficiently. Exergy analysis is a useful engineering tool which assists in improving and optimizing designs because it is a better indicator of nature, causes, and locations of thermodynamic losses in a system when compared to energy analysis [160, 164]. It can be applied in complex integrated systems such as biorefineries and provide a qualitative and quantitative estimation of energy requirements effectively and identify the thermodynamic inefficiencies taking place in the systems [74]. Once the thermodynamic inefficiencies, also known as exergy destructions, are quantified, and located, engineering measures can be taken to enhance the overall exergy efficiency. The exergy destruction can be minimized as the exergy efficiency increases.
4.1.4 Exergy and environmental sustainability
Exergy is closely related to sustainability. Novak [163] commented that a reduction in exergy destruction or depletion is crucial in improving the sustainability of a system [163]. In this context, exergy analysis is a practical approach related to the development of sustainability since it is instrumental in providing insights into exergy destruction. These exergy destructions can cause negative impacts to the environment. By minimizing or eliminating the source of exergy destruction, this can boost the efficiency of the overall process and contribute to reduced environmental damage. Rosen et al. [160] suggested that the utilization of non-renewable resources as a power source should be minimized to prevent harmful emissions to the environment [160].
The relationship among sustainability, exergy efficiency, and environmental impact is represented in Fig. 4. When the exergy efficiency is approaching 100%, the environmental impact is kept at minimal and the sustainability of a system is approaching infinity as the system is experiencing the reversible process [160]. At this stage, the exergy is only converted from one form to another without suffering from exergy destruction. Conversely, when the exergy efficiency is approaching 0%, the system fails to achieve sustainability because energy resources are not utilized properly, resulting in an increasing amount of interfaced exergy destruction to the environment [160].
Relationships between exergy efficiency with sustainability and environmental impact [74]
4.1.5 Exergy analysis of biomass conversion in biorefinery
For the concept of biorefinery, reducing sugar is an essential component for the production of bioethanol and LA. In the conversion of biomass into reducing sugars, the drying and pretreatment process plays the role of minimizing the size of biomass and facilitating the conversion of cellulose to reducing sugar with better accessibility to enzymes, respectively [15]. It is reported that a significant amount of exergy is destroyed due to the heat and mass transfer phenomena during chemical reactions [74]. Thus, the selection of the suitable drying and pre-treatment methods is dependent on its thermodynamic performance instead of its potential yield of reducing sugar. For a fair comparison, exergy analysis would be a useful indicator of thermodynamic performance in a system [160, 164]. The following subsections outlined the research works where exergy-based analyses had been used for assessing the bioconversion of any of the three generations feedstock. Reported studies based on exergy aspects for developed biorefineries are summarized in Table 10.
Ofori-Boateng and Lee [165] carried out exergy analyses on the production of ethanol from oil palm fronds (OPFs) based on three different pretreatment methods, which are steam explosion, organosolv, and microbial pretreatment method [165]. The system which employed the microbial pretreatment method exhibited the highest overall exergy efficiency of 90.93%, followed by organosolv (90.30%), and steam explosion (66.65%), as illustrated in Fig. 5. The exergy destruction in the steam explosion was mainly originated from the high-pressure steam and dryer, which involved high energy consumption. Similarly, the drying unit was also the major contributor toward the exergy destruction in organosolv due to the heavy heat exchange. This is because OPFs have very high moisture content. Therefore, high energy was needed to decrease the moisture content to about 10%. It was recommended to employ sun drying for preliminary drying before the actual drying in dryers as this could reduce the demand for energy. Aside from the dryer being the hotspot for thermodynamic losses, the heavy usage of chemicals such as ethanol and water was also one of the underlying reasons behind the low exergy efficiency of orgonosolv. This limitation could be overcome with the recovery of ethanol to be reused in the process. The generation of wastes was the main cause of thermodynamic losses in the microbial pretreatment method. The conversion of these wastes into value-added products would be advantageous toward the overall performance and environmental sustainability of the system.
Overall exergy efficiency of system with different pre-treatment methods [165]
An exergy analysis was conducted by Silva Ortiz and de Oliveira [166] on four different pre-treatment technologies for the preparation of two types of lignocellulosic biomass with different chemical compositions for the production of bioethanol [166]. The pre-treatment methods were steam explosion (case study A), organosolv (case study B), liquid hot water (case study C), and a combination of steam explosion with hot water liquid (case study D). It was discovered that the lignin-rich part of sugarcane bagasse could lead to a declined exergy performance compared to parts with lower lignin content. Case study D showed the highest overall exergy efficiency as it utilized the exergy contents from outflow of steam explosion in hot water liquid method. The combined method was a breakthrough and should be entitled for an economic and environmental assessment for industrial-scale processing. When evaluated alone, both the hot water liquid method and steam explosion were reported to involve high pressure and temperature. Consequently, high energy was needed which affects its thermodynamic performance.
Modarresi et al. [167] applied exergy analysis for the quantification of thermodynamic efficiencies to evaluate the magnitude of irreversibility within bioethanol production processes from straw [167]. Pinch analysis was first conducted, and the minimum hot and cold utility demands of the plant were optimized by 40%. The exergy efficiency of the plant after optimization was concluded to be higher than the base case due to the effective reduction in demand for steam and cooling water. Following the exergy analysis, it was discovered that the bioethanol production process resulted in the highest exergy efficiency. This was attributed to the useful exergy outflow of stillage since it could be converted into biomethane and combusted in a steam boiler for power generation. On the other hand, the combined heat and power (CHP) process suffered from the high exergy destruction because the unit involved rapid heat losses to the surrounding during combustion and unknown materials which were not reacting correctly.
An exergy analysis was employed by Sanjuan-Acosta et al. [170] to assess the production of agar from macroalgae, Gracilaria sp. [170]. The macroalgae was washed, dried, and pretreated with sodium hydroxide (NaOH). This was followed by neutralization using acid solution and heating to obtain the diluted agar, which was later separated and purified through evaporation from other solid sludge. The cellulose-rich agar, when optimized with acid or alkali pretreatment, could produce reducing sugar, which would be a favorable carbon source for LA fermentation process [171]. The stage with the highest exergy irreversibility (16,552 MJ/h) was the evaporation unit due to the variations in temperature for the separation of agar from water. The second-largest contributor of exergy losses was the Filtration III stage, which resulted from the formation of undesired waste (6146.60 MJ/h) as illustrated in Fig. 6. In terms of exergy efficiency, filtration I associated with low consumption of utilities had the highest exergy efficiency of 98.9%, whereas the evaporation unit had the lowest exergy efficiency of 27.9%. Hence, efforts should be shifted to improve the performance of the evaporation unit and filtration III through better energy integration.
Overview of exergy analysis for production of agar from Gracilaria sp. [170]
Fudholi et al. [169] analyzed the thermodynamic performance of drying red seaweed using the solar system with the conventional energy and exergy analysis [169]. The solar dryer took 15 h to decrease the moisture content of 40 kg red seaweed from 90 to 10%. The energy analysis showed that the specific energy consumption of the system was 2.62 kWh/kg, whereas the maximum efficiency of moisture removal from the biomass was calculated to be 95% by maintaining the drying temperature between 35 and 60 °C. Throughout the day, the solar dryer displayed an exergy efficiency within the range of 1 to 93%, achieving an average of 30%. Thus, this study had proven that the utilization of solar dryer would be a great idea to decrease the exergy losses caused by drying unit for the biorefinery developed by Ofori-Boateng and Lee and Sanjuan-Acosta et al. [165, 170].
A lignocellulosic biorefinery for co-generation of LA and electricity production was exergetically analyzed by Aghbashlo et al. [74]. Aghbashlo et al. [74] discovered that the overall functional exergy efficiency of the system was 44.73% where the boiler contributed the highest exergy destruction rate (129.41 MW) followed by LA production sub-unit [74]. In other words, this study revealed that both sub-units have very poor exergy efficiencies due to the generation of entropy in large amounts. The quantitative results presented in Fig. 7 showed that the boiler had exergetic improvement potential as high as 68.90 MW, indicating the possibility of further optimization in the design to improve the overall efficiency of the process. However, they also learned that the refrigeration sub-unit had the least exergy efficiency rate, which was against the concept of exergy because this sub-unit had a low destruction rate. They further related this to the high consumption of power within the sub-unit. Notably, the results from this exergy analysis highlighted that future optimization can be focused on the steam generation and LA production sub-units.
Exergy destruction rates derived from subsystem of LA Biorefinery. (1) pre-treatment of feedstock; (2) production of cellulase enzyme; (3) saccharification; (4) boiler; (5) water treatment; (6) generation of power and distribution of steam; (7) production of LA; (8) refrigeration; and (9) cooling tower [74]
In another study, Soltanian et al. [172] conducted an exergoeconomic analysis on the lignocellulosic biorefinery system reported by Aghbashlo et al. [74]. Similarly, the steam generation unit accounted for 43.73% of the overall cost rate of the biorefinery, whereas the LA production sub-unit came in the second rank with 20.49%. The high-cost rate in each sub-unit is associated with the high exergy destruction, as mentioned previously. The pre-treatment and saccharification sub-units had the lowest relative cost difference of 0.07, whereas the refrigeration sub-unit which consumed higher energy had a higher relative difference of 20.47. This indicated that the refrigeration sub-unit can be further optimized for improvement whereas both the pre-treatment and saccharification sub-units were over-designed. They suggested that an absorption refrigerator can be a potential alternative for reduction in electrical energy consumption. In conclusion, the result from this exergoeconomic analysis was in line with the exergy analysis where it suggested major optimization actions should be carried out on both the steam generation and LA production sub-units.
The performance of LA production from lignocellulosic biomass was assessed by Gezae Daful and Görgens [173] from the economic and environmental aspects [173]. The study was conducted in six different scenarios where the substrate for S1, S3, and S5 is hemicellulose liquid fraction while S2, S4, and S6 are cellulose-lignin solid. S1 and S2 utilized Ca(OH)2 to neutralize the fermentation broth, which led to the formation of undesired gypsum waste. S3 and S4 isolated thermophilic bacteria, Bacillus coagulans for fermentation where neutralization is not necessary. S5 and S6 replaced Ca(OH)2 with Mg(OH)2 for neutralization. Despite the fact that S1 and S2 provided the highest internal rate of return among other scenarios, both scenarios also accounted for the highest operating cost and total capital investment. The study also found that S1 and S2 resulted in higher environmental impacts as the production of gypsum waste from large quantities of chemicals usage was not avoided. Contrarily, the scenario without gypsum waste, S5 and S6 were the more attractive processes economically and environmentally since both also resulted in a relatively high internal rate of return and relatively lower operating cost compared to S1 and S2. For S3 and S4, the environmental impacts were caused by the nutrients used in the growth medium for Bacillus coagulans. In overall, S5 and S6 were the best scenarios due to their high revenue, low environmental impacts, and low operating costs. The price for LA was determined in the range of US $1.30–5.00/kg.
Another published article by Liu et al. [168] was based on the techno-economic evaluation on l-lactic acid production from corn stover [168]. The corn stover was pre-treated with dilute acid and bio-detoxified before the conversion into LA. The experiment demonstrated an optimal performance by producing l-lactic acid with a concentration of 104.5 g/L and a yield of 0.72 g/g. The analysis reported that the minimum selling price of l-lactic acid was determined at US $0.56/kg. In the overall process, the enzymatic hydrolysis represented the most expensive step. The exploration of cheaper enzymes substitutes should be emphasized in order to achieve a more attractive selling price of the product. Currently, the selling price of l-lactic acid derived from corn stover reported by Liu et al. [168] was able to compete with the current commercial l-lactic acid produced from food crops with the selling price around US $3.00–4.00/kg [25, 168].
In conclusion, the common hotspots for exergy losses in a biorefinery tend to be found at units which consume high energy, especially in the boiler and chiller involved in power generation, as reported by Aghbashlo et al. and Modarresi et al. [74, 167]. The exergy losses in the boiler can be reduced either by reducing the volume of combustion air or introducing intake air enriched with oxygen. For chiller, the replacement of a conventional refrigeration system to an adsorption refrigeration system is highly recommended to promote the recirculation of heat within the system. This can efficiently minimize any wasted energy within the system, thereby enhancing the overall exergy efficiency. It was demonstrated by Soltanian et al. [172] that these energy-intensive hotspots also contributed to the high operating costs along with fermentation units, as reported by Liu et al. [168]. The high cost was owing to the usage of expensive feedstock and enzymes together with the addition of costly chemicals or nitrogen sources for the growth of microorganisms required for LA production [172]. Aside from this, the heavy usage of chemicals and water supply could be influential on the exergy efficiency of the system, as studied by Ofori-Boateng and Lee [165]. Therefore, the recovery and recycling of chemicals for repeated cycles could be a potential solution for reducing the thermodynamic losses.
Apart from that, those units which involved intensive changes in temperature were found to be potential contributors to exergy destructions. Ofori-Boateng and Lee [165] had also expressed their concern on the amount of exergy losses contributed by the drying units to the system in their studies. Recommendations were given that the moisture content of the biomass should be reduced to a certain extent from drying under the sun before the conventional drying process. Ultimately, utility cost could be reduced while benefiting the exergy performance of the system.
Besides, the exergy efficiency of conventional drying processes could be further enhanced with the replacement of a solar dryer. Fudholi et al. [169] had justified the effectiveness of a solar dryer in drying red seaweed, which had achieved a very high exergy efficiency of 93% during sunshine hours [169]. However, it should be noted that the drying time required was relatively long as drying can only be performed during sunny days. At other hours of the day, the exergy efficiency could be as low as 1%. The improvement potential (IP) for this solar dryer was rated in the range of 0.3 and 630 W, where it signified a high potential to enhance the exergy efficiency. Similarly, Sanjuan-Acosta et al. [170] had reported the evaporation unit as being the largest contributor of exergy losses owing to changes in temperature and high consumption of energy [170]. Energy integration using the exergy-based pinch concept can be utilized for enhancement in exergy efficiency. The study also revealed that the residual waste stream from the third filtration unit was one of the primary sources for exergy losses. This exergy destruction could be reduced by processing the waste into fertilizer while simultaneously promoting better environmental performance for this macroalgae-based biorefinery system.
4.2 Life cycle assessment of algae-based biorefinery
Life cycle assessment plays a pivotal role in assessing the potential environmental hotspots of a system in interest based on a framework given by ISO 14040 and 14044 [174, 175]. Four different methodologies of defining goal and scope, collection of inventory data for subsequent analysis, the assessment on impacts toward the system, and interpretation of results from impact analysis are conducted during the whole life cycle assessment (LCA) study. Based on the quantitative output from the LCA study, relevant optimization of the production system to mitigate these impacts in the whole life cycle could be made. This methodology allowed Smullen et al. [176] to identify significant environmental impacts in a bioethanol biorefinery during the pre-hydrolysis stage [176]. The improvement in energy efficiency at that stage was necessary due to the large consumption of chemicals, steam, and electricity. Regardless of the reliability of this approach, this methodology would be time-consuming as a large amount of input data was required to be gathered for the accurate assessment of a system [177].
For LA biorefineries, the application of LCA is essential as this methodology provides an overview on the environmental impacts associated with LA production from terrestrial biomass and algae feedstock for comparison. According to Jung et al. [98], terrestrial biomass is a greater contributor toward climate change compared to algae biomass [98]. The excessive land use for the reproduction of terrestrial biomass intensifies the greenhouse gases (GHG) emissions as forest land is exploited [178]. On the other hand, algae biomass does not require land and is able to grow in extreme growth conditions along with a variety of water environments [64, 105]. Aside from this, terrestrial biomass has lignin, which is distinctively different from the structure of macroalgae biomass. Delignification requires a sequence of pre-treatment techniques employing a large amount of toxic chemicals such as acid and alkali. Smullen et al. [176] employed LCA to evaluate the environmental performance using different types of chemicals to release fermentable sugars from lignocellulosic biomass. The usage of hydrochloric acid and sodium hydroxide resulted in the highest emissions output to the surrounding, contributing to environmental impact categories such as global warming potential, eutrophication, acidification, human, and marine ecotoxicity. Contrarily, these environmental burdens would be kept minimal for algae biomass due to the absence of lignin [51, 77, 78, 103, 104]. Despite the environmental merits of algae biomass during the cultivation and pre-treatment stages, the maturity of current algae biomass-based technology remains a challenge as it is energy intensive which contributes to impact categories, as reported by Ögmundarson et al. [179]. Thereby, LCA is a reliable methodology in pinpointing the relevant environmental hotspots in the current technology and further drives researchers in developing a more environmental-friendly and economical technology. As of now, there are very limited LCA studies performed on algae biorefinery systems, with only two of them related to LA production, which are summarized in Table 11.
Based on a LCA analysis reported by van Oirschot et al. [180], the goal was to explore the potential optimizations in cultivation, harvesting, transport, and drying of seaweed from an environmental standpoint [180]. The study was performed based on two seaweed drying systems, each using 1 ton of protein in the dried seaweed as the functional unit (FU). One of the systems featured a single layer of cultivation strips, and the other featured a double number of cultivation strips in the water column. Overall, the dual-layer cultivation system produced 50% greater yield compared to single layer cultivation system for producing one ton of protein in dried seaweed, while keeping the environmental impact at a lower rate relative to the one-layer system. Out of all the system boundaries, they concluded that the drying process of seaweed to be the highest contributor to the environmental effects in the examined system as 9.9 MJ was required to produce the desired FU due to the large generation of GHG from fuel burning for the energy production.
Meanwhile, the drying process of macroalgae was agreed by a few researchers as the hotspot of the environmental impacts. Sander and Murthy [181] performed a life cycle assessment for biofuel production from algae and indicated that the dewatering process accounted for the largest fossil fuel-derived energy input with high GHG emissions [181]. In such a scenario, the environmental impacts from energy usage and GHG emissions could be reduced with the degradation of algae biomass enzymatically, as stated by Sander and Murthy [181]. They suggested that renewable energy alternatives such as solar dryers should be adopted for the dewatering of seaweed. Besides that, Langlois et al. [182] evaluated the potential environmental impacts resulting from the methane production with offshore-cultivated seaweed and its combustion in an engine [182]. Similarly, they found out that the drying process consumed the most energy in the biorefinery. They believed that the reported energy consumption (920 kWh/t) in the drying process could be reduced with heat recovery or utilizing mechanical compression of steam for evaporation. The replacement of electricity from the grid with electricity generated from wind energy showed significant improvement in environmental impacts from 5.6 to 86.0%.
The environmental impacts of utilizing green macroalgae, Ulva spp. for the production of LA was analyzed using an exploratory LCA study by Helmes et al. [183]. The system boundary was determined as follows: cultivation of macroalgae, pretreatment of feedstock for release of sugars (acid hydrolysis), fermentation of sugars to LA, and the isolation of LA from the fermentation broth. The FU for this study was defined as 1 kg of LA. They discovered that the electricity consumption during the cultivation of macroalgae was the major driver toward the environmental impacts, contributing 97% to the ReCiPe score. The CO2 emission resulted from the acid hydrolysis due to heavy usage of acid contributed 2% to the ReCiPe score, which came in as the second-highest hotspot. The remaining 1% was due to the residual waste generated from the fermentation and purification process. The consumption of electricity could be reduced with an optimized cultivation method and also the utilization of alternative renewable energy sources. The emission of CO2 could be reduced by adjusting the amount of acid for the production of 1 kg of LA whereas the waste could be converted into fertilizer or animal feed, subsequently reducing the impacts they had on the environment.
The LCA study published by Ögmundarson et al. [179] evaluated the environmental impacts in LA production from macroalgae biomass, Laminaria sp. [179]. They presented a LCA study based on the cradle to grave life cycle of LA production from three generations of feedstock, corn, corn stover, and macroalgae in two different scenarios (drying and without drying), respectively. Ögmundarson et al. [179] again found out that the high utility inputs (up to 86%) for drying of biomass was the main environmental hotspot contributing to global warming with regard to LA production from macroalgae. This was possibly due to the technologically immature production system. The environmental impacts in terms of global warming, land use, water consumption, and impact on human health in terms of carcinogenic toxicity were greatly reduced when wet Laminaria sp. biomass for LA production was demonstrated compared to utilization of dried macroalgae [179]. Despite this advantage, the drying process cannot be easily excluded in macroalgae-based biorefinery as it has a significant influence in reducing the contamination risk in the feedstock. In addition, the microorganisms tend to consume more on dry macroalgae biomass [179]. Thus, the application of wet macroalgae biomass in biorefinery stands as a vital challenge to be overcome in the near future.
5 Conclusions and future prospective
In recent years, biodegradable plastic composed of polylactic acid (PLA) has gained immense interest due to the increasingly alarming plastic pollution. This has driven up the demand of LA; it is the main building block for PLA. Commercially, LA production via fermentation is preferred over chemical synthesis as it is proclaimed to be more sustainable with the utilization of renewable resources instead of fossil fuels. However, the biggest obstacle of the fermentation route is the huge production cost relative to the cost of substrate and downstream processes. In accordance with increased demand for high optical purity LA, development in new substrate candidates and downstream processes are vital. This review sheds light on the role of macroalgae as a promising substrate for sustainable LA production due to its rapid growth rate and a wide range of polysaccharide content. The viability of macroalgae-based biorefineries from exergy, economic, and environmental aspects was also explored by approaching the developed biorefineries with exergy-based methods and life cycle assessment studies. Nevertheless, the advances in technology for the processing of macroalgae are to be further explored and optimized for attaining increased yield, productivity, and optical purity of LA at large-scale production. Once the establishment of these technologies is achieved, macroalgae could be the most beneficial feedstock for the production of biofuel and biochemical in the future.
References
Payne J, McKeown P, Jones MD (2019) A circular economy approach to plastic waste. Polym Degrad Stab 165:170–181. https://doi.org/10.1016/j.polymdegradstab.2019.05.014
Plastic Oceans International (2020) The Facts. https://plasticoceans.org/the-facts/
Garside M (2020) Plastics industry - Statistics & Facts. https://www.statista.com/topics/5266/plastics-industry/#:~:text=Since%20the%20start%20of%20itsfifth%20of%20global%20oil%20consumption
Serle J (2020) How long do biodegradable bags take to decompose? https://www.sciencefocus.com/science/how-long-do-biodegradable-bags-take-to-decompose/#:~:text=Biodegradable%20plastics%20take%20three%20tothe%20amount%20of%20moisture%20present
Morão A, de Bie F (2019) Life Cycle Impact Assessment of Polylactic Acid (PLA) Produced from sugarcane in Thailand. J Polym Environ 27(11):2523–2539. https://doi.org/10.1007/s10924-019-01525-9
Msuya N (2017) Poly(lactic-acid) Production_From Monomer to Polymer: A review. Sci-Fed J Polymersci 1
Jem KJ, Tan B (2020) The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv Ind Eng Polymer Res 3(2):60–70. https://doi.org/10.1016/j.aiepr.2020.01.002
Alves de Oliveira R, Komesu A, Vaz Rossell CE, Maciel Filho R (2018) Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem Eng J 133:219–239. https://doi.org/10.1016/j.bej.2018.03.003
Ahmad A, Banat F, Taher H (2020) Enhanced lactic acid production from food waste in dark fermentation with indigenous microbiota. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00801-2
Sharma A, Pranaw K, Singh S, Khare S, Chandel A, Nain P, Nain L (2020) Efficient two-step lactic acid production from cassava biomass using thermostable enzyme cocktail and lactic acid bacteria: insights from hydrolysis optimization and proteomics analysis. 3 Biotech 10. https://doi.org/10.1007/s13205-020-02349-4
Jiang S, Xu P, Tao F (2019) l-Lactic acid production by Bacillus coagulans through simultaneous saccharification and fermentation of lignocellulosic corncob residue. Bioresour Technol Rep 6:131–137. https://doi.org/10.1016/j.biteb.2019.02.005
Neu A-K, Pleissner D, Mehlmann K, Schneider R, Puerta-Quintero GI, Venus J (2016) Fermentative utilization of coffee mucilage using Bacillus coagulans and investigation of down-stream processing of fermentation broth for optically pure l(+)-lactic acid production. Bioresour Technol 211:398–405. https://doi.org/10.1016/j.biortech.2016.03.122
Pleissner D, Neu A-K, Mehlmann K, Schneider R, Puerta-Quintero GI, Venus J (2016) Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Bioresour Technol 218:167–173. https://doi.org/10.1016/j.biortech.2016.06.078
Bustamante D, Tortajada M, Ramón D, Rojas A (2020) Production of D-Lactic Acid by the Fermentation of Orange Peel Waste Hydrolysate by Lactic Acid Bacteria. Fermentation 6(1):1
Tan IS, Lam MK, Foo HCY, Lim S, Lee KT (2020) Advances of macroalgae biomass for the third generation of bioethanol production. Chin J Chem Eng 28(2):502–517. https://doi.org/10.1016/j.cjche.2019.05.012
Abdel-Rahman MA, Sonomoto K (2016) Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J Biotechnol 236:176–192. https://doi.org/10.1016/j.jbiotec.2016.08.008
Komesu A, Oliveira J, Martins L, Maciel M, Filho R (2017) Lactic acid production to purification: a review. BioResources 12:4364–4383. https://doi.org/10.15376/biores.12.2.4364-4383
Vijayakumar J, Thangavelu V, Rajendran A (2008) Recent trends in the production, purification and application of lactic acid. Chem Biochem Eng Q (cabeq@pbf.hr) 22(2):22
Wee Y-J, Kim J-N, Ryu H-W (2006) Biotechnological production of lactic acid and its recent applications Food Technol. Food Technol Biotechnol 44
Ata R, Aladdin A, Othman N, Malek RA, Leng O, Aziz R, El Enshasy H (2015) Lactic acid applications in pharmaceutical and cosmeceutical industries. J Chem Pharm Res 2015:729–735
Chen X, Chen G, Wang G, Zhu P, Gao C (2020) Recent progress on 3D-printed polylactic acid and its applications in bone repair. Adv Eng Mater 22(4):1901065. https://doi.org/10.1002/adem.201901065
Tan J, Abdel-Rahman MA, Sonomoto K (2018) Biorefinery-based lactic acid fermentation: microbial production of pure monomer product. In: Di Lorenzo ML, Androsch R (eds) Synthesis, Structure and Properties of Poly(lactic acid). Springer International Publishing, Cham, pp 27–66. https://doi.org/10.1007/12_2016_11
Patrizi N, Bruno M, Saladini F, Parisi ML, Pulselli RM, Bjerre AB, Bastianoni S (2020) Sustainability assessment of biorefinery systems based on two food residues in Africa. Front Sustain Food Syst 4(166). https://doi.org/10.3389/fsufs.2020.522614
Zhao Z, Xie X, Wang Z, Tao Y, Niu X, Huang X, Liu L, Li Z (2016) Immobilization of Lactobacillus rhamnosus in mesoporous silica-based material: An efficiency continuous cell-recycle fermentation system for lactic acid production. J Biosci Bioeng 121(6):645–651. https://doi.org/10.1016/j.jbiosc.2015.11.010
Ahmad A, Banat F, Taher H (2020) A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ Technol Innov 20:101138. https://doi.org/10.1016/j.eti.2020.101138
Zhang L, Li X, Yong Q, Yang S-T, Ouyang J, Yu S (2015) Simultaneous saccharification and fermentation of xylo-oligosaccharides manufacturing waste residue for l-lactic acid production by Rhizopus oryzae. Biochem Eng J 94:92–99. https://doi.org/10.1016/j.bej.2014.11.020
Pleissner D, Demichelis F, Mariano S, Fiore S, Navarro Gutiérrez IM, Schneider R, Venus J (2017) Direct production of lactic acid based on simultaneous saccharification and fermentation of mixed restaurant food waste. J Clean Prod 143:615–623. https://doi.org/10.1016/j.jclepro.2016.12.065
López-Gómez JP, Alexandri M, Schneider R, Latorre-Sánchez M, Coll Lozano C, Venus J (2020) Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J Clean Prod 247:119165. https://doi.org/10.1016/j.jclepro.2019.119165
Abdel-Rahman MA, Hassan SE-D, Roushdy MM, Azab MS, Gaber MA (2019) Free-nutrient supply and thermo-alkaline conditions for direct lactic acid production from mixed lignocellulosic and food waste materials. Bioresour Technol Rep 7:100256. https://doi.org/10.1016/j.biteb.2019.100256
Li J, Sun J, Wu B, He B (2017) Combined utilization of nutrients and sugar derived from wheat bran for d-Lactate fermentation by Sporolactobacillus inulinus YBS1-5. Bioresour Technol 229:33–38. https://doi.org/10.1016/j.biortech.2016.12.101
Hassan SE-D, Abdel-Rahman MA, Roushdy MM, Azab MS, Gaber MA (2019) Effective biorefinery approach for lactic acid production based on co-fermentation of mixed organic wastes by Enterococcus durans BP130. Biocatalysis Agric Biotechnol 20:101203. https://doi.org/10.1016/j.bcab.2019.101203
van der Pol EC, Eggink G, Weusthuis RA (2016) Production of l(+)-lactic acid from acid pretreated sugarcane bagasse using Bacillus coagulans DSM2314 in a simultaneous saccharification and fermentation strategy. Biotechnol Biofuels 9(1):248. https://doi.org/10.1186/s13068-016-0646-3
Alves de Oliveira R, Schneider R, Vaz Rossell CE, Maciel Filho R, Venus J (2019) Polymer grade l-lactic acid production from sugarcane bagasse hemicellulosic hydrolysate using Bacillus coagulans. Bioresour Technol Rep 6:26–31. https://doi.org/10.1016/j.biteb.2019.02.003
Ahring BK, Traverso JJ, Murali N, Srinivas K (2016) Continuous fermentation of clarified corn stover hydrolysate for the production of lactic acid at high yield and productivity. Biochem Eng J 109:162–169. https://doi.org/10.1016/j.bej.2016.01.012
Chen P-T, Hong Z-S, Cheng C-L, Ng IS, Lo Y-C, Nagarajan D, Chang J-S (2020) Exploring fermentation strategies for enhanced lactic acid production with polyvinyl alcohol-immobilized Lactobacillus plantarum 23 using microalgae as feedstock. Bioresour Technol 308:123266. https://doi.org/10.1016/j.biortech.2020.123266
Chen H, Chen B, Su Z, Wang K, Wang B, Wang Y, Si Z, Wu Y, Cai D, Qin P (2020) Efficient lactic acid production from cassava bagasse by mixed culture of Bacillus coagulans and lactobacillus rhamnosus using stepwise pH controlled simultaneous saccharification and co-fermentation. Ind Crop Prod 146:112175. https://doi.org/10.1016/j.indcrop.2020.112175
Ma X, Gao M, Yin Z, Zhu W, Liu S, Wang Q (2020) Lactic acid and animal feeds production from Sophora flavescens residues by Rhizopus oryzae fermentation. Process Biochem 92:401–408. https://doi.org/10.1016/j.procbio.2020.01.030
Radosavljević M, Pejin J, Kocić-Tanackov S, Mladenović D, Djukić-Vuković A, Mojović L (2018) Brewers' spent grain and thin stillage as raw materials in l-(+)-lactic acid fermentation. J Inst Brew 124(1):23–30. https://doi.org/10.1002/jib.462
de la Torre I, Acedos MG, Ladero M, Santos VE (2019) On the use of resting L. delbrueckii spp. delbrueckii cells for D-lactic acid production from orange peel wastes hydrolysates. Biochem Eng J 145:162–169. https://doi.org/10.1016/j.bej.2019.02.012
Zaini NABM, Chatzifragkou A, Charalampopoulos D (2019) Microbial production of d-lactic acid from dried distiller's grains with solubles. Eng Life Sci 19(1):21–30. https://doi.org/10.1002/elsc.201800077
Li Z, Han L, Ji Y, Wang X, Tan T (2010) Fermentative production of l-lactic acid from hydrolysate of wheat bran by Lactobacillus rhamnosus. Biochem Eng J 49(1):138–142. https://doi.org/10.1016/j.bej.2009.10.014
Kong X, Zhang B, Hua Y, Zhu Y, Li W, Wang D, Hong J (2019) Efficient l-lactic acid production from corncob residue using metabolically engineered thermo-tolerant yeast. Bioresour Technol 273:220–230. https://doi.org/10.1016/j.biortech.2018.11.018
Rawoof SAA, Kumar PS, Vo D-VN, Devaraj K, Mani Y, Devaraj T, Subramanian S (2020) Production of optically pure lactic acid by microbial fermentation: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01083-w
Chen X, Wang X, Xue Y, Zhang T-A, Li Y, Hu J, Tsang YF, Zhang H, Gao M-T (2018) Influence of rice straw-derived dissolved organic matter on lactic acid fermentation by Rhizopus oryzae. J Biosci Bioeng 125(6):703–709. https://doi.org/10.1016/j.jbiosc.2018.01.004
Trakarnpaiboon S, Srisuk N, Piyachomkwan K, Yang S-T, Kitpreechavanich V (2017) l-Lactic acid production from liquefied cassava starch by thermotolerant Rhizopus microsporus: Characterization and optimization. Process Biochem 63:26–34. https://doi.org/10.1016/j.procbio.2017.08.019
Thongchul N (2013) Production of Lactic Acid and Polylactic Acid for Industrial Applications. In: Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers, pp 293–316. https://doi.org/10.1002/9781118642047.ch16
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31(6):877–902. https://doi.org/10.1016/j.biotechadv.2013.04.002
Ilmén M, Koivuranta K, Ruohonen L, Rajgarhia V, Suominen P, Penttilä M (2013) Production of l-lactic acid by the yeast Candida sonorensis expressing heterologous bacterial and fungal lactate dehydrogenases. Microb Cell Factories 12(1):53. https://doi.org/10.1186/1475-2859-12-53
Bintsis T (2018) Lactic acid bacteria: their applications in foods. J Bacteriol Mycol Open Access 6. https://doi.org/10.15406/jbmoa.2018.06.00182
Ma K, Hu G, Pan L, Wang Z, Zhou Y, Wang Y, Ruan Z, He M (2016) Highly efficient production of optically pure l-lactic acid from corn stover hydrolysate by thermophilic Bacillus coagulans. Bioresour Technol 219:114–122. https://doi.org/10.1016/j.biortech.2016.07.100
Juturu V, Wu JC (2016) Microbial production of lactic acid: the latest development. Crit Rev Biotechnol 36(6):967–977. https://doi.org/10.3109/07388551.2015.1066305
Abdel-Rahman MA, Tashiro Y, Zendo T, Sakai K, Sonomoto K (2015) Enterococcus faecium QU 50: a novel thermophilic lactic acid bacterium for high-yield l-lactic acid production from xylose. FEMS Microbiol Lett 362(2):1–7. https://doi.org/10.1093/femsle/fnu030
Kumar R, Kaur M, Garsa AK, Shrivastava B, Pv R, Tyagi A (2015) Natural and Cultured Buttermilk. In. pp 203-225
Rombouts JL, Kranendonk EMM, Regueira A, Weissbrodt DG, Kleerebezem R, van Loosdrecht MCM (2020) Selecting for lactic acid producing and utilising bacteria in anaerobic enrichment cultures. Biotechnol Bioeng 117(5):1281–1293. https://doi.org/10.1002/bit.27301
Abdel-Rahman M, Hassan S, Ms A, Ma G (2016) Effective production of lactic acid by a newly isolated alkaliphilic psychrobacter maritimus BoMAir 5 Strain. J Appl Biotechnol Bioeng 1:00012. https://doi.org/10.15406/jabb.2016.01.00012
Calabia B, Tokiwa Y, Aiba S (2011) Fermentative production of L: -(+)-lactic acid by an alkaliphilic marine microorganism. Biotechnol Lett 33:1429–1433. https://doi.org/10.1007/s10529-011-0573-0
Parra-Ramírez D, Martinez A, Cardona CA (2019) Lactic acid production from glucose and xylose using the lactogenic Escherichia coli strain JU15: Experiments and techno-economic results. Bioresour Technol 273:86–92. https://doi.org/10.1016/j.biortech.2018.10.061
Wang Y, Li K, Huang F, Wang J, Zhao J, Zhao X, Garza E, Manow R, Grayburn S, Zhou S (2013) Engineering and adaptive evolution of Escherichia coli W for l-lactic acid fermentation from molasses and corn steep liquor without additional nutrients. Bioresour Technol 148:394–400. https://doi.org/10.1016/j.biortech.2013.08.114
Tsuge Y, Kato N, Yamamoto S, Suda M, Inui M (2019) Enhanced production of d-lactate from mixed sugars in Corynebacterium glutamicum by overexpression of glycolytic genes encoding phosphofructokinase and triosephosphate isomerase. J Biosci Bioeng 127(3):288–293. https://doi.org/10.1016/j.jbiosc.2018.08.002
Tsuge Y, Kato N, Yamamoto S, Suda M, Jojima T, Inui M (2019) Metabolic engineering of Corynebacterium glutamicum for hyperproduction of polymer-grade l- and d-lactic acid. Appl Microbiol Biotechnol 103(8):3381–3391. https://doi.org/10.1007/s00253-019-09737-8
Alexandri M, Schneider R, Mehlmann K, Venus J (2019) Recent advances in d-lactic acid production from renewable resources: case studies on agro-industrial waste streams. Food Technol Biotechnol 57(3):293–304. https://doi.org/10.17113/ftb.57.03.19.6023
Olszewska-Widdrat A, Alexandri M, López-Gómez J, Schneider R, Mandl M, Venus J (2019) Production and Purification of l-lactic Acid in Lab and Pilot Scales Using Sweet Sorghum Juice. Fermentation 5:36. https://doi.org/10.3390/fermentation5020036
Khoo CG, Dasan YK, Lam MK, Lee KT (2019) Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour Technol 292:121964. https://doi.org/10.1016/j.biortech.2019.121964
Soltanian S, Aghbashlo M, Almasi F, Hosseinzadeh-Bandbafha H, Nizami A-S, Ok YS, Lam SS, Tabatabaei M (2020) A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers Manag 212:112792. https://doi.org/10.1016/j.enconman.2020.112792
Machineni L (2020) Lignocellulosic biofuel production: review of alternatives. Biomass Convers Biorefinery 10(3):779–791. https://doi.org/10.1007/s13399-019-00445-x
Sun Y, Xu Z, Zheng Y, Zhou J, Xiu Z (2019) Efficient production of lactic acid from sugarcane molasses by a newly microbial consortium CEE-DL15. Process Biochem 81:132–138. https://doi.org/10.1016/j.procbio.2019.03.022
Tirpanalan Ö, Reisinger M, Smerilli M, Huber F, Neureiter M, Kneifel W, Novalin S (2015) Wheat bran biorefinery—an insight into the process chain for the production of lactic acid. Bioresour Technol 180:242–249. https://doi.org/10.1016/j.biortech.2015.01.021
Fukushima K, Sogo K, Miura S, Kimura Y (2004) Production of D-lactic acid by bacterial fermentation of rice starch. Macromol Biosci 4(11):1021–1027. https://doi.org/10.1002/mabi.200400080
Komesu A, Oliveira J, Martins L, Maciel M, Filho R (2018) Lactic acid and ethanol: promising bio-based chemicals from fermentation. In. pp 84-115. https://doi.org/10.1002/9781119460381.ch6
Ghaffar T, Irshad M, Anwar Z, Aqil T, Zulifqar Z, Tariq A, Kamran M, Ehsan N, Mehmood S (2014) Recent trends in lactic acid biotechnology: A brief review on production to purification. J Radiat Res Appl Sci 7(2):222–229. https://doi.org/10.1016/j.jrras.2014.03.002
Carpinelli Macedo JV, de Barros Ranke FF, Escaramboni B, Campioni TS, Fernández Núñez EG, de Oliva Neto P (2020) Cost-effective lactic acid production by fermentation of agro-industrial residues. Biocatalysis Agric Biotechnol 27:101706. https://doi.org/10.1016/j.bcab.2020.101706
de la Torre I, Ladero M, Santos VE (2019) Production of D-lactic acid by L. delbrueckii growing on orange peel waste hydrolysates and model monosaccharide solutions: effects of pH and temperature on process kinetics. Biomass Convers Biorefinery 9(3):565–575. https://doi.org/10.1007/s13399-019-00396-3
Yuwa-amornpitak T, Chookietwatana K (2018) Bioconversion of waste cooking oil glycerol from cabbage extract to lactic acid by Rhizopus microsporus. Braz J Microbiol 49:178–184
Aghbashlo M, Mandegari M, Tabatabaei M, Farzad S, Mojarab Soufiyan M, Görgens JF (2018) Exergy analysis of a lignocellulosic-based biorefinery annexed to a sugarcane mill for simultaneous lactic acid and electricity production. Energy 149:623–638. https://doi.org/10.1016/j.energy.2018.02.063
Probst M, Walde J, Pümpel T, Wagner AO, Insam H (2015) A closed loop for municipal organic solid waste by lactic acid fermentation. Bioresour Technol 175:142–151. https://doi.org/10.1016/j.biortech.2014.10.034
Abdul Latif N-IS, Ong MY, Nomanbhay S (2019) Hydrothermal liquefaction of Malaysia's algal biomass for high-quality bio-oil production. Eng Life Sci 19(4):246–269. https://doi.org/10.1002/elsc.201800144
Ramachandra TV, Hebbale D (2020) Bioethanol from macroalgae: Prospects and challenges. Renew Sust Energ Rev 117:109479. https://doi.org/10.1016/j.rser.2019.109479
Dave N, Selvaraj R, Varadavenkatesan T, Vinayagam R (2019) A critical review on production of bioethanol from macroalgal biomass. Algal Res 42:101606. https://doi.org/10.1016/j.algal.2019.101606
Smith VH, Sturm BSM, deNoyelles FJ, Billings SA (2010) The ecology of algal biodiesel production. Trends Ecol Evol 25(5):301–309. https://doi.org/10.1016/j.tree.2009.11.007
Ferreira GF, Ríos Pinto LF, Carvalho PO, Coelho MB, Eberlin MN, Maciel Filho R, Fregolente LV (2019) Biomass and lipid characterization of microalgae genera Botryococcus, Chlorella, and Desmodesmus aiming high-value fatty acid production. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-019-00566-3
Chong TY, Cheah SA, Ong CT, Wong LY, Goh CR, Tan IS, Foo HCY, Lam MK, Lim S (2020) Techno-economic evaluation of third-generation bioethanol production utilizing the macroalgae waste: A case study in Malaysia. Energy 210:118491. https://doi.org/10.1016/j.energy.2020.118491
Ashokkumar V, Salim MR, Salam Z, Sivakumar P, Chong CT, Elumalai S, Suresh V, Ani FN (2017) Production of liquid biofuels (biodiesel and bioethanol) from brown marine macroalgae Padina tetrastromatica. Energy Convers Manag 135:351–361. https://doi.org/10.1016/j.enconman.2016.12.054
Jang S-S, Shirai Y, Uchida M, Wakisaka M (2012) Production of mono sugar from acid hydrolysis of seaweed. Afr J Biotechnol 11(8):1953–1963. https://doi.org/10.5897/AJB10.1681
Trivedi N, Gupta V, Reddy CRK, Jha B (2013) Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour Technol 150:106–112. https://doi.org/10.1016/j.biortech.2013.09.103
Kim D-H, Lee S-B, Jeong G-T (2014) Production of reducing sugar from Enteromorpha intestinalis by hydrothermal and enzymatic hydrolysis. Bioresour Technol 161:348–353. https://doi.org/10.1016/j.biortech.2014.03.078
Kostas ET, White DA, Du C, Cook DJ (2016) Selection of yeast strains for bioethanol production from UK seaweeds. J Appl Phycol 28(2):1427–1441. https://doi.org/10.1007/s10811-015-0633-2
El Harchi M, Fakihi Kachkach FZ, El Mtili N (2018) Optimization of thermal acid hydrolysis for bioethanol production from Ulva rigida with yeast Pachysolen tannophilus. S Afr J Bot 115:161–169. https://doi.org/10.1016/j.sajb.2018.01.021
Borines MG, de Leon RL, Cuello JL (2013) Bioethanol production from the macroalgae Sargassum spp. Bioresour Technol 138:22–29
Yuan Y, Macquarrie DJ (2015) Microwave assisted step-by-step process for the production of fucoidan, alginate sodium, sugars and biochar from Ascophyllum nodosum through a biorefinery concept. Bioresour Technol 198:819–827
Kostas ET, White DA, Cook DJ (2017) Development of a bio-refinery process for the production of speciality chemical, biofuel and bioactive compounds from Laminaria digitata. Algal Res 28:211–219. https://doi.org/10.1016/j.algal.2017.10.022
Soliman R, Younis S, El-Gendy NS, Mostafa S, El-Temtamy S, Hashim A (2018) Batch bioethanol production via the biological and chemical saccharification of some Egyptian marine macroalgae. J Appl Microbiol 125(2):422–440. https://doi.org/10.1111/jam.13886
Wei N, Quarterman J, Jin Y-S (2013) Marine macroalgae: an untapped resource for producing fuels and chemicals. Trends Biotechnol 31(2):70–77. https://doi.org/10.1016/j.tibtech.2012.10.009
Abd-Rahim F, Wasoh H, Zakaria MR, Ariff A, Kapri R, Ramli N, Siew-Ling L (2014) Production of high yield sugars from Kappaphycus alvarezii using combined methods of chemical and enzymatic hydrolysis. Food Hydrocoll 42:309–315. https://doi.org/10.1016/j.foodhyd.2014.05.017
Wu F-C, Wu J-Y, Liao Y-J, Wang M-Y, Shih I-L (2014) Sequential acid and enzymatic hydrolysis in situ and bioethanol production from Gracilaria biomass. Bioresour Technol 156:123–131. https://doi.org/10.1016/j.biortech.2014.01.024
Masarin F, Cedeno FRP, Chavez EGS, De Oliveira LE, Gelli VC, Monti R (2016) Chemical analysis and biorefinery of red algae Kappaphycus alvarezii for efficient production of glucose from residue of carrageenan extraction process. Biotechnol Biofuels 9(1):122. https://doi.org/10.1186/s13068-016-0535-9
Gomez-Zavaglia A, Prieto Lage MA, Jimenez-Lopez C, Mejuto JC, Simal-Gandara J (2019) The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants (Basel, Switzerland) 8(9):406. https://doi.org/10.3390/antiox8090406
Renganathan R, Takriff M (2016) Prospects of algae and their environmental applications in Malaysia: a case study. J Bioremediation Biodegradation 07. https://doi.org/10.4172/2155-6199.1000321
Jung KA, Lim S-R, Kim Y, Park JM (2013) Potentials of macroalgae as feedstocks for biorefinery. Bioresour Technol 135:182–190. https://doi.org/10.1016/j.biortech.2012.10.025
Lobban CS, Wynne MJ (1981) The biology of seaweeds, vol 17. Univ of California Press
Li J, Wang G, Chen M, Li J, Yang Y, Zhu Q, Jiang X, Wang Z, Liu H (2014) Deoxy-liquefaction of three different species of macroalgae to high-quality liquid oil. Bioresour Technol 169:110–118. https://doi.org/10.1016/j.biortech.2014.06.080
Hong IK, Jeon H, Lee SB (2014) Comparison of red, brown and green seaweeds on enzymatic saccharification process. J Ind Eng Chem 20(5):2687–2691. https://doi.org/10.1016/j.jiec.2013.10.056
Sadhukhan J, Gadkari S, Martinez-Hernandez E, Ng KS, Shemfe M, Torres-Garcia E, Lynch J (2019) Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem 21(10):2635–2655. https://doi.org/10.1039/C9GC00607A
Yanagisawa M, Nakamura K, Ariga O, Nakasaki K (2011) Production of high concentrations of bioethanol from seaweeds that contain easily hydrolyzable polysaccharides. Process Biochem 46(11):2111–2116. https://doi.org/10.1016/j.procbio.2011.08.001
Rajak RC, Jacob S, Kim BS (2020) A holistic zero waste biorefinery approach for macroalgal biomass utilization: A review. Sci Total Environ 716:137067. https://doi.org/10.1016/j.scitotenv.2020.137067
Jambo SA, Abdulla R, Mohd Azhar SH, Marbawi H, Gansau JA, Ravindra P (2016) A review on third generation bioethanol feedstock. Renew Sust Energ Rev 65:756–769. https://doi.org/10.1016/j.rser.2016.07.064
Murphy F, Devlin G, Deverell R, McDonnell K (2013) Biofuel Production in Ireland—an approach to 2020 Targets with a Focus on Algal Biomass. Energies 6(12):6391–6412
Bhatia L, Bachheti RK, Garlapati VK, Chandel AK (2020) Third-generation biorefineries: a sustainable platform for food, clean energy, and nutraceuticals production. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00843-6
Zollmann M, Robin A, Prabhu M, Polikovsky M, Gillis A, Greiserman S, Golberg A (2019) Green technology in green macroalgal biorefineries. Phycologia 58(5):516–534. https://doi.org/10.1080/00318884.2019.1640516
Baghel RS, Trivedi N, Gupta V, Neori A, Reddy C, Lali A, Jha B (2015) Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chem 17(4):2436–2443. https://doi.org/10.1039/C4GC02532F
van Hal JW, Huijgen WJJ, López-Contreras AM (2014) Opportunities and challenges for seaweed in the biobased economy. Trends Biotechnol 32(5):231–233. https://doi.org/10.1016/j.tibtech.2014.02.007
Jiang R, Ingle KN, Golberg A (2016) Macroalgae (seaweed) for liquid transportation biofuel production: what is next? Algal Res 14:48–57. https://doi.org/10.1016/j.algal.2016.01.001
Gegg P, Wells V (2017) UK macro-algae biofuels: a strategic management review and future research agenda. J Marine Sci Eng 5(3):32. https://doi.org/10.3390/jmse5030032
Nguyen C, Kim J-S, Hwang H, Park MS, Choi G, Choi Y, Jang K, Kim J-C (2012) Production of L-lactic acid from a green microalga, Hydrodictyon reticulum, by Lactobacillus paracasei LA104 isolated from the traditional Korean food, makgeolli. Bioresour Technol 110:552–559. https://doi.org/10.1016/j.biortech.2012.01.079
Nguyen CM, Kim J-S, Song JK, Choi GJ, Choi YH, Jang KS, Kim J-C (2012) D-Lactic acid production from dry biomass of Hydrodictyon reticulatum by simultaneous saccharification and co-fermentation using Lactobacillus coryniformis subsp. torquens. Biotechnol Lett 34(12):2235–2240. https://doi.org/10.1007/s10529-012-1023-3
Hirayama S, Ueda R (2004) Production of optically pure d-lactic acid by Nannochlorum sp. 26A4. Appl Biochem Biotechnol 119(1):71–77. https://doi.org/10.1385/ABAB:119:1:71
Pedro F, Souza Filho MJT (2020) Integrated Biorefineries for the Production of Bioethanol, Biodiesel, and Other Commodity Chemicals. In: Green Energy to Sustainability, pp 465–488. https://doi.org/10.1002/9781119152057.ch19
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17(1):36. https://doi.org/10.1186/s12934-018-0879-x
t’Lam GP, Vermuë MH, Eppink MHM, Wijffels RH, van den Berg C (2018) Multi-Product Microalgae Biorefineries: From Concept Towards Reality. Trends Biotechnol 36(2):216–227. https://doi.org/10.1016/j.tibtech.2017.10.011
Ruiz HA, Rodríguez-Jasso RM, Fernandes BD, Vicente AA, Teixeira JA (2013) Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew Sust Energ Rev 21:35–51. https://doi.org/10.1016/j.rser.2012.11.069
Garcia-Vaquero M, Rajauria G, O'Doherty JV, Sweeney T (2017) Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res Int 99:1011–1020. https://doi.org/10.1016/j.foodres.2016.11.016
Thompson TM, Young BR, Baroutian S (2019) Advances in the pretreatment of brown macroalgae for biogas production. Fuel Process Technol 195:106151. https://doi.org/10.1016/j.fuproc.2019.106151
Steinbach D, Kruse A, Sauer J (2017) Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production—a review. Biomass Convers Biorefinery 7(2):247–274. https://doi.org/10.1007/s13399-017-0243-0
Kostas E, Wilkinson S, White D, Cook D (2016) Optimization of a total acid hydrolysis based protocol for the quantification of carbohydrate in macroalgae. J Algal Biomass Utilization 2016:21–36
Behera S, Singh R, Arora R, Sharma NK, Shukla M, Kumar S (2015) Scope of algae as third generation biofuels. Front Bioeng Biotechnol 2(90). https://doi.org/10.3389/fbioe.2014.00090
Nanda S, Mohammad J, Reddy SN, Kozinski JA, Dalai AK (2014) Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers Biorefinery 4(2):157–191. https://doi.org/10.1007/s13399-013-0097-z
Sudhakar MP, Merlyn R, Arunkumar K, Perumal K (2016) Characterization, pretreatment and saccharification of spent seaweed biomass for bioethanol production using baker's yeast. Biomass Bioenergy 90:148–154. https://doi.org/10.1016/j.biombioe.2016.03.031
Hong Y, Chen C, Wu Y-R (2019) Biobutanol production from sulfuric acid-pretreated red algal biomass by a newly isolated Clostridium sp. strain WK. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.1820
Taherzadeh MJ, Karimi K (2007) Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2(4):707–738
Jmel MA, Anders N, Yahmed NB, Schmitz C, Marzouki MN, Spiess A, Smaali I (2018) Variations in Physicochemical Properties and Bioconversion Efficiency of Ulva lactuca Polysaccharides After Different Biomass Pretreatment Techniques. Appl Biochem Biotechnol 184(3):777–793. https://doi.org/10.1007/s12010-017-2588-z
Kim SW, Hong C-H, Jeon S-W, Shin H-J (2015) High-yield production of biosugars from Gracilaria verrucosa by acid and enzymatic hydrolysis processes. Bioresour Technol 196:634–641. https://doi.org/10.1016/j.biortech.2015.08.016
Wu Z-Z, Li D-Y, Cheng Y-S (2018) Application of ensilage as a green approach for simultaneous preservation and pretreatment of macroalgae Ulva lactuca for fermentable sugar production. Clean Techn Environ Policy 20(9):2057–2065. https://doi.org/10.1007/s10098-018-1574-7
Hessami MJ, Phang SM, Salleh A, Rabiei R (2018) Evaluation of tropical seaweeds as feedstock for bioethanol production. Int J Environ Sci Technol 15(5):977–992. https://doi.org/10.1007/s13762-017-1455-3
Mansa RF, Chen W-F, Yeo S-J, Farm Y-Y, Bakar HA, Sipaut CS (2013) Fermentation study on macroalgae Eucheuma cottonii for bioethanol production via varying acid hydrolysis. In: Advances in biofuels. Springer, pp 219–240. https://doi.org/10.1007/978-1-4614-6249-1_13
Yuan Y, Macquarrie DJ (2015) Microwave assisted acid hydrolysis of brown seaweed Ascophyllum nodosum for bioethanol production and characterization of alga residue. ACS Sustain Chem Eng 3(7):1359–1365. https://doi.org/10.1021/acssuschemeng.5b00094
Hessami MJ, Cheng SF, Ambati RR, Yin YH, Phang SM (2019) Bioethanol production from agarophyte red seaweed, Gelidium elegans, using a novel sample preparation method for analysing bioethanol content by gas chromatography. 3 Biotech 9(1):25. https://doi.org/10.1007/s13205-018-1549-8
Amamou S, Sambusiti C, Monlau F, Dubreucq E, Barakat A (2018) Mechano-enzymatic deconstruction with a new enzymatic cocktail to enhance enzymatic hydrolysis and bioethanol fermentation of two macroalgae species. Molecules 23(1):174
Saravanan K, Duraisamy S, Ramasamy G, Kumarasamy A, Balakrishnan S (2018) Evaluation of the saccharification and fermentation process of two different seaweeds for an ecofriendly bioethanol production. Biocatalysis Agric Biotechnol 14:444–449. https://doi.org/10.1016/j.bcab.2018.03.017
Ge L, Wang P, Mou H (2011) Study on saccharification techniques of seaweed wastes for the transformation of ethanol. Renew Energy 36(1):84–89. https://doi.org/10.1016/j.renene.2010.06.001
Ravanal MC, Camus C, Buschmann AH, Gimpel J, Olivera-Nappa Á, Salazar O, Lienqueo ME (2019) Chapter 4 - Production of Bioethanol From Brown Algae. In: Hosseini M (ed) Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts. Woodhead Publishing, pp 69–88. https://doi.org/10.1016/B978-0-12-817937-6.00004-7
Koppram R, Nielsen F, Albers E, Lambert A, Wännström S, Welin L, Zacchi G, Olsson L (2013) Simultaneous saccharification and co-fermentation for bioethanol production using corncobs at lab, PDU and demo scales. Biotechnol Biofuels 6(1):2. https://doi.org/10.1186/1754-6834-6-2
Talukder MMR, Das P, Wu JC (2012) Microalgae (Nannochloropsis salina) biomass to lactic acid and lipid. Biochem Eng J 68:109–113. https://doi.org/10.1016/j.bej.2012.07.001
Alkaya E, Kaptan S, Ozkan L, Uludag-Demirer S, Demirer GN (2009) Recovery of acids from anaerobic acidification broth by liquid–liquid extraction. Chemosphere 77(8):1137–1142. https://doi.org/10.1016/j.chemosphere.2009.08.027
Wasewar KL, Yawalkar AA, Moulijn JA, Pangarkar VG (2004) Fermentation of glucose to lactic acid coupled with reactive extraction: a review. Ind Eng Chem Res 43(19):5969–5982. https://doi.org/10.1021/ie049963n
Krzyżaniak A, Leeman M, Vossebeld F, Visser TJ, Schuur B, de Haan AB (2013) Novel extractants for the recovery of fermentation derived lactic acid. Sep Purif Technol 111:82–89. https://doi.org/10.1016/j.seppur.2013.03.031
Lan K, Xu S, Li J, Hu C (2019) Recovery of lactic acid from corn stover hemicellulose-derived liquor. ACS Omega 4(6):10571–10579
Kim YH, Moon SH (2001) Lactic acid recovery from fermentation broth using one-stage electrodialysis. J Chem Technol Biotechnol 76(2):169–178
Madzingaidzo L, Danner H, Braun R (2002) Process development and optimisation of lactic acid purification using electrodialysis. J Biotechnol 96(3):223–239. https://doi.org/10.1016/S0168-1656(02)00049-4
Li Y, Shahbazi A (2006) Lactic acid recovery from cheese whey fermentation broth using combined ultrafiltration and nanofiltration membranes. In: Twenty-Seventh Symposium on Biotechnology for Fuels and Chemicals. Springer, pp 985–996. https://doi.org/10.1385/ABAB:132:1985
Lu Z, Wei M, Yu L (2012) Enhancement of pilot scale production of l(+)-lactic acid by fermentation coupled with separation using membrane bioreactor. Process Biochem 47(3):410–415. https://doi.org/10.1016/j.procbio.2011.11.022
Lee HD, Lee MY, Hwang YS, Cho YH, Kim HW, Park HB (2017) Separation and purification of lactic acid from fermentation broth using membrane-integrated separation processes. Ind Eng Chem Res 56(29):8301–8310. https://doi.org/10.1021/acs.iecr.7b02011
Pal P, Dey P (2013) Process intensification in lactic acid production by three stage membrane integrated hybrid reactor system. Chem Eng Process Process Intensif 64:1–9. https://doi.org/10.1016/j.cep.2012.12.006
Komesu A, Wolf Maciel MR, Rocha de Oliveira JA, da Silva Martins LH, Maciel Filho R (2017) Purification of lactic acid produced by fermentation: focus on non-traditional distillation processes. Sep Purif Rev 46(3):241–254. https://doi.org/10.1080/15422119.2016.1260034
Kumar A, Thakur A, Panesar PS (2019) Lactic acid and its separation and purification techniques: a review. Rev Environ Sci Biotechnol:1–31. https://doi.org/10.1007/s11157-019-09517-w
Kumar A, Thakur A, Panesar PS (2019) A comparative study on experimental and response surface optimization of lactic acid synergistic extraction using green emulsion liquid membrane. Sep Purif Technol 211:54–62. https://doi.org/10.1016/j.seppur.2018.09.048
Kumar A, Thakur A, Panesar PS (2018) Statistical optimization of lactic acid extraction using Green Emulsion Ionic Liquid Membrane (GEILM). J Environ Chem Eng 6(2):1855–1864. https://doi.org/10.1016/j.jece.2018.01.037
Jusoh N, Noah NFM, Othman N (2019) Extraction and recovery optimization of succinic acid using green emulsion liquid membrane containing palm oil as the diluent. Environ Prog Sustain Energy 38(3):e13065. https://doi.org/10.1002/ep.13065
Ahmad A, Buddin MS, Ooi B, Kusumastuti A (2017) Utilization of environmentally benign emulsion liquid membrane (ELM) for cadmium extraction from aqueous solution. J Water Process Eng 15:26–30. https://doi.org/10.1016/j.jwpe.2016.05.010
Demichelis F, Laghezza M, Chiappero M, Fiore S (2020) Technical, economic and environmental assessement of bioethanol biorefinery from waste biomass. J Clean Prod 277:124111. https://doi.org/10.1016/j.jclepro.2020.124111
Luis P (2013) Exergy as a tool for measuring process intensification in chemical engineering. J Chem Technol Biotechnol 88(11):1951–1958. https://doi.org/10.1002/jctb.4176
Rosen MA, Dincer I, Kanoglu M (2008) Role of exergy in increasing efficiency and sustainability and reducing environmental impact. Energy Policy 36(1):128–137. https://doi.org/10.1016/j.enpol.2007.09.006
Vliet LTFRTV (2009) Exergy: The Concept of “Quality Energy”. In: Thermodynamics for the Practicing Engineer, pp 377–390. https://doi.org/10.1002/9780470451595.ch19
Ptasinski KJ (2015) Exergy Analysis. In: Efficiency of biomass energy, pp 37–90. https://doi.org/10.1002/9781119118169.ch2
Novak P (2017) Exergy as Measure of Sustainability of Energy System. Int J EarthEnviron Sci 2. https://doi.org/10.15344/2456-351X/2017/139
Dincer I, Ratlamwala TAH (2013) Importance of exergy for analysis, improvement, design, and assessment. WIREs Energy Environ 2(3):335–349. https://doi.org/10.1002/wene.63
Ofori-Boateng C, Lee KT (2013) Comparative thermodynamic sustainability assessment of lignocellulosic pretreatment methods for bioethanol production via exergy analysis. Chem Eng J 228:162–171. https://doi.org/10.1016/j.cej.2013.04.082
Silva Ortiz P, de Oliveira S (2014) Exergy analysis of pretreatment processes of bioethanol production based on sugarcane bagasse. Energy 76:130–138. https://doi.org/10.1016/j.energy.2014.04.090
Modarresi A, Kravanja P, Friedl A (2012) Pinch and exergy analysis of lignocellulosic ethanol, biomethane, heat and power production from straw. Appl Therm Eng 43:20–28. https://doi.org/10.1016/j.applthermaleng.2012.01.026
Liu G, Sun J, Zhang J, Tu Y, Bao J (2015) High titer l-lactic acid production from corn stover with minimum wastewater generation and techno-economic evaluation based on Aspen plus modeling. Bioresour Technol 198:803–810. https://doi.org/10.1016/j.biortech.2015.09.098
Fudholi A, Sopian K, Othman MY, Ruslan MH (2014) Energy and exergy analyses of solar drying system of red seaweed. Energy Build 68:121–129. https://doi.org/10.1016/j.enbuild.2013.07.072
Sanjuan-Acosta M, Romero K, Ochoa M, Sánchez Tuirán E, González-Delgado A (2018) Computer-aided exergy analysis of agar production process from macroalgae Gracilaria sp. Contemp Eng Sci 11:651–658. https://doi.org/10.12988/ces.2018.7890
Rattanasaensri S, Nunraksa N, Muangmai N, Praiboon J, Chirapart A (2018) Ethanol production from Gracilaria fisheri using three marine epiphytic yeast species. J Appl Phycol 30(6):3311–3317. https://doi.org/10.1007/s10811-018-1527-x
Soltanian S, Aghbashlo M, Farzad S, Tabatabaei M, Mandegari M, Görgens JF (2019) Exergoeconomic analysis of lactic acid and power cogeneration from sugarcane residues through a biorefinery approach. Renew Energy 143:872–889. https://doi.org/10.1016/j.renene.2019.05.016
Gezae Daful A, Görgens JF (2017) Techno-economic analysis and environmental impact assessment of lignocellulosic lactic acid production. Chem Eng Sci 162:53–65. https://doi.org/10.1016/j.ces.2016.12.054
Rebello S, Anoopkumar AN, Aneesh EM, Sindhu R, Binod P, Pandey A (2020) Sustainability and life cycle assessments of lignocellulosic and algal pretreatments. Bioresour Technol 301:122678. https://doi.org/10.1016/j.biortech.2019.122678
Seghetta M, Hou X, Bastianoni S, Bjerre A-B, Thomsen M (2016) Life cycle assessment of macroalgal biorefinery for the production of ethanol, proteins and fertilizers—a step towards a regenerative bioeconomy. J Clean Prod 137:1158–1169. https://doi.org/10.1016/j.jclepro.2016.07.195
Smullen E, Finnan J, Dowling D, Mulcahy P (2019) The environmental performance of pretreatment technologies for the bioconversion of lignocellulosic biomass to ethanol. Renew Energy 142:527–534. https://doi.org/10.1016/j.renene.2019.04.082
Caro D (2019) Carbon Footprint. In: Fath B (ed) Encyclopedia of Ecology, 2nd edn. Elsevier, Oxford, pp 252–257. https://doi.org/10.1016/B978-0-12-409548-9.10752-3
Roy P, Tokuyasu K, Orikasa T, Nakamura N, Shiina T (2012) A review of life cycle assessment (LCA) of bioethanol from lignocellulosic biomass. Japan Agric Res Q 46(1):41–57. https://doi.org/10.6090/jarq.46.41
Ögmundarson Ó, Sukumara S, Laurent A, Fantke P (2020) Environmental hotspots of lactic acid production systems. GCB Bioenergy 12(1):19–38. https://doi.org/10.1111/gcbb.12652
van Oirschot R, Thomas J-BE, Gröndahl F, Fortuin KPJ, Brandenburg W, Potting J (2017) Explorative environmental life cycle assessment for system design of seaweed cultivation and drying. Algal Res 27:43–54. https://doi.org/10.1016/j.algal.2017.07.025
Sander K, Murthy GS (2010) Life cycle analysis of algae biodiesel. Int J Life Cycle Assess 15(7):704–714. https://doi.org/10.1007/s11367-010-0194-1
Langlois J, Sassi J-F, Jard G, Steyer J-P, Delgenes J-P, Hélias A (2012) Life cycle assessment of biomethane from offshore-cultivated seaweed. Biofuels Bioprod Biorefin 6(4):387–404. https://doi.org/10.1002/bbb.1330
Helmes R, López-Contreras A, Benoit M, Abreu M, Maguire J, Moejes F, Burg SWK (2018) Environmental impacts of experimental production of lactic acid for bioplastics from Ulva spp. Sustainability 10:2462. https://doi.org/10.3390/su10072462
Acknowledgement
The authors would like to acknowledge the financial supports given by Fundamental Research Grant Scheme (FRGS/1/2019/TK02/CURTIN/03/2) from Ministry of Higher Education (MOHE), Malaysia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chung, M.R.W.Y., Tan, I.S., Foo, H.C.Y. et al. Potential of macroalgae-based biorefinery for lactic acid production from exergy aspect. Biomass Conv. Bioref. 13, 2623–2653 (2023). https://doi.org/10.1007/s13399-021-01375-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01375-3