Abstract
Ethanol production from acidic-alkaline pretreated cashew apple bagasse (CAB-OH) was investigated using separated hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF) processes. First, a screening of Kluyveromyces strains was conducted by SHF and a maximum ethanol concentration of 24.1 g L−1 was obtained using Kluyveromyces marxianus ATCC36907, which presented similar profiles when compared to results obtained by a Saccharomyces strain. The effect of temperature on ethanol production conducted by SHF using K. marxianus ATCC36907 was investigated, and the maximum ethanol yield (YE/G) was obtained at 40 °C (0.46 g g−1) using a synthetic medium. In the SHF using CAB-OH hydrolysate, the maximum ethanol concentration obtained was 24.9 g L−1, 5.92 g L−1 h−1 of productivity, and ethanol yield of 0.43 g g−1 at 40 °C. Afterwards, K. marxianus ATCC36907 was used in the bioconversion of CAB-OH by SSF, and an ethanol concentration of 41.41 ± 0.2 g L−1 was obtained using 10 % CAB-OH at 40 °C, 150 rpm and 24 h, resulting in a YʹE/G of 0.50 gE gG −1 and an efficiency of 98.4 %, in the process conducted with cellobiase supplementation. SHF and SSF processes using CAB-OH and K. marxianus ATCC36907 can be used to ethanol production, but the SSF process required only one step to achieve the same production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass is an abundant and potentially low-cost renewable source. Therefore, their use allows the production of a valuable biofuel and the utilization of a wide range of residues from domestic, agricultural, and industrial activities [1, 2]. The conversion of biomass into biofuels has drawn much attention from the government and researchers, especially because ethanol is one of the most promising alternatives to conventional petroleum-based transport fuels [1, 3–7].
Cashew apple is a pseudofruit from the northeastern region of Brazil, and the cashew agroindustry has an outstanding role in the local economy due to cashew nut exportation. According to FAOSTAT (Food and Agriculture Organization of the United Nations) data, Brazil is the fifth largest manufacturer of cashew nuts with the production of more than 230,000 tons of cashew apple nut with shells in 2011. On the other hand, the industrial processing of peduncles for juice production results in 15 % (w/w) bagasse, which represents no commercial value and is one of the major sources of waste from the Northeast Brazilian agribusiness. These facts, and the composition of cashew apple bagasse (CAB), 18–21 % of cellulose, 10–19 % of hemicellulose, 33–35 % of lignin, 6–8 % of extractives, and 1.5–1.6 % of ash [8, 9], points the raw material as an alternative and inexpensive (<$0.10/kg) lignocellulosic material for the production of fuel ethanol [10–15].
In general, ethanol production from lignocellulosic biomass comprises the following main steps: pretreatment, hydrolysis of cellulose and hemicellulose, sugar fermentation, and, finally, recovery and purification of ethanol to meet fuel specifications [4]. Among the processes for cellulosic ethanol production, simultaneous saccharification and fermentation (SSF) is an interesting alternative due to the integration of saccharification and fermentation steps. One of the advantages for using SSF is the simplified process, when compared with separate hydrolysis and fermentation (SHF). In addition, the inhibition process of the cellulase enzyme by glucose is avoided, thereby increasing saccharification rate and ethanol yield. However, the main disadvantage of SSF is the lower efficiency of hydrolysis, which should be carried out at a lower temperature in order to be compatible with fermentation, when compared to SHF [5].
In this context, ethanol production at elevated temperatures has received much attention. Industrial ethanol production mostly employs a mesophilic strain S. cerevisiae at a fermentation temperature up to 35 °C. Kluyveromyces marxianus is recognized as a thermotolerant yeast strain; it grows rapidly at temperatures above 40 °C and could be economically explored for the SSF process [16–18]. In addition, ethanol fermentation at a high temperature is a key requirement for effective ethanol production in tropical countries where average daytime temperatures are usually high throughout the year. The advantages of rapid fermentation at a high temperature are not only a decreased risk of contamination but also a reduction in cooling costs [16].
In this work, the screening Kluyveromyces strains was performed in the SHF process for ethanol production using an enzymatic hydrolysate of pretreated cashew apple bagasse (CAB-OH). The influence of temperature on ethanol production by K. marxianus ATCC36907 was evaluated and based on the results obtained by SHF, and the SSF process was conducted using 10 % w/v CAB-OH (corresponding at 7.5 % w/v of glucan).
Material and Methods
Raw material and Pretreatment
Cashew apple (Anacardium occidentale L.) bagasse (CAB) in natura was donated by the Jandaia Industry of Juice (Ceará, Brazil). Before the pretreatment step, CAB in natura was processed as described in a previous work of our research group [15, 19]. It was washed three times with water, dried at 60 °C for 24 h, milled in boil mill (Model R-TE-350, Tecnal, Brazil), and selected particles with size of 0.24–0.85 mm.
A two-stage acidic-alkaline pretreatment was proposed. Dilute sulfuric acid pretreatment was performed in the first stage mainly for hemicellulose removal while the second stage carried out alkaline (NaOH) pretreatment primarily for delignification. Diluted sulfuric acid pretreatment was carried out according to [13]: The pretreatment was conducted in autoclave using 30 % (w/v) solid concentration, 0.6 M H2SO4 at 121 °C for 15 min in loosely covered flasks. The solid residue (designated as CAB-H) was washed with water until the wash water reached a pH of 6.0 ± 0.5; it was then dried at 50 °C for 24 h. The alkaline pretreatment was conducted in autoclave using 7.5 % (w/v) solid concentration, 1.0 M NaOH at 121 °C for 30 min in loosely covered flasks [13]. The solid residue (designated as CAB-OH) was washed with water until the wash water reached pH of 7.0; it was then dried at 50 °C for 24 h. The particles retained on 20# to 80# mesh (0.25-0.84 mm) sieve were used for enzymatic hydrolysis, based in a protocol from the National Renewable Energy Laboratory (NREL) [20].
Compositional Analysis of CAB
Compositional analysis was determined according to the protocol proposed by the NREL [21, 22] in terms of extractives, glucan, xylan, lignin, and ash.
Enzymes and Enzyme Activity
A commercial enzyme extract, Celluclast 1.5 L, EC. 232-734-4 (Novozymes, Bagsvaerd, Denmark) was purchased from Sigma-Aldrich (St Louis, Missouri, USA), and β-glucosidase enzyme (NS 50010, Novozymes, Bagsvaerd, Denmark) was generously donated by Novozymes (Novozymes, Bagsvaerd, Denmark).
The enzymatic activities of cellulase complex and β-glucosidase were determined according with the literature [23] at pH 4.8 and 50 °C. Cellulose activity was expressed as 1 FPU—the quantity of enzyme releasing 1 μmol of glucose from blotting-paper Whatman No. 1 for 1 min. β-glucosidase activity was expressed as 1 CBU—the quantity of enzyme transforming 1 μmol of cellobiose into 2 μmol of glucose per 1 min.
The commercial cellulase preparation had cellulase activity of 128.9 FPU mL−1 and β-glucosidase activity of 5.63 CBU mL−1. Cellobiase enzyme (NS50010-Novozyme) had β-glucosidase activity of 266 U mL−1.
Enzymatic hydrolysis of CAB-OH
The saccharification of CAB-OH was performed with a commercial enzyme extract, Celluclast 1.5 L, EC. 232-734-4 (Novozymes, Bagsvaerd, Denmark), according the NREL procedure of Enzymatic Saccharification of Lignocellulosic Biomass [20] with adaptations: the solid concentration used was 16 % (w/v) [13], corresponding to 11.5 % (w/v) glucan, at 45 °C, with 60 FPU/gglucan for 72 h of enzymatic hydrolysis. After the enzymatic of CAB-OH, the solid was separated by centrifugation (10,000g for 15 min), followed by filtration.
The percentage of glucan digestion was calculated using Eq. 1, and xylan digestion was defined according Eq. 2:
In Eqs. 1 and 2, an anhydrous correction factor of 0.88 was used for xylose and a correction of 0.90 was used for glucose to calculate the concentration of the polymeric sugars cellulose and hemicellulose, respectively.
The glucose-rich supernatant, here named hydrolysate, was later used in the fermentation process for ethanol production.
Microorganism and Inoculum Preparation
The yeast strains used in this work are listed in Table 1. The culture was inoculated on YEPD agar (yeast extract, 10 g L−1; peptone, 20 g L−1; glucose 20 g L−1; agar 20 g L−1) and incubated at 30 °C for 48 h. Inoculum preparation was performed in 250 mL Erlenmeyer flasks with a medium volume of 100 mL of YEPD (yeast extract, 10 g L−1; peptone, 20 g L−1; glucose 20 g L−1). Growth was carried out at 30 °C on an orbital shaker (TE-421, Tecnal, Brazil) for 24 h. After that, cells were centrifuged at 10,000g for 15 min to obtain the initial biomass used in the fermentation assays.
Fermentation Assays
Fermentation of CAB-OH Hydrolysate
First, Kluyveromyces yeasts screening was conducted in order to evaluate its capability of ethanol production using CAB-OH hydrolysate, and the results were compared with the performance of Saccharomyces sp. 1238 strain. For that purpose, experiments were conducted in 250 mL flasks, at 150 rpm, 30 °C (for all yeasts), pH 4.5–5.0 (same pH for all microorganisms) and 5 g L−1 initial cell concentration. The culture medium used in the SHF was prepared by diluting the enzymatic hydrolysate with distilled water up to a glucose concentration of 50 g L−1, which was further supplemented as follows: 5 g L−1 of yeast extract and 1 g L−1 of (NH4)2SO4. It was sterilized at 110 °C for 10 min.
Ethanol Production by K. marxianus ATCC36907 Using a Synthetic Medium and Using the CAB-OH Hydrolysate After Enzyme Hydrolysis: Influence of Temperature
The influence of temperature (30–50 °C) on ethanol production by the selected yeast in the previous step was first evaluated using a synthetic medium with a similar composition of the diluted hydrolysate used in the SHF process. The culture medium was prepared in citrate buffer at 30 mM and pH 4.5–5.0 with 50 g L−1 of initial glucose, 5 g L−1 of yeast extract, and 1 g L−1 of (NH4)2SO4, and it was sterilized at 110 °C for 10 min. Afterwards, the temperature that allowed to achieve best results of ethanol production using the synthetic medium was evaluated using the enzymatic hydrolysate with the same initial concentration of glucose. The initial biomass concentration was fixed at 5 g L−1 in the all experiments. The culture media were sterilized at 110 °C for 10 min.
Simultaneous Saccharification and Fermentation
Simultaneous saccharification and fermentation (SSF) processes were conducted in 250 mL flasks with 10 % w/v CAB-OH (corresponding at 7.5 % w/v of glucan) in 50 mM citrate buffer at pH 4.5–5.0, supplemented with 5 g L−1 of yeast extract and 1 g L−1 of (NH4)2SO4. The culture medium was sterilized at 110 °C for 10 min. Experiments were performed with an initial cell concentration (K. marxianus ATCC36907) of 5 g L−1, with cellulases from Celluclast 1.5 L (30 FPU/gglucan). The process was conducted at 40 °C and 150 rpm. Another experiment, at the same operational conditions, was conducted to evaluate the supplementation of medium with cellobiases (NS 50010) at 60 CBU/gglucan. The ethanol yield (YʹE/G) and ethanol efficiency of the SSF processes (ηʹ) were calculated as described by Eqs. 3 and 4, respectively:
where EtOH t is the concentration of ethanol at time t, EtOH 0 is the initial ethanol concentration, Biomass is the dry biomass concentration at the beginning of fermentation (g L−1), 0.511 is the conversion factor for glucose to ethanol, and 1.11 is the conversion factor for cellulose to glucose.
Analytical Methods
Scanning Electron Microscopy
The morphology and the physical structure of the CAB, CAB-H, and CAB-OH were observed by scanning electron microscopy (SEM) using a Philips XL 30 ESEM microscope, which was operated at 20 kV and 0.7 torr. The samples were coated with gold by electrodeposition under vacuum prior to analysis.
Sugar, Ethanol, and Inhibitor Concentrations
Samples of the fermented broth were centrifuged at 3000g for 15 min, and the supernatant was used for sugar, ethanol, and inhibitor (formic acid, acetic acid, furfural, and hydroxymethylfurfural) analysis by HPLC. A Waters system (Waters, Milford, MA, USA) equipped with a refractive index Waters 2414 detector and an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA) was used. The eluent was 5 mmoL L−1 H2SO4 in MilliQ Water (simplicity 185, Millipore, Billerica, MA) at 65 °C. A flow rate of 0.5 mL min−1 was used for the analysis of sugars and ethanol, and 0.6 mL min−1 for inhibitor quantification. Samples were identified by comparing the retention times with those of inhibitors, carbohydrate and ethanol standards.
Statistical Analysis
The results of ethanol production for Kluyveromyces strain and Saccharomyces sp. 1238 were analyzed for statistical significance by a one-way analysis of variance (ANOVA) at 95 % confidence level (p < 0.05), available in Microcal Origin 8.1 software (Microcal Software Inc, Northampton. MA. USA).
Results and Discussion
Compositional Analysis of Cashew Apple Bagasse and Physical Structural Changes
Table 2 provides the results of the compositional analysis of CAB in natura, CAB-H, and CAB-OH. After the diluted acid pretreatment, the remaining solid fraction was separated from the hydrolysate prior to the alkaline pretreatment. The amount of recovered material corresponded to 61.38 % of the untreated CAB. The alkaline pretreatment resulted in a solid recovery of 9.34 % and a reduction of 49 % in the lignin percentage; it was achieved at 17.07 ± 1.9 %. This lignin reduction has been reported by other authors with lignin content from 24–55 to 20 % after by different alkaline pretreatment (i.e., NaOH, AFEX) of hardwood: [4, 24, 25]. Then, the hydrolysate obtained from alkaline pretreatment step is rich in lignin and the literature reports that it has potential to replace a significant fraction of petrochemicals if combined, in the future, with residual lignin produced at the emerging advanced lignocellulose biorefineries. Other applications for lignin are power/fuel, macromolecules, and aromatic chemicals [26, 27].
As the pretreatment with NaOH removed part of the lignin from CAB, this resulted in an accumulation and subsequent increase in the glucan percentage of CAB-OH (71.93 %). Furthermore, it can be noted in Table 2 that the compositional analysis of CAB results only in 79.5 % of material content, which can be attributed to the presence of other compounds not determined, for example, protein. The literature reports [10, 11], for instance, that a range of 3.0–14.0 % (w/w) protein can be found in cashew apple bagasse.
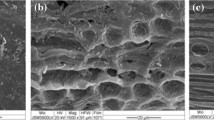
The physical structural changes of CAB, CAB-H, and CAB-OH were imaged on a scanning electron microscope (Fig. 1 in supplementary data - ESI). As shown in Fig. 1A-ESI, the texture of CAB in natura appeared irregular and it is probably covered with a wax layer commonly found in biomass. After acid pretreatment (Fig. 1B-ESI), many granules appeared on the surface and they disappeared after the alkali pretreatment of CAB-H (Fig. 1C-ESI), since the solid CAB-OH became thinner and regular.
Enzymatic Hydrolysis of CAB-OH
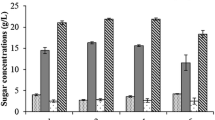
Figure 1 shows the sugar concentrations and glucose and xylose yields during the enzymatic hydrolysis of CAB-OH. An increase in glucose and xylose concentrations, as well as in their yields along time, is observed. The highest glucose (88.07 ± 3.1 g L−1) and xylose (2.88 ± 0.4 g L−1) concentrations were obtained after 96 h of enzymatic hydrolysis. The results of the glucose and xylose yields showed that the enzymatic hydrolysis of CAB-OH led to an increase in their respective yields, 76.5 ± 1.0 and 68.6 ± 0.7 % after 96 h of hydrolysis.
Carbohydrates concentrations and yields obtained from the enzymatic hydrolysis of CAB-OH (16 % CAB-OH, corresponding to 11.5 % w/v of glucan) conducted with Celluclast 1.5 L (60 FPU/gglucan) at 45 °C and 150 rpm. Glucose (filled circle), cellobiose (square), xylose (filled triangle), glucose yield (open circle), and xylose yield (open triangle)
On the other hand, a high cellobiose concentration was identified during the hydrolysis of CAB-OH, i.e., a cellobiose concentration of 52.76 ± 3.5 g L−1 was found at 24 h with a decrease to 30.34 ± 0.8 g L−1 at the end of process. Cellobiose accumulation during enzymatic hydrolysis of CAB-OH can be attributed to a deficiency in cellobiases, which is observed in most of the commercial cellulases complexes available, such as Celluclast 1.5 L (5.63 CBU mL−1 β-glucosidase). In this case, to reduce the presence of cellobiose, some authors supplement the cellobiase complex during the enzymatic hydrolysis step, for example by using Novozymes 188 [28–30]. Inhibitors (HMF and furfural) at concentrations over than 0.001 g L−1 were not detected in the enzymatic hydrolysate.
Wang et al. [31] studied the enzymatic hydrolysis (40 FPU/gcellulose and 70 CBU/gcellobiase) of coastal Bermuda grass (Cynodon dactylon L.) pretreated with NaOH (0.75 % w/v). Although these authors have supplemented the medium with a cellobiase complex, they obtained glucose (90.43 %) and xylose (65.11 %) yields closer to the ones obtained in this work. Haque et al. [6] obtained sugar yields of 87 % after enzymatic hydrolysis of Miscanthus sinensis, also pretreated with NaOH, and their results corroborated with the results obtained with CAB-OH.
At the end of the enzymatic hydrolysis, the hydrolysate composition was 88.07 ± 3.1 g L−1 glucose, 30.34 ± 0.8 g L−1 cellobiose, and 2.88 ± 0.4 g L−1 xylose.
Fermentation of Cashew Apple Bagasse Hydrolysate
Before fermentation, the hydrolysate was first diluted with distilled water to an initial glucose concentration of 50 g L−1, and the potential of Saccharomyces and Kluyveromyces yeast strains was evaluated for ethanol production by the SHF process. Figure 2a, b shows the glucose consumption and ethanol production by Kluyveromyces strains and Saccharomyces sp. 1238. K. marxianus ATCC36907 was the only strain of Kluyveromyces which consumed all glucose from the enzymatic hydrolysate, reaching the stationary phase after 4–5 h of fermentation. It was observed that K. marxianus ATCC36907 consumed all glucose from the hydrolysate after 5 h of fermentation with a maximum ethanol production of 24.1 ± 0.4 g L−1 and a corresponding productivity of 4.4 g L−1 h−1.
Glucose (a) and ethanol (b) concentrations obtained during the screening of K. marxianus strains and by Saccharomyces sp. 1238. Fermentation conditions at 30 °C, pH 4.5–5.0 and 150 rpm. K. marxianus CE025 (filled square), K. marxianus ATCC36907 (filled circle), K. marxianus CCA510 (filled triangle), and Saccharomyces sp. 1238 (open square)
Saccharomyces sp. 1238 provided a maximum ethanol concentration of 23.4 ± 0.9 g L−1 after 4 h of fermentation, similar to the ethanol concentration obtained for K. marxianus ATCC36907, 23.8 g L−1 (at 4 h of fermentation). Other studies with Saccharomyces sp. 1238 [32] showed that it has the potential to produce ethanol (23 g L−1) at 34 °C from an enzymatic hydrolysate of sugarcane bagasse (initial glucose concentration of 59.70 g L−1) after pretreatment by steam explosion followed by delignification with NaOH.
The lower ethanol production (2 g L−1) was observed in the process using K. marxianus CCA510. Different results were obtained by Albuquerque et al. [9]. These authors evaluated the production of xylitol from K. marxianus CCA510, and the authors pointed that the yeast was able to consume both glucose and xylose to ethanol and xylitol production, respectively. In addition, the authors obtained 12 g L−1 of ethanol when using acid hydrolysate from cashew apple bagasse as carbon source.
At present, industrial ethanol production mostly employs strains of S. cerevisiae at a fermentation temperature around 35 °C. K. marxianus is recognized as a thermotolerant yeast strain capable of growing at 52 °C. However, some reports in the literature [17] have cited that lower ethanol productivities were achieved with K. marxianus when compared to S. cerevisiae. This behavior was not observed in this work for K. marxianus ATCC36907.
Table 3 shows the parameters of ethanol yield (YE/G), efficiency (%), and productivity at maximum ethanol concentration of fermentation for K. marxianus ATCC36907, K. marxianus CE025, K. marxianus CCA510, and Saccharomyces sp. 1238. At a significance level of 99.5 %, the results of ethanol yields (YE/G) for K. marxianus ATCC36907 and Saccharomyces sp. 1238 were not significantly different.
According to the results, K. marxianus ATCC36907 shows potential for ethanol production similar to the Saccharomyces strain evaluated here. Therefore, the influence of temperature on ethanol production by K. marxianus ATCC36907 was investigated, aiming further studies of its potential in the SSF process.
Influence of Temperature on Ethanol Production by K. marxianus ATCC36907
Figure 3 shows the influence of temperature on glucose (Fig. 3a) and ethanol (Fig. 3b) concentrations during the growth of K. marxianus ATCC36907. Glucose consumption was only weakly influenced by temperature. Glucose was exhausted after 5 h of fermentation at the temperatures of 30 ºC and 35 °C, while at higher temperatures there was no glucose after 3 h and 4 h for fermentation. It represents an improvement around 20 % of fermentation time. Based on the results of biomass, μmax was calculated and the value was applied to the linearized Arrhenius equation (see supplementary material – Fig. 2 ESI). The value obtained for the constant frequency was 40.45, and the activation energy was 14.50 kJ mol−1 with an adjustment coefficient (R 2) of 0.927. The literature reports (Reeves, 2004) the kinetic analysis of K. marxianus used sweet potato waste as substrate in temperatures of 40 ºC, 45 ºC, and 50 °C. The author obtained activation energy value of 133.05 kJ mol−1 with an adjustment coefficient (R 2) of 0.893. This result may be attributed by the complexity of raw material that influenced the yeast growth when compared with the synthetic media used in this work.
Figure 3b presents the ethanol profiles at the different temperature studied. It can be noted that they are similar, and the yeast was capable of producing ethanol at 40, 45, and 50 °C, achieving maximum concentrations of 24.9 g L−1, 24.3 g L−1, and 23.4 g L−1, respectively. In addition, it was observed that ethanol productivity (see Table 4) increased with increasing temperatures.
Some authors [33] studied the effect of temperature on ethanol production by K. marxianus CECT 10875 at 40–44 °C using a synthetic medium containing glucose supplemented with 5 g L−1 of yeast extract and salts. The authors found that, within the temperature range studied, the temperature did not significantly influenced ethanol production. The authors also obtained a maximum ethanol concentration of 21–22 g L−1 and ethanol yield of 86 %, which is similar to the values obtained in this study.
Table 4 shows the results of fermentation parameters after temperature variation. The values of YE/G indicate that the temperature range studied (30–50 °C) did not have a significant influence on ethanol yields (0.45 g g−1 to 0.46 g g−1), nor on the fermentation efficiency (88–90 %). However, according to Table 4, the productivity of ethanol was influenced by temperature, and maximum productivity was obtained at 40 ºC and 50 °C, 3.96 g L−1 h−1. It is noteworthy that ethanol yields and efficiency at 40 °C were higher than at 50 °C. Furthermore, yields were close to the maximum theoretical value of YE/G (0.511 g g−1).
Based on the results of the temperature study in synthetic medium, ethanol production from K. marxianus ATCC36907 was evaluated using the enzymatic hydrolysate from CAB-OH at 40 °C, prepared as described in 2.6.1 and 2.6.2. Figure 4 shows the results of glucose and ethanol concentrations obtained from enzymatic hydrolysate fermentation at 40 °C. As seen in the results obtained from the synthetic medium, there was no glucose after 4 h of fermentation. The maximum ethanol concentration obtained was 24.9 g L−1, 5.92 g L−1 h−1 of productivity, and ethanol yield of 0.43 g g−1.
Other authors [14] evaluated the influence of temperature (30–40 °C) on ethanol production by K. marxianus CE025 using a hydrolysate obtained from the acid pretreatment of cashew apple bagasse (CAB). The initial sugar concentration (glucose + xylose) was 53 g L−1. The best results of ethanol yield were obtained at 30 °C, with the highest ethanol concentration of 12.36 g L−1, productivity of 0.26 g L−1 h−1, and YE/G and 0.42 g g−1. The YE/G obtained by these authors was closer than the results from this study at higher temperatures of fermentation.
Similar results were achieved by other authors [34], when studying ethanol production by K. marxianus CECT 10875 using steam exploded barley straw. In the SHF process at 42 °C, the authors obtained the highest ethanol concentration of 23 g L−1 at 24 h with 56.4 g L−1 of initial glucose. Pessani et al. [35] performed studies using the SSF process at 37–45 °C and obtained better results at 45 °C with the K. marxianus IMB3 strain. The authors obtained the highest ethanol concentration and yield of 23 g L−1 and 86 %, respectively.
Ethanol Production by K. marxianus ATCC36907 by Simultaneous Saccharification and Fermentation
The results regarding ethanol concentration and sugars (glucose, xylose, and cellobiose) after SSF of CAB-OH using cells of K. marxianus ATCC36907 at 40 °C and cellulase at 30 FPU/gglucan are shown in Fig. 5a. A higher ethanol concentration (25.56 ± 0.9 g L−1) was obtained after 48 h of fermentation, with a productivity of 0.53 g L−1 h−1. The efficiency (ηʹ) of ethanol production was 60.66 %. It can be seen that glucose and cellobiose accumulated during the process, approximately 2.5 g L−1 and 10 g L−1 remained at the medium at 72 h, respectively. Cellobiose concentration (12.61 ± 0.5 g L−1) was maximal at 48 h of fermentation, while at 72 h the concentration decreased to 9.88 ± 0.1 g L−1. Cellobiose accumulation indicates the inhibition of cellulases, which reduced the performance of the reaction. This process influenced glucose production and negatively affected the YʹE/G value (0.31). The present results corroborate with the obtained results for other study, which used different raw lignocellulosic materials and microorganisms, but the same strategy (SSF process), i.e., wheat straw [36], barley straw [34], Kanlow switchgrass (Panicum virgatum var. Kanlow) [37].
Effect of enzyme in the profile of glucose, cellobiase and ethanol in the SSF processes by K. marxianus ATCC36907 using 10 % CAB-OH (7.5 % glucan from CAB-OH) at 40 °C, pH 4.5–5.0 and 150 rpm. a Celluclast 1.5 L (30 FPU/gglucan) and b Celluclast 1.5 L (30 FPU/gglucan) and Cellobiase (60 CBU/gglucan). Glucose (square), cellobiose (circle), and ethanol (triangle) concentrations
Since the commercial complex Celluclast 1.5 L is deficient in cellobiases (5.63 CBU/mL), the SSF process was conducted with cellobiase supplementation (60 CBU/gGLUCAN) and the results are show in the Fig. 5b and Table 5. Cellobiose accumulation did not observe during the process and residual glucose concentrations in the fermentation medium were below 2 g L−1 after 24 h. A higher ethanol concentration (41.41 ± 0.2 g L−1) was obtained after 24 h of fermentation, with a productivity, yield and efficiency of 1.73 g L−1 h−1, 0.50 gE.gG −1, and 98.4 %, respectively (Table 5). These results are higher than the obtained without cellobiase supplementation.
Mass Balance
Figure 6 shows a flowchart with the different processes (SHF and SSF) evaluated in this study. After the pretreatment stage, the glucan was recorded to be 15.57 g after acid pretreatment and 6.72 g after alkali pretreatment, per 100 g of the original starting material. After acid pretreatment, almost 22 g of total sugars were recovered, which has been reported as potential substrate for ethanol and xylitol production [9, 13, 15]. Rocha et al. [13], for instance, reported a maximum ethanol concentration of 10.0 g L−1 in a 4 L bench-scale bioreactor using S. cerevisiae. Albuquerque et al. [9], using K. marxianus CCA 510, produced both ethanol and xylitol, reaching a concentration of 11.89 ± 0.34 g L−1 and 6.76 ± 0 28 g L−1, respectively.
Comparing the results of ethanol yield after SHF and SSF processes (Fig. 6), it can be seen that the amount obtained by SSF (27.8 Kg/ton CAB from SSF using 7.5 % glucan, corresponding at 10 % CAB-OH) was closer (but higher) than the one obtained for SHF (22.1 Kg/ton CAB). Nevertheless, the SSF system required lower initial concentrations of enzyme and cellulose when compared to SHF, which turns SSF an interesting alternative to produce ethanol from CAB-OH. Moreover, the hydrolysate obtained from alkali pretreatment step is rich in lignin, which can be further processed for the production of power/fuel, macromolecules, and aromatic chemicals [28, 29].
Conclusion
The results of the present study show that the enzymatic hydrolysate from the acidic-alkaline pretreated cashew apple bagasse is an alternative glucose source for ethanol production by K. marxianus strains. Among the yeasts studied, K. marxianus ATCC36907 exhibited promising results of ethanol production at different temperatures by SHF process. In this case, the highest ethanol production was obtained at 40 °C corresponding at a concentration and yield of 24.9 g L−1 and 22.1 Kg/ton CAB, respectively. In the SSF process using CAB-OH at 40 °C, the ethanol yield achieved was 27.8 Kg/ton CAB with a concentration of 41.41 g L−1 using 10 % CAB-OH. The SHF and SSF processes demonstrated to be promising alternatives for CAB-OH application in second-generation ethanol production by K. marxianus ATCC36907.
References
Alzate, C. A. C., & Toro, O. J. S. (2006). Energy consumption analysis of integrated flowsheets for production of fuel ethanol from lignocellulosic biomass. Energy, 31(13), 2447–2459. doi:10.1016/j.energy.2005.10.020.
Hu, Z., & Wen, Z. (2008). Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochemical Engineering Journal, 38(3), 369–378. doi:10.1016/j.bej.2007.08.001.
Zhu, S., Wu, Y., Yu, Z., Zhang, X., Wang, C., Yu, F., & Jin, S. (2006). Production of ethanol from microwave-assisted alkali pretreated wheat straw. Process Biochemistry, 41(4), 869–873. doi:10.1016/j.procbio.2005.10.024.
Alvira, P., Tomás-Pejó, E., Ballesteros, M., & Negro, M. J. (2010). Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresource Technology, 101(13), 4851–4861. doi:10.1016/j.biortech.2009.11.093.
Hasunuma, T., & Kondo, A. (2012). Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains. Process Biochemistry, 47(9), 1287–1294. doi:10.1016/j.procbio.2012.05.004.
Haque, M. A., Barman, D. N., Kang, T. H., Kim, M. K., Kim, J., Kim, H., & Yun, H. D. (2013). Effect of dilute alkali pretreatment on structural features and enhanced enzymatic hydrolysis of Miscanthus sinensis at boiling temperature with low residence time. Biosystems Engineering, 114(3), 294–305. doi:10.1016/j.biosystemseng.2013.01.006.
Moreno, A. D., Ibarra, D., Ballesteros, I., González, A., & Ballesteros, M. (2013). Comparing cell viability and ethanol fermentation of the thermotolerant yeast Kluyveromyces marxianus and Saccharomyces cerevisiae on steam-exploded biomass treated with laccase. Bioresource Technology, 135, 239–245. doi:10.1016/j.biortech.2012.11.095.
da Costa, J. A., Marques, J. E., Jr., Gonçalves, L. R. B., Rocha, M. V. P., Marques, J. E., Jr., Gonçalves, L. R. B., & Rocha, M. V. P. (2014). Enhanced enzymatic hydrolysis and ethanol production from cashew apple bagasse pretreated with alkaline hydrogen peroxide. Bioresource Technology, 179, 249–259. doi:10.1016/j.biortech.2014.11.010.
de Albuquerque, T. L., Gomes, S. D. L., Marques, J. E., Jr., Jr Ivanilo, S., & Rocha, M. V. P. (2015). Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catalysis Today, 255, 33–40. doi:10.1016/j.cattod.2014.10.054.
Ferreira, A. C. H., Neiva, J. N. M., Rodriguez, N. M., Lôbo, R. N. B., & de Vasconcelos, V. R. (2004). Valor nutritivo das silagens de capim-elefante com diferentes níveis de subprodutos da indústria do suco de caju. Revista Brasileira de Zootecnia, 33(6), 1380–1385. doi:10.1590/S1516-35982004000600004.
Matias, M. F. O., de Oliveira, E. L., Gertrudes, E., & dos Anjos Magalhães, M. M. (2005). Use of fibres obtained from the cashew (Anacardium ocidentale, L) and guava (Psidium guayava) fruits for enrichment of food products. Brazilian Archives of Biology and Technology, 48(spe), 143–150. doi:10.1590/S1516-89132005000400018.
Rodrigues, T. H. S., Dantas, M. A. A., Pinto, G. A. S., & Gonçalves, L. R. B. (2007). Tannase production by solid state fermentation of cashew apple bagasse. Applied Biochemistry and Biotechnology, 137–140(1–12), 675–688. doi:10.1007/s12010-007-9088-5.
Rocha, M. V. P., Rodrigues, T. H. S., de Macedo, G. R., & Gonçalves, L. R. B. (2009). Enzymatic hydrolysis and fermentation of pretreated cashew apple bagasse with alkali and diluted sulfuric Acid for bioethanol production. Applied Biochemistry and Biotechnology, 155(1–3), 407–417. doi:10.1007/s12010-008-8432-8.
Rocha, M. V. P., Rodrigues, T. H. S., Melo, V. M. M., Gonçalves, L. R. B., & de Macedo, G. R. (2011). Cashew apple bagasse as a source of sugars for ethanol production by Kluyveromyces marxianus CE025. Journal of Industrial Microbiology & Biotechnology, 38(8), 1099–1107. doi:10.1007/s10295-010-0889-0.
Rocha, M. V. P., Rodrigues, T. H. S., de Albuquerque, T. L., Gonçalves, L. R. B., & de Macedo, G. R. (2014). Evaluation of dilute acid pretreatment on cashew apple bagasse for ethanol and xylitol production. Chemical Engineering Journal, 243, 234–243. doi:10.1016/j.cej.2013.12.099.
Limtong, S., Sringiew, C., & Yongmanitchai, W. (2007). Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresource Technology, 98(17), 3367–3374. doi:10.1016/j.biortech.2006.10.044.
Watanabe, T., Srichuwong, S., Arakane, M., Tamiya, S., Yoshinaga, M., Watanabe, I., & Nakamura, T. (2010). Selection of stress-tolerant yeasts for simultaneous saccharification and fermentation (SSF) of very high gravity (VHG) potato mash to ethanol. Bioresource Technology, 101(24), 9710–9714. doi:10.1016/j.biortech.2010.07.079.
Narra, M., James, J. P., & Balasubramanian, V. (2015). Simultaneous saccharification and fermentation of delignified lignocellulosic biomass at high solid loadings by a newly isolated thermotolerant Kluyveromyces sp. for ethanol production. Bioresource Technology, 179, 331–338. doi:10.1016/j.biortech.2014.11.116.
Rodrigues, T. H. S., Rocha, M. V. P., de Macedo, G. R., & Gonçalves, L. R. B. (2011). Ethanol production from cashew apple bagasse: improvement of enzymatic hydrolysis by microwave-assisted alkali pretreatment. Applied Biochemistry and Biotechnology, 164(6), 929–943. doi:10.1007/s12010-011-9185-3.
Selig, M., Weiss, N., & Ji, Y. (2008). Enzymatic saccharification of lignocellulosic Biomass laboratory analytical procedure (LAP). Retrieved from http://www.nrel.gov/docs/gen/fy08/42629.pdf
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2011). Determination of structural carbohydrates and lignin in biomass determination of structural carbohydrates and lignin in biomass. laboratory analytical procedure (LAP) (Vol. 2011).
Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., & Templeton, D. (2008). Determination of extractives in biomass laboratory analytical procedure ( LAP ) Issue Date : 7 / 17 / 2005 Determination of extractives in biomass laboratory analytical procedure ( LAP ). Renewable Energy, (January).
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59(2), 257–268. Retrieved from http://www.degruyter.com/view/j/pac.1987.59.issue-2/pac198759020257/pac198759020257.xml .
Carvalheiro, F., Duarte, L. C., & Gírio, F. M. (2008). Hemicellulose biorefineries: a review on biomass pretreatments. Journal of Scientific & Industrial Research, 67(11), 849–864. Retrieved Oct.17, 2014, from http://nopr.niscair.res.in/bitstream/123456789/2429/1/JSIR.pdf .
Taherzadeh, M. J., & Karimi, K. (2008). Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. International Journal of Molecular Sciences, 9(9), 1621–1651. doi:10.3390/ijms9091621.
Macfarlane, A. L., Mai, M., & Kadla, J. F. (2014). Advances in biorefineries. Advances in Biorefineries Elsevier. doi:10.1533/9780857097385.2.659.
Berlin, A., & Mikhail Balakshin. (2014). Industrial lignins: analysis, properties, and applications. In Bioenergy Research: Advances and Applications (p. 500). Newnes. Retrieved from http://books.google.com/books?id=BWUiAQAAQBAJ&pgis=1
Martins, L. F., Kolling, D., Camassola, M., Dillon, A. J. P., & Ramos, L. P. (2008). Comparison of Penicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Bioresource Technology, 99(5), 1417–1424. doi:10.1016/j.biortech.2007.01.060.
Pierre, G., Maache-Rezzoug, Z., Sannier, F., Rezzoug, S.-A., & Maugard, T. (2011). High-performance hydrolysis of wheat straw using cellulase and thermomechanical pretreatment. Process Biochemistry, 46(11), 2194–2200. doi:10.1016/j.procbio.2011.09.002.
Pierre, G., Sannier, F., Goude, R., Nouviaire, A., Maache-Rezzoug, Z., Rezzoug, S.-A., & Maugard, T. (2011). Evaluation of thermomechanical pretreatment for enzymatic hydrolysis of pure microcrystalline cellulose and cellulose from Brewers’ spent grain. Journal of Cereal Science, 54(3), 305–310. doi:10.1016/j.jcs.2011.06.004.
Wang, L., Templer, R., & Murphy, R. J. (2012). High-solids loading enzymatic hydrolysis of waste papers for biofuel production. Applied Energy, 99, 23–31. doi:10.1016/j.apenergy.2012.03.045.
De Albuquerque Wanderley, M. C., Martín, C., de Rocha Moraes, G. J., & Gouveia, E. R. (2013). Increase in ethanol production from sugarcane bagasse based on combined pretreatments and fed-batch enzymatic hydrolysis. Bioresource Technology, 128, 448–453. doi:10.1016/j.biortech.2012.10.131.
Tomás-Pejó, E., Oliva, J. M., González, A., Ballesteros, I., & Ballesteros, M. (2009). Bioethanol production from wheat straw by the thermotolerant yeast Kluyveromyces marxianus CECT 10875 in a simultaneous saccharification and fermentation fed-batch process. Fuel, 88(11), 2142–2147. doi:10.1016/j.fuel.2009.01.014.
García-Aparicio, M. P., Oliva, J. M., Manzanares, P., Ballesteros, M., Ballesteros, I., González, A., & Negro, M. J. (2011). Second-generation ethanol production from steam exploded barley straw by Kluyveromyces marxianus CECT 10875. Fuel, 90(4), 1624–1630. doi:10.1016/j.fuel.2010.10.052.
Pessani, N. K., Atiyeh, H. K., Wilkins, M. R., Bellmer, D. D., & Banat, I. M. (2011). Simultaneous saccharification and fermentation of Kanlow switchgrass by thermotolerant Kluyveromyces marxianus IMB3: the effect of enzyme loading, temperature and higher solid loadings. Bioresource Technology, 102(22), 10618–10624. doi:10.1016/j.biortech.2011.09.011.
Linde, M., Jakobsson, E., Galbe, M., & Zacchi, G. (2008). Steam pretreatment of dilute H2SO4-impregnated wheat straw and SSF with low yeast and enzyme loadings for bioethanol production. Biomass and Bioenergy, 32(4), 326–332. doi:10.1016/j.biombioe.2007.09.013.
Suryawati, L., Wilkins, M. R., Bellmer, D. D., Huhnke, R. L., Maness, N. O., & Banat, I. M. (2009). Effect of hydrothermolysis process conditions on pretreated switchgrass composition and ethanol yield by SSF with Kluyveromyces marxianus IMB4. Process Biochemistry, 44(5), 540–545. doi:10.1016/j.procbio.2009.01.011.
Acknowledgments
The authors would like to thank the Brazilian research funding agencies ANP, CAPES, and CNPq (Federal). Saccharomyces sp. 1238 was kindly donated by the Laboratory of Biotechnology Process, located at the Federal University of Pernambuco, Recife, Brazil. We also thank Dr. Sandra Ceccato-Antonini from the Laboratory of Agricultural Microbiology, UFSCar – São Paulo – Brazil, for the Kluyveromyces marxianus CCA510 strain. In addition, the authors gratefully acknowledge Novozymes for donating the enzyme complex used in this study.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 338 kb)
Rights and permissions
About this article
Cite this article
Rodrigues, T.H.S., de Barros, E.M., de Sá Brígido, J. et al. The Bioconversion of Pretreated Cashew Apple Bagasse into Ethanol by SHF and SSF Processes. Appl Biochem Biotechnol 178, 1167–1183 (2016). https://doi.org/10.1007/s12010-015-1936-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1936-0