Abstract
Induced prey defenses can be costly. These costs have the potential to reduce prey survival or reproduction and, therefore, prey population growth. I estimated the potential for predators to suppress populations of pea aphids (Acyrthosiphon pisum) in alfalfa fields through the induction of pea aphid predator avoidance behavior. I quantified (1) the period of non-feeding activity that follows a disturbance event, (2) the effect of frequent disturbance on aphid reproduction, and (3) the frequency at which aphids are disturbed by predators. In combination, these three values predict that the disturbances induced by predators can substantially reduce aphid population growth. This result stems from the high frequency of predator-induced disturbance, and the observation that even brief disturbances reduce aphid reproduction. The potential for predators to suppress prey populations through induction of prey defenses may be strongest in systems where (1) predators frequently induce prey defensive responses, and (2) prey defenses incur acute survival or reproductive costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the goals of behavioral ecology has been to link the behaviors of individual animals to patterns observed at the population level. Predator–prey interactions have been well-studied by both behavioral ecologists and population ecologists, and provide a promising avenue towards relating individual behaviors to population patterns (Smith and Sibly 1985; Schoener 1986; Werner 1992; Anholt 1997; Lima 1998a; Schmitz and Suttle 2001; Beckerman et al. 2002). At the level of populations, predators are recognized for their ability to suppress the growth of prey populations, while increasing their own numbers. The suppressive effect of predators on prey populations is generally assumed to occur through consumption of prey individuals.
At the level of individuals, predators are also recognized for their non-consumptive interactions with prey: prey often respond to the presence of predators with defensive changes in their behavior (and other traits as well). These predator-induced changes in prey behavior can be costly, as the behaviors that help reduce the risk of consumption often cause prey to experience decrements in other correlates of fitness (for reviews, see Sih 1987, 1994; Lima and Dill 1990; Karban and Baldwin 1997; Lima 1998a, b; Sih et al. 1998; Tollrian and Harvell 1999; Preisser et al. 2005). In this paper, I test the hypothesis that the costs associated with prey defensive behaviors contribute to predators’ suppression of prey populations (see S1 for a diagram).
There are two complementary ways to address this hypothesis. The first approach is to expose whole populations of prey to cues associated with predation risk, then test whether cues alone affect population growth. By amputating wasp ovipositors, Tamaki et al. (1970) separated the defense-inducing cues of parasitoid wasps from actual parasitism, and found that population growth in pea aphids was reduced in the presence of amputated wasps. My colleagues and I used a similar strategy to isolate the non-consumptive effects of a pea aphid predator by disabling its mouthparts (Nelson et al. 2004). When small populations of aphids were exposed to a non-consumptive predator in field enclosures, they showed reduced population growth compared to populations in enclosures without predators. Thus, deleting the consumptive ability of predators empirically demonstrates that predators can suppress prey populations by means other than consumption. However, this approach does not shed light on the mechanisms by which non-consumptive interactions retard aphid population growth.
The second method of addressing the hypothesis that the costs of induced defense reduce prey population growth is to test whether the mechanisms that could generate such an effect are in place. Two key components that determine the extent to which induced defenses reduce prey population growth are the costliness of the prey’s induced response in terms of survival and reproduction, and the frequency at which predator–prey encounters result in induction (Nelson and Rosenheim 2006). Among the many types of costs that may be imposed by induced prey defenses, reduced or delayed reproductive output are of particular interest because of their relevance to population growth. Reproductive costs have been documented in ciliates (Kuhlmann et al. 1999), cladocerans (Spitze 1992; Tollrian and Dodson 1999), rotifers (Gilbert 1999), and aphids (Tamaki et al. 1970; Roitberg et al. 1979).
We must also consider the frequency at which prey responses are induced: an extremely costly prey defense will have minimal impact on population growth if it is rarely induced, and a defense that imposes small costs may affect population growth nonetheless if it is commonly induced (Nelson and Rosenheim 2006). Induction rates may be expressed as a frequency, as a proportion of the time budget, or as a proportion of the population, depending on whether the prey response is a discrete event such as a disturbance, a graded response such as an activity reduction, or a non-reversible shift such as accelerated development. With measurements of induction rates in one hand, and measurements of induction costs in the other, it is possible to estimate the dynamical consequences of induced defenses for prey populations.
This paper tests the hypothesis that predators affect pea aphid populations through non-consumptive mechanisms by quantifying the components of the inductive interactions between predators and prey, and then calculating the expected reduction in population growth. Specifically, I measured (1) the immediate costs of aphid behavioral shifts in terms of risk of mortality and reduced aphid feeding activity, (2) the long-term cost of reduced aphid feeding on aphid reproduction, and (3) the frequency of predator-induced behavioral shifts. These measurements are combined to estimate the induction-mediated effect of predators on aphid population growth.
Natural history: the pea aphid
Pea aphids (Acyrthosiphon pisum) are phloem-feeding herbivores on alfalfa and other legumes, where they interact with a suite of predators that includes lady beetles, damsel bugs, hover flies, and parasitoid wasps (Rotheray 1989; Snyder and Ives 2003; Nelson and Rosenheim 2006). Pea aphids are relatively large and mobile aphids, and they respond to predators by extracting their mouthparts from the plant and walking away or dropping from the feeding site. Previous studies have set specific expectations regarding the survivorship costs of avoidance behavior to pea aphids. The dropping behavior of pea aphids has been proposed to increase exposure to lethally high ground temperatures (Roitberg and Myers 1979) and ground-foraging predators (Losey and Denno 1998). Pea aphids are also expected to suffer growth and reproductive costs when predator-induced interruptions in feeding lead to reduced nutrient intake (Dill et al. 1990; Kouame and Mackauer 1992). Because aphids must compensate for the low nitrogen content of their food by dedicating much of their time budget to ingesting phloem (Wilkinson and Douglas 1995; Dixon 1998, pp. 15–16; Caillaud and Via 2000) even small losses in feeding time may be physiologically costly.
Aphids are renowned for their high rate of reproduction. From spring through autumn, populations are composed of parthenogenetic, viviparous females. Pea aphids are born as nymphs, and molt into adults about one week later. One or 2 days later they begin reproducing at a rate of 5–10 offspring per day for about 20 days under laboratory conditions (Campbell and Mackauer 1977).
In general, reductions in reproduction do not necessarily lead to reductions in population growth. A population growth rate that is strongly density-dependent will not be affected by the costs of induced defenses. Where prey experience reproductive costs of induced defense, there exists the potential for predators to influence prey population growth via non-consumptive mechanisms; whether this potential is realized will depend on the biology of the population in question. I have two reasons to speculate that population growth rates may be density-independent for the pea aphids in my study fields. First, the fields were mowed monthly for alfalfa hay, effectively re-setting the system. Second, the pea aphids in my study fields generally occurred as singletons and, aside from a few recently-produced nymphs, did not associate in colonies.
Materials and methods
Costs of predator-induced avoidance behavior I: focal observations of disturbed aphids
To determine the immediate consequences of pea aphid predator avoidance behavior, I conducted direct visual observations of pea aphids that were disturbed by predators in the field. Observations were initiated by haphazardly selecting a location in an alfalfa field and searching for a feeding pea aphid. An aphid predator was released 5–50 mm from the focal aphid to elicit aphid predator avoidance behavior. Because my goal was to gain a synoptic view of aphid predator avoidance behavior, I observed various stages of aphids in different fields at different times of year, and used an assortment of predator taxa to induce disturbance.
Observations were conducted during daytime hours in November 1999, and April, May, June, and September of 2000. These months represent the parts of the growing season in which pea aphids are typically abundant in alfalfa (University of California 1985). Observations were conducted on medium and large nymphs, and winged and apterous (non-winged) adult pea aphids (Table 2). Small nymphs were not used because they were unresponsive to predators.
I collected locally abundant predatory insects from the field and held them for 0.1–3.0 h before their release. Predators used included juvenile and adult coccinellids and nabids, and larval chrysopids (Table 2). Because predator release did not always result in a behavioral interaction, predators were re-introduced up to five times at 3-min intervals until the aphid responded. Fifty-nine percent of the aphids responded after one introduction, and 85% had responded by the third predator introduction.
Aphids typically responded to predator presence by walking away from their feeding site or letting go and dropping from the plant. Once the aphid responded to the presence of the predator by removing its mouthparts from the plant, the observation commenced. I continuously recorded aphid behavior, classified as feeding (mouthparts touching plant), resting, or walking, from the time of feeding interruption to the time the aphid had resettled and resumed feeding. Resettlement was defined as 20 min of continuous feeding. Twenty minutes of the mouthparts touching the plant is a good indication that the aphid has accepted the host plant (Caillaud and Via 2000; M. Caillaud, personal communication). Aphid behavior was recorded using a combination of an audio tape recorder and a hand-held computer (Psion Organiser II LZ64) running event recording software (Noldus Observer 3.0). For each aphid, I recorded whether it survived the disturbance, and I recorded its “disturbance period”, the interval from the time the aphid was disturbed to the time it initiated resettlement (but not including the 20 min of feeding that were observed to determine the point of resettlement).
Observations that ended before the aphid had resettled (for example, if the aphid was lost from view) were considered censored observations. For a censored observation, the disturbance period is not known precisely, but it is known to exceed the observation period. The mean and median disturbance period were calculated using survival analysis, which properly accounts for the information contained in censored observations (SAS 1994; Kleinbaum and Klein 2005).
Costs of predator-induced avoidance behavior II: starvation experiment
To measure the reproductive cost of aphid predator-avoidance behavior, I simulated daily disturbance periods by separating aphids from their host plants once per day for different durations and subsequently recorded the aphids’ nymph production. “Starvation” is used here to refer to the experimentally-determined periods of separation from plants; aphids may not necessarily have been feeding immediately before separation, and they may not necessarily have resumed feeding immediately upon return to the plant (McLean and Kinsey 1969). Shorter starvation periods simulated low rates of disturbance; longer periods simulated high disturbance frequencies.
The aphids used in this experiment were the progeny of field-collected apterous aphids, born in the laboratory and reared for three days in field cages to standardize their age and protect them from parasitism. Three days after birth they were transferred to the experimental alfalfa stems, one aphid per stem. Through the course of the experiment, the aphids developed into adults and began producing nymphs. Aphids were constrained to the top 5–10 cm of the stem by a plastic funnel (diameter 10.2 cm, depth 9.2 cm) lined with polytetrafluoroethylene (Fluon AD1, Asahi Glass Fluoropolymers, Lancashire, UK), a material so smooth that insects cannot walk on it. The open top of the funnel cages exposed the aphids to ambient environmental conditions and permitted daily manipulation of the aphids. Aphids were caged on alfalfa plants for 9 days, 1–9 August 2001. Starvation treatments were imposed daily on 8 of the 9 days, from when the aphids were 3–11 days old (except on day 5, when the field was irrigated).
One hundred and eighty aphids were assigned at random to four starvation treatments: 0, 1, 3, or 6 h every day. Aphids in the 0-h treatment were not disturbed; the 1-, 3-, and 6-h aphids were gently disturbed and then removed from the plant using a fine paintbrush and held in a shaded plastic vial for the appropriate time period. I inspected aphids daily and recorded aphid status (alive, dead, or missing), aphid stage (nymph, apterous adult, or winged adult), and the presence or absence of offspring. Other insects that arrived in the cages were removed. When the aphids were 12 days old the aphids and their alfalfa stems were collected and brought to the laboratory, where the aphids were weighed on a microbalance and the nymphs on each stem were counted.
Eighty aphids (44%) died or escaped before the end of the experiment and were excluded from all analyses. Starvation period did not influence the probability of dying or escaping during the experiment (logistic regressions: n died = 46, r 2 < 0.01, P = 0.31; n missing = 34, r 2 < 0.01, P = 0.92). No aphids mummified. Of the remaining 100 aphids (56%), all individuals molted to the adult stage and 95 produced at least one nymph. The effect of daily starvation period (0, 1, 3, or 6 h) on aphid total fecundity was tested by simple linear regression. Because starvation was expected to reduce reproduction, I tested the null hypothesis that the slope of the regression line was equal to or greater than zero against the one-tailed alternative that the slope was less than zero. I evaluated the effect of starvation on aphid development and body mass, also using simple linear regression. Because starvation was anticipated to delay development and reduce body mass, these tests were also one-tailed.
Frequency of predator-induced disturbance in the field: video observations
To estimate the frequency of predator-induced disturbance under natural conditions, pea aphids were monitored in the field using tripod-mounted video cameras. The feeding activity of individual pea aphids was recorded during August and September 2001 in five fields of alfalfa on the campus of the University of California at Davis, California (30°32′N, 121°44′W) (Table 1). At night, subjects were illuminated using the camera-mounted infrared light. Infrared light is not visible to insects (Chapman 1998 p. 599), and the light did not appear to influence the aphids’ behavior. Aphids were checked periodically, and video recordings were stopped when aphids were missing from their feeding location or when videotapes ended. Videotapes were viewed to reveal the times and reasons that aphids stopped feeding. Observations ended (1) when aphids spontaneously stopped feeding, (2) when aphids were disturbed or consumed in encounters with other insects, (3) when cameras ran out of videotape, or (4) at the predetermined cutoff times that ended a 12-h day or night. Three observations in which the view became obscured were discarded because the time and reason for stopping feeding could not be determined.
Observations were summed by field. Thus, for each field, we observed a number of disturbances in a number of hours of observation time. The nature of these data precluded a direct calculation of the mean disturbance rate and the variance of the mean. Rather, the frequency of predator-induced disturbances was calculated for each of the five study fields as the number of predator-induced disturbances observed divided by the total duration of observed aphid feeding activity. The result was scaled to express the number of disturbances per 12 h period. This calculation gave a point estimate of the disturbance rate in each of the five fields (without associated variance). The disturbance rates measured by this calculation approximate the mean disturbance rates experienced by individual aphids (Feller 1966; Heimpel et al. 1998).
Predator densities were measured by counting all insects collected in ten sweeps of a 28-cm diameter insect net swung through one linear meter of alfalfa. Five replicates of ten sweeps each were conducted in four of the five study fields (one field was not sampled). Other results from these video observations are presented elsewhere (Nelson and Rosenheim 2006); the methods are repeated here for clarity.
Estimating population consequences
To estimate aphid reproductive losses incurred by predator-induced disturbances, I combined the results of the three projects described above. The focal observations provided an estimate of the duration of the predator-induced disturbance period (units: minutes of lost feeding time/disturbance). The starvation experiment yielded an estimate of the percent loss in reproduction for 60 min of daily removal from the plant [units: percent loss in reproduction/(60 min lost feeding time/day)]. I multiplied these two estimates together to calculate the estimated percentage loss in reproduction caused by one daily disturbance [units: percent loss in reproduction/(disturbance/day)].
Bootstrapping procedures were used to determine the 95% confidence interval around this value, as follows. Step 1: the 77 focal observations were resampled with replacement to produce a simulated data set, which was then subjected to survival analysis to calculate minutes of lost feeding time/disturbance. This procedure was repeated 250 times. Step 2: the starvation experiment was simulated by resampling with replacement the data from each of the four starvation periods. The simulated results were subjected to simple linear regression and the slope and intercept were used to calculate percent reproductive loss/60 min lost feeding time; this procedure was repeated 250 times. Step 3: each of the 250 values from step 1 was paired with a value from step 2, and the two were multiplied together. The result was 250 simulated estimates of percent reproductive loss/disturbance, whose distribution yielded a 95% confidence interval. Resampling procedures were conducted using Resampling Stats for Excel (Resampling Stats, Arlington, VA, USA, http://www.resample.com).
The video observations generated a point estimate of the disturbance rate in each of five fields (units: disturbances/12-h day). I multiplied the percent loss caused by one daily disturbance (and the 95% confidence limits) by the field-specific disturbance rate to calculate the estimated percent loss in reproduction in a particular field (units: percent loss in reproduction).
Results
Costs of predator-induced avoidance behavior I: focal observations of disturbed aphids
Ninety-four pea aphid observations were initiated. Seventeen observations were excluded from the analysis: 6 because the predator consumed the aphid, and 11 because the predator induced no response in the aphid. Of the remaining 77 observations, 60 observations were completed when aphids resettled and 17 were censored (terminated before aphid resettlement). Of the 77 observations, 57 aphids dropped, 1 flew, 13 walked, and 6 responded to the predator by interrupting their feeding but did not immediately move away from their feeding site. Of the 57 aphids that dropped, 18 were temporarily or permanently lost from view and their landing location was undetermined, 26 landed on alfalfa plant material lower in the canopy, 6 landed on plants other than alfalfa, 5 landed on dead plant material, and 2 landed on the soil surface. The two aphids that fell to the soil walked onto alfalfa within 2 min. No aphids contacted ground-foraging predators. In all cases, aphids survived the predator-induced disturbance.
The calculation of the mean disturbance period underestimates the true mean because it assumes that each observed disturbance period ended by aphid resettlement. This is true for the completed observations, but for the censored observations it is known only that the disturbance period was longer than the observation period. The mean disturbance period (±SD) was 50.4 ± 6.4 min (biased). The median disturbance period was 31.2 min (lower and upper 95% confidence limits: 23.5 and 46.8 min).
Aphid disturbance period did not vary with month of year or aphid stage (survival analyses, log-rank tests: month of year, P = 0.89; aphid stage, P = 0.84). Intriguingly, there was a marginally significant difference in disturbance period among predator types (survival analysis, log-rank test: P = 0.05); however, because the type of predator used was correlated with field and month (Table 1), this effect is merely suggestive.
Costs of predator-induced avoidance behavior II: starvation experiment
Of the 100 aphids present at the end of the experiment, 10 had developed into winged adults and 90 into apterous adults. Winged and apterous aphids showed similar responses to starvation, but because they have distinct life history strategies (Mackay and Wellington 1975; Campbell and Mackauer 1977), I analyzed their responses separately. For winged aphids, fecundity declined as starvation periods lengthened [P = 0.04 (one-tailed test); data not shown]. Apterous aphids also showed increasing losses in reproduction as they spent more time off the plant [P < 0.0001 (one-tailed test), Fig. 1c].
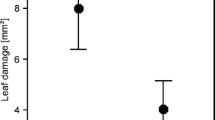
Regressions (means ± SE) of the starvation period in pea aphid (Acyrthosiphon pisum) on a time to adulthood (y = 7.9 + 0.2x; r 2 = 0.23; F = 26.4; P < 0.0001), b adult body weight (y = 2.4 − 0.2x; r 2 = 0.30; F = 35.0; P < 0.0001), and c nymph production (y = 14.2 − 1.3x; r 2 = 0.22; F = 25.4; P < 0.0001). The mean starvation (non-feeding) period resulting from one predator-induced disturbance as determined by focal observations (see text) is 50.4 min or 0.84 h. Regression lines are fit to n = 90 apterous aphids
Starvation caused an increase in development time and a decrease in body weight; (winged aphids: development time, P = 0.015; body weight, P = 0.065; apterous aphids: development time, P < 0.0001; body weight, P < 0.0001; Fig. 1a, b; all tests one-tailed.) The aphids’ loss in fecundity was largely explained by developmental time and body weight. [Stepwise multiple regression showed that nymph production was best explained by body mass and development time, with starvation treatment excluded from the model (r 2 = 0.65, P < 0.0001)].
The results of this experiment offered two ways of estimating the reproductive cost of disturbance. For both methods, I used only the responses of apterous aphids, because they were the numerically dominant morph. The first method is suggested by the focal observations, which showed that disturbed aphids lose, on average, 50.4 min of feeding time. The reproductive cost of one daily disturbance therefore may be most nearly represented by the difference in reproduction between undisturbed aphids and aphids starved for 1 h, which indicates that aphids lose 24% of their total reproduction for every 60 min of daily starvation. The second method considers that the cost per disturbance may not remain constant as aphids are disturbed more than once per day. Therefore, the reproductive costs of multiple disturbances may be best represented by the slope of the regression line relating starvation period to total fecundity, which indicates that total reproduction is reduced by 9.0% for every 60 min of daily starvation. For the purposes of estimating the population consequences of disturbance (see below), I chose to use the more conservative value of 9.0%, bearing in mind that the reproductive costs per daily disturbance are probably higher, particularly for aphids experiencing low disturbance frequencies.
Frequency of predator-induced disturbance in the field: video observations
One hundred and one aphids were observed by video camera for 178.1 h (Table 2). Ninety-seven aphids were observed for 132.9 h during daylight hours, 08:00 to 20:00. Ten aphids were observed for 45.2 h during nighttime hours, 20:00 to 08:00. (Six aphids were observed during both day and night hours.) Observation durations ranged from 1 min to 10 h 29 min. The daytime rate of enemy-induced disturbances measured in the five fields ranged from 0.26 to 4.55 disturbances per 12 h day; the mean (±SE) was 2.03 ± 0.74 (Fig. 2). The nighttime rate of disturbance, measured in two of the five fields, averaged 0.22 ± 0.22 disturbances per 12 h night.
Number of predator-induced disturbances per 12 h day (08:00 to 20:00) experienced by pea aphids living in five alfalfa fields, A–E. Data are from video-recorded observations; see Table 2 for summary statistics. Values were obtained by dividing the total number of predator-induced disturbances by the total duration of observations for each field, then scaling the results to a 12 h period
There was a seasonal component to the variation among fields: fields sampled at later dates had lower daytime disturbance rates (Table 2; linear regression: r 2 = 0.89; P = 0.02). Variation in predator densities may be a possible source of this variation in disturbance rates: linear regression showed that daytime disturbance rates tended to be higher in fields with higher predator densities, but the relationship was not significant [r 2 = 0.68; P = 0.17 (data not shown)].
Estimating population consequences
Population growth depends on births and deaths. As mentioned above (results: focal observations), predator-induced disturbances did not reduce pea aphid survival—so, there is no effect on death rates. Combining the results of the focal observations (50.4 min lost feeding time/disturbance) with the results of the starvation experiment [9.0% reproductive loss/(60 min lost feeding time/day)] generates the prediction that predator-induced disturbances reduce aphid birth rates: aphids experiencing one disturbance per day are expected to suffer a 7.6% loss of reproductive output [95% confidence interval: (4.7%, 10.2%)]. Expected losses are greater in fields where disturbance rates exceed one disturbance per day (and are lower in fields where disturbance rates are less than one disturbance per day). In the five fields where daytime disturbance rates were measured, predator-induced disturbances occurring during daytime hours are projected to cause 2.0–34.6% losses in pea aphid reproduction (Fig. 3). Nighttime disturbance rates were not measured in all fields; to the extent they occur, nighttime disturbances would be expected to cause further losses.
Discussion
The non-consumptive effects of predators on prey populations should be strongest when the cost of prey defense is high, and when the induction of prey defensive behavior is frequent. The studies presented in this paper show that both conditions can be true of pea aphids. First, disturbed pea aphids lose an average of 50.4 min of feeding time—and when aphids lose feeding time, they suffer reductions in fecundity. Second, pea aphids can be disturbed by predators frequently, from 0.26 to 4.55 times per day. Multiplying the reproductive cost per disturbance by the frequency of disturbance yields the key result of this paper: predators, through their effects on aphid behavior, can reduce aphid population birth rates by 2–35%.
This study detected no survival costs for pea aphids disturbed by predators; however, it detected considerable reproductive costs arising from disturbed aphids’ lost feeding time. The high reproductive costs of defense that were detected in the starvation experiment would not have appeared if pea aphids had the ability to compensate for lost feeding time. Removing aphids from their plants for one or more hours per day reduced reproduction approximately linearly, indicating that aphids cannot compensate for lost feeding time by adjusting their activity budgets. Similar starvation regimes imposed in a laboratory study of pea aphids caused similar reductions in fecundity (Kouame and Mackauer 1992). Evidently, reduced phloem intake quickly translates into reduced reproductive output.
One reason that aphids have difficulty compensating for lost feeding time may be that their food source, plant phloem, is nutrient-poor, so the primary constraint on their growth and reproduction is the amount of time spent feeding (Risebrow and Dixon 1987; Dixon 1998 pp. 15–16). Indeed, evidence suggests that, when left undisturbed, aphids devote a large portion of their activity budget to feeding. First, aphids observed for 10 h periods in the laboratory spent an average of 8.5 h in feeding-related activity (Wilkinson and Douglas 1995). Second, undisturbed aphids observed in the video observations reported above occasionally interrupted their feeding spontaneously, about once every 2 h (mean feeding bout duration: 2.0 h). However, separate focal observations of undisturbed aphids show that, after a spontaneous interruption, aphids typically move a short distance and resume probing activity quickly (net displacement: 2.6 ± 0.5 cm; inter-feeding interval: 4.3 ± 1.1 min; means ± SE for 22 aphids, unpublished data), suggesting that aphids do not voluntarily dedicate long periods to non-feeding activity.
Population-level consequences of predator-induced prey defenses should be most visible if, in addition to being costly, prey defensive responses are induced frequently. Video-recorded observations conducted under field conditions showed that predator-induced disturbances are common events: predators can disturb pea aphids from their feeding sites one or more times per day. The frequency of disturbance is a product of predator–aphid encounter rates and aphid behavioral response rates. Encounter rates are expected to vary with predator densities and predator types, both of which vary widely in alfalfa. Sampling efforts in four of the five study fields provided a preliminary indication that predator density may explain some of the field-to-field variation in disturbance rates.
This study joins a host of studies that document the costs of predator avoidance behavior. Although behavioral studies have often shown that induced defenses negatively affect various fitness correlates, population-level approaches to predator–prey interactions generally have not yet incorporated the costliness of prey defense. The studies presented in this paper link together to help translate the effects of predators on individual behavior into effects on prey reproduction and, therefore, prey population growth. This approach was also taken by McPeek and Peckarsky (1998), working with two aquatic insects that reduce their feeding activity in the presence of predators, and subsequently suffer reductions in their body size. McPeek and Peckarsky, using calculations based on the insects’ life history parameters, predicted that predator-induced reductions in feeding activity would influence population growth only in one of the two species. In another version of this mechanistic approach, researchers have constructed life tables for Daphnia reared in the presence or absence of water-borne predator cues, then calculated the intrinsic rate of increase (e.g., Walls and Ketola 1989; Riessen and Sprules 1990; Spitze 1992). When predator cues were present, some clones of Daphnia induced a morphological defense, causing delays and/or reductions in their reproduction and a smaller calculated rate of increase. For pea aphids in alfalfa, the predicted consequences of predator-induced defenses for population growth are generated by the aphids’ behavioral response, the high frequency that it is induced, and the consequent delayed and reduced reproductive output.
The projects described in this paper comprise one half of a two-pronged program testing for induction-mediated predator suppression of pea aphid populations. The reduced aphid population growth that is predicted in this paper was empirically demonstrated in a parallel study of the pea aphid system (Nelson et al. 2004). In two population-level experiments designed to test for disturbance-mediated effects of predators, the growth of caged aphid populations was suppressed by 30% when non-consumptive predators were present, showing that the induction of avoidance behavior can indeed reduce aphid population growth. Together, the population-level experiments and the present study provide the first study system for which we have both a demonstration of non-consumptive predators reducing prey population growth and an understanding of the underlying mechanisms.
The losses in aphid reproduction estimated in this study should be viewed as a qualitative result. The actual reductions in aphid reproduction caused by non-consumptive interactions with predators in my study fields may be quite different from the mean estimates presented in Fig. 3. The video and focal observations are descriptive studies and their results should be fairly general, but the relationship between starvation time and fecundity is specific to the conditions of the starvation experiment. The experiment used young aphids and ran for 9 days, so the measured effects of starvation may pertain to only one portion of aphid life history. Thus, the actual disturbance effects of predators may differ from the estimated effects. However, there are several reasons to think that this study provides a conservative estimate of the reproductive costs of disturbance. First, the starvation experiment modeled multiple disturbance events by increasing the duration of a single starvation period, but three disturbances of 1 h duration may in fact be more costly than one disturbance of 3 h duration; therefore, the starvation experiment may underestimate the costs of multiple disturbances. Second, the “disturbance period” measured by my focal observations included only the time the aphids’ mouthparts were not touching the plant and did not account for the non-feeding time between placing mouthparts on the plant and beginning to ingest phloem; therefore, the focal observations may have underestimated the lost feeding time that follows a predator-induced disturbance. Third, the losses projected for the five study fields are based on predator-induced disturbances that occur during daylight hours. Though the durations and the reproductive costs of nighttime disturbances were not measured in this study, any disturbances induced during nighttime hours would be expected to cause further losses in these fields. Finally, the combined estimates do not account for all possible consequences of disturbance. For example, disturbed aphids may experience greater exposure to secondary predators such as ground beetles, web-building spiders, and entomopathogenic fungi (Losey and Denno 1998; Roy et al. 1998). Any additional costs would make the actual induction-mediated impact of predators greater than that predicted here. Viewed in light of these caveats, the predicted means and their associated bounds provide a useful picture of the range of reproductive loss expected to be caused by predator-induced disturbance.
The calculated estimates of reproductive loss arise from reductions in feeding time, not from mortality. The costs of defense for field-dwelling pea aphids would be still greater if, in addition to reproductive costs, there were also survival costs associated with pea aphid avoidance behavior. However, the nature of survival costs for pea aphids is unclear. Results from other work imply that pea aphids risk dying as a consequence of avoiding predators. Roitberg and Myers (1979) suggest that when pea aphids drop, they might be exposed to high soil temperatures and die of heat stress. Losey and Denno (1998) suggest that fallen pea aphids might become prey to ground-foraging predators. However, the focal observations reported in this study revealed no immediate mortality of disturbed aphids. The pea aphids I observed rarely contacted the ground after responding to a predator, and they never encountered hot soils or ground-foraging predators. The aphids simply lost an average of 50 min potential feeding time and suffered reproductive costs, which have more moderate effects on aphid population growth.
Thus, the calculations presented in this paper show that even in the absence of dramatic, immediately lethal consequences of avoidance behavior, induced defenses against predators can impact population parameters. Costs of anti-predator behaviors have been measured in many organisms and, while short-term mortality costs appear in some species, sublethal costs are more common. Therefore, while immediate mortality is a risk faced by prey avoiding predators in some systems, less dramatic costs such as interruption and disturbance may be much more general consequences of prey defensive strategies.
When the costs of predator-induced interruption and disturbance appear to be small, their impact on prey at the population level depends strongly on their frequency of occurrence. The frequency of predator-induced disturbances depends, in turn, on the predator–prey encounter rate and the prey behavioral response rate. High encounter rates result from high predator–prey ratios. High response rates are expected to result from evolutionary processes when predator cues are both detectable and reliable, and when the cost of responding is low (or the cost of not responding is high). Predictions concerning the consequences of predator-induced disturbances for prey populations will be facilitated when experiments measuring the costs of disturbance are coupled with observations measuring the frequency of disturbance.
The strongest contribution of non-consumptive predator effects to overall prey suppression should occur in systems where prey have frequent encounters with defense-inducing cues, and prey defenses impose substantial reproductive and/or survival costs. In these types of systems, a full understanding of predator impacts on prey populations may require consideration of the non-consumptive, as well as the consumptive effects of predators.
References
Anholt BR (1997) How should we test for the role of behaviour in population dynamics? Evol Ecol 11:633–640
Beckerman A, Benton TG, Ranta E, Kaitala V, Lundberg P (2002) Population dynamic consequences of delayed life-history effects. Trends Ecol Evol 17:263–269
Caillaud MC, Via S (2000) Specialized feeding behavior influences both ecological specialization and assortative mating in sympatric host races of pea aphids. Am Nat 156:606–621
Campbell A, Mackauer M (1977) Reproduction and population growth of the pea aphid under laboratory and field conditions. Can Entomol 109:277–284
Chapman RF (1998) The insects: structure and function, 4th edn. Cambridge University Press, Cambridge
Dill LM, Fraser AHG, Roitberg BD (1990) The economics of escape behavior in the pea aphid, Acyrthosiphon pisum. Oecologia 83:473–478
Dixon AFG (1998) Aphid ecology: an optimization approach, 2nd edn. Chapman and Hall, London
Feller W (1966) An introduction to probability theory and its applications. Wiley, New York
Gilbert JJ (1999) Kairomone-induced morphological defenses in rotifers. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, pp 127–141
Heimpel GE, Mangel M, Rosenheim JA (1998) Effects of time limitation and egg limitation on lifetime reproductive success of a parasitoid in the field. Am Nat 152:273–289
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Kleinbaum DG, Klein M (2005) Survival analysis: a self-learning text, 2nd edn. Springer, Berlin Heidelberg New York
Kouame KL, Mackauer M (1992) Influence of starvation on development and reproduction in apterous virginoparae of the pea aphid, Acyrthosiphon pisum. Can Entomol 124:87–95
Kuhlmann H-W, Kusch J, Heckmann K (1999) Predator-induced defenses in ciliated protozoa. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, pp 142–159
Lima SL (1998a) Nonlethal effects in the ecology of predator–prey interactions. Bioscience 48:25–34
Lima SL (1998b) Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Study Behav 27:215–290
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Losey JE, Denno RF (1998) Positive predator–predator interactions: enhanced predation rates and synergistic suppression of aphid populations. Ecology 79:2143–2152
Mackay PA, Wellington WG (1975) A comparison of the reproductive patterns of apterous and alate virginoparous Acyrthosiphon pisum (Homoptera: Aphididae). Can Entomol 107:1161–1166
McLean DL, Kinsey MG (1969) Probing behavior of the pea aphid, Acyrthosiphon pisum. IV. Effects of starvation on certain probing activities. Ann Entomol Soc Am 62:987–994
McPeek MA, Peckarsky BL (1998) Life histories and the strengths of species interactions: combining mortality, growth, and fecundity effects. Ecology 79:867–879
Nelson E, Rosenheim J (2006) Encounters between aphids and their predators: the relative frequencies of disturbance and consumption. Entomol Exp Appl 118:211–219
Nelson E, Matthews C, Rosenheim J (2004) Predators reduce prey population growth by inducing changes in prey behavior. Ecology 85:1853–1858
Preisser EL, Bolnick DI, Benard MF (2005) Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86:501–509
Riessen HP, Sprules WG (1990) Demographic costs of antipredator defenses in Daphnia pulex. Ecology 71:1536–1546
Risebrow A, Dixon AFG (1987) Nutritional ecology of phloem-feeding insects. In: Slansky F, Rodriguez JG (eds) Nutritional ecology of insects, mites, spiders, and related invertebrates. Wiley, New York, pp 421–448
Roitberg BD, Myers JH (1979) Behavioural and physiological adaptations of pea aphids (Homoptera: Aphididae) to high ground temperatures and predator disturbance. Can Entomol 111:515–519
Roitberg BD, Myers JH, Frazer BD (1979) The influence of predators on the movement of apterous pea aphids between plants. J Anim Ecol 48:111–122
Rotheray GE (1989) Aphid predators: insects that eat greenfly. Richmond Pub. Co. Slough
Roy HE, Pell JK, Clark SJ, Alderson PG (1998) Implications of predator foraging on aphid pathogen dynamics. J Invertebr Pathol 71:236–247
SAS (1994) JMP statistics and graphics guide: version 3. SAS Institute, Cary
Schmitz OJ, Suttle KB (2001) Effects of top predator species on direct and indirect interactions in a food web. Ecology 82:2072–2081
Schoener TW (1986) Mechanistic approaches to community ecology: a new reductionism? Am Zool 26:81–106
Sih A (1987) Predators and prey lifestyles: an evolutionary and ecological overview. In: Kerfoot WC, Sih A (eds) Predation: direct and indirect impacts on aquatic communities. University Press of New England, Hanover, pp 203–224
Sih A (1994) Predation risk and the evolutionary ecology of reproductive behaviour. J Fish Biol 45:111–130
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355
Smith RH, Sibly RM (1985) Behavioural ecology and population dynamics: towards a synthesis. In: Sibly RM, Smith RH (eds) Behavioural ecology: ecological consequences of adaptive behaviour: the 25th symposium of the British Ecological Society, Reading, 1984. Blackwell Scientific, Oxford Oxfordshire, pp 577–591
Snyder WE, Ives AR (2003) Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol. Ecology 84:91–107
Spitze K (1992) Predator-mediated plasticity of prey life history and morphology: Chaoborus americanus predation on Daphnia pulex. Am Nat 139:229–247
Tamaki G, Halfhill JE, Hathaway DO (1970) Dispersal and reduction of colonies of pea aphids by Aphidius smithi (Hymenoptera: Aphidiidae). Ann Entomol Soc Am 63:973–980
Tollrian R, Dodson SI (1999) Inducible defenses in cladocera: constraints, costs, and multipredator environments. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, pp 177–202
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
University of California (1985) Integrated pest management for alfalfa hay. University of California, Division of Agriculture and Natural Resources, Oakland
Walls M, Ketola M (1989) Effects of predator-induced spines on individual fitness in Daphnia pulex. Limnol Oceanogr 34:390–396
Werner EE (1992) Individual behavior and higher-order species interactions. Am Nat 140:S5–S32
Wilkinson TL, Douglas AE (1995) Aphid feeding, as influenced by disruption of the symbiotic bacteria: an analysis of the pea aphid (Acyrthosiphon pisum). J Insect Physiol 41:635–640
Acknowledgments
Thanks to Doug Tallamy for suggesting pea aphids as a study system. The development and execution of the project benefited from discussions with the UC Davis Plant–Insect Group, the Rosenheim Lab Group, and Kai Cha. In the field, I received assistance from Chris Matthews, Kelly Lister, and Kate Chmiel, and our activities were kindly accommodated by Mark Rubio and the UC Davis Animal Science farm crew. I am grateful for the financial support I received from the UC Davis Center for Population Biology, NRI Competitive Grants Program/USDA grants 96-35302-3816 and 2001-35302-10955 to Jay Rosenheim, the San Francisco ARCS Foundation, and Kai Cha. The Rosenheim Lab Group, Rick Karban, Sharon Lawler, and Andy Sih induced changes in this paper by commenting on drafts of the manuscript. The experiments reported here comply with the current USA law.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Oswald Schmitz.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Nelson, E.H. Predator avoidance behavior in the pea aphid: costs, frequency, and population consequences. Oecologia 151, 22–32 (2007). https://doi.org/10.1007/s00442-006-0573-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0573-2