Abstract
When prey encounter predators, they exhibit antipredator responses to reduce their risk of predation. Delayed responses can be fatal. Because prey can assess the risk of predation using predation-related cues, previous exposures to these cues could affect subsequent antipredator responses. We tested this possibility using the pea aphid Acyrthosiphon pisum Harris (Hemiptera: Aphididae) and its predator the Asian ladybird beetle, Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Aphids disperse from their host plants after sensing predators. We investigated whether previous exposures to cues from conspecifics and ladybird beetles affected the dispersal rates of aphids encountering predators. The cues contained visual, chemical, and vibrational information from aphids and ladybird beetles. Aphids that had previously been exposed to these cues increased dispersal rates and, consequently, suffered less predation than unexposed aphids. To clarify how aphids increased their dispersal rate, we examined their feeding times. Aphids that had been exposed to cues reduced feeding times compared with unexposed conspecifics. Therefore, we further tested whether the predator-induced dispersal of non-feeding aphids was greater than that of feeding conspecifics and found correlated differences. Previous exposures to cues from conspecifics and predators may allow prey to tune their antipredator responses to predation risk prior to further predator encounters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When prey encounter predators, their antipredator responses include changes in their morphology (Relyea 2003; Tollrian 1995; Weisser et al. 1999) and behavior (Gross 1993; Lima 1998; Witz 1990). However, prey do not always exhibit antipredator responses immediately after the detection of predators, and these delayed responses can be fatal (Barbour and Clark 2012; Schmitt et al. 2016). Before encountering predators, prey are likely to recognize the presence of predators using cues from conspecifics (Leavesley and Magrath 2005; Pestana et al. 2013) and heterospecifics (Goodale and Kotagama 2008; Hughes et al. 2010) that are being attacked by predators, as well as cues derived from the predators (Dielenberg and McGregor 2001; Luca and Gerlai 2012). In fact, prey exhibit antipredator responses to these cues even if they do not encounter a live predator (Kunert et al. 2005; Pestana et al. 2013; Suraci et al. 2016). The presence of predation cues does not mean that prey will have a subsequent encounter with a predator. In the absence of predators, the prey cease their antipredator responses and restart feeding and reproduction. Prey that have been exposed to predation risks will likely encounter predators at a later point.
The antipredator responses of prey are affected by previous experiences with predators and cues associated with predation (Ayon et al. 2017; Choh et al. 2014; Stephenson 2016). For example, larvae of the western flower thrips, Frankliniella occidentalis Pergande (Thysanoptera: Thripidae), that have previously been exposed to alarm pheromones produce droplets of these pheromones in response to simulated predator attacks faster than conspecific larvae that have no prior exposure (De Brujin et al. 2006). Thus, when prey have previously recognized the risk of predation, the antipredator responses to subsequent encounters with predators may be enhanced. Although studies have suggested that learning plays a role in enhancing antipredator responses in previously exposed prey (Kelley and Magurran 2003; Wisenden et al. 1997), little is known about how this previous exposure affects their responses to subsequent predator attacks.
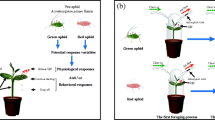
The pea aphid, Acyrthosiphon pisum Harris (Hemiptera: Aphididae), is a model species for testing antipredator responses in prey (Braendle and Weisser 2001; Losey and Denno 1998; Mondor and Roitberg 2004; Tamai and Choh 2018). When pea aphids encounter predators, they respond by dispersing from their host plants by walking and dropping (Dill et al. 1990; Nelson 2007), releasing alarm pheromones (Nault et al. 1973; Vandermoten et al. 2012), and increasing the proportion of winged morphs in the colony (Dixon and Agarwala 1999; Kunert et al. 2005). In nature, aphids live in groups on a host plant, so predators cannot kill all the individuals simultaneously. Therefore, it is possible that aphids could perceive cues from conspecifics being attacked by predators and that some of those aphids that have been exposed to the cues only encounter actual predators later. The cues would contain visual information and volatile substances from ladybird beetles and conspecifics attacked by the predators, such as alarm pheromones and vibration of the aphids and the predators, respectively. To test the effects of previous exposures to such cues on the subsequent predator-induced dispersal of pea aphids, and to elucidate the mechanism by which this occurs, we studied the predator–prey system of pea aphids and larvae of the Asian ladybird beetle Harmonia axyridis Pallas (Coleoptera: Coccinellidae).
We first examined the effects of previous exposures to cues from conspecifics and ladybird beetles on the dispersal of aphids from host shoots when predators were released onto the shoots. We then investigated how long the effects were retained after exposure had ceased. Feeding aphids must pull their stylets out from the host plants to change feeding sites or to migrate. Hence, whether aphids are feeding or not is likely a crucial factor affecting their ability to quickly escape from predators. To test whether aphid feeding was interrupted by exposure to cues from conspecifics and ladybird beetles, we measured the feeding times of aphids with and without exposure to cues from conspecifics and predators. Then, we investigated whether the interruption in feeding resulted in the quick dispersal of aphids in response to predators.
Materials and methods
Plants and insects
Broad bean plants, Vicia faba L. cv. ‘Nintokuissun’ (Fabales: Fabaceae), were reared in plastic pots (70 mm height, 75 mm diameter) filled with soil in an incubator at 25 ± 1 °C and 60 ± 10% relative humidity, with a 16-h:8-h (light:dark) photoperiod. We used plants at 8–12 days after germination for the experiments.
A single female pea aphid was collected from a field of white clover, Trifolium repens L. (Fabales: Fabaceae), on the campus of Chiba University, Matsudo, Japan in April 2012. All the aphids in the experiments were descended from the single female by parthenogenesis and maintained on broad bean plants in a climate-controlled room at 25 ± 1 °C and 60 ± 10% relative humidity, with a 16-h:8-h (light:dark) photoperiod. For experiments, we used apterous adult females (8–12 days after birth) that had been selected randomly from the culture.
Adult ladybird beetles were collected between April and September 2012 from the same field as the aphids and maintained in a climate-controlled room under the same conditions [25 ± 1 °C and 60 ± 10% relative humidity, with a 16-h:8-h (light:dark) photoperiod] (Tamai and Choh 2018). Both adult and larval ladybird beetles were fed daily with Mediterranean flour moth eggs, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae), which are commercially available (Entofood; Arysta Lifescience Corp., Tokyo, Japan). We randomly selected 4th-instars from the culture to use in the experiments. The larvae were starved for 24 h prior to experiments by keeping individuals in 15 mL plastic tubes. We conducted all the experiments in a climate-controlled room at 25 ± 1 °C and 60 ± 10% relative humidity, with a 16-h:8-h (light:dark) photoperiod.
Experiment 1: effects of previous exposure to cues from conspecifics and ladybird beetles on aphid dispersal
We used a plastic container (165 mm height, 120 mm diameter) to expose aphids to cues from conspecifics and ladybird beetles (Tamai and Choh 2018). The container consisted of two compartments: an upper compartment for the source of the cues from conspecifics and predators (20 mm height, 120 mm diameter; the ‘cue chamber’) and a lower compartment for exposing aphids to the cues (145 mm height, 120 mm diameter; the ‘exposure chamber’). The two compartments were divided using nylon gauze to prevent the migration of test insects between the two compartments. They were separated before the start of the experiments. We cut a broad bean shoot (approximately 80 mm high with four leaves) with a razor blade and inserted it into a 10-mL glass vial (46 mm height, 25 mm diameter) filled with water. The vial was sealed with dry cotton wool to prevent test insects from dropping into the water. The sides of the vials were coated with Fluon® (Asahi Glass Co., Tokyo, Japan) to prevent the relocation of insects that dispersed from the shoots. We introduced 10 adult aphids onto the shoot and placed it in the center at the bottom of the exposure chamber. Subsequently, 20 adult aphids and three ladybird beetle larvae were released into the cue chamber. The cue chamber was connected to the exposure chamber and kept there for 1 h to expose the aphids to cues from conspecifics and predators. As a control, we repeated the same experiments but without putting any insects into the cue chamber. We did not observe aphids dispersing from host shoots during exposure.
To exclude the possibility of cues from conspecifics and predators remaining in the exposure chamber and to determine the predator-induced dispersal of aphids, we used another clean plastic container that was of the same size as the exposure chamber. Instead of covering this chamber with nylon gauze, it was capped (the ‘experimental chamber’). We coated the inside of the experimental chamber with Fluon®. The aphids on shoots in the vials were moved from the exposure chamber to the experimental chamber immediately (i.e., 1 min) after exposure. A ladybird beetle larva was released onto the shoot at 1, 15, 30, and 60 min after the exposure using different test insects for each time point. The experimental chambers were randomly assigned to each treatment (1, 15, 30, and 60 min after exposure). We repeated the experiments 18 times.
The numbers of aphids that dispersed and that survived in the experimental chamber were measured 1 h after the release of the predators. We assumed that aphids at the bottom of the experimental chamber were individuals that dispersed from shoots.
Experiment 2: feeding times of aphids after exposure to cues from conspecifics and ladybird beetles
The effects of previous exposures to cues from conspecifics and ladybird beetles on the subsequent feeding times of aphids were examined using a plastic container (30 × 30 × 20 mm). The container consisted of two compartments that were divided by nylon gauze and were separated before the start of the experiments. An expanding broad bean true leaf near a shoot apex was placed on water-saturated cotton wool in the lower component. We placed an adult aphid on the leaf in the lower compartment and then released five adult aphids and a ladybird beetle larva into the upper compartment. Subsequently, the two compartments were connected for 1 h by placing the upper on the lower compartment. Under these conditions, aphids in the lower compartment were exposed to cues from conspecifics and ladybird beetles from the upper compartment. As a control, we repeated the same experiments without putting any insects into the upper compartment.
Feeding time was measured as an indicator of aphid foraging behavior in this experiment. To observe aphids in the lower compartments, the upper compartments were removed immediately (i.e., 1 min) after exposure. In the dispersal experiments, we found effects of exposure to the cues on predator-induced dispersal of aphids at 1, 15, and 30 min, but not at 60 min, after the exposure (Fig. 1a). We focused on two-time points (i.e., 1 min and 60 min after exposure) as representatives. The lower compartments were randomly assigned to each treatment (at 1 and 60 min after exposure). The penetration of aphid stylets into plant tissue (feeding) negatively correlates with the movement of aphid antennae and bodies (Hardie et al. 1992). Consequently, we measured the period when the aphids’ mouthparts were touching the leaves without their antennae and bodies moving as the feeding time. We observed feeding behaviors for 15 min at 1 and 60 min after exposure and measured the total feeding times during these periods. The experiments were replicated 20 times using different test insects for each time point.
Mean numbers (± SEMs) of aphids that a dispersed from host shoots having a ladybird beetle and b survived in the experimental chamber. Aphids were either exposed or not exposed to cues from conspecifics and ladybird beetles, and then the dispersal and survival at 1, 15, 30, and 60 min after exposure were measured. The letters above the bars indicate significant differences among the treatments (GLM, LRT: p < 0.05). The number of aphids that survived was significantly affected by their previous exposure (GLM, LRT: p < 0.05)
Experiment 3: effects of feeding on aphid dispersal
When aphids that had previously been exposed to cues from conspecifics and ladybird beetles encountered predators, they dispersed more rapidly from their shoots and fed for shorter times than conspecifics without such exposure (Figs. 1a, 2). Hence, we tested whether interruptions in feeding lead to increased predator-induced dispersals of aphids. In the absence of predators and cues from conspecifics and predators, almost all the aphids in a colony were feeding on shoots (Tamai, personal observation). Therefore, to obtain feeding and non-feeding aphids, we exposed aphids to cues from conspecifics and ladybird beetles using the same experimental set-up as in the dispersal experiment. An aphid was placed on a broad bean shoot in the exposure chamber and then exposed for 1 h to cues from conspecifics and ladybird beetles, as in the dispersal experiment. The aphid on the shoot was moved from the exposure chamber to the experimental chamber immediately after exposure to cues. Subsequently, a ladybird beetle larva was released onto the petiole of a leaf with the aphid in the experimental chamber.
If interruptions in feeding lead to the quick escape of aphids from predators, then aphids that had not been feeding would disperse more rapidly in response to predators than conspecifics that had been feeding, irrespective of previous exposure. We investigated whether aphids were feeding when they initially encountered predators and recorded the feeding status (i.e., feeding and non-feeding) of aphids that dispersed in response to predators. These behavior were observed for 15 min after the release of predators and repeated 40 times using different test insects for each time point. Aphids that did not disperse were killed by the predator. In eight of the replications, the aphids did not encounter the predators within the 15-min period and, therefore, were discarded.
Statistical analyses
In Experiment 1, the numbers of aphids that dispersed and survived in the experimental chamber were compared as dependent variables, using a generalized linear model (GLM) with a binomial distribution and a logit-link function. The model had two independent variables, exposure to cues and time after exposure, and interactions between these independent variables. The effects of the independent variables and the interactions were tested using the likelihood ratio tests (LRT), which followed a Chi square distribution. We then conducted pairwise comparisons using the LRT, based on the Bonferroni correction, because there was a significant interaction between the independent variables.
In Experiment 2, the total feeding time of an aphid on a leaf in the experimental chamber was compared, as a dependent variable, using ordinary least squares regression with a Gaussian distribution. The model had two independent variables, exposure and time after exposure, and interactions between these independent variables. The effects of the independent variables and the interactions were tested using the LRT as described for the dispersal experiment. We then conducted multiple pairwise comparisons because there were significant differences in the interactions between the independent variables.
In Experiment 3, we compared the proportion of aphids that dispersed (the number of aphids that dispersed/the number of aphids that dispersed + the number of aphids killed) and the number of aphids killed in the cue chamber as dependent variables using a GLM with a binomial distribution and a logit-link function. The model had an independent variable (feeding or non-feeding), and the effects of this independent variable were compared using the LRT. All the analyses were performed with R version 3.3.2 (R Development Core Team 2015).
Results
Experiment 1: effects of previous exposure to cues from conspecifics and ladybird beetles on aphid dispersal
In the cue chamber, ladybird beetles killed 5.33 ± 0.16 aphids during the exposure period. The number of aphids that dispersed from shoots with predators in the experimental chamber was affected by previous exposure to cues from conspecifics and ladybird beetles (χ2 = 607.17, df = 1, p < 0.0001) and the time following exposure (χ2 = 593.09, df = 3, p < 0.01). Furthermore, the dispersal of aphids that had been exposed to the cues was affected by time after exposure (χ2 = 577.37, df = 3, p < 0.01). The predator-induced dispersal of exposed aphids was greater than that of unexposed aphids at 1, 15, and 30 min after exposure (p < 0.01, Fig. 1a), but not after 60 min (p > 0.05, Fig. 1a).
The number of aphids that survived in the experimental chamber was affected by their previous exposure (χ2 = 71.56, df = 1, p < 0.05), but not by the length of time following exposure (χ2 = 65.74, df = 3, p = 0.75). The survival of aphids that had been exposed to cues was not affected by time after exposure (χ2 = 65.24, df = 3, p = 0.92). Aphids exposed to the cues had greater survival rates in the experimental chamber than unexposed aphids (Fig. 1b).
Experiment 2: feeding times of aphids after exposure to cues from conspecifics and ladybird beetles
Ladybird beetles killed 2.03 ± 0.09 aphids during exposure to cues from conspecifics and ladybird beetles. The feeding time of aphids was affected by their previous exposure (χ2 = 97 × 105, df = 1, p < 0.01) and the time after exposure (χ2 = 887 × 104, df = 1, p < 0.01). The feeding time of aphids that had been exposed to the cues was affected by time after exposure (χ2 = 838 × 104, df = 1, p < 0.05). Although the feeding time of exposed aphids was shorter than that of unexposed aphids at 1 min after exposure (p < 0.05, Fig. 2), this difference was not found at 60 min after exposure (p > 0.05, Fig. 2). The feeding time of exposed aphids at 60 min after exposure was longer than that at 1 min after exposure (p < 0.05, Fig. 2).
Experiment 3: effects of feeding on aphid dispersal
When aphids that had been exposed to cues from conspecifics and ladybird beetles encountered predators, 13 individuals were feeding and 19 individuals were not feeding in 32 experimental replications. The feeding status (i.e., feeding and non-feeding) of aphids in the experimental chamber did not affect the number of aphids killed by predators in the cue chamber (5.23 ± 0.39 feeding and 5.26 ± 0.32 non-feeding; χ2 = 16.23, df = 1, p = 0.96). Hence, both feeding and non-feeding aphids had similar predation risks in the cue chamber. When aphids had been exposed to the cues, the proportion of dispersed feeding aphids was lower than that of non-feeding aphids (0.38 feeding and 0.68 non-feeding; χ2 = 887 × 104, df = 1, p < 0.05). This shows that feeding aphids were more likely to be killed by predators than non-feeding aphids.
Discussion
The predator-induced dispersal of aphids that had been exposed to cues from conspecifics and ladybird beetles was greater than that of unexposed aphids. As a result, the survival rate of exposed aphids was greater than that of unexposed aphids. Aphids reduced feeding time soon after exposure and non-feeding aphids increased dispersal rates in response to encounters with predators, compared with feeding conspecifics. Feeding aphids suffered more predation than non-feeding aphids (Experiment 3); therefore, delayed antipredator responses can be fatal to aphids. Thus, by interrupting feeding, exposed aphids could more rapidly escape from predators compared with unexposed conspecifics.
In predator–prey systems, previous experience with predators affects the prey’s antipredator responses during subsequent encounters with predators (Ayon et al. 2017; Choh et al. 2014; Griffin et al. 2001; Stephenson 2016). For example, California ground squirrels, Otospermophilus beecheyi Richardson (Rodentia: Sciuridae), that have encountered live snakes become more sensitive to predator-related cues (Ayon et al. 2017). Learning plays an important role in the antipredator response of prey that have experienced predators (Griffin et al. 2001; Kelley and Magurran 2003; Wisenden et al. 1997). However, it has been unclear how the antipredator responses of prey are altered by previous experiences. Here, we showed that aphids increased their ability to disperse rapidly from shoots by interrupting feeding in response to cues from conspecifics and ladybird beetles.
Prey coexist with several predator species, and the risks of predation by each vary in nature. Therefore, prey may not encounter predator species that they have experienced previously. In fact, some studies have reported that prey show antipredator responses to novel predator species after having experienced another species of predator (Ferrari et al. 2010; Griffin et al. 2001). When aphids interrupted feeding in response to cues from conspecifics and ladybird beetles, they removed their stylets from host plants, leading to their ability to rapidly disperse. The response of removing the stylet is an effective way to respond quickly to predator attack. Hence, exposed aphids may disperse from host plants immediately, even when encountering novel predator species.
The priming effects of cues from conspecifics and ladybird beetles on predator-induced dispersal were observed 1, 15, and 30 min after exposure to the cues, but not after 60 min (Fig. 1a). It is possible that the aphids no longer remembered their experience 60 min after exposure to cues from conspecifics and ladybird beetles. In fact, animal memory is lost with time (Brown et al. 2011; Ferrari et al. 2012; Margulies et al. 2005; Matsumoto and Mizunami 2002; Menzel 2001). For example, when Drosophila melanogaster Meigen (Diptera: Drosophilidae) experience a chemical-odor cue coupled with an electric shock, as a negative reinforcement, the learning effect is reduced by 30–60 min after the experience (Folkers et al. 1993). The retention period of learning depends on the species (Basile and Hampton 2012; Kotler et al. 1992; Němec et al. 2015). Further studies are needed to clarify how the priming effect on predator-induced dispersal is lost.
The aphids reduced their feeding time soon after exposure to cues from conspecifics and ladybird beetles. If aphids did not feed on host shoots for longer periods, then they could starve or reduce their performance and reproduction levels. Although we did not observe the feeding behaviors of aphids during exposure to cues, it is likely that the aphids reduced their feeding times during that period. Nelson (2007) reported that adult female aphids that had experienced starvation for 1 h daily during the nymphal stage have reduced reproduction rates. This suggests that the interruption of feeding can be costly to aphids, even though the starvation period is short (Kouamé and Mackauer 1992; Nelson 2007). Thus, exposed aphids might not show greater dispersal rates than unexposed aphids owing to the restarting of feeding at 60 min after exposure.
In this study, cues from conspecifics and ladybird beetles would contain visual information and volatile substances from ladybird beetles and conspecifics attacked by the predators, such as alarm pheromones and the vibrations of the aphids and the predators, respectively. Aphids disperse from their host plants (Dill et al. 1990; Harrison and Preisser 2016; Roitberg and Myers 1978) and increase the production of winged dispersal morphs (Hatano et al. 2010; Kunert et al. 2005; Podjasek et al. 2005) when exposed to alarm pheromones. In addition, volatile cues from predators themselves play a role in the antipredator responses of prey (Dill et al. 1990; Hermann and Thaler 2014; Oku et al. 2003). Thus, volatile cues released from conspecifics and/or the predators might affect antipredator responses of aphids. When aphids are put very close to artificially crushed conspecifics without contact, they remove their stylets from host plants and then leave the feeding sites (Tamai and Choh, personal observation). However, when aphids are put close to larvae of ladybird beetles, they do not interrupt feeding. Hence, aphids would use volatile cues from conspecifics attacked by ladybird beetles but not visual information and volatile cues from ladybird beetles as possible cues for priming of predator-induced dispersal. Nevertheless, we cannot exclude a possibility that vibration of aphids and ladybird beetles affected feeding of aphids. Further studies are needed to clarify cues involved in the priming effects on antipredator responses of aphids.
The present study demonstrated that the predator-induced dispersal of prey was affected by previous exposure to cues from conspecifics and predators. For organisms that live in groups, such as aphids, the predation risk is not equal for all the individuals. When predators attack an individual prey, other individuals in the same group are likely to perceive information related to predation and prepare for predator avoidance. The heterogeneity of attack rates on each prey individual in a group would weaken the strength of predation by altering the behaviors of surviving prey individuals, and might affect the persistence of predator–prey systems (McCann et al. 1998).
References
Ayon RE, Putman BJ, Clark RW (2017) Recent encounters with rattlesnakes enhance ground squirrel responsiveness to predator cues. Behav Ecol Sociobiol 71:149. https://doi.org/10.1007/s00265-017-2378-1
Barbour MA, Clark RW (2012) Ground squirrel tail-flag displays alter both predatory strike and ambush site selection behaviours of rattlesnakes. Proc R Soc B Biol Sci 279:3827–3833
Basile BM, Hampton RR (2012) Monkeys show recognition without priming in a classification task. Behav Process 93:50–61
Braendle C, Weisser WW (2001) Variation in escape behavior of red and green clones of the pea aphid. J Insect Behav 14:497–509
Brown GE, Ferrari MCO, Malka PH, Oligny MA, Romano M, Chivers DP (2011) Growth rate and retention of learned predator cues by juvenile rainbow trout: Faster-growing fish forget sooner. Behav Ecol Sociobiol 65:1267–1276
Choh Y, Takabayashi J, Sabelis MW, Janssen A (2014) Witnessing predation can affect strength of counterattack in phytoseiids with ontogenetic predator-prey role reversal. Anim Behav 93:9–13
De Brujin PJA, Egas M, Janssen A, Sabelis MW (2006) Pheromone-induced priming of a defensive response in Western flower thrips. J Chem Ecol 32:1599–1603
Dielenberg RA, McGregor IS (2001) Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev 25:597–609
Dill LM, Fraser AHG, Roitberg BD (1990) The economics of escape behaviour in the pea aphid, Acyrthosiphon pisum. Oecologia 83:473–478
Dixon AFG, Agarwala BK (1999) Ladybird-induced life-history changes in aphids. Proc R Soc B Biol Sci 266:1549–1553
Ferrari MCO, Lysak KR, Chivers DP (2010) Turbidity as an ecological constraint on learned predator recognition and generalization in a prey fish. Anim Behav 79:515–519
Ferrari MCO, Vrtělová J, Brown GE, Chivers DP (2012) Understanding the role of uncertainty on learning and retention of predator information. Anim Cogn 15:807–813
Folkers E, Drain P, Quinn WG (1993) Radish, a Drosophila mutant deficient in consolidated memory. Proc Natl Acad Sci USA 60:8123–8127
Goodale E, Kotagama SW (2008) Response to conspecific and heterospecific alarm calls in mixed-species bird flocks of a Sri Lankan rainforest. Behav Ecol 19:887–894
Griffin AS, Evans CS, Blumstein DT (2001) Learning specificity in acquired predator recognition. Anim Behav 62:577–589
Gross P (1993) Insect behavioral and morphological defenses against parasitoids. Annu Rev Entomol 38:251–273
Hardie J, Holyoak M, Taylor NJ, Griffiths DC (1992) The combination of electronic monitoring and video-assisted observations of plant penetration by aphids and behavioural effects of polygodial. Entomol Exp Appl 62:233–239
Harrison KV, Preisser EL (2016) Dropping behavior in the pea aphid (Hemiptera: Aphididae): how does environmental context affect antipredator responses? J Insect Sci 16:1–5
Hatano E, Kunert G, Weisser WW (2010) Aphid wing induction and ecological costs of alarm pheromone emission under field conditions. PLoS One 5:e11188. https://doi.org/10.1371/journal.pone.0011188
Hermann SL, Thaler JS (2014) Prey perception of predation risk: volatile chemical cues mediate non-consumptive effects of a predator on a herbivorous insect. Oecologia 176:669–676
Hughes NK, Korpimäki E, Banks PB (2010) The predation risks of interspecific eavesdropping: weasel-vole interactions. Oikos 119:1210–1216
Kelley JL, Magurran AE (2003) Learned predator recognition and antipredator responses in fishes. Fish Fish 4:216–226
Kotler B, Blaustein L, Brown J (1992) Predator facilitation: the combined effect of snakes and owls on the foraging behavior of gerbils. Ann Zool Fennici 29:199–206
Kouamé KL, Mackauer M (1992) Influence of starvation on development and reproduction in apterous virginoparae of the pea aphid, Acyrthosiphon pisum (Harris) (Homoptera: Aphididae). Can Entomol 124:87–95
Kunert G, Otto S, Röse USR, Gershenzon J, Weisser WW (2005) Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecol Lett 8:596–603
Leavesley AJ, Magrath RD (2005) Communicating about danger: urgency alarm calling in a bird. Anim Behav 70:365–373
Lima SL (1998) Nonlethal effects in the ecology of predator-prey interactions: what are the ecological effects of anti-predator decision-making? Bioscience 48:25–34
Losey JE, Denno RF (1998) The escape response of pea aphids to foliar-foraging predators: factors affecting dropping behaviour. Ecol Entomol 23:53–61
Luca RM, Gerlai R (2012) In search of optimal fear inducing stimuli: differential behavioral responses to computer animated images in zebrafish. Behav Brain Res 226:66–76
Margulies C, Tully T, Dubnau J (2005) Deconstructing memory in Drosophila. Curr Biol 15:700–713. https://doi.org/10.1016/j.cub.2005.08.024
Matsumoto Y, Mizunami M (2002) Temporal determinants of long-term retention of olfactory memory in the cricket Gryllus bimaculatus. J Exp Biol 205:1429–1437
McCann K, Hastings A, Huxel GR (1998) Weak trophic interactions and the balance of enriched metacommunities. Nature 395:794–798
Menzel R (2001) Searching for the memory trace in a mini-brain, the honeybee. Learn Mem 8:53–62
Mondor EB, Roitberg BD (2004) Inclusive fitness benefits of scent-marking predators. Proc R Soc B Biol Sci 271:S341–S343
Nault LR, Edwards LJ, Styer WE (1973) Aphid alarm pheromones: secretion and reception. Environ Entomol 2:101–105
Nelson EH (2007) Predator avoidance behavior in the pea aphid: costs, frequency, and population consequences. Oecologia 151:22–32
Němec M, Syrová M, Dokoupilová L, Veselý P, Šmilauer P, Landová E, Lišková S, Fuchs R (2015) Surface texture and priming play important roles in predator recognition by the red-backed shrike in field experiments. Anim Cogn 18:259–268
Oku K, Yano S, Osakabe M, Takafuji A (2003) Spider mites assess predation risk by using the odor of injured conspecifics. J Chem Ecol 29:2609–2613
Pestana JLT, Baird DJ, Soares AMVM (2013) Predator threat assessment in Daphnia magna: the role of kairomones versus conspecific alarm cues. Mar Freshw Res 64:679–686
Podjasek JO, Bosnjak LM, Brooker DJ, Mondor EB (2005) Alarm pheromone induces a transgenerational wing polyphenism in the pea aphid, Acyrthosiphon pisum. Can J Zool 83:1138–1141
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Roitberg BD, Myers JH (1978) Adaptation of alarm pheromone responses of the pea aphid Acyrthosiphon pisum (Harris). Can J Zool 56:103–108
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation, Vienna. http://www.R-project.org
Schmitt MH, Stears K, Shrader AM (2016) Zebra reduce predation risk in mixed-species herds by eavesdropping on cues from giraffe. Behav Ecol 27:1073–1077
Stephenson JF (2016) Keeping eyes peeled: guppies exposed to chemical alarm cue are more responsive to ambiguous visual cues. Behav Ecol Sociobiol 70:575–584
Suraci JP, Clinchy M, Dill LM, Roberts D, Zanette LY (2016) Fear of large carnivores causes a trophic cascade. Nat Commun 7:1–7. https://doi.org/10.1038/ncomms10698
Tamai K, Choh Y (2018) Antipredator response of pea aphids Acyrthosiphon pisum (Hemiptera: Aphididae): effects of predation risks from an alternative patch on a current patch. Appl Entomol Zool 53:267–274
Tollrian R (1995) Predator-induced morphological defenses: costs, life history shifts, and maternal effects in Daphnia pulex. Ecology 76:1691–1705
Vandermoten S, Mescher MC, Francis F, Haubruge E, Verheggen FJ (2012) Aphid alarm pheromone: an overview of current knowledge on biosynthesis and functions. Insect Biochem Mol Biol 42:155–163
Weisser WW, Braendle C, Minoretti N (1999) Predator-induced morphological shift in the pea aphid. Proc R Soc B Biol Sci 266:1175–1181
Wisenden BD, Chivers DP, Smith RJF (1997) Learned recognition of predation risk by Enallagma damselfly larvae (Odonata, Zygoptera) on the basis of chemical cues. J Chem Ecol 23:137–151
Witz BW (1990) Antipredator mechanisms in arthropods: a twenty year literature survey. Fla Entomol 73:71–99
Acknowledgements
We thank Kiyoshi Nakamuta and Masashi Nomura for their valuable comments. This study was supported by the Japan Society for Promotion of Science (Basic Research C; Grant number 23570018 to YC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tamai, K., Choh, Y. Previous exposures to cues from conspecifics and ladybird beetles prime antipredator responses in pea aphids Acyrthosiphon pisum (Hemiptera: Aphididae). Appl Entomol Zool 54, 277–283 (2019). https://doi.org/10.1007/s13355-019-00623-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-019-00623-3