Abstract
Co-production of bio-char and syngas by gasification is a promising way for biomass comprehensive utilization. In this work, mainly two co-products from pinewood pellet gasification, namely bio-char and syngas were studied on a downdraft reactor. Based on the experiment, a detailed kinetic gasification model was built by Aspen Plus. The influence of temperature, ER, and steam amount was studied. Results of the pyrolysis stage show that bio-char yield during pyrolysis was about 22.8%wt and the initial pore structure was formed with a BET surface area of 36.8 m2/g. The main pyrolysis tar compounds detected by GC/MS were furfural and phenols. The gasification stage results show that H2 concentration reached the maximum of 18.62%vol at ER = 0.3. The maximum concentration of CO was 16.2%vol at ER = 0.25. The syngas yield increased with ER value. At low ER of 0.15, the syngas yield was 1.22 Nm3/kg and increased to 2.26 Nm3/kg at ER of 0.4. The carbon conversion ratio also increased with ER value. When ER = 0.4, the highest carbon conversion ratio reached 91.7%. The bio-char at gasifier outlet was a kind of highly carbonized material and the carbon content was 82.5%wt. During gasification, pore structure of bio-char was enlarged and the BET-specific surface area was about 215 m2/g. Modeling results show that by adjusting the gasification parameters, such as temperature, air equivalent ratio, and steam amount, the product distribution in the gasifier outlet could be effectively controlled. Mass and energy balance evaluation for the downdraft gasification system indicates that the pyrolysis stage and reduction stage are endothermic processes, which adsorb heat of 2.47 kW (Q1) and 2.44 kW (Q2) respectively from the partial oxidation stage. Partial oxidation stage acts as the heat source of the gasifier.
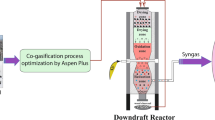
Graphical abstract

Figure. Biochar and syngas cogernation system based on biomass gasification
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biomass is an important renewable resource, which has been used for thousands of years. Traditional biomass utilization methods, such as direct combustion, have low utilization rate and serious pollution. Advanced biomass utilization technologies, including pyrolysis for oil production, gasification, and carbonization, could convert biomass into a variety of highly valuable products, such as bio-oil, syngas, and bio-char [1]. Among them, biomass gasification technology uses/employs gasification agents, such as air, oxygen, or steam, to convert biomass feedstock into syngas (mainly CO and H2). Syngas is cleaner and can be used for power generation as well as production of chemical products [2]. The traditional biomass gasification technology is faced with the problems of low gasification efficiency [3] and high tar content [4]. So the large-scaled commercial application of biomass gasification still has a great bottleneck.
Bio-char is usually obtained by pyrolysis and gasification of biomass at high temperature. It is a porous structure material with large specific surface area and abundant surface functional groups [5]. After further treatment, it can be used as activated carbon and organic fertilizer, etc., which has higher application value. Conventional bio-char production methods include slow pyrolysis, carbonization, hydrothermal pyrolysis, and microwave pyrolysis [6]. The physical and chemical properties of bio-char depend on the type of raw materials and the carbonization conditions, including temperature, carbonization time, reaction atmosphere, and heating rate [7]. The target product of traditional carbonization technology is bio-char, but the utilization of carbonization by-products, such as dry distillation gas and bio-oil, has not been fully treated, which causes pollution and waste of energy [6]. In addition, the traditional biomass gasification technology targets syngas and does not effectively utilize bio-char and other by-products of the gasification process.
Therefore, the use of biomass gasification technology, through adjusting the gasification reaction conditions for co-production of bio-char and syngas, could achieve the maximum economic benefit and effectively promote the industrial application of biomass gasification technology. Gasification polygeneration refers to the reaction of biomass with gasification agent at high temperature to produce bio-char, syngas, and bio-oil [8, 9]. However, at present, more studies are focused on individual bio-char or syngas production, while there are few studies on bio-char and syngas co-production. How to achieve efficient bio-char and syngas co-production and obtain comprehensive economic benefits through the regulation of reaction conditions in the gasification process is the key question.
On the other hand, the process of energy absorption and release in each stage of fixed bed gasification system has an important influence on the operation and control of gasifier. This effect is reflected in the temperature distribution characteristics at each stage of the gasifier. Generally speaking, the drying, pyrolysis, and gasification processes of biomass are endothermic reactions, while the partial oxidation processes are strongly exothermic reactions. How to adjust the heat at each stage to achieve the energy balance in the gasification process is of great significance in the process of cogeneration of bio-char and syngas. However, few studies in this field have been reported.
Modeling methods, including computational fluid dynamicsis (CFD), thermodynamic equilibrium model and artificial neural network (ANN) model, are effective approaches for evaluating biomass gasification process. Among them, Aspen Plus adopts thermodynamic or kinetic models, which can carry out detailed analysis on each stage of biomass gasification and obtain the distribution of product and energy flow in each stage. It has been widely used in the field of biomass gasification. Vikram et al. [10] employed Aspen Plus to study the steam-CO2 gasification of biomass. The Aspen Plus method was proved to be useful for thermodynamic analysis and parametric optimization of biomass gasification system. Pala et al. [11] studied steam gasification of biomass using Aspen Plus model for syngas adjustment. The developed model was based on Gibbs free energy minimization applying the restricted equilibrium method. However, using Aspen plus for modeling of co-production of bio-char and syngas in fixed gasifier has not been considered. In addition to assessing the distribution of gasification products, Aspen Plus model can also evaluate the energy balance of the system. Shang et al. [6] conducted theoretical study of activated carbon production via a two-step carbonization-activation process based on Aspen Plus calculation. The theoretical results showed that utilization of the heat duty of combustion of volatiles and sensible heat from flue gas could supply the necessary energy for carbonization, steam generator (boiler), and activation reactors. However, the evaluation of the energy distribution among different stages of gasification system has rarely been reported. The comprehensive evaluation for gasification products as well as energy balance during co-production of syngas and bio-char has never been reported.
In this paper, the downdraft fixed bed reactor is used for co-production of bio-char and syngas through gasification technology. By adjusting the gasification conditions, such as temperature and gasification agent, the yield and characteristics of syngas and bio-char are adjusted, and the parameters for the preparation of bio-char and syngas are adjusted. Based on the experimental results, the Aspen Plus software was used to model and simulate the process of bio-char and syngas co-production. The influence of reaction conditions on product distribution of bio-char and syngas was studied. Furthermore, the energy balance during each stage in the downdraft gasifier was evaluated. This work will provide theoretical support for the industrial application of co-production via biomass gasification.
2 Experimental and modeling methodology
2.1 The experimental apparatus and methods
In this work, the pinewood pellet was used as the biomass material for co-production experiment. Pinewood pellets were produced from pinewood sawdust using a pelletizer. The sawdust was collected from Shanxi province, China. The pellet diameter was about 5 mm.
The gasification experiments were conducted on a bench-scaled downdraft gasifier, as shown in Fig. 1.
The diameter of the furnace is 100 mm, which is made of 304 stainless steel. The accumulation height of the fuel bed is about 600–800 mm. The thickness of the external thermal insulation layer of the furnace body is about 150 mm. Electric heating is used outside the furnace body to preheat and start the gasification process. Electric heating system was composed of three stages, which is corresponding to pyrolysis, oxidation and reduction zone. The pyrolysis stage was controlled at 500 ℃. When the gasification temperature reaches the set value and can be self-sustained, electric heating is stopped. The feeding rate of pinewood pellet was controlled as 5 kg/h by a feeding valve. The feeding valve adopts multi-bin body design. Its purpose is on the one hand for feeding, on the other hand can prevent air from leaking into the gasifier. Air was chosen as the gasification agent, which was supplied from the top of the gasifier. The ash/bio-char was discharged by a moving grate. The discharged bio-char was stored in a stainless-steel tank and cooled to room temperature with nitrogen sweeping, which prevented oxidation by air. Syngas was purified, cooled, and then analyzed by a flue gas analyzer. A total of four gaseous species, namely H2, CO, CO2, and CH4 were analyzed. The tar sample was collected by cold solvent trapping method [12, 13] and organic compounds were analyzed by GC/MS. The pore structure of the bio-char was analyzed on physical adsorption analyzer (Micromeritics, TriStar II Plus Series) [14].
2.2 The Aspen Plus model building
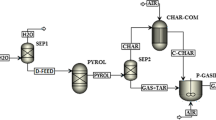
Based on the experimental results, the co-production of syngas and bio-char model was built on Aspen Plus platform. The model is mainly composed of four main stages: the drying and pyrolysis stage, the partial oxidation stage, the reduction stage, and separator stage. The detailed modules used in the co-production system are summarized in Fig. 2 as follows.
The nonconventional biomass feed components are first dried and decomposed in the RYIELD block, which is used to model the pyrolysis stage [11, 15]. In this block, the nonconventional biomass feedstock is decomposed into bio-char, moisture, gaseous compounds, and typical tar compounds. The pyrolysis production distribution was obtained from pyrolysis experiments. The volatiles were fed into a partial oxidation stage (POX). In this stage, the volatiles mix with air and oxidation reactions play a major role, which produces a high temperature zone [16, 17]. At high temperature, the chemical reaction rapidly approaches equilibrium. Therefore, the partial oxidation stage is modeled by a RGibbs block. In addition, the reactions between volatiles and air are homogenous reactions, which are much faster than heterogenous reactions. Therefore, the heterogenous oxidation between bio-char and air were ignored in this work. The flue gas as well as bio-char was fed into the reduction stage, in which the bio-char is gasified and syngas is produced. By controlling the air and steam amount, the degree of gasification reaction can be controlled, and then the proportion of syngas and bio-char can be adjusted. At the gasifier outlet, bio-char and syngas are separated and collected, which is simulated by a SEP block.
The connection and flow sheet of the co-production system are shown in Fig. 3.
With regard to the co-production system, the energy balance was also taken into consideration. In general, pyrolysis and reduction are endothermic processes, which need external heat supply. On the contrary, the partial oxidation stage is an exothermic process, which acts as a heat source for the system.
In order to simplify the reaction process and obtain reasonable results, the basic assumptions of the model are as follows:
-
I.
The pyrolysis reaction was simulated by Ryield module. Pyrolysis products include gases, water, tar, and coke. The gas composition includes CO, CO2, H2, CH4, and N2. The pyrolysis reaction was summarized as follow:
$$\mathrm{Biomass}\:\rightarrow\:0.2182\;{\mathrm H}_2\mathrm O\:+\:0.1301\;\mathrm{Tar}\:+\:0.228\;\mathrm{bio}-\mathrm{char}\:+\:0.4226\;\mathrm{Gas}\;(\mathrm R1)$$ -
II.
Two model tar compounds were used to represent pyrolysis tar mixture, namely furfural and phenol. Naphthalene, benzene, and toluene were considered during the gasification process and were included in the products [18].
-
III.
The bio-char was considered as the mixture of ash and carbon. Hydrogen and oxygen were not considered as the composition of bio-char.
-
IV.
The heat dissipation loss of the system is not considered.
-
V.
The gasification stage was simulated by reaction kinetic mechanism, which is composed of both homogenous and heterogenous reactions. The detailed reactions and kinetic parameters are summarized in Table 1 as follows:
3 Results and discussion
3.1 The pyrolysis product analysis
The proximate analysis was analyzed on a muffle furnace based on the Chinese National Standard GB/T 28,731–2012 [23]. The ultimate analysis was conducted on the elemental analyzer. The results are shown in Table 2 as follows.
In the gasifier, the pyrolysis is the first stage during biomass gasification. During pyrolysis, the raw biomass was decomposed into moisture, gas, tar, and bio-char. Furthermore, the pyrolysis products are mixed with air or oxygen in the partial oxidation zone to release heat and form a hot zone. The pyrolysis bio-char is further gasified by CO2/H2O to produce syngas. Therefore, the initial pyrolysis stage is crucial for the operation of the gasifier. It is necessary to evaluate the product distribution in pyrolysis stage.
Firstly, the pyrolysis products of pinewood pellet were analyzed on the fixed bed reactor. The furnace was heated from room temperature to 500 ℃ at heating rate of 10 ℃/min and maintained at 500 ℃ for 10 min. No gasification agent (air or steam) was supplied. N2 was used as the carrier gas to drive the gaseous products and tar compounds to the outlet of the reactor. When pyrolysis reaction finished, the bio-char sample was cooled down to temperature lower than 50℃. The gaseous compounds, tar and bio-char were sampled and analyzed. The results are summarized in Table 3 as follows.
A total of four gaseous species, namely H2, CO, CO2, and CH4 were analyzed. In the pyrolysis process, besides these four gases, there are also hydrocarbons such as ethylene and ethane, the production of which are relatively small [24]. Therefore, all small molecule hydrocarbons are converted to methane here. The mass yields of gaseous species were calculated based on the N2 concentration in the product gas. The mass of N2 was obtained by multiplication of gas flow rate and concentration. The yield of water was calculated by difference. The yields of bio-char and tar were about 22.8%wt and 13.01%wt, respectively. The initial pore structure was formed in the bio-char by the release of volatiles and the BET-specific surface area of bio-char was about 36.8 m2/g.
The main compounds of the pyrolysis tar analyzed by GC/MS are summarized in Table 4 as follows.
As shown in Table 4, the top ten typical tar compounds with the highest relative concentration (peak area) were identified by GC/MS. Furfural, 2-Furanmethanol, Levoglucosenone, and 1,2-Cyclopentanedione are typical pyrolysis products of cellulose and hemicellulose, which are non-aromatic compounds [25]. The most abundant compounds are phenol and its derivative, namely phenolic compounds. As shown in Table 4, the relative of phenol is about 15.32%. Phenolic components are mainly derived from the pyrolysis of lignin, which is composed of three typical phenolic monomers [26]. Besides the above compounds, formic acid, acetic acid, aldehydes, and ketones are also largely produced during pinewood pyrolysis [27]. However, due to the small molecular weight of these products, they were covered by solvent peaks in GC/MS detection and were not analyzed. Based on the above analysis, the phenol and furfural were used as the model tar compounds during the Aspen Plus modeling.
3.2 The gasification products analysis
Next, the gasification experiments of pinewood pellet were conducted in the pilot reactor. The equivalence ratio (ER) of air was chosen as the adjusting parameter, which varied from 0.15 to 0.4. The syngas gas yield, composition, and tar yield in the outlet of the reactor were measured. Based on the results, the carbon conversion ratio was calculated. The results are summarized in Table 5 as follows.
As shown in Table 5, it can be seen that with the increasing of ER (0.15–0.4), the concentration of CH4 decreased from 7.23 to 1.62%vol, which is attributed to the thermal decomposition and oxidation reactions. CO and H2 are two primary species of syngas. The yields of CO and H2 first increased and then decreased with the increasing of ER. At ER = 0.3, the concentration of H2 reached the maximum of 18.62%. The result is consistent with the conclusion of M. Formica [28]. The maximum concentration of CO was 16.2%vol at ER = 0.25. Too much air would consume CO and H2, which benefited the yield of CO2. Cirillo et al. [29] also arrived at similar conclusion. The syngas yield increased with ER value. At low ER of 0.15, the syngas yield was 1.22 Nm3/kg and increased to 2.26 Nm3/kg at ER of 0.4. The carbon conversion ratio also increased with ER value. The highest carbon conversion ratio of 91.7% is reached when ER = 0.4. On the contrary, the gasification tar yield decreased rapidly with the increase of ER. The partial oxidation enhanced the tar decomposition in gasifier [30, 31]. The conversion characteristics of tar in the gasification process are similar to that of methane. With the increase of oxygen, its oxidative decomposition degree was strengthened; therefore, its concentration decreased. The tar samples are shown in Fig. 4 as follows.
The gasification char at the outlet of the gasifier was collected and analyzed. The elemental analysis, proximate analysis, and pore structure were analyzed. The detailed information of bio-char sample derived at ER = 0.3 is summarized in Table 6 as follows.
It can be seen that compared with raw pinewood material, bio-char produced in gasification is a kind of highly carbonized material. The carbon content is 82.5% wt, while the hydrogen and oxygen content decrease to 15.8% and 1.2% respectively. Almost all volatiles have been released during pyrolysis and gasification, with only 2.4%wt remaining. Most of ash is enriched in bio-char, and its content is up to 13.6%wt. During gasification, the bio-char reacted with gasification agents (H2O and CO2) and the pore structure was enlarged. The BET-specific surface area is about 215 m2/g. Therefore, this kind of bio-char could be further activated to produce activated carbon with largely BET surface area, which is much more valuable acting as an absorbent material. The appearance and SEM results of different char samples are shown in Fig. 5 as follows.
Maneerung et al. [32] also reported that the BET surface area of bio-char residue derived at the outlet of gasifier was 172.24 m2/g. The bio-char residue could be further activated by steam to produce activated carbon with BET surface area of 776.24 m2/g.
3.3 The performance validation of the Aspen Plus gasification model
Next, the Aspen Plus model was validated by the experiment. The reaction temperature of pyrolysis and partial oxidation stages are set as 500 ℃ and 1000 ℃ respectively. The gasification stage temperature was controlled as 800 ℃ according to experimental data. The ER value was chosen as 0.3. The comparison of experimental and modeling is summarized in Table 7 as follows:
There are overestimate for the yield of H2, CO, and CH4, while the concentration of CO2 was underestimated. The largest relative deviation appears for the methane concentration, which is 58.19%. The reason is that the concentration of methane was much lower than other three species. The relative deviation of methane prediction is large. But compared with the thermodynamic equilibrium model, the prediction error of methane is much smaller [11, 33]. The simulation results show overestimation for the yield of H2, CO, and CH4, while the concentration of CO2 was underestimated. The deviation of CH4 has also been reported in previous literature works. Kuo et al. [34] pointed out that the possible reason for high CH4 concentration in syngas in real gasification conditions may be due to the partial thermal cracking of volatiles undergoing pyrolysis. Vikram et al. [10] speculated that the high rate of steam methane reforming reaction (CH4 + H2O → CO + 3H2) may be attributed to the under prediction of CH4 and the over prediction of H2 and CO.
In this work, the gaseous fractions in syngas were determined by the reactions and kinetic parameters. The overestimation for the yield of H2, CO may be attributed to simplification of pyro-char, in which the water gas reaction (R2) and Boudouard reaction (R3) are enhanced. The overestimation of CH4 may be attributed of the methanation reaction (R5) and the reverse reaction of steam methane reforming (R6).
Due to the complexity of biomass gasification process, there are many reasons for the error, including model simplification, dynamic parameters, and data deviation of experiment itself. Although there is some variance for gasification products, the Aspen Plus model is still provide some useful information for evaluating the gasification process. Therefore, the model was used to evaluate the co-production of syngas and bio-char, which could reflect the conversion law of gasification process to some extent and guide engineering application. Therefore, the Aspen Plus model was used for the simulation in the subsequent experiments.
3.4 The modeling of syngas and bio-char co-production
Next, the co-production of syngas and bio-char by biomass gasification was modeled. The gasification parameters, including gasification temperature, air equivalence ratio, and steam/biomass ratio, were taken into consideration.
Firstly, the influence of gasification temperature on the product distribution was studied. In this case, the air equivalence ratio was fixed as constant value of 0.3 and the steam flow rate was zero. The temperature points were 700℃, 750℃, 800℃, 850℃, and 900℃. The syngas composition, syngas yields, and bio-char yields are calculated and summarized in Fig. 6 as follows.
It can be seen that higher temperatures promote syngas yield while lead to a decline in bio-char yields. With temperature increase from 700 to 900 ℃, the bio-char yield decreases from 9.64 to 6.97%wt, while the syngas yield increases from 2.13 to 2.22 Nm3/kg. With regard to the syngas composition, the concentration of CO and H2 increases, while CO2 and CH4 decrease. Higher temperature benefits the water gas reaction (R2), Boudouard reaction (R3), and Steam methane reforming (R6); therefore, the bio-char yield and methane concentration decrease. More CO and H2 are produced at higher temperature.
Next, the influence of air equivalence ratio on product distribution is modeled. In this case, temperature is held constant at 800 °C and the steam/biomass is zero. The air equivalence ratio is varied at range of 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, and 0.4. The modeling results are shown in Fig. 7.
Air plays a role as gasification agent, which converts biomass into syngas. It can be seen that with the increasing of ER, the syngas yield significantly improves from 1.41 to 2.54 Nm3/kg. Conversely, the bio-char yield dropped from 10.31 to 7.47%wt, which is attributed to the water gas reaction (R2) and Boudouard reaction (R3). With regard to the syngas composition, the methane concentration dramatically decreases from 12.55 to 1.62%vol, which is attributed to the drastic oxidation reaction in partial oxidation stage. With the increasing of ER, the concentration of CO and H2 decreases slightly. The results are inconsistent with the experimental results. The reason is that in the model, the gasification temperature is fixed by varying the ER value [17]. However, in gasification experiments, the char bed temperature in gasification stage cannot be constant when changing ER value. In general, the higher the ER is, the more heat is released and the higher the temperature will be achieved. Therefore, there is a maximum point for CO and H2 concentration during varying ER value in experiment. The change of temperature and equivalent ratio is not considered in this work.
Steam is another gasification agent, which is usually added into gasifier together with air or pure oxygen to produce more hydrogen. In this case, the equivalent ratio is fixed as constant of 0.3 and temperature is kept at 800 ℃. The steam/biomass ratio is varied at range of 0, 0.1, 0.2, 0.3, 0.4, and 0.5. The modeling results are summarized in Fig. 8 as follows.
It can be seen that the addition of steam in the gasification stage largely improves the carbon conversion rate and the bio-char yield decreases from 8.08 to 4.58%wt, which is attributed to the water gas reaction (R2). The syngas yield increases from 2.18 to 2.42 Nm3/kg. With regard to the syngas composition, the hydrogen concentration increases from 21.34 to 26.32%vol, while the CO concentration decreases from 17.38 to 14.07%vol, which is attributed to water–gas shift reaction (R4).
Based on the experiments and modeling results, it is evident that by adjusting the gasification parameters, such as temperature, air equivalent ratio, and steam amount, the product distribution in the gasifier outlet could be effectively controlled. With regard to the tar problems, the pyrolysis is largely reduced by partial oxidation and catalytic cracking. The tar yield is as low as 224 mg/kg (ER = 0.3, S/B = 0), which corresponds to 113 mg/Nm3. After simple purification, syngas can be used to generate electricity in internal combustion engines. The bio-char is another high valuable product. The yield of bio-char is largely influenced by ER and steam amount. The BET-specific surface area of bio-char (ER = 0.3, S/B = 0) was about 215 m2/g, which could be used as the raw material for activated carbon production.
Next, the energy balance in the downdraft gasification was evaluated based on the Aspen Plus modeling. The reaction temperature of pyrolysis and partial oxidation stages are set as 500 ℃ and 1000 ℃, respectively. The gasification stage temperature was controlled as 800 ℃ according to experimental data. The ER value was chosen as 0.3. The feeding rate of pinewood pellet is controlled as 5 kg/h. The energy balance in different stages (pyrolysis stage, partial oxidation stage, and reduction stage) was evaluated. The heat flow and connection of each stage are summarized in Fig. 9 as follows:
As shown in Fig. 9, it can be seen that the pyrolysis stage is an endothermic process, which adsorbs heat of 2.47 kW (Q1) from the partial oxidation stage. Partial oxidation stage is the heat source of the gasifier. In partial oxidation zone, the combustion of volatiles released large amount of heat and generate hot zone. Q1 is transferred to the pyrolysis stage by means of radiation and heat conduction. The reduction stage is also an endothermic process, which adsorbs heat of 2.44 kW (Q2) from the partial oxidation stage. Besides, the hot flue gas (1000 ℃) generated in the partial oxidation stage also carries large amount of heat to the reduction zone, which acts as the heat source for the reduction process.
Based on the experiment and modeling results, a bio-char and syngas co-production system based on biomass gasification is shown in Fig. 10 as follows.
In this system, the raw biomass feeding is firstly decomposed into volatiles and bio-char in pyrolysis section. The volatiles are further mixed with air or oxygen for partial combustion to act as the heat source of the system. The tar is also largely decomposed in this section. The pyrolysis bio-char is further gasified by flue gas or external steam to produce syngas. By adjusting gasification parameters (ER, temperature, steam amount), the ratio of syngas and bio-char as well as the product properties are adjusted and controlled for different applications. The bio-char could be further activated to produce higher valuable activated carbon. The system is an efficient and comprehensive utilization of biomass resources and has a high application prospect.
4 Conclusion
Co-production of bio-char and syngas by gasification is a promising way for biomass comprehensive utilization. In this work, mainly two co-products from pinewood pellet gasification, namely bio-char and syngas were studied on a downdraft reactor. Based on the experimental results, a detailed kinetic gasification model was built by Aspen Plus. Pyrolysis results show that bio-char yield during pyrolysis was about 22.8%wt and the initial pore structure was formed with a BET surface area of 36.8 m2/g. The main pyrolysis tar compounds detected by GC/MS were furfural and phenols, which were used as model tar compounds. The gasification results show that H2 concentration reached the maximum of 18.62% at ER = 0.3. The maximum concentration of CO was 16.2% at ER = 0.25. The syngas yield increased with ER value. At low ER of 0.15, the syngas yield was 1.22 Nm3/kg and increased to 2.26 Nm3/kg at ER of 0.4. The carbon conversion ratio also increased with ER value. The highest carbon conversion ratio of 91.7% was reached when ER was 0.4. The bio-char at gasifier outlet was a kind of highly carbonized material and the carbon content was 82.5%wt. During gasification, pore structure of bio-char was enlarged and the BET-specific surface area was about 215 m2/g. The tar was largely removed in the gasifier and the yield was 224 mg/kg biomass at ER = 0.3. The model was validated by the experiment (ER = 0.3). The influence of temperature, ER, and steam amount was studied. Modeling results show that by adjusting the gasification parameters, such as temperature, air equivalent ratio, and steam amount, the product distribution in the gasifier outlet could be effectively controlled. In the end, mass and energy balance evaluation for the downdraft gasification system indicates that the pyrolysis stage and reduction stage are endothermic processes, which adsorb heat of 2.47 kW (Q1) and 2.44 kW (Q2), respectively from the partial oxidation stage. Partial oxidation stage acts as the heat source of the gasifier.
Data availability
All statistics generated and analyzed during our study are included in this article.
References
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86
Rauch R, Hrbek J, Hofbauer H (2014) Biomass gasification for synthesis gas production and applications of the syngas. Wiley Interdisciplinary Reviews: Energy and Environment 3(4):343–362
Kozlov A, Marchenko O, Solomin S (2019) The modern state of wood biomass gasification technologies and their economic efficiency. Energy Procedia 158:1004–1008
Rios MLV, González AM, Lora EES, del Olmo OAA (2018) Reduction of tar generated during biomass gasification: a review. Biomass Bioenerg 108:345–370
Zhao S, Chen L (2020) Utilization of biomass waste for activated carbon production by steam gasification in a rotary reactor: experimental and theoretical approach. Biomass Conversion and Biorefinery 1–11
Shang Z, Yang Z, Ma Y (2021) Theoretical study of activated carbon production via a two-step carbonization-activation process based on Aspen Plus calculation. Biomass Conversion and Biorefinery 1–10.
Liu L, Qian H, Mu L, Wu J, Feng X, Lu X, Zhu J (2021) Techno-economic analysis of biomass processing with dual outputs of energy and activated carbon. Bioresource Technol 319:124108
Kwon G, Cho D-W, Jang H, Lam SS, Song H (2022) Synergistic effects of blending seafood wastes as co-pyrolysis feedstock on syngas production and biochar properties. Chem Eng J 429:132487
He X, Wang C-H, Shoemaker CA (2021) Multi-objective optimization of an integrated biomass waste fixed-bed gasification system for power and biochar co-production. Comput Chem Eng 154:107457
Vikram S, Rosha P, Kumar S, Mahajani S (2022) Thermodynamic analysis and parametric optimization of steam-CO2 based biomass gasification system using Aspen Plus. Energy 241:122854
Pala LPR, Wang Q, Kolb G, Hessel V (2017) Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: an Aspen Plus model. Renewable Energy 101:484–492
Lee SY, Alam T, Kim J-H, Lee J-C, Park S-W (2021) Qualitative analysis of tar based on tar sampling conditions for empty fruit bunch gasification. Biomass Conversion and Biorefinery 1–10
Zhao S, Bi X, Pan X, Su Y, Wu W (2021) The optimization of in-situ tar reduction and syngas production on a 60-kW three-staged biomass gasification system: theoretical and practical approach. Biomass Conversion and Biorefinery 11(5):1835–1846
Al-Yaari M, Saleh TA, Saber O (2021) Removal of mercury from polluted water by a novel composite of polymer carbon nanofiber: kinetic, isotherm, and thermodynamic studies. RSC Adv 11(1):380–389
Al Amoodi N, Kannan P, Al Shoaibi A, Srinivasakannan C (2013) Aspen Plus simulation of polyethylene gasification under equilibrium conditions. Chem Eng Commun 200(7):977–992
Gerun L, Paraschiv M, Vijeu R, Bellettre J, Tazerout M, Gøbel B, Henriksen U (2008) Numerical investigation of the partial oxidation in a two-stage downdraft gasifier. Fuel 87(7):1383–1393
Gai C, Dong Y (2012) Experimental study on non-woody biomass gasification in a downdraft gasifier. Int J Hydrogen Energy 37(6):4935–4944
Abdelouahed L, Authier O, Mauviel G, Corriou J-P, Verdier G, Dufour A (2012) Detailed modeling of biomass gasification in dual fluidized bed reactors under Aspen Plus. Energy Fuels 26(6):3840–3855
Kaushal P, Tyagi R (2017) Advanced simulation of biomass gasification in a fluidized bed reactor using Aspen Plus. Renewable Energy 101:629–636
Acar M, Böke E (2018) Simulation of biomass gasification process using Aspen Plus
Mendiburu AZ, Carvalho JA Jr, Coronado C (2014) Thermochemical equilibrium modeling of biomass downdraft gasifier: stoichiometric models. Energy 66(mar.1):189–201
Cao Y, Wang Q, Du J, Chen J (2019) Oxygen-enriched air gasification of biomass materials for high-quality syngas production. Energy Convers Manag 199:111628
Fang S, Shi C, Jiang L, Li P, Bai J, Chang C (2020) Influence of metal (Fe/Zn) modified ZSM-5 catalysts on product characteristics based on the bench-scale pyrolysis and Py-GC/MS of biomass. Int J Energy Res 44(7):5455–5467
Hsiau S-S, Chen Y-S, Yang S (2022) Numerical modeling of biomass fast pyrolysis by using an improved comprehensive reaction scheme for energy analysis. Renew Energy 181:355–364
Greenhalf C, Nowakowski D, Harms A, Titiloye J, Bridgwater A (2013) A comparative study of straw, perennial grasses and hardwoods in terms of fast pyrolysis products. Fuel 108:216–230
Hatakeyama H, Hatakeyama T (2009) Lignin structure, properties, and applications. Biopolymers 1–63
Kan T, Strezov V, Evans TJ (2016) Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew Sustain Energy Rev 57:1126–1140
Formica M, Frigo S, Gabbrielli R (2016) Development of a new steady state zero-dimensional simulation model for woody biomass gasification in a full scale plant. Energy Convers Manage 120:358–369
Cirillo D, Di Palma M, La Villetta M, Macaluso A, Mauro A, Vanoli L (2021) A novel biomass gasification micro-cogeneration plant: experimental and numerical analysis. Energy Convers Manag 243:114349
Ahrenfeldt J, Egsgaard H, Stelte W, Thomsen T, Henriksen UB (2013) The influence of partial oxidation mechanisms on tar destruction in TwoStage biomass gasification. Fuel 112:662–680
Su Y, Luo Y, Chen Y, Wu W, Zhang Y (2011) Experimental and numerical investigation of tar destruction under partial oxidation environment. Fuel Process Technol 92(8):1513–1524
Maneerung T, Liew J, Dai Y, Kawi S, Chong C, Wang C-H (2016) Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: kinetics, isotherms and thermodynamic studies. Biores Technol 200:350–359
Nikoo MB, Mahinpey N (2008) Simulation of biomass gasification in fluidized bed reactor using Aspen Plus. Biomass Bioenerg 32(12):1245–1254
Kuo P-C, Wu W, Chen W-H (2014) Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis. Fuel 117:1231–1241
Author information
Authors and Affiliations
Contributions
Yanping Zhou is responsible for all matters of the paper.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y. Experimental and Aspen Plus modeling research on bio-char and syngas co-production by gasification of biomass waste: the products and reaction energy balance evaluation. Biomass Conv. Bioref. 14, 5387–5398 (2024). https://doi.org/10.1007/s13399-023-04085-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04085-0