Abstract
The sense of hearing is essential for permitting human beings to interact with the environment, and its dysfunctions can strongly impact on the quality of life. In this context, the cochlea plays a fundamental role in the transformation of the airborne sound waves into electrical signals, which can be processed by the brain. However, several diseases and external stimuli (e.g., noise, drugs) can damage the sensorineural structures of cochlea, inducing progressive hearing dysfunctions until deafness. In clinical practice, the current pharmacological approaches to treat cochlear diseases are based on the almost exclusive use of systemic steroids. In the last decades, the efficacy of novel therapeutic molecules has been proven, taking advantage from a better comprehension of the pathological mechanisms underlying many cochlear diseases. In addition, the feasibility of intratympanic administration of drugs also permitted to overcome the pharmacokinetic limitations of the systemic drug administration, opening new frontiers in drug delivery to cochlea. Several innovative drug delivery systems, such as in situ gelling systems or nanocarriers, were designed, and their efficacy has been proven in vitro and in vivo in cochlear models. The current review aims to describe the art of state in the cochlear drug delivery, highlighting lights and shadows and discussing the most critical aspects still pending in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hearing is one of the most important senses of a human body, since it is strongly involved in the ability of humans to communicate each other. Airborne sounds are collected by a complex anatomical structure, the ear, and translated into electrical signals, which are sent to the occipital lobes of the brain to be processed. According to its anatomy, the ear is conventionally divided in three parts: the outer, middle, and inner ear. The cochlea is the part of inner ear dedicated to the hearing; taking advantage from its snail shell-like structure, it can convert the mechanical stimuli coming from the middle ear to electrical signals.
The cochlea functionality may be affected by external stimuli (e.g., loud sounds), infections, and diseases, which induce alterations of hearing (e.g., tinnitus) and/or reduction of hearing capacity until hearing loss [1, 2]. However, few medicinal products are currently authorized for cochlear indication in humans [3, 4]. Efficacious drug concentrations in the cochlea cannot be easily reached due to the ear anatomy. Similarly, the local drug administration through the outer ear cannot be provided, due to the vacuum space in the middle ear. To overcome this limitation, intratympanic and intracochlear injections are the only possibilities to locally administer therapeutics [4]. In this context, the most promising approaches to improve the drug concentration at the therapeutic site or to decrease the dose regimen appear in situ gelling systems or nanosystems. The need to develop drug delivery systems and novel techniques of administration by these routes is also associated with the identification of novel therapeutic candidates and the study of the cochlear drug distribution.
Based on these premises, the review describes the most critical features of the anatomy and physiology of the inner ear that should be taken in consideration during the design of drug delivery systems intended to be administered by the intratympanic route. The in vitro and in vivo efficacy of several drug substances and drug delivery systems in restoring the physiological functionality of cochlea are discussed based on the articles found in PubMed and Scopus databases. The analysis of information on cochlear implants, other medical devices, and available diagnostic tools is outside the aim of the review. However, the reader can refer to other reviews available in literature [5,6,7,8].
Anatomy of inner ear
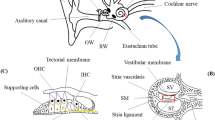
The ear structure is conventionally divided in three parts: the outer, middle, and inner ear (Fig. 1). The outer ear is formed by the auricle, which is the external part of the ear, and the ear channel. Directly in contact with the external environment, this structure amplifies and directs the sound waves from the air to the tympanic membrane, also called eardrum. Placed as separation of the outer ear to the middle ear (i.e., tympanic cavity), the eardrum is a complex membrane formed by three different tissue layers [9]. It is enough flexible to vibrate when stimulated by the sound waves and to translate them in mechanical movements to three ossicles located in the tympanic cavity: the malleus, the incus, and the stapes. The function of these bones is to transfer the mechanical signals induced by the tympanum vibration to the oval window, one of the two openings between the middle and the inner ear. Since the oval window membrane (OWM) is smaller than the tympanic membrane, the vibrations induced by the ossicle movements result amplified when arrive in the inner ear. The cochlea is the part of the inner ear where the mechanical stimuli are translated into electrical signals and, then, sent through the auditory nerves to the occipital lobe of the brain for being processed. High frequencies are detected in the basal region of the cochlea (max 20,000 Hz), whereas low frequencies (100 Hz) near to the helicotrema, which is the apical part of cochlea [10]. The cochlear system can absolve this complex function taking advantage from a snail shell-like structure that includes three compartments: the scala vestibuli, the scala media, and the scala tympani. The scala vestibuli is in direct contact to the OWM and is filled by perilymph, an extracellular fluid with a composition of electrolytes like plasma. The perilymph volume corresponds to the 90% of the total fluid space of cochlea (≈84 μL) [11]. The scala tympani originates from the scala vestibuli near to the helicotrema and ends at the round window, the second opening of the inner ear to the cavity of middle ear. The scala media is located between the two other compartments and contains the organ of Corti, which is the sensory organ of the hearing. The scala media is separated from the scala vestibuli by the Reissner’s membrane and from the scala tympani by the basal membrane. Unlike other cavities, the scala media is filled by endolymph, which differs from perilymph of the higher concentration of potassium ions and the lower of sodium [12]. The different composition of perilymph and endolymph guarantees a positive electric potential of 80–90 mV. The conservation of such electric potential is essential for the correct transduction of mechanical waves in electrical signals and is guaranteed by the negligible permeability of both the Reissner’s and basal membranes and the existence of a sophisticated barrier between endolymph and blood given by the stria vascularis [13]. Indeed, the blood-cochlear barrier, which is likely the blood-brain barrier, is constituted by a continuous capillary endothelium characterized by tight junctions and no fenestrations. The organ of Corti located near the basal membrane of the scala media is formed by the tectorial membrane, the supporting cells, and the hair cells. The last ones are sensorial cells characterized by the presence of stereocilia in the apical surface and connected to nerve synapsis at the basal surface.
When the sound waves induce the stapes movements, the generated perilymphatic waves transfer the vibrations to the basal membrane. The movement of the basal membrane determines the reduction of the gap between the hair cells and the tectorial membrane so that the deflection of stereocilia opens specific mechanical sensitive potassium ion channels in the apical part of the hair cells. The consequent depolarization and repolarization of hair cells permit the translation of the fluid wave movements into electrical signals, which are transferred through nerves to brain for being processed as sounds.
Cochlear diseases and cellular therapeutic targets
Considering the fine structures involved in the hearing process, several pathological mechanisms may cause an impairment on the functionality of cochlea. Indeed, infections [14], drugs [1], autoimmune diseases [15, 16], loud sounds [17, 18], and other external stimuli [19] can affect its ability to transfer the mechanical signals induced by airborne sound waves and to process them in electrical stimuli.
Infections are the most common diseases of the outer and middle ear because they are connected to the external environment and the oral cavity via Eustachian tube, respectively. Otitis media is one of the most frequent disease in infants and children [14]. Taking advantages from the differences in anatomical angles of Eustachian tubes of infants and children with respect to adults, bacteria of the oral cavity can colonize the cavity of middle ear inducing an inflammatory response. Besides the inflammation symptomatology, pain, and risk of eardrum perforation [14], the otitis media can also cause sensorineural hearing loss and an enlargement of endolymphatic spaces, the so-called endolymphatic hydrops [20]. These cochlear complications seem related to the permeation through the round window membrane (RWM) of molecules and bactericidal agents that can be ototoxic [21]. Indeed, several drugs are demonstrated to induce temporary or permanent cochlear dysfunctions, resulting in hearing loss [1]. Aminoglycoside antibiotics (e.g., streptomycin, gentamicin), non-steroidal anti-inflammatory drugs (e.g., salicylates), loop diuretics (e.g., furosemide), and chemotherapy agents (e.g., cisplatin and carboplatin) can alter the functionality of hair cells, although the toxic mechanism may vary according to the drug class. For instance, aminoglycoside antibiotics and cisplatin induce an imbalance in the reactive oxygen species (ROS) in the hair cell cytoplasm [22] and the activation of apoptosis pathways [23, 24]. The ototoxicity of furosemide is due to the interference with the enzymes involved in the ion transport in the stria vascularis, disrupting the barrier properties and altering the endolymph composition with a decrease of the endolymphatic potential [1].
The ROS balance can be also modified by stressful external stimuli, such as loud sounds and chronic noise exposure [17]. High levels of noise weight on the mitochondrial respiratory process of hair cells, producing an excess of free radicals able to alter the cytoplasmic redox balance [25]. In addition, several evidences also suggested that high-frequency sounds impact negatively on the stria vascularis functionality [17, 26], inducing a decrease of the endolymphatic potential [18]. Besides the abovementioned therapeutic targets, different types of ion channels resulted involved in the regulation of ionic fluxes around cochlea [12]. Potassium voltage-gated ion channels (e.g., Kv3) and glutamate receptors are one of the most studied classes for restoring physiological electrical signaling from hair cells to neurons. Indeed, the former channels are directly involved in the repolarization of hair cells [27], whereas the latter ones are overstimulated in stressful conditions (e.g., noise, drugs), inducing a massive calcium influx in neurons [28]. Moreover, nicotinic receptors can be potential candidates for limiting the toxic effects induced by noise [29]. Nicotinic cholinergic receptors (i.e., α9α10 type) are present on the basal surface of hair cells, near to the synapses of the olivocochlear neurons. These neurons are involved in the amplification of sound signaling recorded by hair cells. They can positively modulate the sensitivity of hair cells for sounds, separating them from the background noise and protecting from loud sounds. The activation of α9α10 receptors increases the intracellular calcium concentration, inducing the inhibition of hair cells [30]. The blockage of these receptors can meliorate the control of tinnitus, which is an auditory phantom sensation of ringing in the ears recognized as one of the first clinical signs of damage of the cochlear system [31, 32].
The hearing process can be also affected by immune-mediated diseases [15, 16] and other pathologies with elusive etiology [2]. Among them, Ménière’s disease affects both the vestibular and the cochlear part of inner ear. Its physiopathology seems to be related to the dysfunction in the endolymph homeostasis resulting in the endolymphatic hydrops [33]. The mechanical stress due to the increase in endolymph volume is toxic for the organ of Corti, inducing sensorineural hearing loss and tinnitus. Although the etiopathological mechanism is still unclear, literature evidences suggest an abnormal reduction of endolymph clearance mediated by vasopressin [34]. Indeed, such a hormone controls in physiological conditions the water movements through the endolymphatic sac and the stria vascularis by the modulation of aquaporin expression due to the activation of vasopressin type 2 receptors [35, 36].
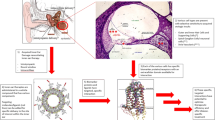
Pharmacokinetics in cochlea and route of administrations
The topical and systemic administration of drugs in the cochlea presents several criticisms due to the peculiar anatomical and histological structures. Indeed, the local drug administration in outer ear results only effective for treating diseases of the outer and middle ear, without reaching therapeutic drug concentrations in cochlear compartments (e.g., perilymph, endolymph) due to the presence of the vacuum of tympanic cavity. The systemic administration presents some issues in the drug distribution. Firstly, the distribution volume of cochlea is limited in comparison to the other physiological compartments, requiring an increase of the administered dose to reach efficacious levels in the scala media. Secondly, the blood-perilymph barrier given by the stria vascularis can also limit the drug distribution in the cochlea [13]. Large and highly charged molecules cannot permeate this barrier, especially in the case of positive-charged molecules due to the endolymph potentials [13]. Therefore, the design of local drug delivery systems is highly desired [5]. Intracochlear drug delivery systems are intended to be injected directly into the cochlear fluids bypassing the absorption step, but their design is critical due to the risk of perilymph leakage from the injection hole and the possible access to the cerebrospinal fluid [37]. Conversely, the intratympanic administration seems to be more promising because the drug product is deposited in the tympanic cavity near to the RWM niche. Although RWM seems to be the main gate of access of drugs to the cochlea from the middle ear, some studies suggested that drug amount could also reach the cochlea via OWM [5, 38]. Thus, it is worthy to underline that an intratympanic drug delivery system should be designed in order to improve the resistance time in the tympanic cavity and to facilitate the drug permeation process through RWM or OWM [5]. Analogously to the eardrum, the RWM is formed by three layers: an outer epithelium in contact with the middle ear cavity, a connective layer, and a squamous epithelium facing the inner ear [21]. The OWM has a similar histology as well. Despite its complex structure, some studies conducted in vivo on animal models suggested that RWM acted as semipermeable membrane. Traces of different types of drugs (e.g., antibiotics, toxin, albumin, anesthetics) or microparticles and nanoparticles could be found in the cochlear fluids after an intratympanic injection [39,40,41,42]. The overall results suggested that several physical or chemical proprieties of molecules or particles could influence the permeation through the RWM via intercellular pathways. The higher the particle size (or the molecular volume), the lower the penetration rate, whereas cationic molecules seem to permeate through the RWM better than anionic ones [41, 43]. In addition, recent published evidences suggested that drug/nanosystem permeation process could be also promoted by endocytosis pathways at the surface of epithelial layer of RWM or OWM [5]. Once a molecule or a particle is in the perilymph, it can reach all the cochlear compartments by passive diffusion through the perilymphatic fluid and partitioning through the cochlear tissues in the different scala. For a more complete description of the pharmacokinetic model of cochlea, the reader is invited to refer to works of Salt and co-workers [4, 44].
Therapeutic molecules
The gold standard in the clinical management of cochlear diseases remains the intratympanic or systemic administration of steroids [44, 45]. Dexamethasone is currently recommended in the treatment of several diseases, such as Ménière’s disease [45], endolymphatic hydrops [46], tinnitus [47], and sudden sensorineural hearing loss [3]. However, the efficacy of both local and systemic steroids in treating cochlear diseases is still matter of debate in medical community, since it has not always supported by extensive and univocal clinical evidences [3, 48, 49].
In the last decades, many efforts were made to propose strategies for preventing the ototoxicity induced by drugs and regenerating the sensorial and supporting cells’ functionality. Tables 1 and 2 report the proof of efficacy of investigated drugs in in vitro and in vivo models, respectively. For an in-depth analysis of the patents recently published on the topic, the reader is invited to refer to work of Nguyen and co-workers [6].
The better comprehension of the etiopathological mechanisms underlying cochlear dysfunctions opened new frontiers in cochlear treatments. Since the drug ototoxicity was mainly induced by the generation of ROS, the effectiveness of several antioxidant agents was tested in vitro [50, 55, 57] and in vivo on animal models [53, 64, 71, 76,77,78, 80] and in human patients [66, 79]. For example, the local or systemic administration of resveratrol or N-acetylcysteine was demonstrated to protect both from drug ototoxicity in animals (e.g., rats, guinea pigs, and zebrafishes) [76, 78] and from noise and loud sounds [77]. However, the mechanism of action of antioxidants is not only limited to a direct effect on cochlear cell lines, as evident in the case of D-methionine and sodium thiosulfate: they prevented the cisplatin-induced ototoxicity, altering the cisplatin pharmacokinetics other than restoring the redox balance in cochlear system [58, 64, 66, 80].
Besides antioxidants, other molecules were proposed to prevent the ROS-mediated and noise-mediated injuries [6, 60, 63, 65, 70, 74]. So and colleagues showed in vitro that flunarizine, a T-type calcium channel blocker, could increase the survival of organ of Corti cells exposed to cisplatin, inhibiting the influx of calcium ions and the lipid peroxidation induced by ROS generation and mitochondrial permeability transition [51]. Moreover, molecules able to modulate the function of ion channel were also investigated to restore the physiological electrolytes’ equilibrium in hair cells and neurons [6]. Activators of Kv3 channels were proposed for treating hearing loss since they are involved in the repolarization of hair cells. Inhibitors of such class of voltage-gated ion channel were studied to treat tinnitus. Molecules able to modulate the activation of nicotinic cholinergic and glutamate receptors were also matter of investigation due to their possible implication on electrical current transmission both in olivocochlear fibers and cochlear hair cells. Boffi and co-workers demonstrated that ascorbic acid acted in vitro as a partial agonist of α9α10 nicotinic cholinergic receptors [83]. Ketamine, a well-known anesthetic drug, resulted effective in reducing the overactivation of N-ethyl-D-aspartate receptors, limiting the toxicity in auditory neurons due to their overstimulation [6]. Another therapeutic strategy investigated for restoring the fitness of neuronal synapsis was the administration of growth factors to cochlea. Indeed, as shown by Suzuki and colleagues, intratympanic delivery of neurotrophin 3 could regenerate the neuronal connections with hair cells after synapsis loss due to high sound exposure [72].
The activation or inhibition of several intracellular metabolic pathways (e.g., Akt/Nrf-2, PI3K, ATM-Chk2-p53) also provided a better resistance of hair cells to damages [56, 75, 84]. For instance, the expression of NADPH oxidases was positively regulated by noise, and the inhibition of their expression might be used as potential therapeutic management of cochlear damages [85]. In this context, the intratympanic administration of a short interfering RNA (siRNA) could reduce the expression of the NOX3 isoform of NADPH oxidase in cells of both organ of Corti and stria vascularis exposed to cisplatin [73]. Moreover, Du and colleagues highlighted that it was possible to regenerate the damaged hair cells using siRNA able to inhibit the expression of gene HES1 [52]. HES1 resulted involved in the downregulation of the gene ATOH1, which encode for a transcription factor involved in differentiation of hair cell. Considering the potential applications in cochlear cell regeneration, many efforts were made for identifying new therapeutic candidates in modulating ATOH1 activation [6]. One of the most promising drug class for the promotion of hair cell regeneration is the inhibitors of γ-secretase, a protein involved in the activation of an inhibitory pathways of ATOH1. In addition, the use of complementary DNA (cDNA) was also investigated to repair genetic dysfunction of hair cells. As an example, intracochlear injection of adenovirus AAV loaded with cDNA of WHRN demonstrated to be effective in restoring the physiological structure of stereocilia of hair cell in a mouse model [82]. However, such a therapeutic solution might be effective only if the sensorial cells resulted only damaged, not destroyed. Since cochlear tissue was not able to restore by itself, the transplantation of stem cells was recently studied to find solutions applicable also in the case of total disruption of hair cells [86]. In this optic, the both embryonic and pluripotent stem cells were proposed and investigated for replacing damaged hair cells, supporting cells, and spinal ganglion neurons [54, 81, 87]. Starting from the demonstration that embryonic stem cells could recreate in vitro the 3D structure of sensorial epithelium of inner ear [87], several studies demonstrated the feasibility of stem cell transplantation via intracochlear injection in different animal models [81]. Although most of the studies showed a good viability and differentiation of stem cells after delivery, several criticisms remain on the round. Firstly, the targeting of the injected cells at the damaged part of the tissues is challenging as well as the regeneration of the complex structure of organ of Corti in vivo. Secondly, the current technologies are not able to target the differentiation of stem cells to preserve the physiological difference in morphological cellular organization in the different part of cochlea [86].
Drug delivery systems
Even if the last decades were characterized by a growing interest to individuate novel therapeutics and technological approaches in the attempt to overcome the biopharmaceutical limitations of cochlea, relatively few information is available in literature. It mainly concerns the design of hydrogels, nanocarriers, and their combinations. The most significant in vitro and in vivo proofs of efficacy of drug delivery systems are summarized in Tables 1 and 2, respectively.
Hydrogels
Although the intratympanic administration results more effective than systemic administration, the biodistribution in the cochlea is strongly influenced by clearance from the RWM surface. Indeed, it significantly reduces the drug residence time of a drug at the adsorption site, as demonstrated in the case of the intratympanic application of methylprednisolone [88]. In order to minimize the formulation washout from RWM, hydrogel formulations made of several natural polymers appeared particularly interesting even if the administrable volume is limited (max 0.4–0.6 mL) [6, 67,68,69, 89, 90]. The efficacy of this approach was demonstrated in the case of hyaluronic acid hydrogel, which could be effective in controlling the delivery of dexamethasone in cochlea of guinea pigs after intratympanic administration [91], as well as in the case of chitosan-based hydrogels containing dexamethasone and gentamicin [92, 93]. Moreover, hydrogels made of gelatin or cross-linked porcine type 1 collagen were effective to deliver proteins, such as insulin-like [68, 69] and hepatocyte growth factors [67].
However, in all these cases, the hydrogel viscosity has to be properly evaluated and selected on the basis of a compromise between the improvement of the resistance time at RWM niche and the injection in the tympanic cavity by a needle lower than G22 [5]. To overcome these limitations, in situ gelling systems, namely injectable formulations that form a macroscopic gel at the site of injection after a stimulus, have been investigated. Engleder et al. designed a thermosensitive hydrogel containing triamcinolone acetonide, taking advantage from the ability of poloxamer 407 to modify its viscosity as function of the temperature [90]. Such a formulation was a solution at room temperature, but it became a gel within few minutes after RWM deposition due to the higher temperature of body (i.e., around 37 °C). Moreover, Yu and co-workers showed that the cochlear distribution of dexamethasone could be significantly improved using a time-sensitive hydrogel made of regenerated silk and polyethylene glycol (PEG) [89]. Such a hydrogel maintained the sol form enough to be intratympanic injected before the sol-gel transition occurred.
Besides the criticisms related to injectability, the technological properties of a hydrogel formulation should be also rationalized to minimize any side effects after deposition in the tympanic cavity. Indeed, some evidences in literature suggested that the intratympanic hydrogels could induce temporary decrease in hearing functionality, particularly when an excessive volume of hydrogel was injected in the tympanic cavity [94]. Such side effect was mainly due to a direct interference of hydrogel with the movements of the three ossicles, and in general, the auditory function was recovered within 7–10 days because of formulation washout from the middle ear [95].
In addition, hydrogels were also studied as vehicles for intracochlear transplantations of stem cells and gene therapy [37, 96]. Since the intracochlear injection required the use of needle of smaller diameter (e.g., G30–G36), the formulation viscosity resulted even more critical in comparison to intratympanic one. However, hydrogel might be more advantageous than solution for preventing the pressure fall in the inner ear due to the RWM perforations, with the risk of an efflux of cerebrospinal fluid through the cochlear aqueduct. Indeed, as demonstrated by Plontke and co-workers, the use of hyaluronic or poloxamer 407 hydrogels preserved the amount of fluorescein that was retained in perilymph of guinea pigs for their ability to seal the RWM perforation [37].
Finally, hydrogels found applications as coating of cochlear implants to prevent the fibrous tissue growth with a consequent worsening of the hearing functionality [97,98,99,100]. To avoid this possible side effect, steroid-loaded hydrogels made of poloxamer 407 were demonstrated to control the release of dexamethasone and prevent fibrous tissue growth in guinea pig model [98]. The use of modified alginate hydrogels was also proposed as coating of cochlear implant to effectively improve its biocompatibility and in vivo performances of the medical device [100].
Nanocarriers
The diffused interest for the therapeutic applications of nanotechnology has also driven the research to novel drug delivery systems intended for both intratympanic and intracochlear administration. Considering the ability of nanoparticles to control and target the drug release, polymeric nanoparticles [40, 61, 62, 101,102,103,104,105,106,107], lipid nanocapsules [59, 108], cubosomes [109], polyplexes [110, 111], metallic core nanoparticles [38, 112,113,114,115], liposomes [43, 116,117,118], and lipoplexes [119] were investigated in vitro and in vivo as drug delivery systems or imaging agents.
Despite the high sensitivity of cochlear structures for external insults, such nanocarriers seem almost safe [108, 109]. As an example, the study of the impact of polymeric nanoparticles, made of poly(d,l-lactide-co-glycolide) (PLGA) and poly(ε-caprolactone)-poly(ethylene glycol) di-block (PCL-PEG), on the in vitro cell viability revealed that such nanocarriers were not toxic for both organ of Corti and stria vascularis cell lines [102]. This result was in agreement with the data provided by Yoon and co-workers on the biocompatibility of different types of oligoarginine-conjugated nanoparticles [104]. In vivo evidences seem to confirm the biocompatibility of polymeric nanocarriers, since they did not alter significantly the auditory functionality of animal models [40, 116]. Analogously, the direct injection of cationic liposomes into guinea pig cochlea did not cause any inflammation or cytotoxic event [119].
Besides the safety profile, another attribute that should be considered as critical is the biodistribution of nanosystems. In fact, if the biodistribution of nanosystems in the cochlea is poor after systemic administration, the intracochlear injection is not easy to perform and only few evidences are reported regarding the efficacy of the drug delivery systems administered by such a route. As an example, Wareing and co-workers demonstrated the feasibility to use cationic liposomes for gene transfection in the cochlear tissue as an alternative to the viral vectors [119]. Indeed, although non-selectively, these nanocarriers could deliver exogenous gene to the spiral limbus, to the spiral ganglia, and to the organ of Corti after a direct injection into the inner ear. Besides these promising results, however, the intratympanic administration of therapeutics remains the most promising alternative [101].
Therefore, the ability of nanocarriers to permeate RWM becomes the most relevant critical issue to assure suitable drug concentration at the therapeutic site. As demonstrated by Ge et al. in chinchilla model, PLGA nanoparticles containing superparamagnetic iron oxide nanoparticles (SPION) could permeate the RWM and distribute in almost all cochlear compartments [107]. Aggregated clusters of nanoparticles were detected in the perilymph fluid and endolymphatic sac near to the organ of Corti. A similar cochlear distribution was also confirmed for other polymeric nanosystems tested in different animal models [40, 106, 109] and for lipid vesicles. The studies conducted by Zou et al. revealed the presence of intact vesicles in the perilymph after intratympanic injection and demonstrated that their permeation through the middle inner ear barriers was size dependent; it was higher for liposomes smaller than 100 nm [43]. In another study, the same research group concluded that RWM was the major pathway for liposome entrance into the inner ear and that liposomes could be retained in cochlea within 6 days after in vivo intratympanic administration [118]. Moreover, liposomes labeled with contrasting agent were traced by magnetic resonance imaging (MRI) after intracochlear and intratympanic administrations in rats, and their distribution in the cochlea was observed within 1 h [117]. Increasing the injected volume, distal portions of the inner ear were also reached by the functionalized nanoparticles, suggesting also a potential exploitation of these systems for vestibular drug delivery.
Besides the obvious applications as imaging tools when loaded in polymeric nanoparticles or liposomes, SPION could be effectively used for promoting the permeation of drugs through the RWM. As an example, Du and co-workers showed that the permeation of dexamethasone was two times increased when SPION-loaded polymeric nanoparticles were used as drug carriers in the presence of a magnetic field [115]. However, despite such interesting biopharmaceutical performances and the possible application in theragnosis, the reduced biodegradability of SPION and the subsequent risk of side effect due to a prolonged biopersistence in cochlea resulted factors that might limit their future applications in humans as therapeutics [120].
The potentialities of drug-loaded nanocarriers in preventing cellular damages induced by ototoxic drugs or for cellular specific targeting were also demonstrated in several preliminary in vivo investigations [111]. Cai et al. showed that PLGA nanoparticles could be effectively used to load and administer different active components present in extract of salvia miltiorrhiza and panax notogineng [105]. Moreover, Sun and co-workers demonstrated that dexamethasone-loaded nanoparticles, made of poly(lactic acid) (PLA)-PEG, could effectively manage adverse effects of cisplatin, regardless of whether they were systemically or locally administered [61, 100]. PLA-PEG nanoparticles resulted more effective than free drug solution to reduce the degeneration of both hair cells and stria vascularis, inflammation processes, and auditory dysfunctions in guinea pig models. In addition, after intratympanic administration, they could improve the drug residence time in the perilymph, suggesting that nanocarriers could be advantageously proposed also to reduce the drug regimen [62].
To improve the effectiveness of nanocarriers in delivering drugs to the therapeutic site, the performances of ligand-functionalized nanoparticles were also investigated. Yoon and colleagues demonstrated that arginine-rich peptides improved the permeation of dexamethasone-loaded polyplexes in cochlear cells, inducing gene expression and anti-inflammatory effect [104]. Accordingly, another cell-penetrating peptide (i.e., PepFect6) resulted effective in favoring the escape of siRNA-loaded polyplexes from the endosomal compartment after cellular uptake in cochlear organotypic culture [110]. Starting from these interesting results on polyplexes, the surface of PCL-PEG nanoparticles was functionalized by a nerve growth factor-derived peptide, demonstrating to favor the in vitro target to the cells involved in the neuronal signaling [111]. Conversely, other cell-penetrating peptides appeared ineffective in targeting lipid nanocapsules as demonstrated by Glueckert and co-workers [107]. Nevertheless, the same authors suggested that antibodies for tyrosine kinase B receptor can effectively improve the in vitro biodistribution and interaction of functionalized silica nanoparticles with positive cell lines in cochlear cell lines [107].
Nanocarrier-loaded hydrogels
Despite the encouraging results obtained in vitro by nanocarriers and the feasibility of their safe administration through the intratympanic route, their residence time in the tympanic cavity remained a critical issue as well as conventional solutions. Thus, to solve this limitation and the possible safety issues related to the administration of too large volume of hydrogel, the combination of these delivery systems seems a very promising strategy. Indeed, the addition of a nanocarrier can solve the issues related to drug loading of the hydrogel, resulting in a significant reduction of the administered volume and in the prolongation of therapeutic drug concentrations in the cochlea, even when the formulation is washed out from the middle ear cavity [116]. Conversely, the viscosity of the hydrogel can improve the resistance time of loading nanosystems in the tympanic cavity. As a consequence, the nanocarrier can benefit by the increase of the resistance time that favors the partition in the OWM or RWM. Although few information is available in literature, some interesting results can be found on the topic [116, 121]. El Kechai and co-workers demonstrated the feasibility of a nanocarrier-loaded hydrogel formulation for intratympanic administration [122]. Liposomes were loaded in a hyaluronic hydrogel, and their composition and concentration were rationalized to obtain a final semisolid preparation with suitable viscosity and elasticity to be injectable. The optimized formulation was used to deliver dexamethasone in cochlea via intratympanic injection. The use of a high viscous hyaluronic acid hydrogel as vehicle permitted to prolong the formulation persistence in the middle ear up to 30 days. When dexamethasone-loaded plain hydrogel (150 μL) was injected in tympanic cavity, drug concentrations were detectable in perilymph up to 15 days. Conversely, when liposome-loaded hydrogel was used, the sustained delivery of dexamethasone was prolonged up to 30 days, even if the volume deposited in the RWM niche was three times lower. Indeed, the results showed a small fraction of intact liposomes in the perilymph, where a great amount of them was retained in the RWM, forming a depot able to sustain the drug release [116]. It also permitted to reach perilymphatic drug concentrations that were very close to the therapeutic level without any negative effects on the hearing functions. Similar results were observed using chitosan hydrogel as vehicle for liposomes [121]. After deposition of hydrogel in the RWM niche, liposomes could diffuse in perilymph and reach the hair cells in mouse model.
Conclusion
The better knowledge of the etiopathological mechanisms underlying cochlear diseases and the development of procedures for the intratympanic drug administration permitted attributing possible new therapeutic indications for well-known active compounds. Indeed, besides the widely use of steroidal anti-inflammatory drugs, antioxidants and other substances were demonstrated to be in vivo effective to prevent the degeneration of organ of Corti cells after exposure to several toxic stimuli, providing a proof of concept of their clinical use in humans. Nevertheless, several issues related to the biopharmaceutical limitations of cochlea still remain on the round. Since the intratympanic injection is invasive and requires specialized medical personnel, the frequency of drug administration should be minimized. However, the continuous fluid drainage from the tympanic cavity may limit the drug residence time at the RWM surface. Moreover, the permeation of the drug through the RWM has to be assured to guarantee its distribution within the cochlea. Among the proposed technological approaches to overcome these issues, the development of nanocarrier-loaded hydrogels appears the most promising. However, the impact of physicochemical properties of vehicles on their injectability, residence time, and permeation of nanocarriers through RWM should be further investigated. Moreover, a better characterization of the biopharmaceutical performances and stability of nanocarriers in the cochlear fluids is highly desirable because many evidences suggested their tendency to aggregate after cochlear biodistribution.
Among the studied nanocarriers, the liposomes appear as the most attractive nanocarriers both for intracochlear and intratympanic injection. Indeed, their high biodegradability, low toxicity, and antigenicity, along with their relatively easy metabolization in vivo, are advantageous attributes to sustain their intracochlear/intratympanic application. Moreover, they can encapsulate different kinds of therapeutics (i.e., steroids [123], chemotherapy drugs [124], and nucleic acids [125, 126]) with very different physicochemical properties. In addition, recent literature evidences suggested that liposome penetrated RWM, forming drug reservoir that could extend the drug release. It may be also underlined that liposome properties can be easily modulated in terms of dimension, surface charge, and fluidity to optimize the permeation performances through the RMW membrane and distribution in cochlear scala. Finally, the liposomal surface can be properly coated with targeting agents to promote active permeation through RWM and cellular targeting.
References
Yorgason JG, Fayad JN, Kalinec F. Understanding drug ototoxicity: molecular insights for prevention and clinical management. Expert Opin Drug Saf. 2006;5(3):383–99.
Vassiliou A, Vlastarakos PV, Maragoudakis P, Candiloros D, Nikolopoulos TP. Meniere’s disease: still a mystery disease with difficult differential diagnosis. Ann Indian Acad Neurol. 2011;14(1):12–8.
Wei BP, Stathopoulos D, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2013; doi:10.1002/14651858.CD003998.pub3.
Salt AN, Plontke SK. Local inner-ear drug delivery and pharmacokinetics. Drug Discov Today. 2005;10(19):1299–306.
El Kechai N, Agnely F, Mamelle E, Nguyen Y, Ferrary E, Bochot A. Recent advances in local drug delivery to the inner ear. Int J Pharm. 2015;494:83–101.
Nguyen K, Kempfle JS, Jung DH, McKenna CE. Recent advances in therapeutic and drug delivery for the treatment of inner ear disease: a patent review (2011-2015). Exp Op Therap Patent. 2017;27(2):191–202.
Litovsky RY, Gordon K. Bilateral cochlear implants in children: effects of auditory experience and deprivation on auditory perception. Hear Res. 2016;338:76–87.
Nguyen S, Cloutier F, Philippon D, Côté M, Bussières R, Backous DD. Outcomes review of modern hearing preservation technique in cochlear implant. Auris Nasus Larynx. 2016;43(5):485–8.
Lim DJ. Structure and function of the tympanic membrane: a review. Acta Otorhinolaryngol Belg. 1995;49(2):101–15.
Williams C. Hearing restoration: Graeme Clark, Ingeborg Hochmair, and Blake Wilson receive the 2013 Lasker~DeBakey Clinical Medical Research Award. J Clin Invest. 2013;123(10):4102–6.
Igarashi M, Ohashi K, Ishii M. Morphometric comparison of endolympatic and perilymphatic spaces in human temporal bones. Acta Otolaryngol. 1986;101(3–4):161–4.
Zdebik AA, Wangemann P, Jentsch T. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology. 2009;24:307–16.
Misrahy GA, Spradley JF, Beran AV, Garwood VP. Permeability of cochlear partitions: comparison with blood-brain barrier. Acta Otolaryngol. 1960;52:525–34.
Principi N, Marchisio P, Rosazza C, Sciarrabba CS, Esposito S. Acute otitis media with spontaneous tympanic membrane perforation. Eur J Clin Microbiol Infect Dis. 2017;36(1):11–8.
Ryan AF, Harris JP, Keithley EM. Immune-mediated hearing loss: basic mechanisms and options for therapy. Acta Otolaryngol Suppl. 2002;548:38–43.
Ruckenstein MJ. Autoimmune inner ear disease. Curr Opin Otolaryngol Head Neck Surg. 2004;12(5):426–30.
Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252(8):504–8.
Ishida A, Sugisawa T, Yamamura K. Effects of high-frequency sound on the guinea pig cochlea. Electrophysiological study using cochlear microphonics, action and endocochlear potential. ORL J Otorhinolaryngol Relat Spec. 1993;55(6):332–6.
Bhandare N, Antonelli PJ, Morris CG, Malayapa RS, Mendenhall WM. Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67(2):469–79.
Paparella MM, Goycoolea MV, Meyerhoff WL. Inner ear pathology and otitis media. A review. Ann Otol Rhinol Laryngol Suppl. 1980;89(3 Pt 2):249–53.
Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121(4):437–47.
Temple MD, Perrone GG, Dawes IW. Complex cellular responses to reactive oxygen species. Trends Cell Biol. 2005;15(6):319–26.
Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226(1–2):157–67.
Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002;164(1–2):115–26.
Jacono AA, Hu B, Kopke RD, Henderson D, Van De Water TR, Steinman HM. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res. 1998;117(1–2):31–8.
Yamane H, Nakai Y, Konishi K, Sakamoto H, Matsuda Y, Iguchi H. Strial circulation impairment due to acoustic trauma. Acta Otolaryngol. 1991;111(1):85–93.
Ruby B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–26.
Bing D, Lee SC, Campanelli D, Xiong H, Matsumoto M, Panford-Walsh R, et al. Cochlear NMDA receptors as therapeutic target of noise-induced tinnitus. Cell Physiol Biochem. 2015;35:1905–23.
Goutman JD, Elgoyhen AB, Gomez-Casati ME. Cochlear hair cells: the sound-sensing machines. FEBS Lett. 2015;589(22):3354–61.
Darbon P, Wright DJ, Evans MG. Conductance properties of the acetylcholine receptor current of Guinea pig outer hair cells. J Assoc Res Otolaryngol. 2011;12(1):59–70.
Plazas PV, Savino J, Kracun S, Gomez-Casati ME, Katz E, Parsons CG, et al. Inhibition of the alpha9alpha10 nicotinic cholinergic receptor by neramexane, an open channel blocker of N-methyl-D-aspartate receptors. Eur J Pharmacol. 2007;566(1–3):11–9.
Eggermont JJ. Tinnitus: neurobiological substrates. Drug Discov Today. 2005;10(19):1283–90.
Salt AN, Plontke SK. Endolymphatic hydrops: pathophysiology and experimental models. Otolaryngol Clin N Am. 2010;43(5):971–83.
Degerman E, In't Zandt R, Palbrink AK, Magnusson M. Vasopressin induces endolymphatic hydrops in mouse inner ear, as evaluated with repeated 9.4 T MRI. Hear Res. 2015;330:119–24.
Nishioka R, Takeda T, Kakigi A, Okada T, Takebayashi S, Taguchi D, et al. Expression of aquaporins and vasopressin type 2 receptor in the stria vascularis of the cochlea. Hear Res. 2010;260(1–2):11–9.
Maekawa C, Kitahara T, Kizawa K, Okazaki S, Kamakura T, Horii A, et al. Expression and translocation of aquaporin-2 in the endolymphatic sac in patients with Meniere’s disease. J Neuroendocrinol. 2010;22(11):1157–64.
Plontke SK, Hartsock JJ, Gill RM, Salt AN. Intracochlear drug injections through the round window membrane: measures to improve drug retention. Audiol Neurootol. 2016;21(2):72–9.
Zou J, Ostrovsky S, Israel LL, Feng H, Kettunen MI, Lellouche JPM, et al. Efficient penetration of ceric ammonium nitrate oxidant-stabilized gamma-maghemite nanoparticles through the oval and round windows into the rat inner ear as demonstrated by MRI. J Biomed Mater Res B Appl Biomater. 2016; doi:10.1002/jbm.b.33719.
Smith BM, Myers MG. The penetration of gentamicin and neomycin into perilymph across the round window membrane. Otolaryngol Head Neck Surg. 1979;87(6):888–91.
Youm I, Musazzi UM, Gratton MA, Murowchick JB, Youan BBC. Label-free ferrocene-loaded nanocarrier engineering for in vivo cochlear drug delivery and imaging. J Pharm Sci. 2016;105(10):3162–71.
Lundman L, Juhn SK, Bagger-Sjoback D, Svanborg C. Permeability of the normal round window membrane to Haemophilus influenzae type b endotoxin. Acta Otolaryngol. 1992;112(3):524–9.
Goycoolea MV, Muchow D, Schachern P. Experimental studies on round window structure: function and permeability. Laryngoscope. 1988;98:1–20.
Zou J, Sood R, Ranjan S, Poe D, Ramadan UA, Pyykko I, et al. Size-dependent passage of liposome nanocarriers with preserved post-transport integrity across the middle-inner ear barriers in rats. Otol Neurotol. 2012;33(4):666–73.
Plontke SK, Salt AN. Simulation of application strategies for local drug delivery to the inner ear. ORL J Otorhinolaryngol Relat Spec. 2006;68(6):386–92.
Phillips JS, Westerberg B. Intratympanic steroids for Ménière’s disease or syndrome. Cochrane Database Syst Rev. 2011; doi:10.1002/14651858.CD008514.pub2.
Liu B, Zhang S, Leng Y, Zhou R, Liu J, Kong W. Intratympanic injection in delayed endolymphatic hydrops. Acta Otolaryngol. 2015;135(10):1016–21.
Albu S, Chirtes F. Intratympanic dexamethasone plus melatonin versus melatonin only in the treatment of unilateral acute idiopathic tinnitus. Am J Otolaryngol. 2014;35(5):617–22.
Chandrasekhar SS. In reference to intratympanic dexamethasone injection for refractory tinnitus: Prospective placebo-controlled study. Laryngoscope. 2014; doi:10.1002/lary.24438.
El Sabbagh NG, Sewitch MJ, Bezdjian A, Daniel SJ. Intratympanic dexamethasone in sudden sensorineural hearing loss: a systematic review and meta-analysis. Laryngoscope. 2016; doi:10.1002/lary.26394.
Kim SK, Im GJ, An YS, Lee SH, Jung HH, Park SY. The effects of the antioxidant alpha-tocopherol succinate on cisplatin-induced ototoxicity in HEI-OC1 auditory cells. Int J Pediatr Otorhinolaryngol. 2016;86:9–14.
So HS, Park C, Kim HJ, Lee JH, Park SY, Lee ZW, et al. Protective effect of T-type calcium channel blocker flunarizine on cisplatin-induced death of auditory cells. Hear Res. 2005;204(1–2):127–39.
Du X, Li W, Gao X, West MB, Saltzman WM, Cheng CJ, et al. Regeneration of mammalian cochlear and vestibular hair cells through Hes1/Hes5 modulation with siRNA. Hear Res. 2013;304:91–110.
Wu CY, Lee HJ, Liu CF, Korivi M, Chen HH, Chan MH. Protective role of L-ascorbic acid, N-acetylcysteine and apocynin on neomycin-induced hair cell loss in zebrafish. J Appl Toxicol. 2015;35(3):273–9.
Bas E, Van De Water TR, Lumbreras V, Rajguru S, Goss G, Hare JM, Goldstein BJ. Adult human nasal mesenchymal-like stem cells restore cochlear spiral ganglion neurons after experimental lesion. Stem Cells Dev. 2014;23:502–14.
Feghali JG, Liu W, Van De Water TR. L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope. 2001;111(7):1147–55.
Youn CK, Jo ER, Sim JH, Cho SI. Peanut sprout extract attenuates cisplatin-induced ototoxicity by induction of the Akt/Nrf2-mediated redox pathway. Int J Pediatr Otorhinolaryngol. 2017;92:61–6.
Bonabi S, Caelers A, Monge A, Huber A, Bodmer D. Resveratrol protects auditory hair cells from gentamicin toxicity. Ear Nose Throat J. 2008;87(10):570–3.
Neuwelt EA, Brummett RE, Remsen LG, Kroll RA, Pagel MA, McCormick CI, et al. In vitro and animal studies of sodium thiosulfate as a potential chemoprotectant against carboplatin-induced ototoxicity. Cancer Res. 1996;56(4):706–9.
Glueckert R, Pritz CO, Roy S, Dudas J, Schrott-Fischer A. Nanoparticle mediated drug delivery of rolipram to tyrosine kinase B positive cells in the inner ear with targeting peptides and agonistic antibodies. Front Aging Neurosci. 2015; doi:10.3389/fnagi.2015.00071.
Astolfi L, Simoni E, Valente F, Ghiselli S, Hatzopoulos S, Chicca M, et al. Coenzyme Q10 plus multivitamin treatment prevents cisplatin ototoxicity in rats. PLoS One. 2016;11(9):e0162106.
Sun C, Wang X, Chen D, Lin X, Yu D, Wu H. Dexamethasone loaded nanoparticles exert protective effects against cisplatin-induced hearing loss by systemic administration. Neurosci Lett. 2016;619:142–8.
Sun C, Wang X, Zheng Z, Chen D, Shi F, Yu D, et al. A single dose of dexamethasone encapsulated in polyethylene glycol-coated polylactic acid nanoparticles attenuates cisplatin-induced hearing loss following round window membrane administration. Int J Nanomedicine. 2015;10:3567–79.
Toplu Y, Sapmaz E, Parlakpinar H, Kelles M, Kalcioglu MT, Tanbek K, et al. The effect of dexpanthenol on ototoxicity induced by cisplatin. Clin Exp Otorhinolaryngol. 2016;9(1):14–20.
Ekborn A, Laurell G, Johnstrom P, Wallin I, Eksborg S, Ehrsson H. D-Methionine and cisplatin ototoxicity in the guinea pig: D-methionine influences cisplatin pharmacokinetics. Hear Res. 2002;165(1–2):53–61.
Uzun L, Kokten N, Cam OH, Tayyar Kalcioglu M, Birol Ugur M, Tekin M, et al. The effect of garlic derivatives (S-allylmercaptocysteine, diallyl disulfide, and S-allylcysteine) on gentamicin induced ototoxicity: an experimental study. Clin Exp Otorhinolaryngol. 2016;9(4):309–13.
Dias MA, Sampaio AL, Venosa AR, Meneses Ede A, Oliveira CA. The chemopreventive effect of Ginkgo biloba extract 761 against cisplatin ototoxicity: a pilot study Int Tinnitus J 2015; 19(2):12–9.
Inaoka T, Nakagawa T, Kikkawa YS, Tabata Y, Ono K, Yoshida M, et al. Local application of hepatocyte growth factor using gelatin hydrogels attenuates noise-induced hearing loss in guinea pigs. Acta Otolaryngol. 2009;129(4):453–7.
Iwai K, Nakagawa T, Endo T, Matsuoka Y, Kita T, Kim TS, et al. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope. 2006;116(4):529–33.
Fujiwara T, Hato N, Nakagawa T, Tabata Y, Yoshida T, Komobuchi H, et al. Insulin-like growth factor I treatment via hydrogels rescues cochlear hair cells from ischemic injury. Neuroreport. 2008;19(16):1585–8.
Demir MG, Altintoprak N, Aydin S, Kosemihal E, Basak K. Effect of transtympanic injection of melatonin on cisplatin-induced ototoxicity. J Int Adv Otol. 2015;11(3):202–6.
Choe WT, Chinosornvatana N, Chang KW. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neuroto. 2004;25(6):910–5.
Suzuki J, Corfas G, Liberman MC. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep. 2016; doi:10.1038/srep24907.
Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, Rybak LP. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13(5):589–98.
Bekmez Bilmez ZE, Aydin S, Sanli A, Altintoprak N, Demir MG, Atalay Erdogan B, et al. Oxytocin as a protective agent in cisplatin-induced ototoxicity. Cancer Chemother Pharmacol. 2016;77(4):875–9.
Benkafadar N, Menardo J, Bourien J, Nouvian R, François F, Decaudin D, et al. Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol Med. 2017;9(1):7–26.
Simsek G, Tokgoz SA, Vuralkan E, Caliskan M, Besalti O, Akin I. Protective effects of resveratrol on cisplatin-dependent inner-ear damage in rats. Eur Arch Otorhinolaryngol. 2012;270(6):1789–93.
Seidman M, Babu S, Tang W, Naem E, Quirk WS. Effects of resveratrol on acoustic trauma. Otolaryngol Head Neck Surg. 2003;129(5):463–70.
Erdem T, Bayindir T, Filiz A, Iraz M, Selimoglu E. The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur Arch Otorhinolaryngol. 2011;269(10):2185–8.
Doolittle ND, Muldoon LL, Brummett RE, Tyson RM, Lacy C, Bubalo JS, et al. Delayed sodium thiosulfate as an otoprotectant against carboplatin-induced hearing loss in patients with malignant brain tumors. Clin Cancer Res. 2001;7(3):493–500.
Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. The J Pharmacol Exp Ther. 2005;314(3):1052–8.
Barboza LCM, Lezirovitz K, Zanatta DB, Strauss BE, Mingroni-Netto RC, Oiticica J, et al. Transplantation and survival of mouse inner ear progenitor/stem cells in organ of Corti after cochleostomy of hearing-impaired guinea pigs: preliminary results. Braz J Med Biol Res. 2016; doi:10.1590/1414-431X20155064.
Chien WW, Isgrig K, Roy S, Belyantseva IA, Drummond MC, May LA, et al. Gene therapy restores hair cell stereocilia morphology in inner ears of deaf whirler mice. Mol Ther. 2016;24:17–25.
Boffi JC, Wedemeyer C, Lipovsek M, Katz E, Calvo DJ, Elgoyhen AB. Positive modulation of the a9a10 nicotinic cholinergic receptor by ascorbic acid. Br J Pharmacol. 2013;168:954–65.
Jadali A, Kwan KY. Activation of PI3K signaling prevents aminoglycoside-induced hair cell death in the murine cochlea. Biol Open. 2016;5(6):698–708.
Vlajkovic SM, Lin SCY, Wong ACY, Wackrow B, Thorne PR. Noise-induced changes in expression levels of NADPH oxidases in the cochlea. Hear Res. 2013;304:145–52.
Okano T, Kelley MW. Stem cell therapy for the inner ear: recent advances and future directions. Trends Amplif. 2012;16:4–18.
Koehler KR, Mikosz AM, Molosh AI, Patel D, Hashino E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature. 2013;500:217–21.
Plontke SK, Mikulec AA, Salt AN. Rapid clearance of methylprednisolone after intratympanic application in humans. Comment on: Bird PA, Begg EJ, Zhang M, et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol 2007;28:1124-30. Otol Neurotol. 2008;29(5):732–3.
Yu D, Sun C, Zheng Z, Wang X, Chen D, Wu H, et al. Inner ear delivery of dexamethasone using injectable silk-polyethylene glycol (PEG) hydrogel. Int J Pharm. 2016;503(1–2):229–37.
Engleder E, Honeder C, Klobasa J, Wirth M, Arnoldner C, Gabor F. Preclinical evaluation of thermoreversible triamcinolone acetonide hydrogels for drug delivery to the inner ear. Int J Pharm. 2014;471(1–2):297–302.
Borden RC, Saunders JE, Berryhill WE, Krempl GA, Thompson DM, Queimado L. Hyaluronic acid hydrogel sustains the delivery of dexamethasone across the round window membrane. Audiol Neurootol. 2011;16(1):1–11.
Paulson DP, Abuzeid W, Jiang H, Oe T, O'MalleyBW, Li D. A novel controlled local drug delivery system for inner ear disease. Laryngoscope. 2008;118(4):706–11.
Lajud SA, Han Z, Chi FL, Gu R, Nagda DA, Bezpalko O, et al. A regulated delivery system for inner ear drug application. J Control Release. 2013;166(3):268–76.
Feng L, Ward JA, Li SK, Tolia G, Hao J, Choo DI. Assessment of PLGA–PEG–PLGA copolymer hydrogel for sustained drug delivery in the ear. Curr Drug Delivery. 2014;11:279–86.
Honeder C, Engleder E, Schopper H, Gabor F, Reznicek G, Wagenblast J, Gstoettner W, Arnoldner C. Sustained release of triamcinolone acetonide from an intratympanically applied hydrogel designed for the delivery of high glucocorticoid doses. Audiol Neurootol. 2014;19:193–202.
Nayagam BA, Backhouse SS, Cimenkaya C, Shepherd RK. Hydrogel limits stem cell dispersal in the deaf cochlea: implications for cochlear implants. J Neural Eng. 2012; doi:10.1088/1741-2560/9/6/065001.
Hütten M, Dhanasingh A, Hessler R, Stöver T, Esser KH, Möller M, et al. In vitro and in vivo evaluation of a hydrogel reservoir as a continuous drug delivery system for inner ear treatment. PLoS ONE. 2014;9(8):e104564.
Honeder C, Zhu C, Schöpper H, Gausterer JC, Walter M, Landegger LD, et al. Effects of sustained release dexamethasone hydrogels in hearing preservation cochlear implantation. Hear Res. 2016;341:43–9.
Wrzeszcz A, Steffens M, Balster S, Warnecke A, Dittrich B, Lenarz T, et al. Hydrogel coated and dexamethasone releasing cochlear implants: quantification of fibrosis in guinea pigs and evaluation of insertion forces in a human cochlea model. J Biomed Mater Res B Appl Biomater. 2015;103(1):169–78.
Chikar JA, Hendricks JL, Richardson-Burns SM, Raphael Y, Pfingst BE, Martin DC. The use of a dual PEDOT and RGD-functionalized alginate hydrogel coating to provide sustained drug delivery and improved cochlear implant function. Biomaterials. 2012;33(7):1982–90.
Tamura T, Kita T, Nakagawa T, Endo T, Kim TS, Ishihara T et al Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope 2005;115(11):2000–5
Musazzi UM, Youm I, Murowchick JB, Ezoulin MJ, Youan B-BC. Resveratrol-loaded nanocarriers: Formulation, optimization, characterization and in vitro toxicity on cochlear cells. Colloids Surf B Biointerfaces. 2014;118(0):234–42.
Wen X, Ding S, Cai H, Wang J, Wen L, Yang F, et al. Nanomedicine strategy for optimizing delivery to outer hair cells by surface-modified poly(lactic/glycolic acid) nanoparticles with hydrophilic molecules. Int J Nanomedicine. 2016;11:5959–69.
Yoon JY, Yang KJ, Kim DE, Lee KY, Park SN, Kim DK, et al. Intratympanic delivery of oligoarginine-conjugated nanoparticles as a gene (or drug) carrier to the inner ear. Biomaterials. 2015;73:243–53.
Cai H, Wen X, Wen L, Tirelli N, Zhang X, Zhang Y, et al. Enhanced local bioavailability of single or compound drugs delivery to the inner ear through application of plga nanoparticles via round window administration. Int J Nanomedicine. 2014;9(1):5591–601.
Kim DK, Park SN, Park KH, Park CW, Yang KJ, Kim JD, et al. Development of a drug delivery system for the inner ear using poly(amino acid)-based nanoparticles. Drug Deliv. 2015;22(3):367–74.
Ge X, Jackson RL, Liu J, Harper EA, Hoffer ME, Wassel RA, et al. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol Head Neck Surg. 2007;137(4):619–23.
Zhang Y, Zhang W, Löbler M, Schmitz KP, Saulnier P, Perrier T, et al. Inner ear biocompatibility of lipid nanocapsules after round window membrane application. Int J Pharm. 2011;404(1–2):211–9.
Bu M, Tang J, Wei Y, Sun Y, Wang X, Wu L, et al. Enhanced bioavailability of nerve growth factor with phytantriol lipid-based crystalline nanoparticles in cochlea. Int J Nanomedicine. 2015;10:6879–89.
Dash-Wagh S, Langel U, Ulfendahl M. Pepfect6 mediated siRNA delivery into organotypic cultures. Methods Mol Biol. 2016;1364:27–35.
Roy S, Johnston AH, Newman TA, Glueckert R, Dudas J, Bitsche M, et al. Cell-specific targeting in the mouse inner ear using nanoparticles conjugated with a neurotrophin-derived peptide ligand: potential tool for drug delivery. Int J Pharm. 2010;390(2):214–24.
Zou J, Hannula M, Misra S, Feng H, Labrador RH, Aula AS, et al. Micro CT visualization of silver nanoparticles in the middle and inner ear of rat and transportation pathway after transtympanic injection. J Nanobiotechnology. 2015;13(1):–5.
Feng H, Pyykkö I, Zou J. Involvement of ubiquitin-editing protein A20 in modulating inflammation in rat cochlea associated with silver nanoparticle-induced CD68 upregulation and TLR4 activation. Nanoscale Res Lett. 2016; doi:10.1186/s11671-016-1430-9.
Kim JW, Lee JH, Ma JH, Chung E, Choi H, Bok J, et al. Magnetic force nanoprobe for direct observation of audio frequency tonotopy of hair cells. Nano Lett. 2016;16(6):3885–91.
Du X, Chen K, Kuriyavar S, Kopke RD, Grady BP, Bourne DH, et al. Magnetic targeted delivery of dexamethasone acetate across the round window membrane in guinea pigs. Otol Neurotol. 2013;34(1):41–7.
El Kechai N, Mamelle E, Nguyen Y, Huang N, Nicolas V, Chaminade P, et al. Hyaluronic acid liposomal gel sustains delivery of a corticoid to the inner ear. J Control Release. 2016;226:248–57.
Zou J, Sood R, Ranjan S, Poe D, Ramadan UA, Kinnunen PK, et al. Manufacturing and in vivo inner ear visualization of MRI traceable liposome nanoparticles encapsulating gadolinium. J Nanobiotechnology. 2010;8:32.
Zou J, Sood R, Zhang Y, Kinnunen PK, Pyykko I. Pathway and morphological transformation of liposome nanocarriers after release from a novel sustained inner-ear delivery system. Nanomedicine. 2014;9(14):2143–55.
Wareing M, Mhatre AN, Pettis R, Han JJ, Haut T, Pfister MHF, et al. Cationinc liposome mediated transgene expression in guinea pig cochlea. Hear Res. 1999;128:61–9.
Tee JK, Ong CN, Bay BH, Ho HK, Leong DT. Oxidative stress by inorganic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:414–38.
Lajud SA, Nagda DA, Qiao P, Takada N, Civantos A, Gu R, et al. A novel chitosan-hydrogel-based nanoparticle delivery system for local inner ear application. Otol Neurotol. 2015;36(2):341–7.
El Kechai N, Bochot A, Huang N, Nguyen Y, Ferrary E, Agnely F. Effect of liposomes on rheological and syringeability properties of hyaluronic acid hydrogels intended for local injection of drugs. Int J Pharm. 2015;487:187–96.
Ozbakir B, Crielaard BJ, Metselaar JM, Storm G, Lammers T. Liposomal corticosteroids for the treatment of inflammatory disorders and cancer. J Control Release. 2014;190:624–36.
Gaviani P, Corsini E, Salmaggi A, Lamperti E, Botturi A, Erbetta A, et al. Liposomal cytarabine in neoplastic meningitis from primary brain tumors: a single institutional experience. Neurol Sci. 2013;34(12):2151–7.
Dan N, Danino D. Structure and kinetics of lipid-nucleic acid complexes. Adv Colloid Interf Sci. 2014;205:230–9.
Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release. 2006;116(2):255–64.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Musazzi, U.M., Franzé, S. & Cilurzo, F. Innovative pharmaceutical approaches for the management of inner ear disorders. Drug Deliv. and Transl. Res. 8, 436–449 (2018). https://doi.org/10.1007/s13346-017-0384-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0384-5