Abstract
Astrocytes regulate local cerebral blood flow, maintain ion and neurotransmitter homeostasis, provide metabolic support, regulate synaptic activity, and respond to brain injury, insults, and infection. Because of their abundance, extensive connectivity, and multiple roles in the brain, astrocytes are intimately involved in normal functioning of the CNS and their dysregulation can lead to neuronal dysfunction. In normal aging, decreased biological functioning and reduced cognitive abilities are commonly experienced in individuals free of overt neurological disease. Moreover, in several age-related CNS diseases, chronic inflammation and altered metabolism have been reported. Since people with HIV (PWH) are reported to experience rapid aging with chronic inflammation, altered brain metabolism is likely to be exacerbated. In fact, many studies report altered metabolism in astrocytes in diseases such as Alzheimer’s, Parkinson’s, and HIV. This review will address the roles of astrocyte activation and altered metabolism in normal aging, in age-related CNS disease, and in HIV-associated neurocognitive disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 35 years, advancements in knowledge and treatment have drastically improved the prognosis of individuals infected with HIV. The diagnosis of HIV has shifted from a life-threatening infection to a manageable chronic illness. The advent of combination antiretroviral therapy (CART) is responsible for the effective suppression of viral replication and prolongs the lives of people with HIV (PWH) to near normal life expectancy (van Sighem et al. 2010). PWH are now becoming an aging population and a new set of HIV-related complications have emerged. Several groups have proposed that HIV infection can lead to accelerated aging (Cassol et al. 2014; Cohen et al. 2015; Cole et al. 2017; Levine et al. 2016; Pfefferbaum et al. 2014). In 2015, the Center for Disease Control and Prevention (CDC) estimated that in the US, 47% of PWH are over the age of 50 and this number continues to climb due to the success of CART (Centers for Disease Control and Prevention. HIV Surveillance Report, 2016). It is projected that by 2020, PWH over 50 years of age will increase to 70% (U.S. Special Committee on Aging, 2013). A large portion of PWH experience cognitive decline prematurely when compared to age-matched HIV negative controls and often more closely resemble the prolife of older adults. Even individuals with undetectable viral loads show decline in memory, attention, psychomotor ability, and executive function. Collectively, these deficits are referred to as HIV-associated neurocognitive disorders (HAND). HAND describes a range of neurological deficits associated with HIV infection with increasing severity, from asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), to HIV-associated dementia (HAD). HAND impacts survival, quality of life, and everyday functioning in PWH and is therefore a major public health concern. Approximately 52% of PWH experience HAND (Heaton et al. 2010).
In normal aging, decreased normal biological function and reduced cognitive abilities are commonly experienced in individuals free of overt neurological disease (Dumas 2015). Healthy older adults show impairments in attention, working memory, and episodic memory relative to younger adults (Verhaeghen and Cerella 2002). Further, advancing age is a major risk factor for the development of neurological disease, with a sharp increase in incidence after 60 years of age (Fjell et al. 2014). Reductions in brain volume, loss of myelin, and region-specific loss of synaptic density are just a few of the many changes observed in aged individuals (Lindenberger 2014; Raz and Rodrigue 2006). Living longer creates opportunities for the population at risk for developing HAND to undergo overlapping pathological changes in biological pathways associated with normal aging. Deviations from the typical trajectory of brain aging are reported in PWH. In fact, it has been reported that PWH on CART have a higher burden of cognitive impairments with advancing age and these disorders present at a younger age than are observed in HIV negative control groups (Mateen et al. 2012). One study of 3945 male participants (2083 HIV negative and 1862 HIV positive) found the median age of first neurologic diagnosis in HIV-positive men receiving antiretroviral therapy was 48 years old compared to 57 years old in the uninfected men (Mateen et al. 2012). Normal brain aging is not associated with significant neuronal loss. Similarly, HAND is often not associated with a drastic loss of neurons leading to the notion that neuronal dysfunction without cell death is likely a main source of cognitive impairments. Even though substantial neuronal death is observed in most other neurodegenerative diseases, there are many commonalities between HAND and other neurodegenerative diseases that have been revealed by studies examining individual disease etiologies. At the brain transcriptome level, there are significant similarities between HAND, multiple sclerosis (MS), and Alzheimer’s disease (AD) (Borjabad and Volsky 2012). One analysis revealed that commonly downregulated genes included those linked to neuronal and synaptic function. This is not surprising since loss of neuronal integrity drives neurodegenerative diseases including HAND. A proteomic analysis of post-mortem frontal cortex from HIV-positive patients with HAD revealed that abnormal expression of proteins involved in glycolysis and oxidative respiration overlap with changes in other neurodegenerative diseases including AD and Parkinson’s disease (PD) (Zhou et al. 2010). Disturbances in energy homeostasis are often observed in various diseases including those of the central nervous system (CNS). Evidence suggests that this may be shared pathologies between HAND and other CNS diseases. Similarities in microglia- and astrocyte-mediated inflammation in the context of AD, MS, and HAND disease progression highlight another avenue in which these disorders converge (Minagar et al. 2002). Given the role of astrocytes in both metabolic support and inflammation, they likely play a critical role in cognitive decline.
A role of astrocytes in health, aging and disease
Astrocytes are stellate cells with elongated processes that are in contact with neurons, synapses, myelin sheath, oligodendrocytes, microglia and blood vessels (Ferrer 2017). Astrocytes constitute a major cellular compartment in the CNS and carry out various functions to maintain normal brain function. It is estimated that a single astrocyte makes contact with between 250,000 and 2 million synapses in the human brain (Oberheim et al. 2006). The heterogeneity of astrocytes allows for vast functional diversity so that their roles in the brain range from regulation of local cerebral blood flow, maintenance of ion and neurotransmitter homeostasis, metabolic support, regulation of synaptic activity, to responses to brain injury, insults and infection. Because of their abundance, extensive connectivity, and multiple roles in the brain, astrocytes are intimately involved in normal functioning of the CNS and their dysregulation can lead to neuronal dysfunction.
Classically, astrocytes are categorized into two major subpopulations: fibrous astrocytes which have long thin processes and are star-like in appearance and protoplasmic astrocytes which have highly complex branching processes that contact synapses and blood vessels (Miller and Raff 1984). Fibrous astrocytes are typically found in the white matter whereas protoplasmic astrocytes are found in the gray matter (Miller and Raff 1984). However, recent morphological and biochemical evidence has proven this classification to be oversimplified. Using a transgenic hGFAP-GFP mouse line, one group identified nine distinct astrocyte classes based on three complementary methods for astrocyte labeling (hGFAP-GFP, GFAP, and s100β) (Emsley and Macklis 2006). From this, the authors concluded that there are considerable region-specific differences in morphology, density, and proliferation rates in astrocytes (Emsley and Macklis 2006). Astrocytes can differ not only in morphology but also in developmental origin, gene expression prolife, physiological properties, and function (Zhang and Barres 2010). Translational profiling approaches and microarray analyses have revealed gene expression can vary substantially between astrocytes from different brain regions (Doyle et al. 2008; Yeh et al. 2009). Even GFAP, which is often considered to be a pan-astrocyte marker, is differentially expressed in various subpopulations of astrocytes (Cahoy et al. 2008). Diversity in gene expression suggests there is likely functional diversity among astrocytes as well. This was highlighted by one group which utilized a fluorescence-activated cell sorting-based approach in combination with cell surface markers to identify five distinct astrocyte subpopulations (John Lin et al. 2017). These subpopulations were further characterized based on their molecular signatures. This analysis revealed one of the astrocyte subpopulations was enriched for genes associated with synapse formation. When directly tested using a co-culture system, compared to the other subpopulations, this specific population of astrocytes had the ability to promote synaptogenesis. Overall, this indicates differences in gene expression profiles can contribute to functional differences in astrocytes. Given the extensive diversity of astrocytes, it is not surprising that the responses of astrocytes in pathological conditions are also very heterogenous. In some conditions, astrocytes become hypertrophic, proliferate, and can release various cytokines (Sofroniew and Vinters 2010). However, under other pathological circumstances, astrocytes can degenerate and lose their normal physiological functions (Scuderi et al. 2013). Recently, the roles of astrocytes in normal brain aging and neurological disease have gained much attention. A delicate balance of downstream signaling is critical to maintain normal brain functioning. In neurodegenerative diseases, deviations in astrocyte homeostasis can manifest differently. In fact, astrogliosis or astrocyte atrophy can contribute to the disease process. Astrocyte atrophy is observed in frontotemporal dementias such as Pick’s disease and frontotemporal lobar degeneration (Broe et al. 2004). In both cases, early and prominent apoptotic death and atrophy of astrocytes is reported, and the degree of astrocyte loss is correlated with disease severity (Broe et al. 2004). However, astrogliosis is more commonly reported in neurological disease and seems to have a leading pathological role in AD, PD, HD, and ALS (Rodríguez et al. 2009).The concept of astrogliosis as a purely pathological response with negative outcomes has been replaced by the concept that astrocyte reactivity is a defense process intended for neuroprotection (Rodríguez-Arellano et al. 2016). Activated astrocytes increase their trophic support for neurons under stress and isolate areas of damage by the formation of a glial scar. However, under certain circumstances, astrocyte activation can become pathological and is referred to as astrocytopathy (Ferrer 2017). Astrocytopathy can manifest as impaired glutamate transport, abnormal glucose and lipid metabolism, and altered calcium signaling (Ferrer 2017). Loss of astrocytic neurotrophic function and activation therefore contributes to brain aging and neurodegenerative disease.
Astrocyte activation in the normally aging brain and age-related CNS diseases
Astrocyte activation is marked by hypertrophic morphology, increased expression of glial acid fibrillary protein (GFAP), secretion of proinflammatory cytokines such as TNF-a and IL-1b, and generation of reactive oxygen species (ROS) (Borjabad et al. 2010). There are signs of age-related increases of astrocyte activation (Lynch et al. 2010; Porchet et al. 2003; Soreq et al. 2017). Myo-inositol, a marker of astrocyte activation, is increased with normal brain aging in humans and in animal models (Harris et al. 2015; Harris et al. 2014; Zhang et al. 2013) supporting aging-related increased astrogliosis. Increased GFAP expression and cell body enlargement is also used as a common indicator of astrocyte activation and like myo-inositol, progressively increases during aging in humans and in animal models of normal aging (Morgan et al. 1999; Nichols et al. 1993; Rodríguez et al. 2014; Weber et al. 2015; Yoshida et al. 1996). Interestingly, this is not the result of greater numbers of astrocytes, instead it is associated with greater cell volume (Middeldorp and Hol 2011). Studies reveal that when neurons are co-cultured with activated astrocytes, there is a significant reduction of synaptic contacts compared to less activated astrocyte co-cultures (Emirandetti et al. 2006). Activated astrocytes increase oxidative stress, upregulate production of proinflammatory cytokines, and facilitate chronic inflammation, all of which contribute to decreased neuronal fitness. In many CNS diseases, increased GFAP expression is a hallmark of neuropathology, establishing a link between astrocyte activation, disease progression, and cognitive deficits.
When compared to younger mice, in the aged mouse brain, a high percentage of astrocytes take on a partially reactive phenotype made apparent by upregulation of potentially harmful reactive genes associated with complement activation, cytokine response, and antigen presentation pathways in brain regions especially vulnerable to neurodegeneration, such as the hippocampus and striatum (Boisvert et al. 2018; Clarke et al. 2018). Reactive astrocytes lose their ability to carry out their normal functions that include the ability to promote neuronal survival, outgrowth, and synaptogenesis (Liddelow et al. 2017). The term inflammaging describes the progressive changes during aging characterized by low-grade chronic upregulation of proinflammatory responses (Franceschi et al. 2007). This process occurs in various organs including the brain, where normal aging is characterized by a variety of neurobiological changes consistent with low-grade chronic neuroinflammation (Lana et al. 2016). Several reports highlight increased levels of proinflammatory cytokines in the hippocampus and cortical areas of the aged brain (Maher et al. 2004; Nolan et al. 2005; Terao et al. 2002). In a microarray study, 35% of the total mRNA that was upregulated in the aged mouse brain were inflammatory-related proteins (Lee et al. 2000). Astrocytes from aged rat brains show increased basal expression of various cytokines including IL-1, IL-6, and TNF-α in the cortex and striatum (Campuzano et al. 2009). Undoubtedly, chronic exposure to astrocyte-derived proinflammatory cytokines can impair neuronal functioning. Adding insult to injury, the aged brain is primed to generate an exacerbated inflammatory response to pathogenic stimuli such as lipopolysaccharide (LPS) and possibly other factors such as low levels of HIV proteins produced in reservoirs such as the CNS (Clarke et al. 2018). This may be of great significance in the context of the aging HIV brain.
AD and PD are age-related neurodegenerative diseases and are often accompanied by astrocyte activation. AD is characterized by progressive memory loss and cognitive decline as a result of widespread neuronal and synaptic loss in the hippocampus, entorhinal area, and cerebral cortex. Pathological features associated with AD are deposits of beta-amyloid into plaques, neurofibrillary hyperphosphorylated tau tangles, and substantial neuronal death (De Strooper and Karran 2016). Several groups have reported that the activity of monoamine oxidase B (MaoB), a mitochondrial membrane protein overexpressed by activated astrocytes, is increased in AD patients indicating ongoing astrogliosis (Gulyás et al. 2011; Saura et al. 1994). Interestingly, increased MaoB activity occurs early during the progression of AD suggesting that astrogliosis contributes in the beginning stages of disease (Carter et al. 2012). Post-mortem AD brain tissue shows signs of astrogliosis (Ingelsson et al. 2004). In AD patients, astrogliosis increases linearly as the disease progresses and is negatively correlated with cortical thickness (Serrano-Pozo et al. 2011). GFAP+ astrocytes cluster around amyloid plaques (Nagele et al. 2004) and can be found in close proximity to neurofibrillary tangle thickness (Serrano-Pozo et al. 2011). Many of the rodent models for AD also exhibit substantial astrocyte activation (Rodríguez et al. 2009). Treatment of astrocytes in vitro with aggregated b-amyloid or with amyloid plaques isolated from human AD brains induced activation made evident by morphological changes and increased GFAP expression (Garwood et al. 2011; Hoppe et al. 2013; Hou et al. 2011). Astrocyte activation is reported to contribute to plaque formation by accumulating large amounts of amyloid-beta (Nagele et al. 2003). Furthermore, amyloid-beta-induced activation of astrocytes, increased proinflammatory cytokine release, and accelerated neurotoxicity were observed in mixed neuronal-astrocyte cultures (Garwood et al. 2011; Jana and Pahan 2010). Neurofibrillary tangle burden in the AD brain is positively correlated with astrocytosis and disease severity (Serrano-Pozo et al. 2011).
Astrocyte activation in the HIV brain
The consensus view is that HIV infection of astrocytes is very limited and does not result in production of mature virions. Thus, HIV neuropathogenesis is mediated largely through the release of virions, neurotoxic viral proteins, and proinflammatory products produced by macrophages and microglia. However, astrocytes are recognized as contributors to HIV neuropathogenesis through their response to these factors. Signs of astrocyte activation, or astrogliosis, are often observed in brain imaging studies of PWH and in post-mortem brain tissues despite the use of antiretroviral therapy (Desplats et al. 2013; Edén et al. 2007; Sabri et al. 2003; Vitkovic and da Cunha 1995; Yang et al. 2016; Young et al. 2014). In fact, even in the absence of detectable viral production, astrocytes show considerable signs of activation in PWH (Edén et al. 2007; Harezlak et al. 2011; Young et al. 2014). Astrocyte activation is detected early in the course of HIV infection of the CNS (Masliah et al. 1996). Similarly, in animal models of HIV infection, astrogliosis is common (Kim et al. 2003; Toggas et al. 1994; Tyor et al. 1993).
Although astrocytes are not capable of productive viral infection, they have been shown to contain and release HIV proteins including Tat, Rev, and Nef (Atwood et al. 1993; Brack-Werner 1999; Ensoli et al. 1993; Ranki et al. 1995). In vitro studies demonstrate that astrocytes become activated when exposed to viral particles or proteins. Tat and gp120 activate astrocytes and stimulate the production of proinflammatory cytokines, chemokines, and nitric oxide, all of which can cause synaptic damage (Ru and Tang 2017). Tat expression increases GFAP expression in astrocytes (Zhou et al. 2004). Subsequently, cell culture supernatants from Tat-expressing astrocytes induce significant neuron death (Zhou et al. 2004). Markers of inflammation continue to be detected in the cerebrospinal fluid (CSF) and brains of PWH even with long-term stable treatment of antiretroviral therapy (Edén et al. 2007; Harezlak et al. 2011). Although HIV-infected astrocytes make up a small fraction of the infected cells of the CNS, they are still a critical population to investigate. HIV-infected astrocytes show higher production of inflammatory mediators in response to LPS suggesting an increased sensitivity to inflammatory triggers (Serramía et al. 2015). Astrocytes are key components of the blood brain barrier (BBB) where they come into direct contact with HIV-infected cells that enter the CNS. Direct infection or exposure to viral proteins at this site can disrupt astrocyte ability to structurally support BBB integrity leading to increased permeability, thereby allowing entrance of additional toxic factors to enter the brain and propagate inflammation and induce greater astrocyte activation. HIV infection or exposure to HIV-related products can significantly alter astrocyte physiology and therefore modulate the essential interactions between astrocytes and neighboring neurons resulting in the deficits associated with HAND.
Altered metabolism in the aging brain and age-related CNS diseases
Brain aging is accompanied by a hypometabolic state that involves decreased glucose uptake (Jiang and Cadenas 2014; Kalpouzos et al. 2009). Under normal conditions, astrocytes rely on anaerobic respiration to generate ATP and lactate. The release of lactate from astrocytes and subsequent uptake by neighboring neurons, known as the astrocyte-neuron lactate shuttle (ANLS), is essential to meet the high-energy requirements of neurons, since they rely on aerobic respiration. Many genes related to mitochondrial bioenergetics and function are upregulated in the brains of aging individuals with mild cognitive impairments relative to age-matched controls (Berchtold et al. 2014). This is in stark contrast to the extensive downregulation of mitochondrial genes in the AD brain (Berchtold et al. 2014), suggesting an intermediate increase in mitochondrial function energy may be an early contributor to a neurodegenerative state and precede hypometabolic conditions. However, due to dynamic glial/neuronal energetic profiles, it can be difficult to tease apart which cell population is driving these changes. Recently, a magnetic resonance spectroscopy study reported decreased neuronal mitochondrial metabolism and increased glial mitochondrial metabolism in healthy adults 76 ± 8 years of age when compared to young volunteers 26 ± 7 years of age (Boumezbeur et al. 2010), indicating that astrocytes are likely responsible for the increased energetics in the aging brain. Astrocyte activation requires enhanced protein synthesis and trafficking thereby increasing cellular energy demands. Upregulation of mitochondrial function may be an energy efficient way for astrocytes to meet their energy demands when under stress. With reports of elevated astrocyte activation in aging individuals, it is likely that astrocytic energy profiles are also altered with age. This was validated by one group that demonstrated an age-dependent metabolic shift from anaerobic metabolism towards increased mitochondrial metabolism and mitochondrial biogenesis in primary cortical astrocytes isolated from 7-, 13-, and 18-month-old rats (Jiang and Cadenas 2014). It is likely that increased mitochondrial metabolism is needed to support metabolically expensive inflammatory responses. Interestingly, considering the intimate coupling of astrocyte anaerobic glycolysis with neuronal bioenergetics, a shift in astrocytes away from anaerobic respiration would reduce lactate production and deprive neurons of this key metabolite. Overall, this may help explain, in part, the hypometabolic state reported in the aged brain. Furthermore, upon aging, the activity of pyruvate carboxylase, a key mitochondrial anaplerotic enzyme, in astrocytes increases and decreasing its activity ameliorated age-related memory impairments in Drosophila (Yamazaki et al. 2014). Overall, enhanced astrocytic mitochondrial function appears to have detrimental effects on the aging brain and may represent a key pathology in neurodegenerative disorders.
Although astrocyte mitochondrial metabolism appears to increase with age, glucose uptake is reduced and glucose transporter 1 (GLUT-1) protein levels are decreased in astrocytes isolated from aged rats compared to those obtained from newborn rats (Souza et al. 2015). Although glucose is utilized as the primary energy source in the brain, fatty acids can also be used (Ebert et al. 2003; Panov et al. 2014). Increased utilization of free fatty acids and ketone bodies has been observed as the brain ages (Kadish et al. 2009; Laranjeira et al. 2016). Fatty acid oxidation is believed to occur most exclusively in astrocytes (Ebert et al. 2003). β-oxidation of fatty acids occurs in the mitochondria and results in the generation of acetyl-CoA that can be used for the TCA cycle. Intrinsic properties of astrocytic mitochondria suggest oxidation of fatty acids may be the preferred substrate for acetyl-CoA generation (Halim et al. 2010; Panov et al. 2014). In summary, increased fatty acid utilization observed during aging likely coincides with a metabolic shift that occurs in astrocytes to support enhanced mitochondrial metabolism.
Various aspects of brain energy homeostasis ranging from dysfunction in glucose metabolism to mitochondrial function coincide with AD disease pathology resulting in an overall hypometabolic state within the CNS (Förster et al. 2012; Yao et al. 2011). Positron emission tomography (PET) studies have demonstrated diminished glucose utilization in AD patients (De Santi et al. 2001; Duara et al. 1986; Ibáñez et al. 1998). In fact, a decline in glucose metabolism can appear prior to histopathological and clinical features (Mosconi et al. 2009). Recently, studies have revealed a connection between brain regions with abnormal glucose metabolism and areas vulnerable to AD pathology (Bero et al. 2011; Oh et al. 2016). Many genes associated with the glycolytic pathway and mitochondrial bioenergetics are altered in AD brains (Berchtold et al. 2014; Brooks et al. 2007). Transcriptome analysis of laser-capture micro-dissected neurons from AD brains revealed that genes influencing mitochondrial energy metabolism have reduced expression (Liang et al. 2008). However another study found the activity of key glycolytic enzymes is altered in AD patients, where glucose 6-phosphate dehydrogenase (G6PDH) activity is significantly reduced in the hippocampus yet phosphofructokinase (PFK), lactate dehydrogenase (LDH), and pyruvate kinase (PK) are increased in the frontal and temporal cortices (Bigl et al. 1999). Increased activity of PFK, LDH, and PK was positively correlated with GFAP levels and colocalized with GFAP+ astrocytes suggesting neuronal metabolism may be reduced, whereas astrocyte metabolism is enhanced (Bigl et al. 1999; Liang et al. 2008). Animal models of AD further delineate metabolic dysregulation within the brain. For example, in transgenic McGill-R-Thy1-APP rats, reduced TCA cycle turnover was detected in hippocampal and cortical neurons as well as cortical astrocytes suggesting mitochondrial metabolism is perturbed in both cell populations (Nilsen et al. 2014).
Astrocyte involvement in AD has recently gained significant attention (Cai et al. 2017). In vitro, amyloid-beta has been shown to disrupt normal astrocyte metabolic function in multiple ways. For example, in mouse hippocampal astrocytes, glucose uptake is impaired upon treatment with amyloid-beta peptides (Abeti et al. 2011). Impaired glycolysis in human fetal astrocytes promotes increased amyloid aggregation and internalization (Fu et al. 2015), which would therefore contribute to the formation of amyloid deposits. Furthermore, amyloid-beta was found to trigger calcium (Ca2+) release from the endoplasmic reticulum leading to transient elevations in intracellular (Ca2+) (Alberdi et al. 2013) and induce loss in mitochondrial potential (Abramov et al. 2004). This subsequently results in reduced mitochondrial oxidative consumption and metabolic failure in astrocytes (Abeti et al. 2011). In line with this, amyloid-beta treatment of U87 glioblastoma cells diminished mitochondrial membrane potential and reduced ATP generation (Yao et al. 2018). Taken together, changes in brain energy homeostasis likely stem from astrocyte dysfunction and loss of their innate ability to support neuronal energy demands ultimately causing neuronal dysfunction and loss. It is possible that this phenomenon may also occur during HIV infection.
Altered metabolism in the HIV brain
In PET studies of PWH on CART, varying levels of reduced glucose uptake have been reported in several brain regions (Andersen et al. 2010; Towgood et al. 2013). Additionally, CSF metabolomics in PWH on CART reveal abnormal changes in brain bioenergetics associated with HIV infection (Cassol et al. 2014; Dickens et al. 2015). For example, PWH with neurocognitive impairments displayed alterations in the Krebs cycle, mitochondrial electron transport chain, and ketone body metabolism, corresponding to mitochondrial dysfunction, and the accumulation of metabolic waste products (Cassol et al. 2014). Interestingly, many of the CSF metabolites altered in PWH under 50 years of age overlapped with those found in advanced age HIV-negative controls over 50 years of age, indicating accelerated aging may occur in PWH (Cassol et al. 2014). Enhanced aerobic glycolysis marked by accumulation of TCA cycle intermediates seems to contribute to declining cognitive status in PWH (Dickens et al. 2015). Increased creatine, which is tightly linked to aerobic glycolysis, along with the accumulation of citrate and acetate, provides added support that mitochondrial dysfunction is augmented in cognitive impairment in PWH (Dickens et al. 2015). Conversely, signs of enhanced anaerobic metabolism and lactate production are associated with improved cognitive status in PWH (Dickens et al. 2015). Proteomic analysis of brain tissue from HAD patients indicates a large percentage of the proteins altered are involved in the glycolytic and oxidative phosphorylation pathways, thereby highlighting the role of bioenergetic pathways in cognitive impairment (Zhou et al. 2010). In HIV-1 transgenic rats, which express seven out of nine HIV proteins, there are significant changes in synaptic mitochondria isolated. These abnormalities included altered expression of electron transport chain (ETC) complex subunits, increases in protein expression of TCA cycle, and fatty acid metabolic processes (Villeneuve et al. 2016). However, some conflicting data have been reported. At the transcript level, brain gene expression analyses reveal that HAND is related to gene pathways involved in mitochondrial functioning being significantly downregulated (Levine et al. 2013). Taken together, this indicates that global brain mitochondrial functioning is perturbed during HIV infection. However, changes in mitochondrial activity caused by HIV may vary across distinct cellular compartments.
It is well established that various HIV proteins cause disruptions in neuronal energy homeostasis (Avdoshina et al. 2016; De Simone et al. 2016; Fitting et al. 2014; Norman et al. 2007; Rozzi et al. 2017; Shah and Kumar 2016; Stevens et al. 2014). A recent report found that gp120 and Tat induce mitochondrial fragmentation and decrease mitochondrial membrane potential in human primary neurons (Teodorof-Diedrich and Spector 2018). Moreover, gp120, Vpr, and Tat all cause mitochondrial depolarization and reduce ATP production in neuronal cultures (Kitayama et al. 2008; Wang et al. 2017). However, the impact of HIV on mitochondrial function in astrocytes is understudied. Exposure of astrocytes to free virus induced mitochondrial permeability transition pore (mPTP) opening and loss of mitochondrial membrane potential (Borgmann and Ghorpade 2018). Similarly, gp120 was found to increase mitochondrial membrane depolarization in astrocytes (Yang et al. 2010). Recently, our group was the first to report that Tat protein induces a metabolic shift in astrocytes from glucose utilization to fatty acid oxidation leading to enhanced mitochondrial respiration, increased ATP levels, and reduced lactate production (Natarajaseenivasan et al. 2018) (Fig. 1). Here, we show that in human fetal astrocytes treated with HIV-1 Tat (1-101aa) over the course of 48 h, there is a significant increase expression of CPT1A (carnitine palmitoyltransferase 1A), a key enzyme for the activation of fatty acid oxidation (Fig. 2). This indicates that enhanced aerobic respiration in astrocytes may contribute to HAND by misappropriating energy substrates essential to neurons and ultimately leading to reduced neuronal mitochondrial metabolism.
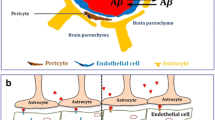
HIV-Tat increases β-oxidation in human primary astrocytes. Astrocytes were exposed to 50 ng/ml recombinant HIV-1 Tat for 48 h and assessed by immunofluorescence for GFAP (red) and carnitine palmitoyltransferase 1 (CPT1) (green), a rate-limiting enzyme for β-oxidation. Reprinted with permission from Natarajanseenivasan et al., Cell Death & Disease, 9: 415, 2018, http://creativecommons.org/licenses/by/4.0/
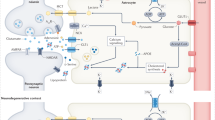
Schematic representation of the proposed metabolic switch in astrocytic metabolism during treatment with recombinant HIV-1 Tat protein. Left panel: Under neurotrophic conditions, astrocytes primarily utilize anaerobic glycolysis resulting in the conversion on pyruvate to lactate by lactate dehydrogenase (LDH) which then is released from the cell via monocarboxylate transporter 4 (MCT4) into the extracellular compartment. Right panel: Under conditions of cell stress such as HIV-1 Tat insult, astrocytes take on a neurotoxic phenotype in which pyruvate is no longer converted to lactate. Instead, pyruvate is converted into acetyl-CoA. Additionally, there is an increase in intracellular calcium into the mitochondria via the mitochondrial calcium uniporter (MCU) and an upregulation of fatty acid oxidation (FAO), providing an additional source of acetyl-CoA, which fuels the TCA cycle and drives mitochondrial metabolism within astrocytes. Overall, this results in increased production of mitochondrial ROS (mROS) and reduced supply of lactate
Conclusion
Chronic low-grade inflammation and metabolic disturbances seem to be a point of convergence for HAND and normal brain aging resulting in cognitive impairments (Fig. 3). Although the causes of inflammation in aging and HAND are quite different, as individuals approach later decades of life, the physiology of cells in the CNS can become augmented. It is plausible that pathways of inflammation and metabolism can influence one another in several ways. Energy homeostasis is likely disrupted during states of inflammation. The maintenance of even low-level inflammation requires energy levels to exceed the normal baseline requirements. In the aging or injured brain, the capacity to meet these energy demands is often compromised as seen by aberrant glucose utilization and mitochondrial dysregulation. The fundamental role of astrocytic input in neuronal metabolic homeostasis and inflammation makes this cell population of great significance to CNS fitness. Astrocytes play roles in most aspects of normal brain functioning, in particular in the uptake of glucose from the periphery and supplying key metabolites to neurons. Loss of these functions, because of age-related physiological changes or insult, have detrimental impacts on the whole brain. In astrocytes, chronic inflammation is observed as a state of activation. The evidence presented in this review from aging populations and PWH suggests that astrocyte activation is increased and is maintained through a metabolic switch from glucose utilization and lactate production towards beta-oxidation of fatty acids and enhanced mitochondrial respiration. This appears to provide astrocytes with additional ATP to support upregulation of a plethora of proinflammatory mediators, increased GFAP, and myo-inositol expression. However, this shift results in the production of oxidative stress and reduced metabolic support to neurons both of which are deleterious to overall brain functioning and cognitive health. Investigating the mechanisms leading to these changes and identifying ways to mitigate these metabolic shifts hold significant therapeutic potential for age-related disorders and PWH. Viral eradication, the ultimate cure for HIV infection, remains elusive. Therefore, efforts to restore the energy balance in the brain through approaches which increase energy substrate bioavailability including but not limited to implementation of a ketogenic diet (Cunnane et al. 2016; Hui et al. 2012; Stafstrom and Rho 2012), intranasal insulin treatment (Mamik et al. 2016), or exercise (Dufour et al. 2013) coupled with use of anti-inflammatory drugs may provide therapeutic benefits to PWH.
References
Abeti R, Abramov AY, Duchen MR (2011) Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain 134:1658–1672
Abramov AY, Canevari L, Duchen MR (2004) Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci 24:565–575
Alberdi E, Wyssenbach A, Alberdi M, Sánchez-Gómez MV, Cavaliere F, Rodríguez JJ, Verkhratsky A, Matute C (2013) Ca(2+)-dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid β-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell 12:292–302
Andersen AB, Law I, Krabbe KS, Bruunsgaard H, Ostrowski SR, Ullum H, Højgaard L, Lebech A, Gerstoft J, Kjaer A (2010) Cerebral FDG-PET scanning abnormalities in optimally treated HIV patients. J Neuroinflammation 7:13
Atwood WJ, Tornatore CS, Meyers K, Major EO (1993) HIV-1 mRNA transcripts from persistently infected human fetal astrocytes. Ann N Y Acad Sci 693:324–325
Avdoshina V, Fields JA, Castellano P, Dedoni S, Palchik G, Trejo M, Adame A, Rockenstein E, Eugenin E, Masliah E, Mocchetti I (2016) The HIV protein gp120 alters mitochondrial dynamics in neurons. Neurotox Res 29:583–593
Berchtold NC, Sabbagh MN, Beach TG, Kim RC, Cribbs DH, Cotman CW (2014) Brain gene expression patterns differentiate mild cognitive impairment from normal aged and Alzheimer’s disease. Neurobiol Aging 35:1961–1972
Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM (2011) Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci 14:750–756
Bigl M, Brückner MK, Arendt T, Bigl V, Eschrich K (1999) Activities of key glycolytic enzymes in the brains of patients with Alzheimer’s disease. J Neural Transm (Vienna) 106:499–511
Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ (2018) The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep 22:269–285
Borgmann K, Ghorpade A (2018) Methamphetamine augments concurrent astrocyte mitochondrial stress, oxidative burden, and antioxidant capacity: tipping the balance in HIV-associated neurodegeneration. Neurotox Res 33:433–447
Borjabad A, Volsky DJ (2012) Common transcriptional signatures in brain tissue from patients with HIV-associated neurocognitive disorders, Alzheimer’s disease, and multiple sclerosis. J NeuroImmune Pharmacol 7:914–926
Borjabad A, Brooks AI, Volsky DJ (2010) Gene expression profiles of HIV-1-infected glia and brain: toward better understanding of the role of astrocytes in HIV-1-associated neurocognitive disorders. J NeuroImmune Pharmacol 5:44–62
Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF (2010) Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab 30:211–221
Brack-Werner R (1999) Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1–22
Broe M, Kril J, Halliday GM (2004) Astrocytic degeneration relates to the severity of disease in frontotemporal dementia. Brain 127:2214–2220
Brooks WM, Lynch PJ, Ingle CC, Hatton A, Emson PC, Faull RL, Starkey MP (2007) Gene expression profiles of metabolic enzyme transcripts in Alzheimer’s disease. Brain Res 1127:127–135
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278
Cai Z, Wan CQ, Liu Z (2017) Astrocyte and Alzheimer’s disease. J Neurol 264:2068–2074
Campuzano O, Castillo-Ruiz MM, Acarin L, Castellano B, Gonzalez B (2009) Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J Neurosci Res 87:2484–2497
Carter SF, Schöll M, Almkvist O, Wall A, Engler H, Långström B, Nordberg A (2012) Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med 53:37–46
Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D (2014) Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS 28:1579–1591
Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A 115:E1896–E1905
Cohen RA, Seider TR, Navia B (2015) HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther 7:37
Cole JH, Underwood J, Caan MW, De Francesco D, van Zoest RA, Leech R, Wit FW, Portegies P, Geurtsen GJ, Schmand BA, Schim van der Loeff MF, Franceschi C, Sabin CA, Majoie CB, Winston A, Reiss P, Sharp DJ, collaboration C (2017) Increased brain-predicted aging in treated HIV disease. Neurology 88:1349–1357
Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, Croteau E, Castellano CA (2016) Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann N Y Acad Sci 1367:12–20
De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, Wang GJ, Schlyer D, Fowler J (2001) Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 22:529–539
De Simone FI, Darbinian N, Amini S, Muniswamy M, White MK, Elrod JW, Datta PK, Langford D, Khalili K (2016) HIV-1 Tat and cocaine impair survival of cultured primary neuronal cells via a mitochondrial pathway. J NeuroImmune Pharmacol 11:358–368
De Strooper B, Karran E (2016) The cellular phase of Alzheimer’s disease. Cell 164:603–615
Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Ellis R, Cherner M, Grant I, Masliah E (2013) Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology 80:1415–1423
Dickens AM, Anthony DC, Deutsch R, Mielke MM, Claridge TD, Grant I, Franklin D, Rosario D, Marcotte T, Letendre S, McArthur JC, Haughey NJ (2015) Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS 29:559–569
Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N (2008) Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135:749–762
Duara R, Grady C, Haxby J, Sundaram M, Cutler NR, Heston L, Moore A, Schlageter N, Larson S, Rapoport SI (1986) Positron emission tomography in Alzheimer’s disease. Neurology 36:879–887
Dufour CA, Marquine MJ, Fazeli PL, Henry BL, Ellis RJ, Grant I, Moore DJ, Group H (2013) Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. J Neuro-Oncol 19:410–417
Dumas JA (2015) What is normal cognitive aging? Evidence from task-based functional neuroimaging. Curr Behav Neurosci Rep 2:256–261
Ebert D, Haller RG, Walton ME (2003) Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 23:5928–5935
Edén A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslén M (2007) Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis 196:1779–1783
Emirandetti A, Graciele Zanon R, Sabha M, de Oliveira AL (2006) Astrocyte reactivity influences the number of presynaptic terminals apposed to spinal motoneurons after axotomy. Brain Res 1095:35–42
Emsley JG, Macklis JD (2006) Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol 2:175–186
Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC (1993) Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol 67:277–287
Ferrer I (2017) Diversity of astroglial responses across human neurodegenerative disorders and brain aging. Brain Pathol 27:645–674
Fitting S, Knapp PE, Zou S, Marks WD, Bowers MS, Akbarali HI, Hauser KF (2014) Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na+ influx, mitochondrial instability, and Ca2+ overload. J Neurosci 34:12850–12864
Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Initiative ADN (2014) What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog Neurobiol 117:20–40
Förster S, Grimmer T, Miederer I, Henriksen G, Yousefi BH, Graner P, Wester HJ, Förstl H, Kurz A, Dickerson BC, Bartenstein P, Drzezga A (2012) Regional expansion of hypometabolism in Alzheimer’s disease follows amyloid deposition with temporal delay. Biol Psychiatry 71:792–797
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128:92–105
Fu W, Shi D, Westaway D, Jhamandas JH (2015) Bioenergetic mechanisms in astrocytes may contribute to amyloid plaque deposition and toxicity. J Biol Chem 290:12504–12513
Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W (2011) Astrocytes are important mediators of Aβ-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis 2:e167
Gulyás B, Pavlova E, Kása P, Gulya K, Bakota L, Várszegi S, Keller E, Horváth MC, Nag S, Hermecz I, Magyar K, Halldin C (2011) Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem Int 58:60–68
Halim ND, Mcfate T, Mohyeldin A, Okagaki P, Korotchkina LG, Patel MS, Jeoung NH, Harris RA, Schell MJ, Verma A (2010) Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia 58:1168–1176
Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B, Consortium HN (2011) Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 25:625–633
Harris JL, Yeh HW, Swerdlow RH, Choi IY, Lee P, Brooks WM (2014) High-field proton magnetic resonance spectroscopy reveals metabolic effects of normal brain aging. Neurobiol Aging 35:1686–1694
Harris JL, Choi IY, Brooks WM (2015) Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front Aging Neurosci 7:202
Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75:2087–2096
Hoppe JB, Rattray M, Tu H, Salbego CG, Cimarosti H (2013) SUMO-1 conjugation blocks beta-amyloid-induced astrocyte reactivity. Neurosci Lett 546:51–56
Hou L, Liu Y, Wang X, Ma H, He J, Zhang Y, Yu C, Guan W, Ma Y (2011) The effects of amyloid-β42 oligomer on the proliferation and activation of astrocytes in vitro. In Vitro Cell Dev Biol Anim 47:573–580
Hui L, Chen X, Bhatt D, Geiger NH, Rosenberger TA, Haughey NJ, Masino SA, Geiger JD (2012) Ketone bodies protection against HIV-1 Tat-induced neurotoxicity. J Neurochem 122:382–391
Ibáñez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, Rapoport SI, Schapiro MB, Horwitz B (1998) Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology 50:1585–1593
Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC (2004) Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 62:925–931
Jana A, Pahan K (2010) Fibrillar amyloid-beta-activated human astroglia kill primary human neurons via neutral sphingomyelinase: implications for Alzheimer’s disease. J Neurosci 30:12676–12689
Jiang T, Cadenas E (2014) Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13:1059–1067
John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, Mohila CA, Ahmed N, Patel AJ, Arenkiel BR, Noebels JL, Creighton CJ, Deneen B (2017) Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20:396–405
Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW (2009) Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci 29:1805–1816
Kalpouzos G, Chételat G, Baron JC, Landeau B, Mevel K, Godeau C, Barré L, Constans JM, Viader F, Eustache F, Desgranges B (2009) Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging 30:112–124
Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ (2003) Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol 162:1693–1707
Kitayama H, Miura Y, Ando Y, Hoshino S, Ishizaka Y, Koyanagi Y (2008) Human immunodeficiency virus type 1 Vpr inhibits axonal outgrowth through induction of mitochondrial dysfunction. J Virol 82:2528–2542
Lana D, Iovino L, Nosi D, Wenk GL, Giovannini MG (2016) The neuron-astrocyte-microglia triad involvement in neuroinflammaging mechanisms in the CA3 hippocampus of memory-impaired aged rats. Exp Gerontol 83:71–88
Laranjeira A, Schulz J, Dotti CG (2016) Genes related to fatty acid β-oxidation play a role in the functional decline of the Drosophila brain with age. PLoS One 11:e0161143
Lee CK, Weindruch R, Prolla TA (2000) Gene-expression profile of the ageing brain in mice. Nat Genet 25:294–297
Levine AJ, Miller JA, Shapshak P, Gelman B, Singer EJ, Hinkin CH, Commins D, Morgello S, Grant I, Horvath S (2013) Systems analysis of human brain gene expression: mechanisms for HIV-associated neurocognitive impairment and common pathways with Alzheimer’s disease. BMC Med Genet 6:4
Levine AJ, Quach A, Moore DJ, Achim CL, Soontornniyomkij V, Masliah E, Singer EJ, Gelman B, Nemanim N, Horvath S (2016) Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neuro-Oncol 22:366–375
Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, Niedzielko TL, Schneider LE, Mastroeni D, Caselli R, Kukull W, Morris JC, Hulette CM, Schmechel D, Rogers J, Stephan DA (2008) Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci U S A 105:4441–4446
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487
Lindenberger U (2014) Human cognitive aging: corriger la fortune? Science 346:572–578
Lynch AM, Murphy KJ, Deighan BF, O'Reilly JA, Gun'ko YK, Cowley TR, Gonzalez-Reyes RE, Lynch MA (2010) The impact of glial activation in the aging brain. Aging Dis 1:262–278
Maher FO, Martin DS, Lynch MA (2004) Increased IL-1beta in cortex of aged rats is accompanied by downregulation of ERK and PI-3 kinase. Neurobiol Aging 25:795–806
Mamik MK, Asahchop EL, Chan WF, Zhu Y, Branton WG, McKenzie BA, Cohen EA, Power C (2016) Insulin treatment prevents neuroinflammation and neuronal injury with restored neurobehavioral function in models of HIV/AIDS neurodegeneration. J Neurosci 36:10683–10695
Masliah E, Ge N, Mucke L (1996) Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol 10:57–67
Mateen FJ, Shinohara RT, Carone M, Miller EN, McArthur JC, Jacobson LP, Sacktor N, Investigators MACSM (2012) Neurologic disorders incidence in HIV+ vs HIV- men: multicenter AIDS cohort study, 1996-2011. Neurology 79:1873–1880
Middeldorp J, Hol EM (2011) GFAP in health and disease. Prog Neurobiol 93:421–443
Miller RH, Raff MC (1984) Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci 4:585–592
Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C (2002) The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 202:13–23
Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE (1999) The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 89:687–699
Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ (2009) FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging 36:811–822
Nagele RG, D'Andrea MR, Lee H, Venkataraman V, Wang HY (2003) Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res 971:197–209
Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC (2004) Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging 25:663–674
Natarajaseenivasan K, Cotto B, Shanmughapriya S, Lombardi AA, Datta PK, Madesh M, Elrod JW, Khalili K, Langford D (2018) Astrocytic metabolic switch is a novel etiology for cocaine and HIV-1 Tat-mediated neurotoxicity. Cell Death Dis 9:415
Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE (1993) GFAP mRNA increases with age in rat and human brain. Neurobiol Aging 14:421–429
Nilsen LH, Witter MP, Sonnewald U (2014) Neuronal and astrocytic metabolism in a transgenic rat model of Alzheimer’s disease. J Cereb Blood Flow Metab 34:906–914
Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, Mills KH, Lynch MA (2005) Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem 280:9354–9362
Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA (2007) HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J Immunol 178:869–876
Oberheim NA, Wang X, Goldman S, Nedergaard M (2006) Astrocytic complexity distinguishes the human brain. Trends Neurosci 29:547–553
Oh H, Madison C, Baker S, Rabinovici G, Jagust W (2016) Dynamic relationships between age, amyloid-β deposition, and glucose metabolism link to the regional vulnerability to Alzheimer’s disease. Brain 139:2275–2289
Panov A, Orynbayeva Z, Vavilin V, Lyakhovich V (2014) Fatty acids in energy metabolism of the central nervous system. Biomed Res Int 2014:472459
Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Zahr NM, Sullivan EV (2014) Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging 35:1755–1768
Porchet R, Probst A, Bouras C, Dráberová E, Dráber P, Riederer BM (2003) Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer’s disease. Proteomics 3:1476–1485
Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K (1995) Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 9:1001–1008
Raz N, Rodrigue KM (2006) Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 30:730–748
Rodríguez JJ, Olabarria M, Chvatal A, Verkhratsky A (2009) Astroglia in dementia and Alzheimer’s disease. Cell Death Differ 16:378–385
Rodríguez JJ, Yeh CY, Terzieva S, Olabarria M, Kulijewicz-Nawrot M, Verkhratsky A (2014) Complex and region-specific changes in astroglial markers in the aging brain. Neurobiol Aging 35:15–23
Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A (2016) Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 323:170–182
Rozzi SJ, Avdoshina V, Fields JA, Trejo M, Ton HT, Ahern GP, Mocchetti I (2017) Human immunodeficiency virus promotes mitochondrial toxicity. Neurotox Res 32:723–733
Ru W, Tang SJ (2017) HIV-associated synaptic degeneration. Mol Brain 10:40
Sabri F, Titanji K, De Milito A, Chiodi F (2003) Astrocyte activation and apoptosis: their roles in the neuropathology of HIV infection. Brain Pathol 13:84–94
Saura J, Luque JM, Cesura AM, Da Prada M, Chan-Palay V, Huber G, Löffler J, Richards JG (1994) Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience 62:15–30
Scuderi C, Stecca C, Iacomino A, Steardo L (2013) Role of astrocytes in major neurological disorders: the evidence and implications. IUBMB Life 65:957–961
Serramía MJ, Muñoz-Fernández M, Álvarez S (2015) HIV-1 increases TLR responses in human primary astrocytes. Sci Rep 5:17887
Serrano-Pozo A, Mielke ML, Gómez-Isla T, Betensky RA, Growdon JH, Frosch MP, Hyman BT (2011) Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol 179:1373–1384
Shah A, Kumar A (2016) HIV-1 gp120-mediated mitochondrial dysfunction and HIV-associated neurological disorders. Neurotox Res 30:135–137
van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F, study Anoc (2010) Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 24:1527–1535
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Soreq L, Rose J, Soreq E, Hardy J, Trabzuni D, Cookson MR, Smith C, Ryten M, Patani R, Ule J, Consortium UBE, Consortium NABE (2017) Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep 18:557–570
Souza DG, Bellaver B, Raupp GS, Souza DO, Quincozes-Santos A (2015) Astrocytes from adult Wistar rats aged in vitro show changes in glial functions. Neurochem Int 90:93–97
Stafstrom CE, Rho JM (2012) The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol 3:59
Stevens PR, Gawryluk JW, Hui L, Chen X, Geiger JD (2014) Creatine protects against mitochondrial dysfunction associated with HIV-1 Tat-induced neuronal injury. Curr HIV Res 12:378–387
Teodorof-Diedrich C, Spector SA (2018) Human immunodeficiency virus type 1 gp120 and Tat induce mitochondrial fragmentation and incomplete mitophagy in human neurons. J Virol 92:e00993-18
Terao A, Apte-Deshpande A, Dousman L, Morairty S, Eynon BP, Kilduff TS, Freund YR (2002) Immune response gene expression increases in the aging murine hippocampus. J Neuroimmunol 132:99–112
Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L (1994) Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367:188–193
Towgood KJ, Pitkanen M, Kulasegaram R, Fradera A, Soni S, Sibtain N, Reed LJ, Bradbeer C, Barker GJ, Dunn JT, Zelaya F, Kopelman MD (2013) Regional cerebral blood flow and FDG uptake in asymptomatic HIV-1 men. Hum Brain Mapp 34:2484–2493
Tyor WR, Power C, Gendelman HE, Markham RB (1993) A model of human immunodeficiency virus encephalitis in scid mice. Proc Natl Acad Sci U S A 90:8658–8662
Verhaeghen P, Cerella J (2002) Aging, executive control, and attention: a review of meta-analyses. Neurosci Biobehav Rev 26:849–857
Villeneuve LM, Purnell PR, Stauch KL, Callen SE, Buch SJ, Fox HS (2016) HIV-1 transgenic rats display mitochondrial abnormalities consistent with abnormal energy generation and distribution. J Neuro-Oncol 22:564–574
Vitkovic L, da Cunha A (1995) Role for astrocytosis in HIV-1-associated dementia. Curr Top Microbiol Immunol 202:105–116
Wang Y, Santerre M, Tempera I, Martin K, Mukerjee R, Sawaya BE (2017) HIV-1 Vpr disrupts mitochondria axonal transport and accelerates neuronal aging. Neuropharmacology 117:364–375
Weber M, Wu T, Hanson JE et al (2015) Cognitive deficits, changes in synaptic function, and brain pathology in a mouse model of normal aging (1,2,3). eNeuro 2(5):ENEURO.0047-15
Yamazaki D, Horiuchi J, Ueno K, Ueno T, Saeki S, Matsuno M, Naganos S, Miyashita T, Hirano Y, Nishikawa H, Taoka M, Yamauchi Y, Isobe T, Honda Y, Kodama T, Masuda T, Saitoe M (2014) Glial dysfunction causes age-related memory impairment in Drosophila. Neuron 84:753–763
Yang Y, Yao H, Lu Y, Wang C, Buch S (2010) Cocaine potentiates astrocyte toxicity mediated by human immunodeficiency virus (HIV-1) protein gp120. PLoS One 5:e13427
Yang L, Yao H, Chen X, Cai Y, Callen S, Buch S (2016) Role of sigma receptor in cocaine-mediated induction of glial fibrillary acidic protein: implications for HAND. Mol Neurobiol 53:1329–1342
Yao J, Rettberg JR, Klosinski LP, Cadenas E, Brinton RD (2011) Shift in brain metabolism in late onset Alzheimer’s disease: implications for biomarkers and therapeutic interventions. Mol Asp Med 32:247–257
Yao Y, Huang JZ, Chen Y, Hu HJ, Tang X, Li X (2018) Effects and mechanism of amyloid β1-42 on mitochondria in astrocytes. Mol Med Rep 17:6997–7004
Yeh TH, Lee DY, Gianino SM, Gutmann DH (2009) Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia 57:1239–1249
Yoshida T, Goldsmith SK, Morgan TE, Stone DJ, Finch CE (1996) Transcription supports age-related increases of GFAP gene expression in the male rat brain. Neurosci Lett 215:107–110
Young AC, Yiannoutsos CT, Hegde M, Lee E, Peterson J, Walter R, Price RW, Meyerhoff DJ, Spudich S (2014) Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology 83:1592–1600
Zhang Y, Barres BA (2010) Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20:588–594
Zhang X, Wu J, Liu H (2013) Age- and gender-related metabonomic alterations in striatum and cerebellar cortex in rats. Brain Res 1507:28–34
Zhou BY, Liu Y, Kim B, Xiao Y, He JJ (2004) Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci 27:296–305
Zhou L, Diefenbach E, Crossett B, Tran SL, Ng T, Rizos H, Rua R, Wang B, Kapur A, Gandhi K, Brew BJ, Saksena NK (2010) First evidence of overlaps between HIV-associated dementia (HAD) and non-viral neurodegenerative diseases: proteomic analysis of the frontal cortex from HIV+ patients with and without dementia. Mol Neurodegener 5:27
Acknowledgements
This work was supported by NIH P01 DA037830 to Kamel Khalili and Dianne Langford, NIH R01 MH107340 to Dianne Langford, NIH K99/R00 HL138268 to Santhanam Shanmughapriya, the Comprehensive NeuroAIDS Center to Kamel Khalili (NIH P30 MH09217), and Bianca Cotto was supported by T32MH079785.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cotto, B., Natarajaseenivasan, K. & Langford, D. Astrocyte activation and altered metabolism in normal aging, age-related CNS diseases, and HAND. J. Neurovirol. 25, 722–733 (2019). https://doi.org/10.1007/s13365-019-00721-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-019-00721-6