Abstract
When a solid fuel particle is injected into a hot fluidized bed, the reactivity of fuel char in gasification reactions (between char carbon and steam and CO2) plays a significant role for reaching a good carbon conversion. In this paper, the gasification reactivity data of some solid waste recovered fuels (SRF) obtained from thermogravimetric analysis (TGA) experiments is presented. Gas mixtures (H2O, H2, CO2, CO), were used in the experiments to find the inhibitive effects of CO and H2. Average char gasification reactivity values are determined from the TGA results. Kinetic parameters for char carbon gasification reactivity correlations are determined from this data. The Uniform Conversion model is used to account for the change of gasification reaction rate as function of carbon conversion. Some discrepancies, due to complicated ash–carbon interactions, are subjects of further research. In the carbon conversion predictor, laboratory measured reactivity numbers are converted into carbon conversion numbers in a real-scale fluidized bed gasifier. The predictor is a relatively simple and transparent tool for the comparison of the gasification reactivity of different fuels in fluidized bed gasification. The residence times for solid fuels in fluidized bed gasifiers are simulated. Simulations against some pilot-scale results show reasonable agreement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In fluidized bed gasification of solid fuels, carbon conversion should be maximized, since it contributes to the efficiency of the gasifier. The stages of gasification of a solid fuel are drying, pyrolysis and char oxidation. The slowest part of fuel conversion in a gasifier is the oxidation of the fuel char by the gasification products CO2 and H2O [1]. The oxidation rate of the char is affected by process temperature, pressure, the behavior of ash, the partial pressures of reactant gases CO2 and H2O and the gaseous components H2 and CO, which have some inhibitive effects [2, 3]. The most significant char gasification reactions are the following:
Barrio et al. [4] studied the reactivity of biomass chars in a thermogravimetric analysis (TGA) and determined the kinetic parameters for the following correlations:
where k 1f (in per second per bar), k 1b (in per second per bar) and k 3 (in per second) are kinetic constants and P CO2, P CO, P H2O and P H2 are the partial pressures (bar) of CO2, CO, H2O and H2. Equation 3 refers to char carbon reaction with steam (Eq. 1) and Eq. 4 to the char carbon reaction with CO2 (Eq. 2).
In the recent review about gasification rates of lignocellulosic chars, Di Blasi [5] states that char from lignocellulosic fuel pyrolysis is highly affected by size and heating rate/temperature of the solid particles. Also, char reactivity decreases when the retention time for fuel pyrolysis increases. Moilanen et al. [2, 6] and Konttinen et al. [7] concluded that the method of slow pyrolysis of the fuel particles used by Barrio [4], results in too low reactivity values. Higher values with fast pyrolysis technique for the same fuel can be obtained, as was shown by Konttinen et al. [7]. It was proposed [5] that the reason for higher rates is that under fast heating, particle undergoes a molten phase with the consequent creation of smoother surfaces and spherical cavities. The faster pyrolysis rate is closer to the reality when fuel particles are fed into hot gasification reactor fluidized bed. In a fluidized bed gasifier, fuel is fed typically through a screw conveyor and when the fuel particles reach the bed, they are heated up very rapidly.
Using Eqs. (3) and (4) to describe rates of char gasification reactions is a simplified approach, assuming the control of the chemical surface reaction only. Di Blasi [5] gives an extensive review of the gasification rate models and kinetic parameters, derived from laboratory-scale tests, including many models with description of the structure or structural changes of the char particles. However, di Blasi [5] has criticism towards these all approaches and thus further work is still needed to generate a general useful model.
1.1 Work reported earlier by the authors
The measurements of the gasification reactivity for have been carried out by using a thermobalance (TGA) system which has been described earlier in [2]. During the course of gasification reactions of a wood sample, its weight decreases. The balance registers the changes in the weight, which are then electronically recorded as function of testing time. The instantaneous reactivity values from the TGA results are determined using the following equation [8]:
where dw is the weight change (mg) at time t and w is the instantaneous ash-free weight (in milligrams). As a simplified approach for determining the kinetic parameters [3], the instantaneous rate is averaged over the majority of the carbon conversion, neglecting changes near the end, Fig. 1, and used for determining the rate constants.
The kinetic parameters determined for birch fuel were reported previously by Konttinen et al. [7]. The parameter fitting equation is as follows:
where R Cexp are the reaction rates from TGA tests (wt %/min) and R Cmodel are corresponding rates using Eqs. (3) and (4). and The sum of Eq. (6) was used for finding the optimum frequency factors (k 01f, k 01b and k 03) and activation energies (E 1f, E 1b and E 3) for kinetic parameters of wood char [7]. The numerical procedure used for fitting the parameters was the second-order Levenberg–Marquardt algorithm for least-squares parameter estimation [9].
Table 1 shows the kinetic parameters that were determined for birch fuel. The results of determining the experimental reaction rates for solid recovered fuels (SRFs) are presented in Chapter 3.
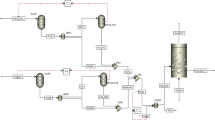
Konttinen et al. [3] reported the principles and structure of the carbon conversion predictor. In the predictor, laboratory measured reactivity numbers are converted into carbon conversion numbers in a real-scale fluidized bed gasification reactor. The predictor is a relatively simple and transparent tool for the comparison of the gasification reactivity of different fuels in fluidized bed gasification. The principles of the carbon conversion predictor model are shown in Fig. 2.
Carbon conversion predictor model principles [3]
In the predictor, as a simplification, fuel drying, pyrolysis and oxidation of volatile carbon and hydrogen (with O2 in the feed air) are taken into account assuming thermodynamic equilibrium. In the submodel “Equilibrium” of Fig. 2 the streams of gaseous O2, N2 and H2O and fuel volatiles (C, H and O) are treated [3] by assuming that they form a composition according the chemical equilibrium of the water gas shift reaction:
In the char gasification fluidized bed submodel (“FBG” in Fig. 2) [3], the char carbon inventory in the gasification fluidized bed is divided into i = 1 … N vertical sections, according to Fig. 3.
The material balance and symbols used for the gasification fluidized bed submodel [3]
The balance of Fig. 3 can be expressed [3] as:
where n Ctot is the total inventory of carbon in the gasification zone fluidized bed (mol). n H2Oeq(i−1) and n CO2eq(i−1) are the flows (in moles per second) of H2O and CO2 from the previous section. X gH2O(i) and X gCO2(i) are the fractional molar conversions of H2O and CO2 in gasification reactions (1) and (2) in Section 1. R C-H2O(i) and R C-CO2(i) (1 per second) describe the reactivity of solid carbon in gasification reactions (1) and (2) and are obtained from Eqs. (3) and (4), using the experimentally determined kinetic parameters. More details about the structure of the carbon conversion predictor can be found in [3].
This kind of simplified modelling approach avoids some of the challenges of more complicated models, while providing a usable model for predicting carbon conversion in a fluidized bed gasifier. Many models in the literature are based on thermodynamic equilibrium calculations to predict the composition of gases and solids exiting the gasifier [10–13]. This Gibbs free energy minimization method fails to predict the formation of certain components, such as unconverted solid carbon and gaseous hydrocarbons, since the formation of these are controlled by kinetics and certain physical phenomena in the fluidized bed [14]. To eliminate this problem, the chemical equilibrium calculation results are usually adjusted using empirical parameters or correlations to match the measured data from gasification reactors, without any information related with fuel reactivity.
Another group of fluidized bed gasification models are based on theories on fluidization hydrodynamics, such as the two-phase theory [14], coupled with chemical kinetics and heat and mass transfer phenomena for fuel in the fluidized bed [15–18]. The fuel-related phenomena include drying, volatilization, partial combustion by oxygen and gasification of char carbon with steam and CO2. In addition, homogeneous reactions in the gas phase are considered. Konttinen et al. [3] concluded that taking all these phenomena into account leads to the use of numerous empirical correlations and assumptions on the chemical and physical phenomena occurring in different phases (bubble and emulsion) of the fluidized bed. The use of fluidization correlations makes the models specific to only one certain type of fluidized bed gasification application. The models usually lead to a group of differential balance equations which have to be solved using numerical methods, which increase the mathematical complexity. For model validation, the experimental data available usually includes the composition of the gaseous and solid streams at the exit of a gasifier. A common conclusion is that model results agree reasonably well with this data and the authors state that the model is validated. However, this is not enough to justify the conclusion that the complicated model structure is correct. For the validation of model structure, measured data inside the gasifier bed, such as the gas composition and temperature profiles, should be available, which is usually not the case. These conclusions are more or less in agreement with the conclusions by Gómez-Barea and Leckner [19], in their recent review on models for biomass gasification in a fluidized bed. They conclude that most of the FB biomass gasification models fit reasonably well experiments selected for validation, despite the various formulations and input data. However, there are few measurements available for comparison with detailed model results. Another phenomenon that can have significance to the carbon conversion in fluidized bed gasification, is the escape of unconverted carbon from the bed with fly ash [3, 19]. The phenomena of fuel fragmentation, attrition and elutriation, which lead to the escape of carbon particles, are not very well known for biomass-type fuels. No common correlations exist that could be useful in a simplified fluidized bed model.
Konttinen et al. [3] compared the modelling results against some pilot-scale data, using fuels coal and biomass. Figure 4 shows the comparisons between model and experimental data, which is obtained from Kurkela et al. [20, 21]. Despite the simplified structure, the results of Fig. 4 indicate reasonable agreement.
In this paper, char gasification reaction rate data from TGA experiments and the derived char reactivity kinetic parameters are shown for two types of solid recovered fuels (SRF1 and SRF2). First, the highlights of the work previously published by the authors were described, as well as the relevant literature references were reviewed in the area of modelling of char gasification and biomass gasification in a fluidized bed. Then, the yet-unpublished experimental work in TGA on two solid recovered fuels and the obtained reaction rate data and kinetic parameters are presented. Using the carbon conversion predictor [3, 22], the predicted carbon conversion numbers are presented and these are compared with previous similar results for some other fuels. The relevance of the numbers obtained is discussed and compared with some experimental pilot-scale gasification test results.
2 Experimental
The properties of solid fuels used in the experiments and modelling in this paper are shown in Table 2, including the previously reported [3] coal and birch wood. SRF refers to solid waste recovered fuels, a waste where inert materials, such as glass and metals, have been removed [23].
The char carbon, as a result of fuel pyrolysis, is the amount of fixed carbon in fuels:
“Combustibles” in Table 2 is the amount of dry ash-free fuel. The measurements of the gasification reactivity for SRF char samples in this paper were carried out by using a thermobalance system and method, described elsewhere [2, 6, 8]. During the course of gasification reactions of a biomass char sample, its weight decreases. The balance registers the changes in the weight, which are then electronically recorded as function of testing time. In the tests, the SRF fuel chars were pyrolyzed with fast pyrolysis technique and chars were gasified using gas mixtures containing H2O, H2, CO2, CO. The reasons for using fast pyrolysis were discussed in Chapter 1. Measurements were carried out at constant temperature. The fuel sample is located in a sample holder and is lowered down to a reaction tube. The main part of the weight loss caused by pyrolysis takes place during this lowering period. Recording of the weight starts when the sample holder is at its lowest position and the rest of heating and pyrolysis takes place within 30–60 s. This first part of the results (pyrolysis) is removed from data evaluation. The heating rate of the sample is estimated to be approximately 20°C/s. Char gasification reactivity can be determined from this data, when knowing the fuel properties such as those in Table 2.
Examples of the obtained TGA curves are shown in Fig. 5.
The weight loss as function of testing time in TGA. Gas temperature 850°C for both SRF samples and 800°C for wood. Gas composition either 100 % H2O or 100 % CO2 [22]
The instantaneous char reactivities as function of fuel char carbon conversion can be obtained using Eq. (5), as shown in Fig. 6. The conversion of fuel char carbon begins between 80 % and 90 % of fuel carbon conversion because 80–90 % of the fuel carbon has been released during pyrolysis.
Moilanen [2] reports the immediate reactivities as function of fuel conversion. Fuel (dry ash-free) conversion and carbon conversion can be determined using Eqs. (10) and (11). It is assumed that in the gasification TGA tests (Fig. 6) all unreacted material in the sample is char carbon.
where m Fuel is initial dry mass of the fuel sample in TGA (in milligrams), m C0 the initial mass of carbon in fuel (in milligrams), m Comb the initial mass of combustibles in fuel (in milligrams), m Cunr is the mass of unreacted carbon (in milligrams). w comb is the weight fraction of combustibles in dry fuel (−), w C the weight fraction of carbon in fuel (−). X Carbon is fuel carbon conversion (%) and X Fuel is fuel overall (combustibles) conversion. Equations 10 and 11 can be combined to give:
Corresponding equations can be derived for char carbon conversion. In case of wood and SRF fuels of Table 2, most of the fuel carbon is converted after the release of volatiles.
3 Parameter fit for the char reactivity model
Examples of the conversion averaged reactivity values for the SRF fuels are listed in Table 3. The method for this conversion was described in Section 1.1.
The kinetic parameters for SRF1 and SRF1 fuels were determined using the experimental TGA data and correlations (3)–(6).
Tables 4 and 5 give the values for the kinetic parameters for SRF fuels obtained in this way.
Figures 7 and 8 show the Arrhenius plots for SRF 1 and SRF 2 fuels.
Figure 9 shows the influence of H2 and CO gases in the gasification with H2O and CO2. In agreement with the earlier results by Barrio [4] and Moilanen and Saviharju [8], CO and H2 have inhibiting effect on the gasification reactivity. Some of the measured reactivities have been removed from the data that was used for the fitting. In the case of CO2 gasification inhibited by CO, the model gives a linear dependence on CO which is a bit different from experimental observations. The reasons for this difference can be attributed to the limitations of parameter fitting with the rate Eqs. (3 and 4; Fig. 10).
Figures 11 and 12 show the influence of CO2 and H2O partial pressures on char gasification reactivity. The non-linear trend of the dependence of gasification reactivity on CO2 partial pressure seems to be followed well by the parameter fit.
The experimental data here is from atmospheric pressure conditions, and it can be expected [2] that the inhibition effect of product gases is probably not that strong as it can be at elevated pressures, such as at 20 bar. The work to investigate data from elevated pressures is in progress.
4 Comparison of the obtained rate parameters with literature
Di Blasi [5] presented a thorough review of the gasification kinetic rate parameters for woody-type biomass fuels. The comparison of the parameters obtained by the authors for wood samples [3, 6], against some parameters from literature is shown in Figs. 13 and 14.
Figure 13 indicates that in case of steam gasification of wood, the parameters presented by the authors do not produce significantly higher rates than those of Barrio [4]. In case of CO2 gasification of Fig. 14, the difference is more pronounced. In both cases, significantly higher reaction rates have been obtained by some other authors. However, this comparison indicates the parameters obtained by the authors for wood are at the same range as the data from literature. Due to the lack of literature references, it was not possible to perform a similar comparison for waste-type (SRF) fuels.
5 Change of char reactivity as function of char carbon conversion
To compensate for the change of char gasification reaction rate as function of carbon conversion, the Uniform Conversion model (UCM) with chemical surface reaction control [14, 24], can be applied. The reasons for not using more complicated models [5] were discussed in Chapter 1. UCM was used by Moilanen and Konttinen [2] previously. In this application, UCM can be written as:
where X C,char is fractional conversion of char carbon (−), t is time (in seconds) and r″ are the average reactivity parameters, either r C-H2O or r C-CO (1/s) which numbers can be obtained using the derived kinetic parameters in correlations (3) and (4). The variable w i is the initial weight of the char sample in the TGA and w is the instantaneous weight at time t.
Using the Eqs. 3, 4, 13 and 14, the TGA results shown in Fig. 5 can be simulated. Figure 15 shows examples of the fit of the UCM to some TGA results, which have also been used in the determination of averaged rate constants of Tables 1, 4 and 5.
UCM model simulations versus TGA char gasification test results (shown in Fig. 5)
Figure 15 indicates that using the UCM approach reasonable fits can be obtained and therefore, this simplified approach can be justified to compensate for the change of char gasification reaction rate as function of fuel carbon conversion. However, some disagreement between model and experimental data can be found towards the end of carbon conversion, especially in the case of birch wood. In the experiments, it has been observed that ash components of biomass fuels can have strong influence on biomass char gasification reaction rates [2]. As was shown more pronounced in Fig. 6, there is a large increase in reactivity for some of the fuels near full conversion followed by a precipitous drop in rate. Both of these phenomena may be related to ash behavior, as already observed in [25, 26]. The metals of ash can act as catalysts as they are exposed in the char matrix towards the end of char carbon conversion to gases. In the work in progress, the changes in the ash will be studied in more detail to be able to explain and predict the observed changes in char reactivity as carbon conversion nears 100 %.
6 Using the laboratory measured data in the carbon conversion predictor
The carbon conversion predictor results for a coal, a biomass and two solid recovered fuels (SRF1 and SRF2) are compared in Fig. 16. The figure shows the comparison as carbon conversion versus the residence time parameter (τ C), which is defined here as [3]:
where M Cbed is the total carbon inventory of the gasification fluidized bed in gasification zone (in grams) and m Cf is the carbon in fuel feed (in grams per second).
Carbon conversion in fluidized bed gasification as function of the carbon residence time parameter (τ C). Wood, coal and SRF fuels at temperatures 850–1,000°C, λ = 0.27 and 1.013 bar. Preliminarily reported in [22]
The results of Fig. 16 show that the tested SRF require the shortest residence time for char particles in fluidized bed gasifier to reach reasonable carbon conversion. The longest residence time is required by coal char particles. This is largely due to high volatiles yield of SRF and biomass. When comparing the curves of wood and SRF, char gasification rates seem to have some importance, resulting in slightly lower residence times for wood to reach 100 % conversion.
In the predictor, it is assumed that all volatile carbon reacts instantaneously (i.e., residence time of 0 s), which form the initial level of fractional carbon conversion, marked separately with a thick horizontal line in Fig. 16 for wood (about 0.56), for coal (0.28) and for and SRF 1 (0.83) and SRF 2 (0.78).
A project is underway to improve the carbon conversion predictor by adding terms in the kinetic equations to account for the effect of the interactions between char carbon and fuel ash. Dupont et al. [25] has done similar work to account for the interaction of K and Si. It can be expected that these kinds of interactions will decrease the required residence times to reach 100 % carbon conversion, from those presented in Fig. 16.
7 Conclusions
In fluidized bed gasification of solid fuels, carbon conversion should be maximized, since it contributes to the efficiency of the gasifier. When a solid fuel particle is injected into the hot fluidized bed, the reactivity of fuel char in gasification reactions (between char carbon and steam and CO2) plays a significant role for reaching a good carbon conversion. In this paper, the gasification reactivity data of some solid waste recovered fuels (SRF) obtained from TGA experiments is presented. In addition to pure H2O and CO2, gas mixtures (H2O, H2, CO2, CO), were used in the experiments to find the inhibitive effects of CO and H2. Average char gasification reactivity values are determined from the TGA results. The fit between the average reactivity values and correlations using the fitted parameters is shown in more detail.
The char reactivity kinetic parameters and predicted carbon conversion numbers are presented for two types of solid recovered fuels (SRF1 and SRF2) and these are compared with previous similar results for some other fuels. It is also shown that the change of fuel char gasification reaction rate as function of fuel conversion can be reasonably well compensated using the Uniform Conversion Model. However, some disagreement can be found towards the end of fuel conversion. In a new project, the changes in the ash will be studied in more detail to be able to explain and predict the observed changes in char reactivity as char conversion nears 100 %.
The kinetic parameters and correlations for char carbon gasification reactivity are used as submodels in the carbon conversion predictor. In the predictor, laboratory measured reactivity numbers are converted into carbon conversion numbers in a real-scale fluidized bed gasifier. The modelling results show that the solid recovered fuels SRFs require the shortest residence time for char particles in fluidized bed gasifier to reach reasonable carbon conversion. The longest residence time is required by coal char particles. The modelling results have also been compared against some pilot-scale data, using fuels coal and biomass. Despite of the simplified structure, the results indicate reasonable agreement.
References
Konttinen J, Reinikainen M, Oasmaa A, Solantausta Y (2011) Thermochemical conversion of forest biomass (Chapter 8). In: Alén R (ed) Papermaking Science and Technology, Book 20: biorefining of forest resources. Paper Engineer’s Association. Bookwell Oy, Porvoo, pp 262–304. ISBN 978-952-5216-39-4
Moilanen A (2006) Thermogravimetric characterisations of biomass and waste for gasification processes. Academic dissertation, Abo Akademi University. VTT Publications 607, Espoo, 103 p. + app. 97 p
Konttinen J, Moilanen A, Hupa M, Kurkela E (2006) Carbon conversion predictor for fluidized bed gasification of biomass fuels—model concept. Paper P071. In: Bridgwater AV, Boocock DGB (eds) Science in thermal and chemical biomass conversion. Vol. 1. CPL, Newbury, pp 590–604
Barrio M (2002) Experimental investigation of small-scale gasification of woody biomass. Academic dissertation. The Norwegian University of Science and Technology, Faculty of Engineering Science and Technology, Department of Thermal Energy and Hydropower. Trondheim, Norway
Di Blasi C (2009) Combustion and gasification rates of lignocellulosic chars. Prog Energy Combust Sci 35:121–140
Moilanen A Vepsäläinen J, Kurkela E, Konttinen J (2004) Gasification reactivity of large biomass particles. In: Proceedings of the Science in Thermal and Chemical Biomass Conversion (STCBC) Conference. Victoria, Vancouver Island, BC, Canada. 30.8.–2.9
Konttinen J, Moilanen A, Vepsäläinen J, Kallio S, Hupa M, Kurkela E (2003) Modelling and experimental testing of gasification of biomass char particles. In: Proceedings of the European Combustion Meeting 2003, Orleans, France, October 26–29
Moilanen A, Saviharju K (1996) Gasification reactivities of biomass fuels in pressurised conditions and product gas mixtures. In: Bridgewater A, Boocock D (eds) Developments in thermochemical biomass conversion. Banff, 20–24 May 1996. Blackie, London, pp 828–837
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J SIAM 11:431–441
Li XT, Grace JR (2004) Biomass gasification in a circulating fluidized bed. Biomass Bioenergy 26:171–193
Mathieu P, Dubuisson R (2002) Performance analysis of a biomass gasifier. Energ Convers Manag 43:1291–1299
Ruggiero M, Mantrida G (1999) An equilibrium model for biomass gasification processes. Renew Energ 16:1106–1109
Schuster G, Löffler G, Weigl K, Hofbauer H (2002) Biomass steam gasification—an extensive parametric modelling study. Bioresour Technol 77:71–79
Kunii D, Levenspiel O (1991) Fluidization engineering, 2nd edn. Butterworth-Heinemann, USA
Fiaschi D, Michelini M (2001) A two-phase one-dimensional biomass gasification kinetics model. Biomass Bioenergy 21:121–132
Hamell S, Krumm W (2001) Mathematical modelling and simulation of bubbling fluidized bed gasifiers. Powder Technol 120:105–112
Sadaka SS, Ghaly AE, Sabbah MA (2002) Two phase biomass air–steam gasification model for fluidized bed reactors. Part I-model development. Biomass Bioenergy 22:439–462
Yan H-M, Heidenreich C, Zhang D-K (1998) Mathematical modelling of a bubbling fluidised-bed coal gasifier and the significance of ‘net flow’. Fuel 77:1067–1079
Gómez-Barea A, Leckner B (2010) Modeling of biomass gasification in fluidized bed. Prog Energy Combust Sci 36:444–509
Kurkela E, Ståhlberg P, Laatikainen J (1993) Pressurized fluidized-bed gasification experiments with wood, peat and coal at VTT in 1991–1992. Part 1. Test facilities and gasification experiments with sawdust. VTT Publications: 161, Finland. ISBN 951-38-4399-8
Kurkela E, Ståhlberg P, Laatikainen-Luntama J (1995) Pressurised fluidised-bed gasification experiments with wood, peat and coal at VTT in 1991–1994. Part 2. Experiences from peat and coal gasification and hot gas filtration. VTT Publications 249, Finland. ISBN 951-38-4787-X
Konttinen J, Moilanen A, DeMartini N and Hupa M Carbon conversion predictor for fluidized bed gasification of biomass fuels—from TGA measurements to char gasification particle model. In: Hofbauer H, Fuchs M (eds) ICPS11 International Conference on Polygeneration Strategies. August 30—September 1, Vienna, Austria. Proceedings pp. 107–112
Solid recovered fuel (2000). Quality control system. SFS 5875. Finnish Standards Association. 29 p. In Finnish
Levenspiel O (1989) The chemical reactor omnibook. OSU, Oregon. ISBN 0-88246-164-8
Dupont C, Nocquet T, Da Costa J, Verne-Tournon C (2011) Kinetic modeling of steam gasification of various woody biomass chars: influence of inorganic elements. Bioresour Technol. doi:10.1016/j.biortech.2011.07.016
Mitsuoka K, Hayashi S, Amano H, Kayahara K, Sasaoaka E, Uddin Md (2011) Gasification of woody biomass char with CO2: the catalytic effects of K and Ca species on char gasification reactivity. Fuel Process Technol 92:26–31
Acknowledgments
The financial support of TEKES—The Finnish Funding Agency for Technology and Innovation (research project PERUSKAASU), Carbona Oy, Condens Oy, Fortum Oil and Gas Oy, Foster Wheeler Energia Oy, Pohjolan Voima Oy, Vapo Oy is gratefully acknowledged. The ongoing project GASIFREAC is financed by the Academy of Finland, which support is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konttinen, J.T., Moilanen, A., DeMartini, N. et al. Carbon conversion predictor for fluidized bed gasification of biomass fuels—from TGA measurements to char gasification particle model. Biomass Conv. Bioref. 2, 265–274 (2012). https://doi.org/10.1007/s13399-012-0038-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-012-0038-2