Abstract
Different strategies for simultaneous saccharification and fermentation (SSF) process were developed to enhance the productivity of ethanol by Kluyveromyces marxianus ATCC36907 as a part of biorefinery concept using cashew apple bagasse (CAB) as biomass. CAB was pretreated with 4.3% (v/v) alkaline hydrogen peroxide at pH 11.5 (CAB-AHP). Batch SSF conducted at 45 °C using 10% (w/v) CAB-AHP reported the highest concentration and ethanol yield. In the fed-batch SSF, the highest production (7.91 g ethanol/100gCAB) and ethanol yield (84.69%) were achieved using 10% (w/v) CAB-AHP initial solids load, 4% solid feeding at 24 h, and the feeding of enzymes only in the beginning of the process. The recycling of pretreatment inputs decreased 50% of the batch-SSF efficiencies, saving the consumption of water, H2O2, and NaOH in 66.67%, 48.44%, and 66.67%, respectively. Lignin was extracted from the hydrolysate with yield of 39.2%. Thus, the proposed biorefinery concept utilizes CAB to produce ethanol with high efficiency by fed-batch SSF, pretreatment input recycling, and the utilization of the extracted lignin for future applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulosic materials represent a promising option as feedstock to second-generation biofuel production. These materials are composed of cellulose, hemicellulose, and lignin in an intricate structure, which is recalcitrant to decomposition [1]. Cashew apple bagasse (CAB), a by-product of the cashew apple juice industry, is an example of lignocellulosic material. CAB has no commercial value, and it is often disposed by local industries [2, 3].
Cashew apple bagasse can be converted into ethanol by performing the following steps: pretreatment, hydrolysis, fermentation, and distillation. During the pretreatment, the break of the polymeric structures of lignin and/or hemicellulose occurs, enhancing the accessibility of enzymes to the biomass during the hydrolysis step [4]. Different pretreatments are performed and the alkaline hydrogen peroxide (AHP) pretreatment has been studied to prepare lignocellulosic substrates from a wide range of raw materials [5,6,7,8,9,10,11]. The oxidative action of the H2O2-derived radicals acts into the cellulose decrystallization, and it also contributes to the depolymerization and high solubilization of lignin [6, 7, 12].
Innovative practices have been employed to lower pretreatment costs, one of them being the liquid phase recycling. The recycling of the liquid phase from the pretreatment has the advantages of reusing chemical agents that did not react with biomass in the initial pretreatment and reduces the consumption of water and energy [13], contributing to the reduction of negative impacts on the production chain of biofuels. Until the present moment, few studies report the pretreatment solution recycling using AHP. Alencar et al. [14] evaluated the influence of liquid fraction pretreatment recycling with AHP on the corn stover enzymatic hydrolysis. There is no report on recycling technology for pretreatment with AHP applied to SSF.
Cellulotics enzymes hydrolyze pretreated biomass, and the hydrolysate containing carbohydrate is metabolized by yeast to produce the ethanol for the yeast. The process of ethanol production can be conducted using a different reaction configuration, such as the integrated process of hydrolysis and fermentation. This process aims to simplify and reduce the integrated costs of the process, being the simultaneous saccharification and fermentation (SSF) an excellent choice for this reaction configuration. The main benefits of this process are as follows: they decrease the problems caused by end-product inhibition of the enzymatic hydrolysis; they reduce the equipment cost since they do not require separate reactors to conduct the saccharification and fermentation; and they require a lower enzyme load. Despite these advantages, the usefulness of SSF has been limited due to the difference in the optimal temperatures for cellulolytic enzyme activities and yeast fermentation [11, 15,16,17]. Also, yeast recycling is very difficult in SSF. Some different SSF configurations are evaluated, for example, (i) conduct a pre-saccharification step; (ii) conduct the process in fed-batch, that makes possible the increase of total substrate content [18]; (iii) conduct an integrated process of high cell density culture combined with SSF for ethanol production [19]; and (iv) perform a fed-batch process with fed of a nitrogen source [20]. The pre-saccharification step is conducted to partly hydrolyze the cellulose to glucose before the yeast addition that it enables higher temperatures during the initial hydrolysis, increasing the enzymatic activity and decreasing the viscosity of the medium [21, 22].
The increase of biomass loading is a traditional strategy used to reach high ethanol concentration, but ethanol yields usually decrease due to poor mass transfer and accumulated inhibitors [23, 24]. Thus, different strategies to conduct the SSF process were studied to improve the SSF performance because this process has a number of well-known advantages, which are undesired effects in fermentation, such as substrate inhibition, and can be minimized with higher productivity, less time, and extension of process control.
To enhance the ethanol production from cashew apple bagasse, we propose a novel design to utilize each component of lignocellulosic biomass for ethanol and bio-chemicals productions, selecting the alkaline hydrogen peroxide pretreatment to produce a cellulose-rich solid with good recovery of lignin for future application. Then, recycling pretreatment inputs for improvement of the industrial plant as a part of the whole biorefinery concept.
In this context, the aim of this study was to develop fed-batch simultaneous saccharification and fermentation to enhance ethanol titers and yields comparing different strategies. The influence of recycling the liquid fraction from AHP pretreatment on the delignification of CAB was also evaluated, and how recycling affects the SSF and reduces the use of inputs.
2 Material and methods
2.1 Lignocellulosic material and pretreatment
Cashew apple bagasse, scientific name Anacardium occidentalis L, was supplied by Jandaia Juice Industry (Ceará, Brazil). The initial preparation of CAB was conducted according to Correia et al. [6]. The alkaline hydrogen peroxide pretreatment was conducted with the best conditions obtained by Correia et al. [6]. A load of solids of 5% (w/v) from CAB was pretreated using 4.3% (v/v) H2O2 solution, pH adjusted to pH 11.5 using 6 mol·L−1 NaOH, in an orbital shaker (Tecnal TE-421, Brazil) at 35 °C for 6 h and 250 rpm. Then, the solid and liquid fractions were separated by filtration. Pretreated CAB was washed three times with water, reaching a pH 7.0 ± 0.5, and oven-dried at 60 °C for 24 h and named as CAB-AHP. The composition analysis of CAB-AHP was determined and solids were used in the SSF processes. The liquid fraction was used in the study of recycling and recovery of lignin.
2.2 The liquid phase of pretreatment recycling
The liquid fraction obtained from the pretreatment was recovered and the volume and pH were measured. Then, 5% (w/v) of CAB was added and a new pretreatment cycle was conducted in the same condition previously described. Subsequently, the pretreated biomass was dried at 60 °C for 24 h to determine the recovered biomass content and then it was used in the batch SSF process. The content of soluble lignin in the liquid fraction of each cycle was determined. The recovered solids were analysed by Fourier-transform infrared spectroscopy (FTIR) using the Varian 660 spectrometer with a resolution of 8 cm−1, spectral range of 4000–650 nm, and background scan of 32.
2.3 Lignin recovery
Lignin was extracted from liquid fraction obtained by acid precipitation (using 50% (v/v) H2SO4 at pH 2), and the precipitated lignin mass was calculated on a dry mass basis [3].
2.4 Characterization of untreated and pretreated CAB
The cellulose, hemicellulose, and lignin content of the CAB and CAB-AHP were determined according to NREL/TP-510-42618 Laboratory Analytical Procedures–LAP [25]. The extractable total solids and ash were analysed according to the NREL/TP-510-42619 [26] and NREL/TP-510-42621 Laboratory Analytical Procedures–LAP [27], respectively. The effect of pretreatment on the CAB structure was also evaluated by FTIR (Varian 660 spectrometer), with a resolution of 8 cm−1, spectral range of 4000–650 nm, and background scan of 32.
2.5 Simultaneous saccharification and fermentation
Kluyveromyces marxianus ATCC36907 was maintained on a YEPD-slanted agar medium (composed of 10 g L−1 yeast extract, 20 g L−1peptone, 20 g L−1 glucose, and 20 g L−1 agar) at − 80 °C.
The yeast was activated before of the SSF processes, and the cell biomass required to conduct the SSF processes was performed according to Correia et al. [3].
The SSF processes were conducted in 250-mL flasks using different loadings of CAB-AHP in a 50 mM citrate buffer at pH 4.5–5.0 supplemented with 1 g·L−1 yeast extract and 1 g. ·L−1 (NH4)2SO4. The medium was sterilized in an autoclave at 110 °C for 10 min before adding the enzymes. The enzymatic loading of 30 FPU/gCAB-AHP (68 FPU/gCellulose) of the Cellic CTec2 (kindly provided by Novozymes with 232.4 FPU/mLExtract of initial enzymatic activity) and 5 g/L initial cell concentration was used. SSF processes were performed in triplicate at 150 rpm using different solids loading and temperatures for 72 h. Samples were collected and then filtered for chromatographic analysis of carbohydrates and ethanol by HPLC. The theoretical ethanol yield was calculated according to Rodrigues et al. [28].
Solid loading effect in SSF: Four loadings were tested: 2.0, 3.0, 4.0, and 4.4 gcellulose/100 mL corresponding at 4.53, 6.79, 9.06, and 10.0 gCAB-AHP/100 mL (solid loads based on the study by Correia et al. [3]), and the other conditions were the same as previously described and the temperature was previously selected.
Temperature effect in SSF: The processes were performed in the range of 37 °C to 50 °C using a biomass load of 4 gcellulose/100 mL corresponding at 9.06 gCAB-AHP/100 mL and the other conditions were the same as previously described.
In all experiments, the ethanol yield (YE/C) and ethanol efficiency (η) of the SSF processes were calculated as described by Eqs. (1) and (2), respectively:
where Ct is the concentration of ethanol at time t, C0 is the initial ethanol concentration, mBiomass is the dry CAB-AHP biomass concentration at the beginning of fermentation (g L−1), 0.511 is the conversion factor for glucose to ethanol, and 1.11 is the conversion factor for cellulose to glucose. Productivity was also determined at the time that obtained the highest ethanol concentration in each process.
2.6 Prehydrolysis and simultaneous saccharification and fermentation
The pre-saccharification was conducted before the SSF for a different time: 12 h or 24 h, using 10 gCAB-AHP/100 mL in a 50-mM citrate buffer at pH 4.5–5.0 supplemented with 1 g·L−1 yeast extract and 1 g·L−1 (NH4)2SO4, and a catalyst loading of 30 FPU/gCAB-AHP (68 FPU/gCellulose) at 45 °C and 150 rpm. After prehydrolysis, 5 g/L cell biomass was inoculated and the process progressed up to 72 h. Samples were withdrawn and filtered for carbohydrates and ethanol chromatographic analysis, and the experiments performed in triplicate.
2.7 Fed-batch SSF
Fed-batch experiments were conducted with different initial and final solid loads, and feeding times ranging from 6 to 48 h. These assays started with 5% (w/v), 6% (w/v) or 10% (w/v) of CAB-AHP. The addition times were fixed at 6 h, 12 h, 24 h, 36 h, and/or 48 h depending on the feeding strategy, with different batches being supplied to increase the substrate loading up to 18% (w/v). There was no supplementation of enzymes during feeds; only in the control experiment, that enzyme was supplemented together with the substrate. The operation conditions were at 45 °C and 150 rpm using 30 FPU/gCAB-AHP of enzyme loading. All experiments were performed in triplicate and statistically analysed at the 95% confidence level.
2.8 Sample analysis
Chromatographic analysis was determined by HPLC using a Water system (Waters, Milford, MA, USA), equipped with a refractive index Waters 2414 detector and an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA). The procedure described by Rocha et al. [29] was used: the eluent was 5 mmoL L−1 H2SO4 in MilliQ Water (simplicity 185, Millipore, Billerica, MA) at 65 °C and 0.5 mL min−1 flow rate. The injection volume of the samples was 20 μL.
2.9 Statistical analysis
The results of ethanol concentrations and efficiencies were analysed for statistical significance by analysis of variance (ANOVA) at 95% confidence level (p < 0.05), using the Microcal Origin software (Microcal Software Inc., Northampton. MA. USA).
3 Results and discussion
3.1 Composition changes of cashew apple bagasse after pretreatment and its application on the area of biorefinery
The CAB was pretreated using AHP to reduce the recalcitrant structure. After the pretreatment, the liquid was then recycled for a new pretreatment of CAB and went through three successive cycles. Then, lignin was extracted from the residual liquid, which can be used to dye adsorption, binders, carbon fibers, motor fuel, plastic material, and adsorbents. The obtained solids were analysed and subjected to the SSF process. Different process configurations were performed to enhance the yield of ethanol.
The CAB-AHP was composed by 44.2% ± 0.3% of cellulose, 18.3% ± 0.9% of hemicellulose, 2.9% ± 0.1% of lignin, 4.9% ± 0.3% of extractable and 5.9% ± 0.9% of ashes, with a yield of 37.3% of the pretreated solid (Table 1). Rodrigues et al. [28] and Barros et al. [30] obtained a yield of around 9.34% of pretreated acidic-alkaline CAB, a lower yield compared with the result obtained in this study. The pretreatment slightly decreased the moisture of the fiber. The moisture contents were 12.5% and 10.8% for the raw CAB and pretreated CAB, respectively. This decrease was due to the fact that hydrogen peroxide is a strong oxidizing agent and forms some oxidation over products, among them is oxygen, gas, and water. The radicals derived from H2O2 have an oxidative action that promotes the cleavage of the ether linkages and the lignin-carbohydrate complex bonds, fragmenting the lignin to low-molecular-weight compounds [6, 12]. The AHP pretreatment affects predominantly lignin components; 91.7% of the original lignin was solubilized (resulting in a low solid yield) decreasing the lignin contents of solids from 35.3 to 2.9%, which caused an increase of cellulose and hemicellulose of 20.6 to 44.2% and 10.2 to 18.3%, respectively (Table 1). On the other hand, regarding the content of the untreated CAB (100 g), a loss of 4.1 g and 3.4 g of cellulose and hemicellulose, respectively, was observed.
From the liquid fraction obtained after pretreatment, 12.69 g of lignin was extracted for each 100 g of CAB.
Lignin is considered a source of aromatic compounds with potential application to the production of value-added chemicals and fuels [31]. Extracted lignin has the potential to produce, for example, polymers, carbon fibers, adhesives, and resins.
AHP pretreatment is more effective for lignin solubilization than acid-alkaline pretreatment reported by Rodrigues et al. [28] and Barros et al. [30]. Therefore, the next step was to evaluate whether CAB-AHP is a potential biomass for ethanol production, and the first configuration studied was the SSF process conducted in batch, evaluating the influence of different conditions.
3.2 Batch simultaneous saccharification and fermentation studies
3.2.1 Effects of solid loading and temperature on the SSF
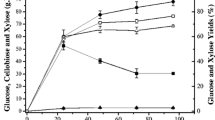
SSFs were performed using four solids loading aiming to enhance ethanol production. The relationships of solid loading to the consumption of carbohydrates and the production of ethanol were summarized and can be seen in Fig. 1A–D and Table 2. The produced glucose was converted to ethanol with a small accumulate in the first hours of the process, which implies that a lag phase adaptation of the microorganism occurred. The glucose becomes consumed after 8 h, and after 24 h only a small amount of glucose was detected in the lower solid loading. Pessani et al. [16] evaluate the ethanol production by SSF from switchgrass, and the authors reported an accumulation of glucose in the first 6 h, and the glucose measured was negligible after 24 h.
Profile for ethanol (■), glucose (●) and xylose (▲) during SSF with Kluyveromyces marxianus ATCC36907 at 45 °C, 150 rpm using 30 FPU cellulase/gCAB-AHP and the different loads of solid: (A) 2.0, (B) 3.0, (C) 4.0, and (D) 4.4 gcellulose/100 mLmedium corresponding to 4.52, 6.79, 9.05, and 10.0 gCAB-AHP /100 mL medium
K. marxianus yeast did not metabolize xylose to ethanol and this carbohydrate was accumulated in the medium, probably due to a repression glucose [12, 32]. Hua et al. [33] proved by gene disruption that hexokinase 1 (KmHXK1) and sucrose non-fermenting 1 (KmSNF1) are involved in the glucose repression of xylose utilization by K. marxianus.
The glucose concentration dropped in all cases, while the ethanol concentration increased rapidly (Fig. 1A–D), achieving the ethanol concentrations of 10.9 g/L, 15.4 g/L, 20.9 g/L, and 21.0 g/L at 4.53%, 6.79%, 9.06%, and 10.00% (w/v) of CAB-AHP, respectively, corresponding to 96.27%, 90.54%, 92.20%, and 83.84% of the theoretical yield (Table 2), respectively. A significant increase in the ethanol concentration was observed with increasing solids load from 6.79% (w/v) to 9.06% (w/v), and the assays using 9.06% and 10% (w/v) of solid loading did not show a significant difference in the ethanol concentration. In the assays using 10% (w/v) of solid loading, the ethanol was produced faster, and its lower efficiency was caused by glucose accumulation in the medium (approximately 4 g/L glucose, Fig. 1D). A higher concentration of solid contains a higher cellulose content that can be hydrolyzed to glucose by cellulolytic enzymes and the glucose converted to ethanol by the action of yeast. However, a high load can cause diffusion problems and decrease the efficiency of the process.
The effects of temperature in the metabolism of K. marxianus ATCC 36907 and in the activity of cellulolytic enzymes during the SSF process were evaluated. In temperatures between 37 °C to 45 °C, the activity of microbial cells can be maintained and the final ethanol concentrations are higher than the obtained concentration at 50 °C. For all evaluated temperatures (except 50 °C), after 30 h of process, an accumulation of glucose was not observed and the ethanol concentration remained constant (Figs. 1D, 2A–C), indicating, possibly, the end of the fermentation process. The cell metabolism of the yeast decreased significantly at 50 °C and there was an accumulation of glucose (Fig. 2C). Xylose remained unchanged during the process, proving that the microorganism does not metabolize xylose to produce ethanol or other byproducts (Figs. 1D, 2A–C).
Profile of ethanol (■), glucose (●), and xylose (▲) during SSF with Kluyveromyces marxianus ATCC36907 using 10.0 gCAB-AHP/100 mL medium (4.4 gcellulose/100 mL medium) and 30 FPU cellulase/gCAB-AHP at 150 rpm and different temperatures: (A) 37 °C, (B) 40 °C, and (C) 50 °C. The experiment at 45 °C is shown in Fig. 1D
After 72 h, the ethanol concentrations were 15.8 g/L, 16.9 g/L, 20.9 g/L, and 11.0 g/L at 37 °C, 40 °C, 45 °C, and 50 °C, respectively. The efficiency of the process increased considerably from 69.80% to 83.84% increasing the temperature of 37 to 45 °C (Table 2). Then, the temperature of 45 °C was selected.
K. marxianus used in this research is a thermotolerant microorganism, that is gaining recognition due to its capability of growing and producing ethanol at temperatures above 40 °C, close to the optimum temperature of enzymatic hydrolysis, which can lead to an integration of both saccharification and fermentation processes [16, 34]. Another advantage of SSFs being conducted at 45 °C is to reduce the possibility of contamination by mesophilic microorganisms [16].
One way to increase ethanol production by SSF is to conduct the process in the proper temperature, and/or by performing at a higher substrate concentration. Nevertheless, this leads to a poorer stirring of the CAB-AHP in the reactor, significantly decreasing the overall ethanol yield or even the cessation of ethanol production. Moreover, in this research it was not possible to increase the solids loading in the SSF process due to the hygroscopic potential from CAB-AHP, causing mass transfer problems.
Thus, as the ethanol concentration cannot be enhanced by simply increasing the substrate concentration, the process must be modified to produce more ethanol. Then, the possible methods are to conduct the process in fed-batch mode, by employing prehydrolysis prior to the fermentation step, or pre-fermenting the liquid fraction of the pretreatment slurry before the SSF [35]. These configurations can improve stirring or decrease the viscosity of the reactional medium. Then, the SSF with prehydrolysis and fed-batch SSF were performed.
3.2.2 Prehydrolysis and simultaneous saccharification and fermentation
A prehydrolysis step was carried out to increase ethanol production, and decrease the viscosity of the medium, thereby facilitating mass transfer during fermentation [30, 36]. In the present study, the prehydrolysis tests were performed at 45 °C for 12 h and 24 h, using 10.0% (w/v) CAB-AHP, the best conditions obtained in the studies of solids loads and temperature, and the results can be seen in Fig. 3A and B.
The prehydrolysis step performed for 12 h and 24 h provided 15.81 g/L and 15.10 g/L of glucose, corresponding at 40.3% and 38.5% of the digestibility of cellulose, respectively. A high digestibility can be obtained with a high hydrolysis time [3], but it is necessary to inoculate the yeast to start the fermentation. The ethanol yields were only 66.54% and 60.23% in the prehydrolysis for 12 h and 24 h, respectively. Then, this configuration did not enhance ethanol production. In the SSF conducted by prehydrolysis, the enzymes were used during a longer time and at a higher temperature, which could lead to a higher degree of deactivation and thus lower hydrolysis and ethanol production at the end of the SSF. Kluyveromyces marxianus may have decreased its efficiency by changes in the osmotic pressure that cause yeast cells to make physiological changes. Some authors also had a negative effect when comparing the ethanol production process with and without the addition of prehydrolysis step [22, 30]. Barros et al. [30] evaluated the prehydrolysis in the simultaneous saccharification and fermentation of cashew apple bagasse pretreated by acidic-alkaline pretreatment using K. marxianus ATCC36907 and the prehydrolysis of 12 h prior to SSF did not increase the overall ethanol yield. Ögren et al. [22] studied PSSF processes for ethanol production by commercial Saccharomyces cerevisae using corn stover as lignocellulosic material. The authors performed studies with different enzyme complexes and they conducted the step of hydrolysis at optimum temperature for enzymes. After the pre-saccharification time, the temperature was reduced to 35 °C for the start of the SSF process. The authors concluded that PSSF processes with longer prehydrolysis time did not provide an increase in ethanol production compared to SSF processes. Also, this step reduces the simplicity of the process as a whole and increases the risk of contamination in industrial processes [30].
3.3 Fed-batch SSF
The fed-batch SSF was conducted evaluating four feeding strategies, those are presented in Table 3. Gradual substrate feeding may be an alternative to increase the ethanol concentration and minimize inhibition problems related to high substrate concentrations in the batch-SSF, such as poor mass transfer [35, 37], since new solid addition is made during liquefaction of the biomass initially added to the reaction, resulting in minimized content of insoluble solids in the medium. The SSF process is a heterogeneous reaction, a system that depends also on different parameters that influence the mass transfer rate and the efficiency of the process such as porosity of the substrate, degree of swelling of the substrate, geometry of the reactor and mixing.
The concentrations of glucose, xylose, and ethanol for the feeding strategies FB1 to FB4 can be seen in Fig. 4. The feeding was performed with the addition of CAB-AHP, and the enzymatic complex was added at the beginning of the process based on the initial substrate loading. Similar glucose profiles were achieved for all strategies and the total glucose produced was metabolized by yeast. An increase in the ethanol production was observed with biomass feeding, but the ethanol productions remained stable after 24 h. Moreover, the yield was lower, indicating that a portion of cellulose content added to the process was not converted into glucose, due to the low enzymatic load.
Profile for ethanol (■), glucose (●) and xylose (▲) obtained by different strategies of fed-batch (FB1, FB2, FB3 e FB4) using Kluyveromyces marxianus ATCC36907, 4.4 gcellulose/100 mL medium corresponding to 10.0 gCAB-AHP/100 mL medium, 30 FPU cellulase/gCAB-AHP at 45 °C, 150 rpm for 72 h. The dashed lines correspond to the feed points of the CAB-AHP
According to Table 3, the feeding strategies (FB1, FB2, FB3, and FB4) propitiated similar results regarding the concentration of ethanol. However, the strategies FB3 and FB4, in which 5% (w/v) CAB-AHP were fed after 6 h and 12 h of SSF, respectively, achieved a production of around 12 g·L−1 and efficiency of 48%. These results did not present significant differences, but they were significantly different when compared with the efficiencies of FB1 and FB2 strategies.
When comparing the productions achieved by batch (using 10% (w/v) CAB-AHP, Fig. 2 and Table 2) and fed-batch (FB1 to FB4, Fig. 4), it is clear that the feeding does not favor a greater ethanol production. A reduction in the efficiency of 83.84 to 48% was verified. The high feeding of solids caused mixing and diffusion problems due to the biomass being fibrous, decreasing the hydrolysis efficiency.
Thus, new strategies with fewer solids loading feeding were conducted using 6% (w/v) or 10% (w/v) of initial solids load and obtained a final load of 18% (w/v). The results can be seen in Fig. 5 and Table 3.
Profile for ethanol (■), glucose (●), and xylose (▲) produced in different strategies of fed-batch (FB5, FB6, FB7, FB8, FB9, FB10, and FB11) with 6% and 10% of initial solids load and 14% and 18% of final load using Kluyveromyces marxianus ATCC36907 at 45 °C, 4.4 gcellulose/100 mL medium corresponding to 10.0 gCAB-AHP/100 mL medium, 150 rpm and 30 FPU cellulase/gCAB-AHP. The dashed lines correspond to the feed points of the CAB-AHP
In the strategies FB5 and FB6 were added 6% (w/v) CAB-AHP at the beginning of the SSF, with four feedings of 2% (w/v) for FB5 and 3% (w/v) for FB6, the final load totaling 14% (w/v) and 18% (w/v), respectively. The ethanol concentration reached a maximum of 14.2 g·L−1 (FB5) and 15.3 g·L−1 (FB6), with an efficiency of 40.49% (FB5) and 33.93% (FB6). Enzymatic conversion of feed CAB-AHP occurred in these strategies, but the glucose produced was not metabolized by the yeast, resulting in an accumulate of this carbohydrate until the end of the process (Fig. 5).
Then, the other five feeding strategies (FB7, FB8, FB9, FB10, and FB11) were investigated using the best solids loading achieved in the batch. The feeding with 4% (w/v) CAB-AHP at 12 h (FB8) was not favorable to the process, perhaps due to the amount of non-hydrolyzed solids present, the addition of more bagasse interfered in the mass transfer and hindering the enzymatic action. Similar results were obtained in the strategy FB10, with the feeding of 4% (w/v) CAB-AHP at the 12 h and 24 h, in which the use of high solids during the feeding negatively affected the process. Wang et al. [38] reported that the slower formation of the product in hydrolysis was associated with a decrease of the enzymes binding capacity to the cellulose due to slower three-dimensional diffusion of enzymes in the environment.
The highest ethanol concentration (29.7 g·L−1) and efficiency (84.69%) was obtained in the FB9 strategy, with a single feed of 4% (w/v) CAB-AHP at the 24 h. A higher ethanol concentration will reduce the downstream distillation cost. The FB11 strategy was performed based on the FB9 strategy, in which 4% (w/v) CAB-AHP was feeding also at 48 h of the process, but there was a reduction of 84.69% (FB9) to 61.88% (FB11) in the efficiency. Low glucose and ethanol concentrations were obtained in the FB11 strategy, probably due to an increase in solid load from 14 to 18% (w/w) and the low enzymatic loading (based on the initial load of CAB-AHP in the reactor) decreased the conversion of the cellulose to glucose, hence the conversion to ethanol by yeast did not occur (Fig. 5).
Regardless of the initial CAB-AHP loading and feeding strategies, in all experiments, the final load of 14% (w/v) or 18% (w/v) showed a constant accumulation of glucose and xylose from the 48 h until the end of the process. Barros et al. [30] also carried out a batch-SSF using cashew apple bagasse pretreated with acid followed by alkali, and a sugar accumulation was observed in the process. They concluded that this effect occurred due to the continuity of hydrolytic activity instead of the cease of cell metabolism.
These results indicate that the time and load of solids feed are variables that influence the fed-batch SSF. The hydrolysis of the solid added at the beginning of the process is necessary, and this fact helps the diffusion of solids and reduces the inhibitory effects of the substrate before the CAB-AHP feed.
The results are corroborated by Gomes et al. [39] and Kossatz et al. [40] that evaluated the production of ethanol from sugarcane bagasse and triticale straw, respectively. They also observed a negative effect on high solids feeding, which could be attributed to rheological difficulties suffered by the fiber in the liquefaction or to an increase of the inhibitory effects that affected the metabolism of enzymes and/or yeast.
The results obtained in the best strategy (FB9), the concentration (29.7 g/L), and yield (84.7%) of ethanol obtained were similar or higher to some studies cited in the literature. Zheng et al. [20] reported an ethanol yield of 86.56% of the theoretical maximum using 15% (w/w) of unwashed corncob residues with tea-seed cake. Shi et al. [19] obtained the highest ethanol titer of 11 g/L, corresponding 65.41% theoretical yield at 72 h by SSF using a pretreated corn stover.
Performing a simple mass balance analysis starting from 100 g of cashew apple bagasse, performing the AHP pretreatment, 37.3 g of pretreated solid is recovered with 79.9% of the cellulose. The highest ethanol production was achieved using 14% (w/v) CAB at 45 °C for 24 h by fed-batch SSF, with a higher yield of 7.91 gethanol/100 gCAB, and this production corresponds to 100.3 L of ethanol per 1000 kg of CAB.
Cashew apple bagasse used in this work has been a source of study to produce second-generation ethanol by other authors, and lower yields were reported. Then, this study enhanced the ethanol production of this feedstock. For example, Rodrigues et al. [28] investigated the ethanol production by SSF process using CAB pretreated with acid followed by alkali and the same microorganism, K. marxianus ATCC 36907, at 40 °C, and the authors reported a yield of 2.21 gethanol/100 gCAB. Moreover, our research adds knowledge to new fed-batch alternatives to achieve technical and economically feasible processes for the production of ethanol from cashew apple bagasse, mainly with a focus in decrease the volume of the enzymatic complex used in the previous studies.
3.4 Recycle of pretreatment study
The recycling of the liquid fraction obtained from AHP pretreatment was conducted in this step, and then the evaluation of its influence on the delignification of CAB, in the reduction of the use of inputs, and how it affects the batch SSF.
There was a tendency to increase in recovery of solids and a decrease in pH values with the successive reuse cycles (Table 4). These parameters are important because the recovery solid will be used in SSF, and a decrease in the pH affects the formation of reactive radicals such as OH− and O2−, which react with the lignin, reducing its removal [14, 41, 42].
There was no significant difference in recovery volume during the evaluated cycles (Table 3). This parameter is important because the liquid contains the inputs (water, hydrogen peroxide, and sodium hydroxide) necessary to conduct the new pretreatment, and it can affect the efficiency of the ethanol production [43].
The FTIR was used to characterize CAB and CAB-AHP obtained without and with the recycling of the alkaline H2O2 solution during the pretreatment stages (see Supplementary material).
Bands in the range of 4000–1800 cm−1 represent O–H and C–H groups. The peaks near 3284 cm−1 correspond to O–H stretching. The peak intensity increased after the pretreatment, indicating that the bonds between lignin and polysaccharides may have been broken and more O–H of cellulose became exposed [44].
The CAB spectrum exhibited vibrations at 1319, 1230, 1148, and 1017.6 cm−1, which are attributed to carbohydrates [45, 46]. The intensity of the bands at 1439 cm−1 and 1319 cm−1 increased after pretreatment. They are associated with C=C and C–O, respectively, corresponding at aromatic skeletal vibrations of lignin [45]. The band at 1230 cm−1 presented an increase of the intensity assigned to an increase of O–H contribution, but also for the band at 1017 cm−1, corresponding to the aliphatic O–H group [46]. A C–O asymmetric band was observed near to 1148 cm−1. This behavior was attributed to the break of lignin aliphatic side chains connected with cellulose and hemicellulose and consequently, the characteristic bands of carbohydrate molecules were more exposed. However, comparing the obtained spectrum of CAB-AHP after the third cycle with the CAB spectrum, the intensities are close. During the first reuse cycles, there was a high removal of the lignin by H2O2 action, increasing the cellulose content in the bagasse.
The intensities of bands at 2922–2890 cm−1 present in CAB spectrum were modified after the pretreatment, but the intensities in the CAB-AHP spectrum were closer to obtained in the CAB spectrum with the increase in the number of cycles, due to lower removal of lignin through of the recycles. These bands are attributing to asymmetric and symmetric C–H stretching of CH, CH2, and CH3. There was an accumulation of certain amounts of alkaline-soluble lignin and phenolic compounds in the hydrolysate, what might bring a negative effect on the reuse for continuous biomass pretreatment [6, 14, 47].
Then, the efficiency of AHP pretreatment was decreasing along with the successive cycles. However, the water and hydrogen peroxide volumes and sodium hydroxide mass can be reduced by up to 66.67%, 48.44%, and 66.67%, respectively, considering the three reuse cycles (Fig. 6A). Applying the CAB-AHP obtained by recycles in the batch-SSF, the obtained ethanol concentration decreased 51.79% between the first and third recycles. The recycles hurt the biomass delignification efficiency, but there was no significant difference in the ethanol concentrations obtained in the second and third recycle (Table 4). Evaluating the amount of ethanol produced by volume or mass of inputs, it was verified that this ratio is higher with the recycling process (Fig. 6B). Thus, the recycling of the hydrolysate (liquid fraction) from AHP pretreatment shows technical and economic advantages to produce ethanol by SSF using CAB as feedstock.
Comparison of the quantity of (A) water, hydrogen peroxide, and sodium hydroxide used in the pretreatment stages, and (B) volume of ethanol produced per gram of quantity of inputs (water, hydrogen peroxide or sodium hydroxide): dark gray bars represent the results without recycling; light gray bars represent the results with recycling of alkaline hydrogen peroxide solution
4 Conclusion
The cashew apple bagasse pretreated by alkaline hydrogen peroxide proved to be adequate for the ethanol production by K. marxianus ATCC36907. The recycling of the liquid fraction from pretreatment showed advantages and lignin can be recovered for future applications. The efficiency of batch SSF was affected by initial solids loading and temperature, and the pre-saccharification stage did not provide an increase in efficiency. The highest ethanol production was obtained by fed-batch SSF, with efficiency and yield (YE/C) of 85% and 0.48 g/g, respectively, and this configuration demonstrated an alternative to achieve technical and economically feasible processes for the production of ethanol from cashew apple bagasse.
References
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafic GH, Gholami M, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sust Energ Rev 27:77–93
Silva JS, Mendes JS, Correia JAC, Rocha MVP, Micoli L (2018) Cashew apple bagasse as new feedstock for the hydrogen production using dark fermentation process. J Biotechnol 286:71–78
Correia JAC, Junior JEM, Gonçalves LRB, Rocha MVP (2015) Enhanced enzymatic hydrolysis and ethanol production from cashew apple bagasse pretreated with alkaline hydrogen peroxide. Bioresour Technol 179:249–259
Chen H-Z, Liu Z-H (2017) Enzymatic hydrolysis of lignocellulosic biomass from low to high solids loading. Eng Life Sci 17:489–499
Banerjee G, Car S, Scott-Craig J, Hodge DB, Walton JD (2011) Alkaline peroxide pretreatment of corn stover: effects of biomass, peroxide, and enzyme loading and composition on yields of glucose and xylose. Biotechnol Biofuels 4:16
Correia JAC, Junior JEM, Gonçalves LRB, Rocha MVP (2013) Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: study of parameters. Bioresour Technol 139:249–256
Karagöz P, Rocha IV, Özkan M, Angelidaki I (2012) Alkaline peroxide pretreatment of rapeseed straw for enhancing bioethanol production by same vessel saccharification and co-fermentation. Bioresour Technol 104:348–357
Alvarez-Vasco C, Zhang X (2017) Alkaline hydrogen peroxide (AHP) pretreatment of softwood: enhanced enzymatic hydrolysability at low peroxide loadings. Biomass Bioenergy 96:96–102
Yuan Z, Wen Y, Kapu NS (2018) Ethanol production from bamboo using mild alkaline pre-extraction followed by alkaline hydrogen peroxide pretreatment. Bioresour Technol 247:242–249
Qiu J, Tian D, Shen F, Hu J, Zeng Y, Yang G, Zhang Y, Deng S, Zhang J (2018) Bioethanol production from wheat straw by phosphoric acid plus hydrogen peroxide (PHP) pretreatment via simultaneous saccharification and fermentation (SSF) at high solid loadings. Bioresour Technol 268:355–362
Liu L, Zhang Z, Wang J, Fan Y, Shi W, Liu X, Shun Q (2019) Simultaneous saccharification and co-fermentation of corn stover pretreated by H2O2 oxidative degradation for ethanol production. Energy 168:946–952
Selig MJ, Vinzant TB, Himmel EM, Decker SR (2009) The effect of lignin removal by alkaline peroxide pretreatment on the susceptibility of corn stover to purified cellulolytic and xylanolytic enzymes. Appl Biochem Biotechnol 155:397–406
Wang W, Chen X, Tan X, Wang Q, Liu Y, He M, Yuan Z (2017) Feasibility of reusing the black liquor for enzymatic hydrolysis and ethanol fermentation. Bioresour Technol 228:235–240
Alencar BRA, Reis ALS, Souza RFR, Morais MA Jr, Menezes RSC, Dutra ED (2017) Recycling the liquid fraction of alkaline hydrogen peroxide in the pretreatment of corn stover. Bioresour Technol 241:928–993
Liu Z-H, Chen H-Z (2017) Two-step size reduction and post-washing of steam exploded corn stover improving simultaneous saccharification and fermentation for ethanol production. Bioresour Technol 223:47–58
Pessani NK, Atiyeh HK, Wilkins MR, Bellmer DD, Banat IM (2011) Simultaneous saccharification and fermentation of Kanlow switchgrass bythermotolerant Kluyveromyces marxianus IMB3: the effect of enzyme loading, temperature and higher solid loadings. Bioresour Technol 102:10618–10624
Saha BC, Nuchols NN, Qureshi N, Kennedy GJ, Iten LB, Cotta MA (2015) Pilot scale conversion of wheat straw to ethanol via simultaneous saccharification and fermentation. Bioresour Technol 175:17–22
Phukoetphim N, Salakkam A, Laopaiboon P, Laopaiboon L (2017) Improvement of ethanol production from sweet sorghum juice under batch and fed-batch fermentations: effects of sugar levels, nitrogen supplementation, and feeding regimes. Electron J Biotechnol 26:84–92
Shi X, Liu Y, Dai J, Liu X, Dou S, Teng L, Meng Q, Lu J, Ren X, Wang R (2019) A novel integrated process of high cell-density culture combined with simultaneous saccharification and fermentation for ethanol production. Biomass Bioenergy 121:115–121
Zheng T, Yub H, Liu S, Jiang J, Wang K (2020) Achieving high ethanol yield by co-feeding corncob residues and tea-seed cake at high-solids simultaneous saccharification and fermentation. Renew Energy 145:858–866
Boonsawang P, Subkaree Y, Srinorakutara T (2012) Ethanol production from palm pressed fiber by prehydrolysis prior to simultaneous saccharification and fermentation (SSF). Biomass Bioenergy 40:127–132
Öhgren K, Vehmaanperä J, Siika-Aho M, Galbe M, Viikari L, Zacchi G (2007) High temperature enzymatic prehydrolysis prior to simultaneous saccharification and fermentation of steam pretreated corn stover for ethanol production. Enzym Microb Technol 40(4):607–613
Liu Z-H, Lei Qin L, Zhu J-Q, Li B-Z, Yuan Y-J (2014) Simultaneous saccharification and fermentation of steam-exploded corn stover at high glucan loading and high temperature. Biotechnol Biofuels 7:167
Zhang M, Wang F, Su R, Qi W, He Z (2010) Ethanol production from high dry matter corncob using fed-batch simultaneous saccharification and fermentation after combined pretreatment. Bioresour Technol 101(13):4959–4964
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocke D (2012) Determination of structural carbohydrates and lignin in biomass, Laboratory Analytical Procedure (LAP) Issue: 3/31/2008 Technical Report NREL/TP-510- 510-42618 Revised August 2012
Sluiter A Ruiz R, Scarlata C, Sluiter J, Templeton D (2008a) Determination of extractives in biomass. NREL – Laboratory Analytical Procedure (LAP), Issue: 7/17/2–05 Technical Report NREL/TP-510-42619 Revised January 2008
Sluiter A, Hames B, Hyman D, Payne C, Ruiz R, Scarlata C, Sluiter A, Sluiter J, Templeton D, Wolfe J (2008b) Determination of total solids in biomass and total dissolved solids in liquid process samples laboratory analytical procedure (LAP) Issue: 3/31/2008. Technical report NREL/TP-510-42621 Revised March 2008
Rodrigues THS, Barrros EM, Brígido JS, Silva WM, Rocha MVP, Gonçalves LRB (2016) The bioconversion of pretreated cashew apple bagasse into ethanol by SHF and SSF processes. Appl Biochem Biotechnol 178:1167–1183
Rocha MVP, Rodrigues THS, Melo VMM, Gonçalves LRB, Macedo GR (2011) Cashew apple bagasse as a source of sugars for ethanol production by Kluyveromyces marxianus CE025. J Ind Microbiol Biotechnol 38:1099–1107
Barros EM, Carvalho VM, Rodrigues THS, Rocha MVP, Gonçalves LRB (2017) Comparison of strategies for the simultaneous saccharification and fermentation of cashew apple bagasse using a thermotolerant Kluyveromyces marxianus to enhance cellulosic ethanol production. Chem Eng J 307:939–947
De Albuquerque TL, Silva JS, De Macedo AC, Gonçalves LRB, Rocha MVP (2019) Biotechnological strategies for the lignin-based biorefinery valorization. In: Reference module in chemistry, molecular sciences and chemical engineering, 1st edn. Elsevier, Amsterdãm
Albuquerque TL, Gomes SDL, Junior JEM, Silva IJ Jr, Rocha MVP (2015) Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catal Today 255:33–40
Hua Y, Wang J, Zhu Y, Zhang B, Kong X, Li W, Wang D, Hong J (2019) Release of glucose repression on xylose utilization in Kluyveromyces marxianus to enhance glucose-xylose co-utilization and xylitol production from corncob hydrolysate. Microb Cell Factories 18:1–24
Moreno AD, Ibarra D, Ballesteros I, González A, Ballesteros M (2013) Comparing cell viability and ethanol fermentation of the thermotolerant yeast Kluyveromyces marxianus and Saccharomyces cerevisiae on steam-exploded biomass treated with laccase. Bioresour Technol 135:239–245
Hoyer H, Galbe M, Zacchi G (2013) The effect of prehydrolysis and improved mixing on high-solids batch simultaneous saccharification and fermentation of spruce to ethanol. Process Biochem 48:289–293
García JF, Anchez S, Bravo V, Cuevas M, Rigal L, Gaset A (2011) Xylitol production from olive-pruning debris by sulphuric acid hydrolysis and fermentation with Candida tropicalis. Holzforschung 65:59–65
Paulová L, Patáková P, Rychtera M, Melzoch K (2014) High solid fed-batch delayed inoculation for improved production of bioethanol from wheat straw. Fuel 122:294–300
Wang W, Kang L, Wie H, Arora R, Lee YY (2011) Study on the decreased sugar yield in enzymatic hydrolysis of cellulosic substrate at high solid loading. Appl Biochem Biotechnol 164(7):1139–1149
Gomes AC, Moysés DN, Anna LMMS, Castro A (2018) Fed-batch strategies for saccharification of pilot-scale mild-acid and alkali pretreated sugarcane bagasse: effects of solid loading and surfactant addition. Ind Crop Prod 119:283–289
Kossatz HL, Rose SH, Viljoen-Bloom M, Zyl WHV (2017) Production of ethanol from steam exploded triticale straw in a simultaneous saccharification and fermentation process. Process Biochem 53:10–16
Gould JM (1985) Studies on the mechanism of alkaline peroxide delignification of agricultural residues. Biotechnol Bioeng XXVII:225–231
Li Y, Cui J, Zhang G, Liu Z, Guan H, Hwang H, Aker WG, Wang P (2016) Optimization study on the hydrogen peroxide pretreatment and production of bioethanol from seaweed Ulva prolifera biomass. Bioresour Technol 214:144–149
Gerbrandt K, Chu PL, Simmonds A, Mullins KA, MacLean HL, Griffin WM, Saville BA (2016) Life cycle assessment of lignocellulosic ethanol: a review of key factors and methods affecting calculated GHG emissions and energy use. Curr Opin Biotechnol 38:63–70
Xu Y, Lia J, Zhang M, Wang D (2018) Modified simultaneous saccharification and fermentation to enhance bioethanol titers and yields. Fuel 215:647–654
Reis CLB, Silva LMA, Rodrigues THS, Félix AKN, Santiago-Aguiar RS, Canuto KM, Rocha MVP (2017) Pretreatment of cashew apple bagasse using protic ionic liquids: enhanced enzymatic hydrolysis. Bioresour Technol 224:694–701
Lopes AM, João KG, Rubik DF, Bogel-Lukasik E, Duarte LC, Andreaus J, Bogel-Lukasik R (2013) Pre-treatment of lignocellulosic biomass using ionic liquids: wheat straw fractionation. Bioresour Technol 142:198–208
Cao W, Sun C, Qiu J, Li X, Liu R, Zhang L (2016) Pretreatment of sweet sorghum bagasse by alkaline hydrogen peroxide for enhancing ethanol production. Korean J Chem Eng 33:873–879
Acknowledgments
The authors thank the Novozymes Group and Jandaia Juice Industry (Ceará, Brazil).
Funding
The authors are grateful for the financial support provided by the Brazilian Research Agencies CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 80 kb).
Rights and permissions
About this article
Cite this article
da Costa Correia, J.A., de Sousa Silva, J., Gonçalves, L.R.B. et al. Different design configurations of simultaneous saccharification and fermentation to enhance ethanol production from cashew apple bagasse pretreated with alkaline hydrogen peroxide applying the biorefinery concept. Biomass Conv. Bioref. 12, 2767–2780 (2022). https://doi.org/10.1007/s13399-020-00796-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00796-w