Abstract
The polysaccharides in the primary plant cell wall are a renewable energy source for biofuel production. However, these polysaccharides are not readily available for bioconversion, and large enzyme sets are required to deconstruct them. Here, we aimed to improve the glucan conversion using recombinant hemicellulases and esterase as a treatment in exploded and sugarcane bagasses (SCB), followed by the addition of commercial CBH I to prevent its inhibition by hemicellulases products. A high secretion level of the recombinant enzymes was observed on SDS-PAGE. The highest activities were verified at a temperature and pH ranging from 40 to 55 °C and 4.5 to 6.0, respectively. The released reducing sugar analysis showed that all enzymes act better on SCB, with xylanase C (XynC) presenting the best activity (0.54 U/mg of protein). The high-performance liquid chromatography (HPLC) analysis demonstrated that 24 h of pretreatment was enough to reach maximum glucan conversion. The best synergy was achieved between XynC and CBH I on SCB, 1.4%. All results showed that the enzymes acted better on SCB, which can be related to the biomass composition and its molecular structure. The enzymatic pretreatment of SCB with XynC was essential to improve the glucan conversion by CBH I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The change from a fossil-based energy matrix to a renewable one is increasingly necessary. The use of feedstock waste as an alternative energy source has been the subject of several studies on biofuel research. In this context, bioethanol, a form of renewable energy produced from biomass, has received greater attention. The plant cell wall, a structure rich in complex polysaccharides, can be degraded into fermentable monosaccharides and subsequently converted into ethanol via fermentation process [1, 2]. In Brazil, sugarcane is the most important biomass, and the bagasse generated after its processing has been seen as a source to enhance ethanol production.
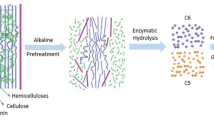
The main constituent of sugarcane primary cell wall is glucuronoarabinoxylan, a hemicellulose polymer consisting of β-1,4-xylose in the main carbon chain, with the addition of arabinose and glucuronic acid side chains [3]. Other components are cellulose, xyloglucan, mannan, glucomannan, and pectin, but the last four components represent a small part of it [4]. Cellulose is the most abundant polymer in nature and the most relevant for the 2G ethanol industry. It consists of thousands of β (1→4)-linked D-glucose monomers, which will be directed for conversion into ethanol by yeasts [5,6,7]. Another polymer considered unique to this plant group is β-glucan, whose main chain consists of glucose, containing β-1-3 and β-1-4 bonds, which is involved in cell growth [8]. Although less mentioned, the secondary cell wall is represented in large amounts on grasses, with lignin as the main component, whose monomers are coniferyl and sinapyl alcohols, and p-coumaryl alcohol in low amounts. Its main function is to promote the plant cell wall rigidity by filling up the pores within the polysaccharide matrix [8].
In order to convert biomass into a more accessible and reactive form, pretreatment technologies have emerged. Their main goals comprise lignin and hemicellulose disruption (or even the removal of them), reduction of cellulose crystallinity, and increase in the porosity of these materials [9]. However, conventional pretreatment methods have technical problems such as the high cost of reagents, loss of sugars that could be used in saccharification, and formation of environmentally harmful compounds and products toxic to microorganisms used in subsequent fermentation processes [10, 11]. After pretreatment, the biomass is ready for the enzymatic saccharification process. In this step, the biomass is treated with cellulolytic, hemicellulolytic, and pectinolytic enzymes. They attack specific polysaccharides in the biomass, which remained unhydrolyzed after physico-chemical pretreatments, improving the release of sugars that will be used in the fermentation process.
However, many sugars released by the action of hemicellulases can inhibit the action of cellulases, which are the main ones in the lignocellulosic biomass conversion process. Therefore, enzymes that can work synergistically and tolerate diverse types of molecules are either bioprospected from nature or produced via genetic manipulation. Another strategy, for biochemically uncharacterized enzymes, is the separation of enzymatic pretreatment between hemicellulases and cellulases. This last strategy was widely used for commercial enzymatic blends. Novozymes, for example, marketed the cellulase cocktail, Cellic® Ctec 2, and suggested its complementation with Cellic® Htec 2, a hemicellulase cocktail. Only recently are state-of-the-art cocktails being marketed with all enzymes in one blend [12,13,14].
Filamentous fungi are the preferred source of industrial enzymes because of their excellent capacity for extracellular protein secretion with a wide spectrum of action and substrate specificity [15]. They are one of the largest groups of eukaryotes that are natural decomposers of organic matter and the primary decomposers of most decayed plant material [16, 17], as they have the enzymatic repertoire necessary for the decomposition of the plant cell wall. Therefore, improving the production of fungal enzymes is of great interest in biotechnology.
In this context, the heterologous protein expression by microbial systems is attractive because of the fast growth on low-cost substrates; they are genetically and physiologically well characterized, and a large number of cloning vectors and host strains are widely available [18]. Furthermore, the use of filamentous fungi as hosts for heterologous protein expression has advantages, as these microorganisms secrete high quantities of protein and have the apparatus for efficient folding and post-transduction modifications [19]. Thus, this work aimed to evaluate the use of an efficient enzymatic cocktail, with recombinant hemicellulases and esterase, prior to the action of a commercial cellulase. The effect of this enzymatic pretreatment was evaluated for sugarcane bagasse and steam-exploded sugarcane bagasse.
2 Materials and methods
2.1 Microorganism strain cultivation and maintenance
The fungal strains Talaromyces funiculosus ATCC 62998, Aspergillus niger ATCC 1015, and Trichoderma reesei ATCC 56765 (American Type Culture Collection, Manassas, VA) were cultured in potato dextrose agar medium (PDA – Difco™) at 30 °C until sporulation. In addition, the auxotrophic mutant Aspergillus nidulans A773 was cultured in minimal medium (MM), supplemented with pyridoxine, as previously described by Segato et al. [20]. For filamentous fungi maintenance, the spores were collected from Petri dishes, transferred to a solution of glycerol 20% and lactose 10%, and stored at − 80 °C. Shot® TOP10 chemically competent E. coli (Invitrogen) were inoculated into plates containing LB medium and maintained at 37 °C until visualization of colonies. For E. coli maintenance, one colony was propagated in liquid LB medium, 120 rpm, and grown overnight at 37 °C, and then 500 μL of the bacterial suspension was added to 500 μL of 80% glycerol solution and stocked at − 80 °C.
2.2 Genomic DNA extraction, plasmids, and gene cloning
For the genomic DNA (gDNA) extraction, the fungal strains (Table 1) were grown in MM-glucose media at 30 °C for 24 h. The mycelial mass was filtered, and 50 mg were frozen in liquid nitrogen and ground with the pistil and mortar followed by the addition of 600 μL of lysis buffer (10% EDTA 0.5 M and 1% SDS). The suspension was heated at 68 °C for 10 min and centrifuged at 13,000 g for 5 min, and the supernatant was transferred into a new tube. A volume of 40 μL of KOAc 5 M was added, mixed by inversion, and incubated on ice for 10 min, followed by new centrifugation at 13,000 g for 5 min. The supernatant was transferred into a fresh tube, and 2.5 volumes of EtOH 95% were added, followed by centrifugation step as previously described. The pellet was washed twice with EtOH 70%. The precipitate was dried at room temperature and then resuspended in 50 μL of TE (Tris-HCl 10 mM, EDTA 1 mM, pH 8.0) containing 50 mg/mL of ribonuclease A.
The gDNA (around 50 ng/μL) were used to amplify the genes of interest (Table 1), with the touchdown polymerase chain reaction (PCR) using Taq DNA polymerase (Sigma). The cycle parameters were as follows: denaturing at 95 °C for 40 s, annealing at 60 °C for 30 s, and extension at 72 °C with the time varying according to the fragment size. This previous cycle was repeated with the exception that the annealing temperature was decreased in 2 °C each cycle until reaching 52 °C and then 28 cycles of 95 °C for 30 s, 52 °C for 30 s, and 72 °C with the time varying according to the fragment size, followed by a final extension at 72 °C for 10 min. All fragments carry a NotI and XbaI restriction sites that allow cloning the genes in the correct orientation into pEXPYR [20]. The cloning of XynC and abfB genes was reported in previous studies by Gonçalves et al. [21].
2.3 Transformation and protein heterologous expression
The successful cloning into pEXPYR was confirmed by sequencing. The positive plasmid for the gene of interest was used to transform A. nidulans A773 as describe by Segato et al. [20]. Positive transformants were selected by the reversion in auxotrophy marker allowing to grow up in absence of uracil and uridine. Approximately 107–108 spores/mL were inoculated in 500 mL of liquid MM supplemented with pyridoxine and 5% maltose distributed into stainless steel cafeteria trays and incubated in stationary conditions at 37 °C for 2 days. The experimental control was carried out with the auxotrophic mutant transformed with an empty pEXPYR vector. The protein expression was checked by the supernatant culture analysis in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
2.4 Enzymatic activity determination
The arabinofuranosidase activity quantification was performed using Kersters-Hilderson method [19, 22]. Arabinanase and xylanase activities were determined using the DNS method with some modification [23]. For the first assay, 25 μL of 0.25% p-nitrophenyl-arabinofuranoside (pNP-ara) was used as the substrate in 25 mM sodium acetate buffer at pH 6.0, and 25 μL of enzyme solution were added. The reaction mixture was incubated at 45 °C for 10 min and stopped with 50 μL of 0.2 M Na2CO3. The p-nitrophenol (pNP) formed was quantified by a spectrophotometer at 405 nm. The standard curve was constructed with (pNP) (0.09 to 0.54 μmols). The activity unit (U) was defined as the amount of enzyme able to release 1 μmol of pNP/min/mL. The assay for arabinanase and xylanase was carried out at 45 °C for 10 min. The reaction mixture consisted of 25 μL of 1% debranched arabinan and xylan from beechwood, respectively, in 25 mM sodium acetate buffer, pH 6.0, and 25 μL of enzyme for and stopped with 50 μL of DNS. The absorbance was read at 540 nm. Arabinose and xylose were used as the standard, and the activity unit (U) was defined as the amount of enzyme that releases 1 μmol of reducing sugar/min/mL, under the assay conditions.
For the feruloyl esterase activity determination, the reaction mixture consisted of 25 μL of 1% insoluble wheat arabinoxylan at pH 6.0 and 25 μL of enzyme solution. The reaction mixture was incubated at 45 °C for 10 min. Then, the mixture was centrifuged and the supernatant used for the activity determination. The spectrophotometer measurements were performed at 310 nm using a standard curve of ferulic acid. The unit of enzyme activity was defined as the amount of enzyme capable of releasing 1 μmol of product/min/mL [24].
A blank reaction was performed for each enzymatic assay. The protein concentration was determined at 595 nm according to Bradford [25], using bovine serum albumin (BSA) as standard.
2.5 Effect of temperature and pH on the recombinant enzyme activity
The optimal temperature and pH were determined at different temperatures (25 to 100 °C) and pH (2.5 to 9.5). The enzymatic reaction was performed as previously described above.
2.6 Biomass pretreatment
The sugarcane bagasse explosion pretreatment was optimized by the Laboratório Nacional de Biorrenováveis (LNBR), as described by Gouveia et al. [26]. In summary, the sugarcane bagasse (SCB) was subjected to steam explosion in 5000 L reactor for 7 min at 200 °C. This material was thoroughly washed (until the total removal of soluble solids from the hydrolysis), air dried, and properly stored. A small amount of this material (3 different samples) was characterized by their chemical composition. The hydrolysis products from SCB were evaluated by high-performance liquid chromatography (HPLC).
2.7 Enzymatic pretreatment
The enzymatic lignocellulosic biomass saccharification was performed in duplicate as described by Selig et al. (2008), from which the procedures for calculating the amount of biomass, the volume of buffer, as well as the concentration of azide used to conduct the experimental assay were taken [27]. Thus, the total amount of SCB and SSE bagasse used in this experimental assay was 0.1 g and 0.9 g, respectively, containing approximately 1% glucan content, based on the composition of each biomass.
The enzymes chosen to perform the hydrolysis experiments are the main enzymes involved in the degradation of lignocellulosic biomass. In this way, the catalysts applied were endo-1,4 β-xylanase (XynC), α-L-arabinofuranosidase (Abf1), feruloyl esterase (FaeA), endo-1,5–α–L–arabinanase (AbnA), and the commercial cellobiohydrolase I (CBH I) (Table 1). All of them were used in different combinations.
The enzymatic load suitable for the biomass saccharification was adjusted according to previous experiments, carried out on Eppendorf scale, in which different concentrations were tested, without the final reaction volume being changed, the reaction buffering being guaranteed, and the effect of the saccharification being maintained. The experiment was then scaled to the final volume of 5 mL. In addition, the biomass hydrolysis time was chosen according to the preservation time of the catalytic activity of XynC, the main enzyme that guaranteed the best biomass digestibility effect (data not shown).
The reaction mixture consisted of 100 μL of sodium azide 2%, to avoid microbial contamination, and 0.75 mg of total protein and buffered in 5 mL of sodium acetate 25 mM, which was incubated at 45 °C, for 24 or 48 h. Then, the samples were centrifuged (10 min, 13,000 g), and the pellet was dried at 100 °C. All samples were weighed, and 1 mL of the total volume (5 mL) was filtered on a Millipore filter of 0.22 μm. The filtrate was analyzed using HPLC in an HPX-87p column (Bio-Rad, Sunnyvale, CA) with a RI detector, flow rate of 6 mL/min at 85 °C, water as the mobile phase, and 10 μL of injection volume.
After this process, the commercial CBH I was added in all tubes in a volume of 4 mL under the same conditions as previously described. For each enzymatic reaction, a blank was performed. Arabinose, xylose, cellobiose, and glucose were used as the standard pattern.
3 Results and discussion
3.1 Protein expression
The expression of XynC, Abf1, AbnA, and FaeA kept high activity levels at 45 °C (Table 2). The commercial enzyme CBH I showed an activity of 7.4 U/g of protein at 45 °C. The SDS-PAGE of the expressed proteins showed a variation in the amount of secreted enzymes (Fig. 1). The variable number of gene copies that integrated into the host genome and the respective gene integration sites might be responsible for this variation. This factor, sometimes, can also lead to the non-expression of desired proteins [28].
Another biological phenomenon guaranteed by the expression system used here is the glycosylation, a post-translational process that occurs first in the endoplasmic reticulum and later in the Golgi complex. This process is described in the literature as being critical to maintaining the biological activity of the proteins [29]. All enzymes studied kept high activity level, showing that the expression system used here ensured the correct protein folding and satisfactory levels of glycosylation and allowed the perfect catalytic functioning of the proteins.
Fungi are natural decomposers of organic matter and carry a vast enzymatic repertoire. Many of the proteins produced commercially for the plant cell wall hydrolysis are from fungi, as the case of the enzyme CBH I from T. longibrachiatum used in this study [30]. Furthermore, the microorganisms providing the genes in this study are filamentous fungi recognized in the literature for their high ability to secrete potent cellulases (T. reesei, T. funiculosus) and hemicellulases (A. niger) [31]. For instance, T. reesei has become the major cell factory in the enzyme industry and the benchmark organism for the production of cellulases with significant cellulose degradation ability [32].
3.2 Temperature and pH effect
The optimum pH and temperature for all enzymes were evaluated in order to proceed to the hydrolysis experiments. The most suitable temperature to keep a relatively high activity of all enzymes and also to preserve the enzyme activity overtime was 45 °C (Fig. 2). The highest levels of all enzyme activities were observed under the pH 6.0 (Fig. 3).
3.3 Physical biomass pretreatment
Comparing the two sugarcane bagasse used in this research, the SCB showed cellulose as a predominant macromolecule with 43.8% in its composition. Other macromolecules were represented by 25.8% of hemicellulose, 22.1% of total lignin, 6.1% of extractives, and 1.4% of ashes (Fig. 4). The sugarcane bagasse pretreated with steam explosion (SSE) had an increased proportion of cellulose to 51.7–58.4% and lignin to 32–34.3%, but the hemicellulosic fraction decreased to 6.5–8.9% (Fig. 4).
A range of biomass pretreatments has been proposed to make the complex plant cell wall structure more accessible to the enzymatic bioconversion processes. An important strategy is the steam explosion, a physical treatment that increases the biomass surface area and consequently improves further treatments, such as enzymatic hydrolysis [20]. Many authors have shown that a sequential biomass treatment is necessary for the enzymatic hydrolysis of sugarcane bagasse and bioenergy generation [3, 33, 34].
3.4 Enzymatic cocktail
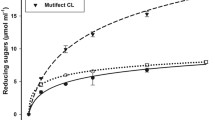
The DNS analysis of each recombinant enzyme tested on the sugarcane bagasse showed a good performance of XynC, followed by FaeA. For the SSE, the best enzymatic activity was for XynC, followed by FaeA. Comparing the enzymatic pretreatment of two studied biomass, the SCB yielded more reducing sugars than exploded bagasse (Fig. 5). This difference was expected since the explosion pretreatment removes part of hemicellulose and cellulose compared with SCB (Fig. 4).
The HPLC analysis showed a high yield of released glucose which can be correlated with the enzymatic pretreatment with XynC helping the commercial CBH I to access its specific substrate, cellulose. However, the hemicellulases’ combined action did not improve this access. The hydrolysis time is essential for the increase in the release of sugar by both bagasses, SSE and SCB. Because of structural changes caused by the explosion pretreatment, less glucose yield using SSE was observed (Fig. 6).
The release of arabinose was not observed in the experimental assay. Since recombinant arabinanase and arabinofuranosidase were used, the absence of arabinose was probably because of the low arabinan concentration in this biomass or by the low access to the branched arabinoxylans considering the sugarcane bagasse architecture.
The low xylose detection can be explained based on the xylanase action mechanism. This enzyme attacks the xylan backbone forming xylose, but also xylooligosaccharides, such as xylobiose and xylotrioses, whose standards were not used in this experimental assay.
The high xylan concentration in this biomass favored the endoxylanases’ participation in the bagasse deconstruction. These data were confirmed by the combinatory analysis, where XynC played a crucial role in the glucose release observed by HPLC. The feruloyl esterase participation could also contribute to the hydrolysis, as observed by De Souza et al. [35].
4 Conclusion
It is clear that other enzymes are necessary to create an efficient cocktail for an enzymatic deconstruction of the biomass. The data obtained here confirm the hierarchical enzymatic attack characteristic of the biomass deconstruction. The action of esterases and hemicellulases prior to the cellulases favored the exposition of the cellulose backbone, leading to an increase of glucose released by the last group of hydrolases. As the energy from fossil compounds is not renewable and highly polluting, alternative energy sources emerge as an attempt to reduce the dependence on fossil fuels. The use of energy from biomass is one favorable cost-benefit option to try to overcome these two big universal problems: energy shortages and nature pollution.
References
Rezende CA, de Lima MA, Maziero P, De Azevedo ER, Garcia W, Polikarpov I (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4:1–18
Santos FA, Queiróz JH, Colodette JL, Fernandes SA, Guimarães VM, Rezende ST (2012) Potencial da palha de cana-de-açúcar para produção de etanol. Quim Nova 35:1004–1010
Carpita NC (1996) Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol 47:445–476
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annual Rev Plant Biol 61:263–289
Heinze T (2015) Cellulose: structure and properties. Adv. Polym. Sci. In: Rojas O (eds) Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials. Advances in Polymer Science, vol 271. Springer, Cham
Trache D, Hazwan Hussin M, Haafiz MK, Thakur VK (2017) Recent progress in cellulose nanocrystals: sources and production. Nanoscale. 9:1763–1786
Gupta PK, Raghunath SS, Prasanna DV, Venkat P, Shree V, Chithananthan C, Choudhary S, Surender K, Geetha K (2019) An update on overview of cellulose, its structure and applications, Cellulose. In: Pascual A R (ed) Cellulose, vol 1. IntechOpen, Lon
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3:1–30
Canilha L, Milagres AMF, Silva SS, Silva JBA, Felipe MGA, Rocha GJM, Ferraz A, Carvalho W (2009) Sacarificação da biomassa lignocelulósica através de pré-hidrólise ácida seguida por hidrólise enzimática: uma estratégia de desconstrução da fibra vegetal. Rev Anal 44:48–54
Wheals A, Basso LC, Alves DMG, Amorim V (1999) Fuel ethanol after 25 years. Tibtech. 17:482–487
Gray KA, Zhao L, Emptage M (2006) Bioethanol. Bioethanol Curr Opin Chem Biol 10:141–146
Lucas RC, Cereia M, Coral MA (2015) Large scale production of cellulases for biomass degradation. In Nascimento R (ed) Fungal biotechnology for biofuel. 1st edn. Bentham Science Publishers, Sharjah, U.A.E. pp316–328
Rana V, Rana D (2017) Use of commercial enzymes to boost on-site enzyme efficiency. In: renewable biofuels. SpringerBriefs in Applied Sciences and Technology. Springer, Cham, pp 87–104
Cellulosic Ethanol Novozymes Cellic® CTec3 - secure your plant’s lowest total cost. Novozyme. Novozymes A/S · Luna No. 2012–01394-01. 6/6. Application Sheet https://s3.amazonaws.com/zanran_storage/bioenergy.novozymes.com/ContentPages/2546502386.pdf .Acessed 09 Jan 2020
Oliveira TB, Gomes E, Rodrigues A (2015) Thermophilic fungi in the new age of fungal taxonomy. Extremophiles 19:31–37
McLaughlin DJ, Spatafora JW (2014) The Mycota 7, Systematics and evolution part A. Springer, Switzerland, Heidelberg
López SC, Sietiö OM, Hildén K, de Vries RP, Mäkelä MR (2016) Homologous and heterologous expression of basidiomycete genes related to plant biomass degradation. In: Schmoll M, Dattenböck C (eds) Gene expression systems in fungi: advancements and applications. Fungal Biology. Springer, Cham, pp 119–160
Ben Mabrouk S, Ayadi DZ, Ben Hlima H, Bejar S (2013) Thermostability improvement of maltogenic amylase MAUS149 by error prone PCR. J Biotechnol 168:601–606
Gomes E, de Souza AR, Orjuela GL, Da Silva R, de Oliveira TB, Rodrigues A (2016) Applications and benefits of thermophilic microorganisms and their enzymes for industrial biotechnology. In: Schmoll M, Dattenböck C (eds) Gene expression systems in fungi: advancements and applications. Fungal Biology. Springer, Cham, pp 459–492
Segato F, Damásio AR, Gonçalves TA, de Lucas RC, Squina FM, Decker SR, Prade RA (2012) High-yield secretion of multiple client proteins in Aspergillus. Enzym Microb Technol 51:100–106
Gonçalves TA, Damasio AR, Segato F, Alvarez TM, Bragatto J, Brenelli LB, Citadini AP, Murakami MT, Ruller R, Paes Leme AF, Prade RA, Squina FM (2012) Functional characterization and synergic action of fungal xylanase and arabinofuranosidase for production of xylooligosaccharides. Bioresour Technol 119:293–299
Kersters-Hilderson H, Claeyssens M, Van Doorslaer E, Saman E, De Bruyne CK (1982) Beta-D-xylosidase from Bacillus pumilus. Methods Enzymol 83:631–639
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–429
Faulds CB, Williamson G (1994) Purification and characterization of a ferulic acid esterase (FAE-III) from Aspergillus niger specificity for the phenolic moiety and binding to microcrystalline cellulose. Microbiology. 140:779–787
Bradford MM (1976) A rapid and sensitive method for quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:1105–1112
Gouveia ER, Nascimento RT, Souto-Maior AM, Rocha GJM (2009) Validação de metodologia para a caracterização química do bagaço de cana-de-açúcar. Quim Nova 32(6):1500–1503
Selig M, Weiss N, JI Y (2008) Enzymatic saccharification of technical lignocellulosic biomass. LAP, NREL/TP-510-42629 https://wwwnrelgov/docs/fy15osti/63351pdf Acessed 09 Jan 2020
Ichioka D, Itoh T, Itoh I (2001) An Aspergillus nidulans uvsC null mutant is deficient in homologous DNA integration. Mol Gen Genet 264(5):709–715
Skropeta D (2009) The effect of individual N-glycans on enzyme activity. Bioorganic med. Chem. 17(2): 2645–2653
De Castro AM, Pedro KC, Da Cruz JC, Ferreira MC, Leite SG, Pereira JRN (2010) Trichoderma harzianum IOC-4038: a promising strain for the production of a cellulolytic complex with significant beta-glucosidase activity from sugarcane bagasse cellulignin. Appl Biochem Biotechnol 162:2111–2112
Selig MJ, Knoshaug EP, Decker SR, Baker JO, Himmel ME, Adney WS (2008) Heterologous expression of Aspergillus niger beta-D-xylosidase (XlnD): characterization on lignocellulosic substrates. Appl Biochem Biotechnol 146:57–68
Paloheimo M, Haarmann T, Mäkinen S, Vehmaanperä J (2016) Production of industrial enzymes in Trichoderma reesei. In: Schmoll M, Dattenböck C (eds) Gene expression systems in fungi: advancements and applications. Fungal Biology. Springer, Cham, pp 23–57
Vogel J (2008) Unique aspects of the grass cell wall. Curr Opin Plant Biol 11:301–307
De Souza AP, Leite DCC, Pattathil S, Hahn MG, Buckeridge MS (2012) Composition and structure of sugarcane cell wall polysaccharides: implications for second-generation bioethanol production. Bioenerg Res 6:564–579
Acknowledgments
We are thankful for Dr. George Jackson de Moraes Rocha, from the Laboratório Nacional de Ciência e Tecnologia do Bioetanol (CTBE), who gave us the biomass used in this research. Dr. Mark R. Wilkins and Dr. Michael Mueller cordially provided laboratory support and help with the HPLC analyses that were performed in the Biosystems and Agricultural Engineering department at the Oklahoma State University.
Funding
This work was funded by the Brazilian CNPq fellowship (Grant No. 141133/2009-0) to RCL and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) post-doctoral fellowship to TBO (Grant No. 2017/09000-4).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research did not involve human participants and/or animals.
Informed consent
All authors have read the manuscript and have approved the submitted version.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Lucas, R.C., de Oliveira, T.B., Lima, M.S. et al. Effect of enzymatic pretreatment of sugarcane bagasse with recombinant hemicellulases and esterase prior to the application of the cellobiohydrolase CBH I Megazyme®. Biomass Conv. Bioref. 12, 491–499 (2022). https://doi.org/10.1007/s13399-020-00719-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00719-9