Abstract
Addictive stimulant drugs, such as cocaine, are known to increase the risk of exposure to HIV-1 infection and hence predispose towards the development of AIDS. Previous findings suggested that the combined effect of chronic cocaine administration and HIV-1 infection enhances cell death. Neuronal survival is highly dependent on the health of mitochondria providing a rationale for assessing mitochondrial integrity and functionality following cocaine treatment, either alone or in combination with the HIV-1 viral protein Tat, by monitoring ATP release and mitochondrial membrane potential (ΔΨm). Our results indicate that exposing human and rat primary hippocampal neurons to cocaine and HIV-1 Tat synergistically decreased both mitochondrial membrane potential and ATP production. Additionally, since previous studies suggested HIV-1 infection alters autophagy in the CNS, we investigated how HIV-1 Tat and cocaine affect autophagy in neurons. The results indicated that Tat induces an increase in LC3-II levels and the formation of Parkin-ring-like structures surrounding damaged mitochondria, indicating the possible involvement of the Parkin/PINK1/DJ-1 (PPD) complex in neuronal degeneration. The importance of mitochondrial damage is also indicated by reductions in mitochondrial membrane potential and ATP content induced by HIV-1 Tat and cocaine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to estimates by WHO and UNAIDS, 36.9 million people around the world are known to be infected by HIV-1 as of 2014 (http://www.who.int/features/qa/71/en/). Among these, more than 12 million people are from the US, and 1 out of 8 is unaware of being infected. Since highly active antiretroviral therapy (HAART) became the main treatment for HIV-1 infection, the average life span of HIV+ individuals has increased significantly. However, despite the reduced morbidity and mortality from HIV infection due to HAART therapy, up to 70 % of seropositive patients develop neurologic complications in the central and peripheral nervous system (Bilgrami and O’Keefe 2014), as well as premature cardiovascular disease (Chu and Selwyn 2011). All these morbidities are thought to be due to the combination of the effects of an aging HIV-infected population together with the long-term effects of the infection itself and long-term antiretroviral therapy (McArthur et al. 2005). HIV can induce primary and secondary neurological disorders: the former includes dementia in adults, encephalopathy in children, HIV-associated (vacuolar) myelopathy, and distal peripheral polyneuropathy. As for the latter, these disorders may be due to opportunistic infections resulting from HIV-induced immunosuppression (Tan et al. 2012) or the neurotoxic effects of HAART (Stavros and Simpson 2014).
Among all of the HIV proteins, at least nine are known to induce neuronal cell death (Mocchetti et al. 2012; Avdoshina et al. 2013). Among these, HIV-1 Tat protein, the transactivator of transcription which regulates both HIV transcription initiation and elongation, is released from HIV-infected cells (Ensoli et al. 1993). HIV-1 Tat negatively affects neuronal survival through several mechanisms, among which is the impairment of mitochondrial function (Kruman et al. 1998). It is widely accepted that psychoactive drugs, including cocaine, increase the risk of exposure to HIV-1 infection and may influence the development of AIDS (Friedman et al. 2006). Similar to HIV-1, cocaine is known to promote progressive neurocognitive disorders (Dahal et al. 2015). Specifically, chronic self-administration of cocaine is associated with early cognitive decline, brain atrophy and gradual loss of grey matter (Connolly et al. 2013). Furthermore, studies have shown that chronic cocaine administration results in neuronal necrosis and atrophy, and decrease in cell proliferation in the hippocampus (Barroso-Moguel et al. 2002; Yamaguchi et al. 2005). Thus, the combined effects of chronic cocaine administration and HIV-1 infection could aggravate neuronal cell injury and promote cell death. Neuronal survival is highly dependent on the integrity and functionality of mitochondria, which provide cellular energy by producing ATP through the coupled electron transport chain and oxidative phosphorylation (OxPhos) (Sheng 2014). The presence of ATP is essential for neuronal cell survival and proper functioning since it allows synapse assembly (Lee and Peng 2008), generation of action potentials (Attwell and Laughlin 2001) and synaptic transmission (Verstreken et al. 2005). The dysregulation of OxPhos and impairment of mitochondrial function caused by cocaine use and HIV-1 infection may result in substantial neuronal damage.
Even though HIV-1 does not infect neurons, a major aspect of HAND is neuronal damage and apoptosis (reviewed in Kovalevich and Langford 2012). HIV transactivator of transcription (Tat) plays an important role in the pathogenesis of HIV-1 neurocognitive disorders such as dementia (HAD) (Langford and Masliah 2002). Tat production in the hilar area of the rat hippocampus was shown to induce extensive neuronal damage, synaptic alterations, and glial activation both in dentate gyrus and in CA3/4 areas (Bruce-Keller et al. 2003). In addition, it was shown that the hippocampus was vulnerable to damage during HIV-infection of the human brain (Torres-Muñoz et al. 2001). Furthermore, Tat protein mediates cognitive and behavioral abnormalities in animal models of HIV disease (Carey et al. 2012; Harricharan et al. 2015). Cocaine abuse is known to aggravate the neurotoxicity of HIV-1 infection and HIV-1 Tat. The neurotoxic effects of Tat have been reported to be oxidative stress-dependent. In this regard, HIV-1 Tat was found to trigger mitochondrial depolarization (Régulier et al. 2004; Lecoeur et al. 2012) and increased intracellular generation of reactive oxygen species (ROS) resulting in neuronal degeneration in primary cultures of rat hippocampal rat cells (Aksenov et al. 2006). Previous studies reported the combined action of cocaine exposure and Tat released from infected glia are neurotoxic in the CNS (Wayman et al. 2015). In line with these findings, our results show that treating rat hippocampal neurons with either cocaine alone or in combination with HIV-1 Tat, strongly decreased mitochondrial ATP production. Moreover, the mitochondrial membrane potential (ΔΨm) is critical for the generation of ATP and loss of ΔΨm leads to ATP depletion within the cell thereby contributing to neuronal death (Joshi and Bakowska 2011). Our results indicate that Tat and cocaine, either alone or in combination, suppress mitochondrial function by decreasing ΔΨm. Previous studies also suggested that HIV-1 infection alters autophagy function in the CNS, but the specific mechanisms remain unclear. Here we investigated how HIV-1 Tat and cocaine interfere with both autophagy and mitophagy in neurons. The results obtained show that Tat induces an increase in LC3-II levels indicating increased autophagy.
With respect to mitophagy, it is well established that proper degradation of aged and damaged mitochondria through the mitophagic machinery is important in cell homeostasis. The PINK1/Parkin pathway plays a key role in ensuring mitochondrial integrity and function. Specifically, Parkin translocation from the cytosol to damaged mitochondria is known to trigger mitophagy and Parkin-targeted mitochondria then accumulate in the neuronal somatodendritic region where mature lysosomes are predominantly located. Interestingly, Parkin translocation to damaged mitochondria is well established in many non-neuronal cell types, however this translocation is controversial in primary neurons (Ashrafi and Schwarz 2015). Here we found that Parkin relocalizes from the cytosol to the mitochondria and appears to form Parkin-ring-like structures surrounding mitochondria indicating the possible involvement of Parkin in the formation of the Parkin/PINK1/DJ-1 (PPD) complex, which leads to the ubiquitination and degradation of Parkin substrates, including Parkin itself. Taken together, these findings will help decipher the underlying molecular mechanisms responsible for the augmented neuropathological effects of cocaine abuse in the setting of HIV-1 infection.
Materials and Methods
Primary Neuronal Cultures
Neuronal cell culture methods were as we have recently described (Darbinyan et al. 2013). Briefly, for primary hippocampal neurons, hippocampal tissue was dissected from embryonic day 18 Sprague Dawley rats (Animal assurance number A3085-01) or human fetal brain tissue (embryonic age 16 weeks) and dissociated with mild mechanical trituration. Dissociated cells were seeded at 5 × 105 cells/well in serum-free neurobasal medium containing B27 (50:1), 2 mM glutamax, and 1 % antibiotic at 37 °C and 5 % CO2. One day later the cultures were refed with fresh serum-free neurobasal medium. After 5 days, the cultures consisted of 90 % neuron-specific nuclear-protein-immunoreactive neurons. Primary dopaminergic rat neurons were harvested from E14 Sprague Dawley rat embryos by dissecting the ventral midbrain and dissociating with a mild mechanical trituration. Dissociated neurons were plated on Poly-D-Lysine coated 96 well plates in serum-free neurobasal medium containing B27 (50:1), 2 mM glutamax, and 1 % antibiotic at 37 °C and 5 % CO2.
Adenoviral Transduction
Four days after plating, neurons were transiently infected for 2 h with either Adeno-GFP, as an internal control, or Adeno-Tat (Mukerjee et al. 2008) with a multiplicity of infection of one.
Treatment of Neurons with Cocaine and Tat for Cell Viability Assay
Rat primary hippocampal or dopaminergic neurons were cultured on poly-D-lysine-coated 96-well plates for 4 days and then treated with different concentrations of cocaine (0.25, 0.5, 1 and 2 μM) in sodium citrate (pH 5.0) for 48 h. Rat primary hippocampal neurons cultured on poly-D-lysine-coated 96-well plates were transiently transduced for 2 h with either Adeno-GFP or Adeno-Tat as described in the earlier section with a multiplicity of infection of one. After infection, supernatants were removed and replaced with fresh serum-free neurobasal medium. Subsequently, 48 h post-transduction, the cultured neurons were treated with 1 μM cocaine in sodium citrate (pH 5.0) for 48 h. As a positive control neurons were treated with 10 μM of ionomycin for 48 h. The viability of rat primary neurons was determined by the MTT assay (Sigma-Aldrich) following the manufacturer’s instructions.
Treatment of Neurons with Tat and Cocaine for Analysis of Autophagy
Rat primary hippocampal neurons cultured on poly-D-lysine-coated 12-well plates were transiently transduced for 2 h with either Adeno-GFP or Adeno-Tat with a multiplicity of infection of one. Subsequently, 48 h post-transduction, the cultured neurons were treated with 1 μM cocaine in sodium citrate (pH 5.0) for an additional 48 h and then cells were harvested for protein extraction as described in the section on Western blot. For analysis of autophagic flux, Bafilomycin A1 (Sigma-Aldrich, St. Louis, MO, USA) was used at a concentration of 0.5 μM for 4 h prior to harvesting the cells for protein extraction.
Western Blot
Neurons were washed once with warm 1X phosphate-buffered saline (PBS) and lysed using TNN lysis buffer (40 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM DTT, 1 mM EDTA, 1 % NP40, and 1 % protease/phosphatase inhibitor cocktail). Protein extracts were eluted with Laemmli sample buffer, heated at 95 °C for 10 min, resolved by SDS–PAGE and transferred to reinforced supported nitrocellulose membranes (Whatman™, Germany) for 2 h at 4 °C in a transfer buffer containing 25 mM Tris (pH 7.4), 200 mM glycine, and 20 % methanol. Membranes were blocked for 1 h at room temperature with 10 % nonfat dry milk in 1X PBS with 0.1 % Tween-20 (PBST), washed and incubated with primary antibodies for 2–4 h in 5 % nonfat dry milk at room temperature. The blots were subsequently washed three times with 1X PBS and incubated with IRDye® 800CW goat anti-mouse and IRDye® 680RD goat anti-rabbit secondary antibodies and visualized with an Odyssey® CLx Imaging System (LI-COR, Inc., Lincoln, NE). The following antibodies were used for Western blot: Rabbit polyclonal antibodies to LC3 (Sigma, St. Louis MO), GAPDH (Santa Cruz Biotechnologies. Dallas TX), β-tubulin (LI-COR, Lincoln NE), HIV-1 Tat (NIH AIDS Reagents, Germantown MD), Parkin, Tomm20 and PINK1 (Abcam, Cambridge MA). Goat polyclonal antibody to VDAC1 and mouse monoclonal to cytochrome C were from Santa Cruz.
Immunocytochemistry
Tomm20 is the translocase of the outer mitochondrial membrane and is used as a marker for mitochondria. For Parkin and Tomm20 labeling, neurons were fixed for 30 min with 4 % paraformaldehyde at room temperature. The fixed cells were permeabilized with 1 % Triton X100 for 30 min, followed by labeling with Parkin and Tomm20 antibody. Briefly, after blocking with 1 % BSA in PBS 0.1 %Tween 20, slides were incubated with rabbit anti-Tomm20 (1:1000) and Mouse anti-Parkin (1:1000), washed in PBS (0.1 M) and incubated with secondary antibody.
Mitochondrial Isolation
Human primary neurons were cultured in poly-D-lysine-coated T-25 tissue culture flasks. Following treatment, neurons were scraped off the plates in cold 1X PBS, pelleted by centrifugation at 1000 rpm. Cell pellets were washed, repelleted and the supernatants were removed. Pellets were subjected to differential centrifugation as we have previously described (Sen et al. 2015). Following the isolation procedures, the resulting mitochondrial-enriched pellets were immediately resuspended and lysed in a small volume of TNN lysis buffer (40 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM DTT, 1 mM EDTA, 1 % NP40, and 1 % protease/phosphatase inhibitors cocktail). Protein concentrations were then determined by the Bradford method.
Mitochondrial Membrane Potential Assay
Permeabilized neurons (20 μg/ml digitonin) were gently stirred and 3 mM JC-1 (Enzo Life Sciences) was added or 30 min in a 5 % CO2 incubator at 37 °C to monitor Δψm and then washed with PBS. Fluorescence signals were monitored at 490-nm excitation (ex)/535-nm emission (em) for the monomer, 570-nm ex/595-nm em for the J- aggregate of JC-1. 10 μM Ca2+ boluses were added every 60 s for 9 boluses. At 20s JC-1 was added to monitor Δψ. Fluorescence signals for JC-1 were monitored at 490 ex/535 em for the monomer and 570ex/595em for the J-aggregate. The protonophore, CCCP, was added to collapse the proton gradient. All experiments were conducted at 37 °C and recorded on a PTI spectrofluorometer.
Measurement of Mitochondrial ATP Levels
Human fetal neurons were treated with Tat, cocaine or rotenone, harvested and the mitochondrial fraction was isolated by differential centrifugation. Finally, the mitochondrial pellet isolated as described earlier was lysed in RIPA buffer. ATP content was measured using the ATP Determination Kit (Molecular Probes, Eugene, OR), a bioluminescence assay for quantitative determination of ATP with recombinant firefly luciferase and substrate D-luciferin, following the manufacturer’s directions.
Statistical Analysis
The data in Figs. 1 and 5 were analyzed by a Student’s two-tailed t-Test, which returns the probability that two samples are likely to have come from the same two underlying populations that have the same mean. When the results from comparing a data point to the control data (lane 1) show that the probability is less than 5 % (p < 0.05), this is taken as a statistically significant difference and marked by an asterisk. For Western blots, each was repeated 2–3 times and a representative experiment is presented.
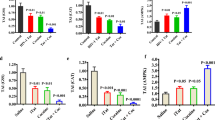
a. MTT Viability assay of dose-dependent effect of cocaine on rat primary hippocampal neurons. Rat primary hippocampal neurons were harvested from E18 rat embryos and plated on Poly-D-Lysine-coated 96 well plates. Cells were then treated with cocaine at concentrations ranging between 0.25 and 2 μM for 48 h. Neuronal viability was assessed by MTT assay, which revealed a substantial dose dependent decrease in cell viability, starting at a concentration of 1 μM cocaine. b. Viability assay of effect of HIV-1 Tat and cocaine, alone or in combination in rat primary hippocampal neurons. Rat primary hippocampal neurons were harvested from E18 rat embryos and plated on Poly-D-Lysine-coated 96 well plates. Cells were then transiently transduced for 2 h with either Adeno-GFP or Adeno-Tat at moi = 1. After 48 h transduction, the neurons were untreated (C-) or treated with 1 μM cocaine in sodium citrate (pH 5.0) or 10 μM ionomycin as a positive control (C+). Cell viability was then analyzed 48 h after cocaine treatment by the MTT assay. The data show a decrease in cell viability induced by both Tat and cocaine. c. Phase contrast photomicrographs of the treated rat primary hippocampal neurons showing the cytopathic effects of Tat and cocaine. The scale bar is 40 μm. d. Viability assay of dose-dependent effect of cocaine on rat primary dopaminergic neurons. Experimental conditions were as for A but rat dopaminergic neurons were utilized. Neuronal viability was assessed by MTT assay, which revealed a substantial dose dependent decrease in cell viability, starting at a concentration of 0.25 μM cocaine. Asterisks indicate that the difference is statistically different from the control in lane 1 (P < 0.05)
Results
Dose-Dependent Effects of Cocaine on Neuronal Viability
The first set of experiments aimed to investigate the dose-dependent effect of cocaine on cell viability, followed by the time-dependent effects of Tat and cocaine on the viability of rat primary neurons. As shown in Fig. 1a, we determined the levels of cell death using the MTT viability assay in rat primary hippocampal neurons after administration of various concentrations of cocaine. Cytotoxicity was observed at concentrations of 1 μM cocaine and above. In order to monitor the combined effects of Tat and cocaine on neuronal death, a viability assay was performed to show the time-dependent response to the treatments. Cell viability was then analyzed at 48 h after cocaine treatment by the MTT assay. Transduction of rat primary hippocampal neurons with Adeno-Tat greatly reduces cell viability as did treatment with cocaine both in the presence and absence of Tat (Fig. 1b). Phase contrast photomicrographs of the neurons treated with cocaine and/or Tat for 48 h are shown in Fig. 1c. We also performed these experiments with rat primary dopaminergic neurons with similar results except that they were more sensitive to low concentrations of cocaine with significant cytotoxicity at 0.25 μM cocaine and above (Fig. 1d).
Effects of Tat and Cocaine on Autophagic Flux
Next, we investigated whether rat primary hippocampal neurons undergoing HIV-1 Tat and cocaine treatment exhibited an increase in autophagic signaling and whether such treatments affected expression of LC3-II. Microtubule-associated protein 1A/1B-light chain 3 (LC3) is a 17 kDa protein found ubiquitously in mammalian cells. Concomitantly with autophagy, a cytosolic form of LC3 (LC3-I) is conjugated to phosphatidylethanolamine to form LC3-phosphatidylethanolamine conjugate (LC3-II), which serves as a marker for autophagosomes (Tanida et al., 2008). Our results indicate that Tat alone or in combination with cocaine, but not cocaine alone, induces an increase in LC3-II levels (Fig. 2a and b). In addition to LC3, SQSTM1/p62, serves as a link between LC3 and ubiquinated substrates (Katsuragi et al. 2015) and a decrease in SQSTM1/p62 levels are associated with autophagy activation in certain conditions (Klionsky et al. 2016). Our results indicate that either Tat alone or cocaine alone or Tat in combination with cocaine, did not induce degradation of SQSTM1/p62 (Fig. 2c and d). Other reports have noted that SQSTM1/p62 changes are cell type specific and do not always correlate with autophagy induction (Klionsky et al. 2016).
Expression of LC3-II and SQSTM1/p62 in primary hippocampal neurons treated with HIV-1 Tat and cocaine, alone or in combination. Rat primary hippocampal neurons were harvested from E18 rat embryos and plated on Poly-D-Lysine-coated 12-well plates. Cells were then transiently transduced for 2 h with either Adeno-GFP or Adeno-Tat at moi = 1. After 48 h following transduction, the cultured neurons were treated with 1 μM cocaine in sodium citrate (pH 5.0). a. Western blots for Tat, LC3 and GAPDH (loading control). The LC3-II band is indicated by an arrowhead. b. Quantification of the LC3-II band intensity to that of GAPDH for Panel A. The data show an increase in LC3-II after Tat and/or cocaine treatment. c. Western blots for SQSTM1/p62 and GAPDH (loading control). d. Quantification of the p62 band intensity to that of GAPDH for Panel c. The data show no alteration in p62 after Tat and/or cocaine treatment. e. Effect of Bafilomycin A on expression of LC3-II in primary hippocampal neurons treated with HIV-1 Tat and cocaine, alone or in combination. Rat primary hippocampal neurons were harvested from E18 rat embryos and plated on Poly-D-Lysine-coated 12-well plates. Cells were then transiently transduced for 2 h with either Adeno-GFP or Adeno-Tat at moi = 1. After 48 h, the cultured neurons were treated with 1 μM cocaine in sodium citrate (pH 5.0) and/or 0.5 μM Bafilomycin A for 4 h prior to harvest. Cells were harvested and analyzed by Western blot for LC3 and GAPDH (loading control). The position of the LC3-II band is indicated by an arrowhead. The band intensities of the LC3-II and GAPDH for each condition were quantified and the ratios are shown underneath the Westerns. HIV-1 Tat increased LC3-II levels compared to both control and GFP but had no effect on bafilomycin-induced LC3-II level, while cocaine alone reduced the induction of LC3-II by bafilomycin
To investigate if Tat and cocaine are acting on the autophagic flux, we employed an autophagic flux assay. The assay detects whether processed LC3-II accumulates in response to autophagy inhibition using bafilomycin A1, which is a specific inhibitor of the vacuolar type lysosomal H+-transporting ATPase (V-ATPase) in cells, and inhibits the acidification of organelles containing this enzyme, such as lysosomes and endosomes (Yamamoto et al. 1998). Rat primary neurons were transiently transduced and/or treated with 1 μM cocaine as previously described and also treated with or without bafilomycin A1 (0.5 μM) for 4 h prior to harvesting of cells. As shown in Fig. 2e, the conversion of LC3-I to LC3-II increased upon bafilomycin A1 treatment (compare lanes 1, to lane 3) suggesting that bafilomycin A1 blocks basal autophagic flux. HIV-1 Tat transduction increased LC3-II levels compared to both control and GFP (compare lanes 1 and 3 to lane 5) but had no effect on bafilomycin-induced LC3-II level (compare lane 4 to lane 6). Interestingly, cocaine alone reduced the induction of LC3-II by bafilomycin (compare lane 4 to lane 8).
Effects of Tat and Cocaine on Mitophagy
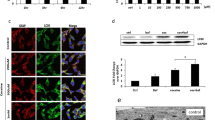
Evidence in mammalian cell lines has implicated Parkin and PINK1 in the mitophagic degradation of dysfunctional depolarized mitochondria (Grenier et al. 2013). Specifically, decreased Parkin levels are associated with mitochondrial dysfunction. However, the role of Parkin in neurons still remains unclear (Grenier et al. 2013). Interestingly, Parkin is slightly downregulated in whole cell extracts of cocaine-treated neurons (Fig. 3a), with translocation of Parkin from the cytosol to the mitochondria in neurons treated with Tat and cocaine (Fig. 3b). PINK1 expression is also slightly downregulated in cell extracts of cocaine alone and cocaine and Tat treated neurons (Fig. 3c). VDAC, also known as mitochondrial porin, is a voltage dependent anion channel, located in the mitochondrial outer membrane. VDAC functions as a gatekeeper, regulating the traffic of mitochondrial metabolites. VDAC is also known to specifically interact with Parkin on damaged mitochondria, and therefore plays a key role in the onset of mitophagy. Our data indicate that both Tat and cocaine treatment induced an upregulation of VDAC expression levels (Fig. 3d), suggesting a response to neuronal stress induced by exposure to cocaine and Tat. Changes in VDAC expression may therefore represent another indication of increased mitophagy signaling in rat hippocampal neurons.
Expression of VDAC, PINK1 and Parkin in primary hippocampal neurons treated with HIV-1 Tat and cocaine, alone or in combination. Rat primary hippocampal neurons were harvested from E18 rat embryos and plated on Poly-D-Lysine-coated 12-well plates. Cells were then transiently transduced for 2 h with either Adeno-GFP or Adeno-Tat (moi = 1). After 48 h, the cultured neurons were treated with 1 μM cocaine in sodium citrate (pH 5.0) or 10 μM ionomycin as a positive control. Western blots were performed for Parkin (a). To examine the subcellular distribution of Parkin, the cultured neurons were fractionated into cytosol and mitochondrial fractions as described and analyzed by Western blot for Parkin, cytochrome c (mitochondrial marker) and GAPDH (cytosolic marker) as shown in Panel b. Western blots of total cell extracts were also performed for PINK1 (c) and VDAC (d). Quantification for each Western is shown in the right hand side of Panels a, c and d. Parkin is slightly downregulated in cocaine-treated neurons and there is translocated from cytosol to mitochondria after treatment with Tat and cocaine. PINK1 is also slightly downregulated with cocaine alone and cocaine plus Tat. VDAC was upregulated by both Tat and cocaine suggesting a possible response to neuronal stress and may indicate increased mitophagy signaling
In order to investigate whether Tat and cocaine treatment can be involved in the regulation of Parkin, immunocytochemistry was performed in neurons treated with cocaine and/or Tat (Fig. 4). This approach did not reveal marked differences in the translocation of Parkin to the mitochondria, but interestingly, Parkin-ring-like structures were visible surrounding fragmented mitochondria of hippocampal neurons transduced with Adeno-Tat (Fig. 4, arrows). These findings are in accord with the findings of Cai et al. (2012), where the authors reported that Parkin translocation to mitochondria in neurons is slower than in non-neuronal cells and Parkin was selectively recruited to depolarized mitochondria to form ring-like structures. In this study, it was also reported that Parkin translocation only occurred in a small percentage of neurons and was restricted to somatodendritic regions, where mature and acidic lysosomes are predominantly localized. This spatial and dynamic process allows neurons to efficiently eliminate dysfunctional mitochondria by selective and dynamic Parkin translocation to depolarized mitochondria and subsequent degradation via the autophagy-lysosomal pathway in live neurons.
Analysis of the effect of HIV-1 Tat and cocaine, alone or in combination on Parkin and Tomm20 subcellular distribution in primary hippocampal neurons by immunocytochemistry. Primary rat hippocampal neurons were harvested from E18 rat embryos and plated on Poly-D-Lysine-coated chamber slides. Cells were then transiently transduced for 2 h with either Adeno-GFP or Adeno-Tat (moi = 1). After 48 h, the cultured neurons were treated with 1 μM cocaine in sodium citrate (pH 5.0). For labeling of Parkin (green) and Tomm20 (a mitochondrial marker, red), immunocytochemistry was performed as described in Materials and Methods with rabbit anti-Tomm20 (1:1000) and mouse anti-Parkin (1:1000). Nuclei are stained with DAPI (blue). Arrows indicate endogenous Parkin-ring-like structures surrounding fragmented mitochondria. Parkin-ring-like structures were visible around fragmented mitochondria of cells treated with Tat. The scale bar is 40 μm
Tat and Cocaine Modulates Mitochondrial Bioenergetics in Primary Human Neurons
To examine whether HIV-1 Tat and cocaine treatments are capable of altering mitochondrial bioenergetics, an ATP determination assay was performed on human primary hippocampal neurons. ATP levels decreased significantly after cocaine and Tat treatments indicating that they strongly affect the mitochondrial respiratory chain (Fig. 5a).
a & b. ATP determination assay and the effects of HIV-1 Tat and cocaine, alone or in combination in hippocampal neurons. Human primary neurons were treated with Tat, cocaine or rotenone, harvested and the mitochondrial fraction was isolated by differential centrifugation. Finally, the mitochondrial pellet was lysed in RIPA buffer and analyzed for ATP by ATP-luciferase assay as described. ATP levels decreased significantly after cocaine and Tat treatments indicating that they strongly affect OxPhos. For each histogram, asterisks indicate that the difference is statistically different from the control in lane 1 (P < 0.05). c. Effects of HIV-1 Tat and cocaine on the mitochondrial membrane potential of human primary neurons. Neurons were treated with Tat and cocaine, alone and in combination, for 48 h. Representative traces of permeabilized (20 μg/ml digitoxin) neurons were loaded with JC-1, a novel cationic carbocyanine dye that accumulates in mitochondria and is a sensitive marker for mitochondrial membrane potential. Carbonyl cyanide m-chlorophenyl hydrazone (CCCP), a highly toxic ionophore and decoupler of the respiratory chain, was added at the conclusion of every experiment to collapse the proton gradient. Quantification of the membrane potential before the addition of CCCP is shown. Data represent the mean ± SEM (n = 3). * - p < 0.05 compared to the control. Results show mitochondrial membrane depolarization with both Tat and cocaine, alone and in combination
It is known that cocaine potentiates the neurotoxic effect of the HIV-1 Tat protein, leading to neurodegeneration in the brain. Specifically, it has been shown that Tat generates mitochondrial-reactive oxygen species (ROS). Oxidative stress caused by reactive oxygen species (ROS) is known to induce rapid depolarization of the inner mitochondrial membrane potential (Δψm). To elucidate whether HIV-Tat and cocaine, alone or in combination, can trigger mitochondrial depolarization, we monitored changes in mitochondrial membrane potential by using Δψ-sensitive fluorescent probe, JC-1 (Molecular Probes). Once loaded into the mitochondria, JC-1 forms red fluorescent (emission at 590 nm) aggregates in the regions of high membrane potential. At low membrane potential JC-1 exists as a green fluorescent (emission at 528 nm) monomer. Therefore, red-to-green JC-1 fluorescence ratios can be used to assess changes in Δψ following different experimental treatments with either Tat or cocaine. Results showed a significant increase in mitochondrial membrane depolarization in hippocampal neurons treated with both Tat and cocaine, alone and in combination (Fig. 5b). These data support a role for the disruption of mitochondrial membrane potential in the neurotoxic effects of HIV-1 Tat and cocaine.
Discussion
Previous studies suggested that the combined effects of chronic cocaine administration and HIV-1 infection are neurotoxic and cause injury to neurons in the CNS (Wayman et al. 2015). The use of cocaine can aggravate the neurotoxic effects of HIV-1 proteins such as HIV-1 Tat and HIV-1 Tat is believed to play an important role in the pathogenesis of HIV-1 neurocognitive disorders such as HAD. HIV-1 Tat is neurotoxic and evidence suggests that these toxic effects are oxidative stress-dependent. In this regard, HIV-1 Tat was found to trigger mitochondrial depolarization and increased intracellular generation of reactive oxygen species (ROS) resulting in neuronal degeneration in primary cultures of rat hippocampal neurons (Aksenov et al. 2006). In the same study, a pharmacologically relevant dose of cocaine (1.5 μM) was non-toxic by itself but significantly enhanced Tat-induced oxidative stress and neurotoxicity in rat hippocampal cell cultures and that cocaine-mediated augmentation of Tat neurotoxicity is related to its ability to potentiate Tat-induced oxidative stress (Aksenov et al. 2006). In contrast, our data indicate that cocaine alone is toxic to rat hippocampal neurons and also confirm the neurotoxic effects of Tat on rat hippocampal neurons (Fig. 1).
Autophagy is a basic physiologic process contributing to the maintenance of cellular homeostasis and encompasses pathways targeting cytosolic proteins and damaged organelles. Deregulation of autophagy plays a pivotal role in the etiology and progress of many neurodegenerative disorders (Ghavami et al. 2014). Monitoring conversion of LC3-I to LC3-II by western blot is widely used to monitor autophagy since the amount of LC3-II is clearly correlated with the number of autophagosomes (Mizushima and Yoshimori 2007). The autophagic flux analysis of SQSTM1/p62 in our studies did not show any changes in the degradation of p62. In this context, it has been reported that in some cell types, there is no change in the levels of p62 despite induction of autophagy (see review, Klionsky et al. 2016). Furthermore, a decrease in p62 might also be an indicator of blockage of autophagy as a result of cleavage of p62 by caspases or calpains (El-Khoury et al. 2014). Our data indicate the induction of autophagy by HIV-1 Tat alone or combined with cocaine but not by cocaine alone. Interestingly, Tat and cocaine also perturbed the level of VDAC and subcellular localization of Parkin, two proteins that are involved in the regulation of autophagy.
The E3 ubiquitin ligase Parkin and the serine/threonine kinase PINK1 are neuroprotective proteins, which are involved in the mitochondrial quality control pathway providing key regulation of the clearance of depolarized and damaged mitochondria by autophagy, a process known as mitophagy (Seirafi et al. 2015). Parkin translocation from the cytosol to depolarized and damaged mitochondria is a trigger for mitophagy. However, much of the work characterizing the Parkin pathway was carried out in immortalized cell lines, which overexpress high levels of Parkin. Thus, while Parkin translocation to damaged mitochondria is well established in many non-neuronal cell types, the occurrence of this translocation in primary neurons is controversial (Ashrafi and Schwarz 2015). Nevertheless, mitochondrial dysfunction is observed in virtually every neurodegenerative disease and genetic results have begun to elucidate the underlying mechanisms of mitochondrial involvement in these diseases, pointing to the importance of defects in the recycling of impaired mitochondria and mitochondrial dysfunction in neurodegeneration (Dupuis 2014). Our data presented here (Figs. 3d and 4) did not reveal marked differences in the translocation of Parkin to the mitochondria upon Tat and cocaine treatment of rat hippocampal neurons, but we did observe Parkin-ring-like structures surrounding fragmented mitochondria of neurons transduced with Adeno-Tat. Likewise, Cai et al. (2012) reported that Parkin translocation to mitochondria is slow but Parkin was selectively recruited to depolarized mitochondria to form ring-like structures, which only occurred in a small percentage of neurons and was restricted to somatodendritic regions. This allows neurons to efficiently eliminate dysfunctional mitochondria by selective and dynamic Parkin translocation and subsequent degradation by the autophagosomes/lysosomes in live neurons (Cai et al. 2012).
Finally, since the neurodegeneration caused by HIV-1 Tat and cocaine involves mitochondria, we analyzed the effects on mitochondrial bioenergetics. HIV-1 Tat alone decreased ATP levels as did cocaine alone but the effect of both Tat and cocaine were more pronounced. Interestingly, HIV-1 Tat alone and cocaine alone had only slight effects on mitochondrial membrane potential but both Tat and cocaine together caused a marked depolarization of the mitochondrial membrane potential. Stevens et al. (2014) reported HIV-1 Tat induced decreases in ATP levels and mitochondrial hypopolarization in primary cultures of mouse cerebral cortical neurons. Creatine was protective against both of these and the authors suggested that creatine might be a useful adjunctive therapy against HAND (Stevens et al. 2014).
Taken together, our data indicate that the neurotoxic effects of both HIV-1 and cocaine are mediated by pathways involving mitochondrial dysfunction and the induction of autophagy offering new insight into the neuropathology of HIV-1/AIDS and cocaine use comorbidity.
References
Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM (2006) Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology 27:217–228
Ashrafi G, Schwarz TL (2015) PINK1- and PARK2-mediated local mitophagy in distal neuronal axons. Autophagy 11:187–189
Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21:1133–1145
Avdoshina V, Bachis A, Mocchetti I (2013) Synaptic dysfunction in human immunodeficiency virus type-1- positive subjects: inflammation or impaired neuronal plasticity? J Intern Med 273:454–465
Barroso-Moguel R, Mendez-Armenta M, Villeda-Hernandez J, Nava-Ruiz C, Santamaria A (2002) Brain lesions induced by chronic cocaine administration to rats. Prog Neuropsychopharmacol Biol Psychiatry 26:59–63
Bilgrami M, O’Keefe P (2014) Neurologic diseases in HIV-infected patients. Handb Clin Neurol 121:1321–1344
Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A (2003) Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci 23:8417–8422
Cai Q, Zakaria HM, Simone A, Sheng ZH (2012) Spatial Parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr Biol 22:545–552
Carey AN, Sypek EI, Singh HD, McLaughlin JP (2012) Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 229:48–56
Chu C, Selwyn PA (2011) Complications of HIV infection: a systems-based approach. Am Fam Physician 83:395–406
Connolly CG, Bell RP, Foxe JJ, Garavan H (2013) Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PLoS One 8:e59645
Dahal S, Chitti SV, Nair MP, Saxena SK (2015) Interactive effects of cocaine on HIV infection: implication in HIV-associated neurocognitive disorder and neuroAIDS. Front Microbiol 6:931
Darbinyan A, Kaminski R, White MK, Darbinian N, Khalili K (2013) Isolation and propagation of primary human and rodent embryonic neural progenitor cells and cortical neurons. Methods Mol Biol 1078:45–54
Dupuis L (2014) Mitochondrial quality control in neurodegenerative diseases. Biochimie 100:177–183
El-Khoury V, Pierson S, Szwarcbart E, Brons NH, Roland O, Cherrier-De Wilde S, Plawny L, Van Dyck E, Berchem G (2014) Disruption of autophagy by the histone deacetylase inhibitor MGCD0103 and its therapeutic implication in B-cell chronic lymphocytic leukemia. Leukemia 28:1636–1646
Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC (1993) Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol 67:277–287
Friedman H, Pross S, Klein TW (2006) Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol 47:330–342
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Łos MJ (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49
Grenier K, McLelland GL, Fon EA (2013) Parkin- and PINK1-dependent mitophagy in neurons: will the real pathway please stand up? Front Neurol 4:100
Harricharan R, Thaver V, Russell VA, Daniels WM (2015) Tat-induced histopathological alterations mediate hippocampus-associated behavioural impairments in rats. Behav Brain Funct 11:3
Joshi DC, Bakowska JC (2011) Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J Vis Exp 51:e2704
Katsuragi Y, Ichimura Y, Komatsu M (2015) p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J 282:4672–4678
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12:1–222
Kovalevich J, Langford D (2012) Neuronal toxicity in HIV CNS disease. Futur Virol 7:687–698
Kruman I, Nath A, Mattson MP (1998) HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol 154:276–288
Langford D, Masliah E (2002) Role of trophic factors on neuroimmunity in neurodegenerative infectious diseases. J Neurovirol 8:625–638
Lecoeur H, Borgne-Sanchez A, Chaloin O, El-Khoury R, Brabant M, Langonné A, Porceddu M, Brière JJ, Buron N, Rebouillat D, Péchoux C, Deniaud A, Brenner C, Briand JP, Muller S, Rustin P, Jacotot E (2012) HIV-1 Tat protein directly induces mitochondrial membrane permeabilization and inactivates cytochrome c oxidase. Cell Death Dis 3:e282
Lee CW, Peng HB (2008) The function of mitochondria in presynaptic development at the neuromuscular junction. Mol Biol Cell 19:150–158
McArthur JC, Brew BJ, Nath A (2005) Neurological complications of HIV infection. Lancet Neurol 4:543–555
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3:542–545
Mocchetti I, Bachis A, Avdoshina V (2012) Neurotoxicity of human immunodeficiency virus-1: viral proteins and axonal transport. Neurotox Res 21:79–89
Mukerjee R, Deshmane SL, Fan S, Del Valle L, White MK, Khalili K, Amini S, Sawaya BE (2008) Involvement of the p53 and p73 transcription factors in neuroAIDS. Cell Cycle 7:2682–2690
Régulier EG, Reiss K, Khalili K, Amini S, Zagury JF, Katsikis PD, Rappaport J (2004) T-cell and neuronal apoptosis in HIV infection: implications for therapeutic intervention. Int Rev Immunol 23:25–59
Seirafi M, Kozlov G, Gehring K (2015) Parkin structure and function. FEBS J 282:2076–2088
Sen S, Kaminiski R, Deshmane S, Langford D, Khalili K, Amini S, Datta PK (2015) Role of hexokinase-1 in the survival of HIV-1-infected macrophages. Cell Cycle 14:980–989
Sheng ZH (2014) Mitochondrial trafficking and anchoring in neurons: new insight and implications. J Cell Biol 204:1087–1098
Stavros K, Simpson DM (2014) Understanding the etiology and management of HIV-associated peripheral neuropathy. Curr HIV/AIDS Rep 11:195–201
Stevens PR, Gawryluk JW, Hui L, Chen X, Geiger JD (2014) Creatine protects against mitochondrial dysfunction associated with HIV-1 Tat-induced neuronal injury. Curr HIV Res 12:378–387
Tan IL, Smith BR, von Geldern G, Mateen FJ, McArthur JC (2012) HIV-associated opportunistic infections of the CNS. Lancet Neurol 11:605–617
Tanida I, Ueno T, Kominami E (2008) LC3 and autophagy. Methods Mol Biol 445:77–88
Torres-Muñoz J, Stockton P, Tacoronte N, Roberts B, Maronpot RR, Petito CK (2001) Detection of HIV-1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains by laser capture microdissection. J Neuropathol Exp Neurol 60:885–892
Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ (2005) Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47:365–378
Wayman WN, Chen L, Persons AL, Napier TC (2015) Cortical consequences of HIV-1 Tat exposure in rats are enhanced by chronic cocaine. Curr HIV Res 13:80–87
Yamaguchi M, Suzuki T, Seki T, Namba T, Liu J, Hori T, Shiga T (2005) Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse 58:63–71
Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y (1998) Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23:33–42
Acknowledgements
We thank Dr. Manish Gupta and other past and present members of the Department of Neuroscience and Center for Neurovirology for their insightful discussion and sharing of ideas and reagents. This study utilized services offered by Lewis Katz School of Medicine Comprehensive NeuroAIDS Center at Temple University. This work was supported by grants P01 DA037830 and P30 MH092177 awarded to KK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All studies were reviewed and approved by the Katz School of Medicine IACUC.
No human subjects were used in these studies.
Funding
This study was funded by grant number P01DA037830 and P30MH092177 awarded to K. Khalili by NIH.
Conflict of Interest
F.I. De Simone declares that she has no conflict of interest.
N. Darbinian declares that she has no conflict of interest.
S. Amini declares that she has no conflict of interest.
M.K. White declares that he has no conflict of interest.
J. Elrod declares that he has no conflict of interest.
P. Datta declares that he has no conflict of interest.
D. Langford declares that she has no conflict of interest.
K. Khalili declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
De Simone, F.I., Darbinian, N., Amini, S. et al. HIV-1 Tat and Cocaine Impair Survival of Cultured Primary Neuronal Cells via a Mitochondrial Pathway. J Neuroimmune Pharmacol 11, 358–368 (2016). https://doi.org/10.1007/s11481-016-9669-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-016-9669-6