Abstract
Background
Sensorineural hearing loss (SNHL) is a disorder that significantly affects human quality of life. Current treatment for SNHL is still limited to hearing aids and cochlear implants. A better understanding of the etiological mechanisms of SNHL will facilitate the elucidation of the oto-protective efficacy of various novel therapeutic molecules. Intratympanic (IT) administration appears to be an attractive route for the delivery of these agents into the inner ear due to its advantages over systemic and cochlear administration. However, this administration route is limited by Eustachian tube clearance, transport capacity through the round window membrane (RWM), and the intrinsic structure of the cochlea, leading to the necessity of developing advanced drug delivery systems to improve the efficacy of IT administration.
Area covered
In this review, we summarize and discuss various drug delivery systems applied to IT administration, including conventional formulations and combinations of hydrogels and nanoparticles such as polymers, lipids, inorganic, and hybrid nanoparticles.
Expert opinion
A variety of innovative injectable systems have been prepared, and they have been demonstrated in several research models to have a significantly better ability to deliver drugs to the inner ear than that of conventional dosage forms. Hydrogels take advantage of prolonged residence time in the middle ear, while nanoparticles can enhance drug stability and cellular uptake, and allow drug targeting to specific cells. The combination of these two strategies is a promising area for future investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the five senses, hearing is one of the most important in the interaction between humans and the external environment. Humans sense surrounding airborne sounds based on a complex anatomical structure called the ear. The ear provides the process for converting sound waves into electrical signals that the brain can process. The organ responsible for hearing is the cochlea, which is embedded in the innermost part of the ear. The well-maintained structural integrity and cellular organization of the cochlea play a pivotal role in converting mechanical stimuli into electrical signals (Ekdale 2016; Fettiplace 2017).
SNHL is considered a global problem and a significant handicap that greatly affects quality of life. In 1985, the worldwide disease prevalence of SNHL was first reported by the World Health Organization (WHO) with approximately 1% of the world’s population (42 million people) estimated to have moderate to severe hearing disorders or impaired hearing (Davis and Hoffman 2019). From 1990 to 2019, the number of people suffering from moderate to complete hearing loss increased from 225.3 million to 403.3 million (Haile et al. 2021). According to WHO statistics, the most recent estimate shows that more than 1.5 billion people (nearly 20% of the world’s population) live with hearing loss, of whom 430 million have hearing loss (over 5% of the world’s population) (WHO 2021a). This estimate predicts that nearly 2.5 billion people (1 in 4 people) will live with hearing loss, and at least 700 million people (1 in 10 people) worldwide will develop hearing loss by 2050 (WHO 2021b). Hearing impairment can negatively affect an individual’s social and emotional development as well as communication and education. People with hearing impairments tend to avoid social situations and experience with increased rates of depression, anxiety, and feelings of inadequacy because they have difficulty acquiring surrounding information.
With the increasing prevalence of hearing loss worldwide, it is necessary to explore and develop treatments for hearing loss due to the negative impact of this condition on quality of life. SNHL can result from several diseases and external stimuli that damage the sensorineural structures of the cochlea (Cohen et al. 2014; Yang and Chung 2016; Vona and Haaf 2016; Cardin 2016; Rizk, et al. 2020; Gacek 2021). The treatment of such inner ear disorders has been a challenge for physicians, as there are few drugs on the market for cochlear indications and it is difficult to achieve effective drug concentrations in the cochlea (Nyberg et al. 2019). In the last two decades, IT administration, a safe procedure that can overcome the pharmacokinetic limitations of systemic administration for the treatment of inner ear diseases, has attracted considerable attention as a potential method for drug delivery to the cochlea. Many conventional dosage forms of medication on the market (e.g., dexamethasone solution 4 mg/mL or 5 mg/mL; prednisolone solution 0.25 mg/mL; methylprednisolone solution 62.5 mg/mL; triamcinolone acetonide suspension 10 mg/mL or 40 mg/mL) are currently used in clinical practice by IT injection instead of systemic application (Lechner et al. 2019; Roßberg et al. 2020). However, although IT administration helps drugs reach the inner ear at a greater level than that achieved through the systemic route, it still has several limitations that make it very difficult to deliver drugs to the cochleae by conventional formulations (including short residence time in the middle ear and low passage of drug through the RWM) (Szeto et al. 2020). These limitations indicate the need for the development of new strategies to improve the efficiency of IT drug delivery systems. A variety of innovative systems have been researched and evaluated in in vitro studies, in vivo studies and clinical trials (Pyykkö et al. 2011; Pararas et al. 2012; El Kechai et al. 2015; Rathnam et al. 2019; Freitas et al. 2021).
The purpose of this review is to provide an overview of drug delivery systems for IT administration. First, we describe the most critical features of the anatomy and physiology of the inner ear that should be considered during the design of drug delivery systems for IT administration. Next, promising systems for future clinical applications and current obstacles to overcome for efficient inner ear treatment are discussed.
Structure of the inner ear and auditory transduction mechanism
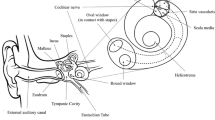
Anatomically, the ear is divided into three parts: the external ear, the middle ear, and the inner ear (Fig. 1A). The external ear consists of the pinna, the auditory canal, and the tympanic membrane (eardrum). The middle ear consists of the tympanic cavity including three auditory ossicles (the malleus, the incus, and the stapes), the round window (RW), the oval window (OW), and the Eustachian tube. The external and middle ears are responsible for guiding and conducting sound waves to the inner ear. The inner ear is an anatomically isolated structure comprising the vestibular system responsible for balance and the spiral cochlea responsible for auditory perception. The cochlea consists of three constitutively fluid-filled cavities. The middle cavity filled with endolymph is called the scala media (SM) and is separated from the superior cavity (scala vestibuli—SV) and inferior cavity (scala tympani – ST) by the vestibular membrane and basilar membrane, respectively. The SV communicates with the middle ear via an OW, whereas the ST communicates with the middle ear via an RW; and both of these cavities are filled with perilymph. The scala media contains the organ of Corti (OC), an essential organ considered to be an auditory receptor that converts sound vibrations into nerve signals (Fig. 1B). The OC is composed of sensory hair cells (inner hair cells (IHC), which are the actual sensory cells, and outer hair cells (OHC), which are cochlear signal amplifiers), supporting cells, basilar membrane, tectorial membrane as well as nerve fibers connecting the organ to the nearby spiral ganglion (SG) (Fig. 1C) (Ekdale 2016). Each auditory hair cell has the potential to detect a distinctive range of sound frequencies varying tonotopically along the cochlear axis. In addition, the scala media includes another vital structure called the stria vascularis that is responsible for producing and maintaining the ionic composition of the endolymph. For sounds to be detected, sound waves must first travel across the external ear canal and middle ear and through the inner ear fluids to stimulate the mechanosensory hair cells housed in the OC to release the neurotransmitters, which are then transferred to the spiral ganglion neurons (SGNs) and further propagated to the auditory cortex of the brainstem, where they are processed to cause auditory sensation (Fettiplace 2017).
The anatomic structure of ear and organ of corti. A The anatomic structure of mammalian ear composed of external ear, middle ear and inner ear, B the cross section of cochlea including 3 compartments [scala vestibuli (SV), scala media (SM) and scala tympani (ST)], and C the anatomic of organ of corti (OC) indicates the relation of cochlear hair cells, tectorial membrane, nerve fibers
Cochlear diseases, molecular mechanism, and therapeutic treatment
Any damage to sensory cells or auditory nerves can cause SNHL, which is the most frequent inner ear impairment in humans. This sensory deficit can originate from external stimuli (such as excessive noise (Yang and Chung 2016), infections (Cohen et al. 2014), and ototoxic drugs (Rizk, et al. 2020)) or intrinsic causes such as genetic mutations (Vona and Haaf 2016), aging (Cardin 2016) and Meniere’s disease (Gacek 2021). Overexposure to ototoxic compounds (for example, aminoglycoside antibiotics (gentamicin, streptomycin, etc.), chemotherapy agents (cisplatin, etc.), and acoustic injury as well as aging affect the distinct cellular signaling pathways to activate apoptosis leading to the degeneration of hair cells and SGNs. Moreover, both infections and Meniere’s disease can cause endolymphatic hydrops, inducing SNHL.
A more comprehensive understanding of the molecular mechanisms related to the degeneration and regeneration of sensory cells and the auditory nerve is of paramount importance in identifying the therapeutic candidates to prevent and treat inner ear diseases (Fig. 2). Most causes of SNHL induce cell death in response to the overproduction of reactive oxygen species (ROS) in both hair cells and SGNs. The high level of ROS activates the MAPK-JNK pathway, triggering the production of pro-apoptotic mediators and pro-inflammatory cytokines, leading to apoptosis or necroptosis (Kurabi et al. 2017). Antioxidants and apoptosis inhibitors as well as anti-inflammatory agents have been investigated to improve the defense capacity of hair cells and SGNs against damage to hinder cell death. On the other hand, the excessive generation of ROS mediates the activation of AMPK to inhibit mTOR, the central regulator of cell growth and cell proliferation, which plays a crucial role not only in cell survival but also in cell regeneration (Zhao et al. 2020; Cortada et al. 2021). Therefore, the stimulation of cell survival which can be achieved by using growth factors is another strategy to prevent hair cells and spiral ganglion nerves from damaging elements. A further approach is the application of molecular candidates (such as ATOH1 and neutrophins) or stem cells for the enhancement of cell regeneration to rescue injured hair cells and SGNs. In addition, gene therapy is a prospective means for treating hearing loss related to the functional deficits caused by genetic mutations (Table 1).
Cellular mechanism of hearing loss. AMPK, AMP-activated kinase; AP-1, activator-protein 1; CytC, cytochrome c; GF, growth factor; GFR, growth factor receptor; JNK, a-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-alpha; m-TOR, mammalian target of rapamycin
Intratympanic administration
There are two main approaches to the treatment of inner ear disorders: systemic drug delivery and local drug delivery. The presence of the blood labyrinth barrier (BLB), which inhibits the exchange of blood and inner ear fluids, is a major factor in the difficulty delivering drugs into the cochlea with sufficient concentration via systemic administration (Nyberg et al. 2019). The necessity of a more effective delivery route that can maximize the drug concentration in the inner ear and minimize the undesired side effects caused by systemic drug delivery has increased interest in local drug deliveries (including IT and intracochlear administration (Salt and Plontke 2009) from many researchers. Intracochlear drug delivery is rarely applied in clinical practice, as it requires surgical access to the cochlea for the sole purpose of local drug delivery. Therefore, IT administration, which is minimally invasive, opens new horizons for drug delivery to the inner ear for the treatment of SNHL.
In IT administration, the drug is transported directly into the middle ear cavity to diffuse the drug through the RWM into the cochlea. The discovery and development of IT administration over time is presented in Fig. 3. In 1956, this route was introduced by Schuknecht to treat Meniere’s disease with gentamicin (Schuknecht 1956). However, it was not until 1996 that IT injections were used in patients with sudden hearing loss (Silverstein et al. 1996), and this drug delivery route has received much attention in the treatment of various inner ear diseases since then. Over the past two decades, much evidence regarding the efficacy of this approach has been reported for the clinical application of steroids, gentamicin, and several other therapeutic candidates in animal studies. More details are described in the section “Drug delivery system for IT administration”.
Pharmacokinetics and challenges in intratympanic drug delivery
Similar to the pharmacokinetic processes that occur in the human body after systemic administration, the pharmacokinetics of drugs after IT delivery to the inner ear also include liberation, absorption, distribution, metabolism, and elimination (Fig. 4). Liberation refers to the release of a drug from an administered dosage form; absorption is the entry of drug into the internal fluid from the administration site through an RWM and an OW; distribution is the process by which drugs spread within the inner ear fluid (perilymph and endolymph) and are delivered to cochlear tissues; metabolism is a chemical alteration of drugs catalyzed by the enzymes in the inner ear fluid; and elimination includes removal of the drug and its metabolites from the inner ear and clearance through the Eustachian tube from the middle ear cavity (Salt and Plontke 2009, 2018). The bioavailability of a drug is determined primarily by the permeability of the drug through the RWM, the residence time of the drug in the middle ear, and the basal to peak concentration gradient of the drug within the cochlea, which are the leading challenges for drug delivery into the cochlea via the IT route.
Pharmacokinetic processes from application position to therapeutic targets including absorption, distribution and clearance of drug after IT administration by direct injection into middle ear cavity. Red arrows indicate the path of the drug into the ear from the middle ear to the cochlea and Eustachian tube. TM, tympanic membrane; RWM, round window membrane
Round window membrane
The RWM that allows drugs in the middle ear to penetrate into the perilymph can be considered the main pathway for drug delivery to the cochlea; however, it is also the fundamental obstacle to this administration route. The RWM, which consists of 3 layers, acts as a semipermeable membrane that allows the passage of low-molecular-weight molecules (such as antibiotics, local anesthetics, and corticosteroids). The permeability of the RWM is affected not only by the characteristics of drugs (lipophilicity and charges) but also by their properties (morphologic strength, thickness, and pathological condition) (Goycoolea and Lundman 1997).
Eustachian tube clearance
The Eustachian tube, which connects the middle ear cavity with the nasopharynx to equalize the middle ear pressure (Smith et al. 2016), also removes drugs administered to the middle ear. This drug loss reduces the residence time of the drugs in the middle ear and consequently decreases the amount of drug that diffuses through the RWM into the inner ear (Plontke et al. 2008b).
Base-to-apex concentration gradient in the cochlea
The base-to-apex concentration gradient occurs due to diffusion of the drug in the spiral structure of the cochlea after IT administration. This phenomenon poses a challenge to the treatment of disorders in the apex due to the difficulty of drug transport into the apical turn as a therapeutic concentration (Plontke et al. 2007, 2008a).
Strategies for overcoming the challenges of intratympanic administration
To overcome the above hurdles and effectively transport drugs to the treatment site, two parameters affecting drug concentration in the perilymph after IT injection should be considered: (1) residence time in the middle ear and (2) passage of drug through the RWM. Various strategies for the development of devices and drug delivery systems have been studied to increase the residence time of a drug in the middle ear cavity and to enhance drug delivery through the RWM. The former goal can be achieved using either several devices (in particular, microwicks, microcatheters, gelfoam, and Seprapack) (Pararas et al. 2012) or gelling systems (Rathnam et al. 2019). To achieve the latter, there are two main strategies: increasing the permeability of the three-layer barrier by a microneedle or penetrating facilitators and improving cellular uptake based on nanoparticle systems (Pyykkö et al. 2011). Although drug delivery devices are not mentioned in this review, we focus on the advanced technologies applied in preparation for intratympanically administered drug delivery systems.
Drug delivery system for intratympanic administration
Conventional drug delivery systems are still being used to treat inner ear diseases through the IT route, but the development of innovative drug delivery systems that overcome the limitations of these conventional dosage forms has been an attractive field for many researchers. A drug delivery system suitable for this route is a system that provides a prolonged residence time and/or sustained release to achieve a therapeutic concentration of drug in the inner ear with minimal invasiveness. In addition, it should be easily injected, biodegradable, and biocompatible. Various systems including hydrogels, nanoparticles, and combinations thereof, have been designed in recent years.
Conventional delivery systems
Conventional delivery systems, including solutions and suspensions, are the first dosage forms applied intratympanically to deliver streptomycin and dexamethasone into the middle ear cavity for the treatment of Meniere’s disease and sudden hearing loss, respectively (Schuknecht 1956; Silverstein et al. 1996). Although these conventional formulations have better therapeutic efficacy than systemic administration, frequent repeated injections (usually 2–5 times per week (Alles et al. 2006)) cause the typical undesired side effects of local injections such as pain, brief caloric vertigo, otitis media or lingering tympanic membrane perforation (Rauch 2011). These limitations are due to the difficulty of reaching therapeutic concentrations in the inner ear fluid due to the rapid drainage of intratympanically injected fluids via the Eustachian tube and the low transportability of the drug through the RWM. In this context, Eustachian tube clearance is the most important route that significantly shortens drug residence time at the absorption site, as demonstrated in the research of Plontke et al. on the IT use of methylprednisolone in humans (Plontke et al. 2008b). Another limitation of treatment with conventional dosage forms is the morphological change of the RWM after some therapeutic agents (e.g., hydrocortisone) come into direct contact with middle ear structures (Spandow et al. 1990; Nordang et al. 2003). Despite these drawbacks, transtympanic injection of conventional solutions or suspensions is widely used to manage common inner ear diseases. Aqueous solutions or suspensions of some therapeutic molecules, such as gentamicin sulfate (Liu et al. 2015a), dexamethasone (Liu et al. 2015a), methylprednisolone (Özel et al. 2016), N-acetyl cysteine (Somdaş et al. 2020), triamcinolone acetonide (Salt et al. 2019), and ciprofloxacin (Mair et al. 2016), have been investigated for the treatment of inner ear impairments through IT application.
Hydrogels
To prevent unintentional leakage via the Eustachian tube described above, viscous hydrogels seem to be a possible strategy to prevent rapid flow through the Eustachian tube, thereby increasing the residence time of the drug in the middle ear cavity and consequently improving the biodistribution of the drug in the cochlea. However, the delivery of formulations to the middle ear is conducted using a long and fine needle (smaller in diameter than G22) (El Kechai et al. 2015). Therefore, the residence time and injectability are two key parameters to consider when developing a hydrogel delivery system for IT administration. Injectability is the ability of a formulation to be injected into the tympanic cavity through a needle with an acceptable force, and residence time is the period of time at the application site calculated from the time the formulation is injected into the middle ear until it is washed out from the middle ear. In general, either injectability or residence time is affected by the viscosity of the hydrogel. The higher the viscosity, the longer the residence time and the harder it is to pass through the needle.
Conventional hydrogels
Several conventional hydrogel-based delivery systems investigated for IT drug delivery have been formulated from a variety of natural polymers including collagen, gelatin, chitosan, and hyaluronic acid (shown in Table 2). Collagen and gelatin are biodegradable polymers that can increase the viscosity of liquid dosage forms. However, since collagen or gelatin alone has limitations in thermal and mechanical stability and aqueous solubility, it cannot be accommodated in the site of administration for a long period of time. For this reason, glutaraldehyde is used as a crosslinking agent for collagen and gelatin, which reduces the degradation rate of collagen and gelatin, enabling long-term applications (Graziola et al. 2016; Peng et al. 2017). Hydrogels generated by glutaraldehyde crosslinking of gelatin or porcine type I collagen have proven effective for the delivery of some proteins, including brain-derived neurotrophic factor (BDNF) (Endo et al. 2005), insulin-like growth factor 1 (IGF-1) (Iwai et al. 2006; Lee et al. 2007; Fujiwara et al. 2008), and hepatocyte growth factor (HGF) (Inaoka et al. 2009). Chitosan is a nontoxic biodegradable cationic polymer that is prepared by alkaline n-deacetylation of chitin obtained from the exoskeleton of crustaceans (Sreenivas and Pai 2008). The positive charge of chitosan prolongs the residence time in the middle ear due to its electrostatic interaction with the negatively charged mucosal surface. In a study by Saber and coworkers, three types of chitosan were investigated to deliver neomycin to the inner ear. In the middle ear, low/medium-molecular-weight chitosan (Ch1 and Ch2) with lower solubility could be observed 7 days after application, while oligomeric chitosan (Ch3) with higher solubility was not observed at the same time. All formulations successfully delivered neomycin to the cochlear and chitosans have been demonstrated to be resistant to cochlea tissues (Saber et al. 2010). Hyaluronic acid is an anionic polymer present in the extracellular matrix of many tissues in the body and has mucoadhesive properties, biodegradability, and biocompatibility. The effect of drug delivery into the inner ear by the hyaluronic acid hydrogel was evidenced by the loss of hair cells after placing the neomycin-loaded hydrogel into the RWM, while there was no significant difference between the results obtained with neomycin solution (Saber et al. 2009). Hyaluronic acid can form highly viscous aqueous hydrogels that can extend residence time up to 72 h, as demonstrated in the study by Borden and colleagues (Borden et al. 2011). However, the high viscosity of the hyaluronic acid hydrogel does not affect the syringeability due to the shear-thinning and self-healing property of this polymeric system (Kechai et al. 2016). In addition to the achievement of higher drug concentrations in the perilymph based on prolonged contact with RWM, hyaluronic acid has been reported to facilitate drug diffusion to the inner ear based on its osmotic effect on the perilymph (Bagger-Sjöbäck et al. 1993) and RWM permeability modulating potential (Selivanova et al. 2003). Most conventional hydrogels mentioned above are in liquid form both before and after injection. It is difficult to deliver hydrogels with very high viscosity to the middle ear via the RWM with the aim of a very long residence time. To overcome these limitations, an in-situ gelling system was investigated.
In situ hydrogels
An in-situ hydrogel is a system that remains in a liquid state before entering the body for easy injection and then undergoes sol–gel transition process to form a semisolid gel at the site of administration after a stimulus or a period of time. The unique viscosity-adjustable property makes in situ hydrogels easy to handle compared to conventional hydrogels. The in-situ gelling hydrogel systems studied for IT administration include thermosensitive hydrogels and time-dependent hydrogels (shown in Table 3).
In-situ thermosensitive hydrogels that retain fluidity at room temperature and gel at body temperature are very attractive for inner ear drug delivery via transtympanic injection. Such thermoreversible systems can be generated from synthetic copolymers such as poloxamer 407 (P407) and PLGA-PEG-PLGA. Both P407 and PLGA-PEG-PLGA copolymers are triblock copolymers composed of a hydrophobic central chain and two hydrophilic side chains. Because of these amphiphilic properties, these copolymers tend to form micelles in water with a hydrophobic core and a hydrophilic shell above the critical micelle concentration (CMC). Intermicellar crosslinking between different micelles at concentrations higher than the critical gel concentration (CGC) leads to the creation of a gel matrix. Heating reduces the CMC and CGC, causing gelation of the thermosensitive copolymer solution (Gong et al. 2012; Fakhari et al. 2017; Russo and Villa 2019). The hydrogels made of either P407 or PLGA-PEG-PLGA are biodegradable, biocompatible, stable, and suitable for controlled release. The concentration of P407 used in hydrogels is typically in the range of 16–20% (w/v). The obtained hydrogel has a gelation temperature of 37℃ (physiological temperature) or less. P407 hydrogel has been extensively investigated as a drug delivery system for the IT administration of several therapeutic agents, such as dexamethasone, methylprednisolone, triamcinolone, and n-acetyl cysteine (Wang et al. 2009, 2011; Piu et al. 2011; Honeder et al. 2014; Engleder et al. 2014; Gausterer et al. 2020). The PLGA-PEG-PLGA solution dissolves at temperatures of 2 to 15℃ and turns into a gel at body temperature. The CGC of PLGA-PEG-PLGA solution can be controlled by changing the length of the PLGA or PEG block (Gong et al. 2012; Wang et al. 2017). The PLGA-PEG-PLGA system was reported to be effective in controlling the delivery of dexamethasone acetate, cidofovir, and levothyroxine (Gao et al. 2010; Feng et al. 2014; Liu et al. 2014; Kamali et al. 2020).
Chitosan glycerophosphate (CGP), a chitosan derivative, also has the ability to form a temperature-sensitive gelling matrix. However, unlike the abovementioned copolymers, the mechanism of gel formation in CGP solutions is driven by hydrophobic association of the chitosan molecules with increasing temperature. At low temperatures, the CGP system remains in a liquid state based on a hydrated protective layer of glycerophosphate around the chitosan chains created through weak hydrogen bonds. As the temperature increases, this layer breaks down, allowing the chitosan molecules to interact through stronger hydrophobic bonds and form hydrogels at body temperature. The gelation temperature and gelation rate of the CGP solution are affected by the deacetylation degree of chitosan, the concentration of chitosan, and the concentration of glycerophosphate (Gong et al. 2012; Liu et al. 2016; Rahmanian-Devin et al. 2021). Moreover, CGP hydrogels have a high affinity for mucosa due to their positively charged nature and have the potential to provide a sustained release delivery system due to their slow degradation over time by lysozyme available in the middle ear. Studies by Paulson et al. and Luo et al. demonstrated that the CGP hydrogel system can deliver dexamethasone and gentamicin to the cochlear fluid of guinea pigs with a steady release profile after IT injection (Paulson et al. 2008; Luo and Xu 2012).
In addition, a time-sensitive silk-polyethylene glycol (PEG) hydrogel that remains in the sol state for sufficient time for injection into the middle ear before the sol–gel transition, has also been investigated for the introduction of dexamethasone into the cochlea (Yu et al. 2016; Chen et al. 2019). Gelation occurs due to the structural transition of silk fibroin from the random coil-dominant structure of the silk solution to the β-sheet structure of the silk hydrogel when the silk solution is incorporated with a polymer (such as PEG). The gelation time depends on the concentration of the silk solution and the type and concentration of PEG (Wang et al. 2015).
In general, due to the advantages of improving residence time and providing sustained release in the RWM, a single IT injection of hydrogel resulted in higher drug levels in the inner ear fluid over an extended period of time that the injection of drug solution. This hydrogel has been proven to be safe and well tolerated. In most studies, only mild conductive transient hearing loss was observed due to interference of the viscous hydrogel with ossicular chain mobility, and no histological damage to the RWM and cochlea after IT administration was observed. However, drug permeability through the RWM is still highly dependent on the drug properties as they do not alter the physicochemical properties of the drug loaded into the gel system. Furthermore, hydrogels cannot stabilize drugs (especially biotherapeutic agents) from degradable reactions or provide targeted delivery. These limitations can be overcome by the application of nanotechnology.
Nanoparticles
Nanoparticle (NP)-based systems have been extensively explored for the IT drug delivery of various therapeutics for the treatment of inner ear disorders due to their potential to stabilize loaded materials, improve diffusion kinetics through RWM, and enhance cellular uptake and specific targeting. NPs encapsulate the drug into a structure, providing a barrier to protect the drug from degradative reactions and providing a controlled and sustained release profile. Likewise, due to the encapsulation of the drug into NPs, the drug properties are modulated by the NPs and the diffusion kinetics of the drug depend on the particle size and surface modification of the NPs. Their small size (typically in the range of tens to hundreds of nanometers in diameter) and surface modifications (including charged, hydrophobic, and tethered cell-penetrating peptides) have been shown to improve the transport of NPs across the row window barrier and increase the uptake and accumulation of drugs in certain cell types of the cochlea. In addition, NP labeling with ligands that interact with specific cellular receptors including tropomyocin receptor kinase B (TrkB) receptor (Bitsche et al. 2011), trisialoganglioside clostridial toxin (GT1b) receptors (Santi et al. 1994), and prestin (Zheng et al. 2000; Liberman et al. 2002), provides targeted delivery to specific cell types such as auditory hair cells and spiral ganglion cells. The advantages of NP-mediated drug delivery mentioned above reduce the dose required and cause unwanted side effects. Many types of NP-based systems for transtympanic administration that can be classified into four main groups: polymer-based, lipid-based, inorganic, and hybrid (Fig. 5).
Polymer-based nanoparticles
Polymer-based NPs are biocompatible, degradable, and maintainable systems prepared from natural and synthetic materials (including preformed polymers and monomers) by a variety of techniques (e.g., emulsification, precipitation, ionic gelation, and microfluidics) for application in the drug delivery of numerous hydrophilic and hydrophobic compounds. The loading efficiency and release kinetics of these drugs can be controlled by modulating formulation parameters such as composition, stability, and surface charge. Nanosystems of this category have been used in many studies to deliver drugs to the inner ear via IT administration, and according to their structure, they can be further divided into four subsets: polymersomes, polymeric NPs, dendrimers, and micelles.
Polymersomes
Polymersomes are a subset of polymer-based NPs with a core–shell structure with a central aqueous core surrounded by an outer bilayer shell composed of a hydrophobic membrane and hydrophilic corona formed by the self-assembly of an amphiphilic copolymer in an aqueous solution. Polymersomes have the ability to encapsulate hydrophilic and hydrophobic drugs with an aqueous core and hydrophobic membrane, respectively, to facilitate codelivery. They are resistant to the immune system due to their biomimetic structure (Lee and Feijen 2012). Many polymersomes studied for transtympanic drug delivery have been manipulated from the di-block copolymer poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG-b-PCL), which showed the ability to penetrate into the inner ear and distribute in various cochlear tissues (Buckiová et al. 2012; Roy et al. 2012). After conjugation to peptides that specifically bind to the surface receptors in selected cell lines (including nerve growth factor-derived peptide (hNgf_EE) and prestin-binding peptides (665 and A666), functionalized PEG-b-PCL polymersomes can specifically target SGNs and outer hair cells, respectively (Roy et al. 2010; Surovtseva et al. 2012). Polymersomes composed of poly(2-hydroxyethyl aspartamide) (PHEA) with or without ligand binding have also been examined to evaluate safety and permeability through RWM (Kim et al. 2015);(Yoon et al. 2015, 2016) (shown in Table 4).
Polymeric nanoparticles
Polymeric NPs are the most commonly used subset of polymer-based NPs with solid matrix structures created from various types of natural and synthetic polymers (shown in Table 5). One of the most extensively investigated polymers is poly(lactic-co-glycolic acid) (PLGA) because of its biodegradability and biocompatibility, approval by the Food and Drug Administration (FDA) and European Medicines Agency (EMA), surface modification ability, and ability to carry either hydrophilic or hydrophobic therapeutics (Tabatabaei Mirakabad et al. 2014). After it was demonstrated in 2005 that PLGA NPs containing rhodamine can cross the RWM after local injection into the middle ear (Tamura et al. 2005), much focus has been placed on PLGA NP systems for drug delivery into the inner ear. PLGA NPs can be used for the delivery of a single drug such as dexamethasone (Sun et al. 2016), or multiple therapeutic candidates such as the combination of salvianolic acid B, tanshinone IIA, and total panax notoginsenoside (Cai et al. 2014) for the purpose of synergistic actions. The accumulation of PLGA NP-based systems can be increased using surface-modified NPs alone or in combination with cell penetrating peptides (CPPs) (Cai et al. 2017; Wen et al. 2016).
Other polymers of interest for IT drug delivery have also been studied, including bovine serum albumin (BSA) and chitosan. BSA is a biodegradable and nontoxic protein capable of forming spherical NPs that can remain in the middle ear cavity for long periods and can penetrate through the RWM and internalize in the cochlea (Yu et al. 2014). Chitosan NPs can be prepared from chitosan by a different technique from chitosan hydrogels (Agnihotri et al. 2004). The application of chitosan NPs has the potential to successfully introduce drugs into the inner ear fluid in a controlled manner, mainly via the oval window (Ding et al. 2019).
Dendrimers
Dendrimers are synthetic polymers with a hyperbranched architecture whose size and shape van be controlled by the creation of repeating units. Dendrimers consist of a hydrophobic core surrounded by hydrophilic branches, and functional groups in hydrophobic core allow it to trap hydrophobic drugs and interact with functional groups to modify the surface (Chauhan 2018). The dendrimers used in otology for IT delivery are commonly formed by polyamidoamine (PAMAM) and hyperbranched poly-l-lysine (HBPL) (shown in Table 6) (Zhang et al. 2011a; Roy et al. 2012; Wu et al. 2013). Activation by heat coupling for surface functionalization with sodium-carboxymethyl-β-cyclodextrin (CM-β-CD) gives dendrimer NPs a higher transfection efficiency for DNA delivery (Wu et al. 2013).
Micelles
Another polymer-based NP with core–shell structure is polymeric micelles prepared by the self-association of amphiphilic block copolymers in an aqueous solution, which have many advantages for drug delivery including biocompatibility, low toxicity, and relatively high stability (Ahmad et al. 2014). NPs of a PEG-PLA micellar structure containing Dex prolonged the circulation time of Dex in the perilymph compared to Dex solution (≥ 48 h vs. < 12 h) and were more resistant to cisplatin-induced damage, as evaluated by the ABR test and hair cell counting test (Yu et al. 2015).
Lipid-based nanoparticles
In addition to polymer-based nanoparticles, lipid-based nanoparticle systems have been widely studied to deliver drugs from the middle ear to the inner ear via RWM, including liposomes, lipid nanoparticles, and cubosomes. As a delivery system, lipid-based NPs have many benefits including biocompatibility, large payload, and the ability to regulate their surface properties.
Liposomes
Liposomes are a subcategory of lipid-based NPs with a core–shell structure typically composed of phospholipids, a major component of the phospholipid bilayer membrane surrounding an aqueous central core. Similar to polymersomes, liposomes can deliver either hydrophilic therapeutics in the aqueous core or hydrophobic therapeutics in the phospholipid bilayer. Because of their cell membrane-like structure, liposomes are highly resistant. The stability of liposomes is controlled by the particle size, surface charge, lipid composition, number of lamellae, and spatial effect of surface modification (Alavi et al. 2017). Liposomes labeled with traceable agents were observed to have the ability to pass through the middle-inner ear barrier and distribute in the cochlea (Zou et al. 2010a, 2012; Buckiová et al. 2012). Permeation through the RWM of liposomes is size dependent, and smaller NPs have higher transport efficiency (Zou et al. 2012). Many in vivo studies have shown that liposomes can deliver genes for expression in various cochlear organs (Wareing et al. 1999; Jero et al. 2001; Maeda et al. 2007). Surface functionalization with A371 (a TrkB ligand) revealed greater targeted accumulation of therapeutic agents in SGNs and inner hair cells than was achieved by unmodified liposomes (Zou et al. 2009) (shown in Table 7).
Lipid nanoparticles
Many other lipid-based NPs lacking an aqueous core-bilayer shell structure, such as liposomes are considered attractive delivery systems for carrying hydrophobic drugs to the inner ear, including lipid nanocapsules (LNCs), solid lipid nanoparticles (SLNs), and cubosomes (shown in Table 8).
LNCs consist of a liquid lipid core composed of triglycerides and mineral oil and an amphiphilic shell composed of nonionic surfactants. It was reported that LNCs could penetrate through the RWM and distribute throughout the cochlear cell populations of human and Sprague–Dawley rat ears without damaging the inner ear structure and function (Zou et al. 2008; Zhang et al. 2011b; Roy et al. 2012). The uptake of LNCs can be altered by the hydrophilicity of the surface. Cationic-PEGylated NPs had the highest cellular uptake in both HEI-OC1 cells and organotypic cultures, which resulted in significantly better otoprotective effects (Yang et al. 2018).
SLNs are solid-core lipid nanocarriers composed mainly of fatty acids or mono-, di- or tri-glycerides, which maintain the solid state of the systems at normal body temperature. SLNs have various advantages over liposomes and LNCs: biocompatibility, biodegradability, high stability, extended drug release, and better target delivery ability (Paliwal et al. 2020). SLNs have been reported to be effective in delivering some glucocorticoids (dexamethasone, dexamethasone acetate, hydrocortisone) and antioxidants (edaravone, clozapine) to the inner ear and provide significant protection against ototoxicity induced by noise or cisplatin overexposure (Chen et al. 2008; Gao et al. 2015; Yang et al. 2018; Cervantes et al. 2019; Wang et al. 2020).
Cubosomes are another type of lipid NP that are bicontinuous cubic phase liquid crystalline systems. Due to the honeycomb structure with bicontinuous domains of water and lipid, cubosomes can load hydrophilic, hydrophobic, and amphiphilic drugs and provide sustained release of the entrapped drug (Naveentaj and Muzib 2020). The tested cubosomes composed of amphiphilic lipids (such as phytantriol and glyceromonooleate) exhibit low toxicity and prospective transport ability across the RWM into the inner ear (Liu et al. 2013b, a, 2015b).
Inorganic NPs
Inorganic NPs are also attractive for application in drug delivery because of their unique physical, electrical, magnetic, and optical properties. Inorganic NPs can be prepared from metals, silica, and hydroxyapatite (Table 9). However, since metallic NPs alone cannot encapsulate drugs, metallic NPs are used in combination with another type of NP for drug delivery.
Silica-based NPs are biocompatible, biodegradable, porous, and spherical NPs. Silica NPs are the most studied inorganic NPs for drug delivery, enabling a high payload of drug within the pores due to their unique porous structure. In addition, silica NPs release drugs loaded by the pore gating strategy and can be easily surface modified (Şen Karaman and Kettiger 2018). Praetorius et al. reported that silica NPs can cross the RWM and distribute in SGNs and inner hair cells without any harmful impacts (Praetorius et al. 2007), and Wise and colleagues demonstrated an effective strategy for delivering BDNF to the inner ear to improve spiral ganglion neuron survival (Wise et al. 2016). In addition, hollow mesoporous silica NPs have been tested as a system carrying gentamycin for the treatment of Meniere’s disease (Xu et al. 2018).
Hydroxyapatite NPs (HAT-NPs) are another widely studied inorganic NP for drug delivery. Hydroxyapatite (a formula formulation of Ca10(PO4)6(OH)2) is the principal constituent of bones and hard tissues, and exhibits excellent biocompatibility, biodegradability, bioresorbability, osteogenesis, osteoconductivity, and osteoinductivity. The proprietary properties of large surface area, high loading capacity, and intracellular transportation capacity make HAT-NPs preferred for use in drug delivery systems (Syamchand and Sony 2015; Murata et al. 2018; Ghiasi et al. 2019). Many studies have shown that HAT-NP can mediate NT3 gene transfection into SGNs in both in vitro and in vivo models (Jiang et al. 2007; Sun et al. 2008; Wu et al. 2012).
Hybrid NPs
Hybrid nanosystems are aimed integrating the advantages of these components into nanoscale systems that can combine materials with different properties, including organic-organic, organic–inorganic, and inorganic-inorganic blends. Compared with nonhybrid NPs, hybrid NPs have several advantages, such as improved circulation time, high stability, and outstanding improvements in drug targeting. In addition, the combination of various available ingredients can avoid the costly and time-consuming synthesis of new molecules (Hadinoto et al. 2013; Tahir et al. 2020; Ferreira Soares et al. 2020). Inorganic magnetic NPs combined with another inorganic constituent such as silica (Dormer et al. 2005; Kopke et al. 2006) or polymers, such as PLGA and poloxamer 407 (Kopke et al. 2006; Barnes et al. 2007; Ge et al. 2007; Zou et al. 2010b; Du et al. 2013a) have also been tested for otologic treatment by IT application (shown in Table 10).
Despite these advantages, similar to conventional delivery systems, the use of nanoparticles alone has some limitations with respect to rapid clearance from the middle ear cavity through the Eustachian tube, reducing the contact of nanoparticles with the RWM for permeation.
Hydrogels combined with nanoparticles
As described above, hydrogels and nanoparticles are beneficial for drug delivery to the inner ear via IT injection. However, there are still individual limitations to the single use of each system. That is, hydrogels are hampered by RWM permeability, whereas nanoparticles are limited by leakage through the Eustachian tube. Therefore, synergistically increasing the control of drug delivery by incorporating hydrogel and nanoparticles in a single formulation is a highly promising strategy to utilize both hydrogel and nanoparticle systems and overcome the drawbacks of each system used alone. Many studies have been conducted to evaluate the effectiveness of the combination of NPs and hydrogels for inner ear drug delivery (shown in Table 11). SPIONs in hydrogel form can pass through the RWM and can be prominently located in the perilymphatic fluid space and over the cochlear tissues in either in vitro or ex vivo model studies without the use of magnetic forces (Thaler et al. 2011). The combination of liposomes and hyaluronic acid hydrogels significantly prolonged the residence time (30 days) compared to liposomes alone (15 days), which facilitated dexamethasone transport into the inner ear (El Kechai et al. 2016). Liposomes could also be entrapped into CGP hydrogels and exhibit higher fluorescence intensity colocalized to inner ear cells than NPs alone (Lajud et al. 2015). The integration of PLGA NPs and CGP hydrogels has the advantage of a longer mean residence time in the perilymph than is achieved by either NPs or hydrogels alone (Dai et al. 2018). In addition, enhancement of the residence time of particle-based carriers in the middle ear cavity is also achieved by using microsphere carriers in combination with film forming agents that can create a dry film to retain drug-loaded particles in the RWM (Dormer et al. 2019). A recent study of our research group on thermosensitive poloxamer hydrogels containing dexamethasone-loaded PLGA NPs also demonstrated the superiority of the combination between PLGA NPs and hydrogel versus PLGA NPs alone for delivering dexamethasone into the inner ear. In vivo studies in BALB/c mice showed that NP-gel remained in the application position for more than 2 days, which led to significantly greater absorption of dexamethasone in the cochleae (7.20 ± 1.76 ng versus 1.83 ± 0.14 ng and 2.71 ± 0.53 ng of dexamethasone solution and dexamethasone-NPs, respectively) (Kim et al. 2021).
Current status, development, and future perspectives of the application of the intratympanic route
The most commonly used therapeutic agents currently administered through the IT route in the treatment of inner ear disorders are steroids prepared in conventional dosage forms commercially available on the market (for example, dexamethasone solution 4 mg/mL or 5 mg/mL; prednisolone solution 0.25 mg/mL; methylprednisolone solution 62.5 mg/mL; triamcinolone acetonide suspension 10 mg/mL or 40 mg/mL) (Lechner et al. 2019; Roßberg et al. 2020). However, these conventional formulations have limitations of a short residence time in the middle ear and a low degree of passage of drugs through the RWM, as described in the above section. To overcome these limitations, many advanced drug delivery systems have been developed with preeminent characteristics for the effective delivery of drugs into the inner ear. Hydrogels with a variety of tunable behaviors that allow prolonged retention in the middle ear cavity can serve as a reservoir for sustained release from the site of application to support drug transport through the RWM. A suspension of ciprofloxacin in poloxamer 407 hydrogel (OTO-201) was developed and approved by the FDA as Otiprio for otitis treatment (Edmunds 2017). In addition, some other products are currently in clinical trials and are expected to enter the market, such as AM-101 (clinical trial Phase III for treatment of Tinnitus of a hyaluronic hydrogel containing esketamine hydrochloride) (van de Heyning et al. 2014), AM-111 (clinical trial Phase III for treatment of acute unilateral sudden deafness of a hyaluronic hydrogel containing brimapitide – a JNK inhibitor) (Staecker et al. 2019), and OTO-104 (clinical trial Phase IIb for treatment of Meniere’s disease of a poloxamer 407 hydrogel containing dexamethasone) (Lambert et al. 2012). In addition to the hydrogel product, a lipid-based formulation of gacyclidine – an N-methyl-D-aspartic acid (NMDA) receptor antagonist (OTO-313) exhibiting sustained delivery was also undergoing phase 1/2 clinical evaluation for the treatment of tinnitus (Maxwell et al. 2021). Although no clinical trials are currently underway for nanoparticles for IT administration, these systems are still considered potential delivery vehicles for the future based on their ability to enhance stability, cellular uptake, and drug targeting to specific cells. In addition, the integration of various drug delivery systems to form a versatile platform with superior properties that increase the efficacy and accuracy of targeted drug delivery is also a promising approach.
Conclusion
In conclusion, ae profound understanding of the etiopathological mechanisms underlying inner ear impairments contributes to the development of new therapies as well as better strategies for the treatment and prevention of hearing loss. A deep understanding of the anatomical structure of the ear and inner ear helps researchers recognize the obstacles for delivering drugs into the inner ear through the IT administration route and to develop formulations with unique properties to overcome these obstacles to reach the targets more effectively. Various efforts have been made to develop an innovative injectable system that can replace conventional formulations for drug delivery into the inner ear with the goals of effectively treating inner ear damage with minimal invasiveness, improving the development of possible new therapeutic candidates, and utilizing the feasibility of IT administration. Many studies on advanced systems, such as hydrogels, nanoparticles and hydrogel-nanoparticles combinations, have accomplished many achievements, laying the foundation for future research concerning IT drug delivery systems.
References
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release 100:5–28. https://doi.org/10.1016/j.jconrel.2004.08.010
Ahmad Z, Shah A, Siddiq M, Kraatz H-B (2014) Polymeric micelles as drug delivery vehicles. RSC Adv 4:17028–17038. https://doi.org/10.1039/C3RA47370H
Alavi M, Karimi N, Safaei M (2017) Application of various types of liposomes in drug delivery systems. Adv Pharm Bull 7:3–9. https://doi.org/10.15171/apb.2017.002
Alles MJRC, Gaag MA, Stokroos RJ (2006) Intratympanic steroid therapy for inner ear diseases, a review of the literature. Eur Arch Oto-Rhino-Laryngol 263:791–797. https://doi.org/10.1007/s00405-006-0065-3
Asplund MS, Lidian A, Linder B et al (2009) Protective effect of edaravone against tobramycin-induced ototoxicity. Acta Otolaryngol 129:8–13. https://doi.org/10.1080/00016480802008199
Bagger-Sjöbäck D, Holmquist J, Mendel L, Mercke U (1993) Hyaluronic acid in middle ear surgery. Otol Neurotol 14:501–506. https://doi.org/10.1097/00129492-199309000-00016
Barboza LCM Jr, Lezirovitz K, Zanatta DB et al (2016) Transplantation and survival of mouse inner ear progenitor/stem cells in the organ of Corti after cochleostomy of hearing-impaired guinea pigs: preliminary results. Braz J Med Biol Res. https://doi.org/10.1590/1414-431X20155064
Barnes AL, Wassel RA, Mondalek F et al (2007) Magnetic characterization of superparamagnetic nanoparticles pulled through model membranes. Biomagn Res Technol 5:1. https://doi.org/10.1186/1477-044X-5-1
Barreto MA, Ledesma AL, de Oliveira CA, Bahmad F Jr (2016) Corticosteroide intratimpânico para perda súbita da audição: isso realmente funciona? Braz J Otorhinolaryngol 82:353–64
Bas E, Van De Water TR, Lumbreras V, et al (2014) Adult human nasal mesenchymal-like stem cells restore cochlear spiral ganglion neurons after experimental lesion. Stem Cells Dev 23:502–514. https://doi.org/10.1089/scd.2013.0274
Benkafadar N, Menardo J, Bourien J et al (2017) Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol Med 9:7–26. https://doi.org/10.15252/emmm.201606230
Bitsche M, Dudas J, Roy S et al (2011) Neurotrophic receptors as potential therapy targets in postnatal development, in adult, and in hearing loss-affected inner ear. Otol Neurotol 32:761–773. https://doi.org/10.1097/MAO.0b013e31821f7cc1
Bonabi S, Caelers A, Monge A et al (2008) Resveratrol protects auditory hair cells from gentamicin toxicity. Ear Nose Throat J 87:570–573
Borden RC, Saunders JE, Berryhill WE et al (2011) Hyaluronic acid hydrogel sustains the delivery of dexamethasone across the round window membrane. Audiol Neurotol 16:1–11. https://doi.org/10.1159/000313506
Brownell WE (1997) How the ear works—natures’s solution for listening. Volta Rev 99:9–28
Buckiová D, Ranjan S, Newman TA et al (2012) Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine 7:1339–1354. https://doi.org/10.2217/nnm.12.5
Cai H, Liang Z, Huang W et al (2017) Engineering PLGA nano-based systems through understanding the influence of nanoparticle properties and cell-penetrating peptides for cochlear drug delivery. Int J Pharm 532:55–65. https://doi.org/10.1016/j.ijpharm.2017.08.084
Cai H, Wen X, Wen L et al (2014) Enhanced local bioavailability of single or compound drugs delivery to the inner ear through application of PLGA nanoparticles via round window administration. Int J Nanomed. https://doi.org/10.2147/IJN.S72555
Campbell K, Claussen A, Meech R et al (2011) d-methionine (d-met) significantly rescues noise-induced hearing loss: timing studies. Hear Res 282:138–144. https://doi.org/10.1016/j.heares.2011.08.003
Cardin V (2016) Effects of aging and adult-onset hearing loss on cortical auditory regions. Front Neurosci. https://doi.org/10.3389/fnins.2016.00199
Cervantes B, Arana L, Murillo-Cuesta S et al (2019) Solid lipid nanoparticles loaded with glucocorticoids protect auditory cells from cisplatin-induced ototoxicity. J Clin Med 8:1464. https://doi.org/10.3390/jcm8091464
Chauhan A (2018) Dendrimers for drug delivery. Molecules 23:938. https://doi.org/10.3390/molecules23040938
Chen G, Hou S-X, Hu P et al (2008) In vitro dexamethasone release from nanoparticles and its pharmacokinetics in the inner ear after administration of the drug-loaded nanoparticles via the round window. Nan Fang Yi Ke Da Xue Xue Bao 28:1022–1024
Chen Y, Gu J, Liu J et al (2019) Dexamethasone-loaded injectable silk-polyethylene glycol hydrogel alleviates cisplatin-induced ototoxicity. Int J Nanomed 14:4211–4227. https://doi.org/10.2147/IJN.S195336
Chien WW, Isgrig K, Roy S et al (2016) Gene therapy restores hair cell stereocilia morphology in inner ears of deaf whirler mice. Mol Ther 24:17–25. https://doi.org/10.1038/mt.2015.150
Choe W-T, Chinosornvatana N, Chang KW (2004) Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neurotol 25:910–915. https://doi.org/10.1097/00129492-200411000-00009
Cohen BE, Durstenfeld A, Roehm PC (2014) Viral causes of hearing loss: a review for hearing health professionals. Trends Hear 18:233121651454136. https://doi.org/10.1177/2331216514541361
Coleman JKM, Littlesunday C, Jackson R, Meyer T (2007) AM-111 protects against permanent hearing loss from impulse noise trauma. Hear Res 226:70–78. https://doi.org/10.1016/j.heares.2006.05.006
Cortada M, Levano S, Bodmer D (2021) mTOR Signaling in the inner ear as potential target to treat hearing loss. Int J Mol Sci 22:6368. https://doi.org/10.3390/ijms22126368
Dahm V, Nieratschker M, Riss D et al (2019) Intratympanic triamcinolone acetonide as treatment option for idiopathic sudden sensorineural hearing loss. Otol Neurotol 40:720–727. https://doi.org/10.1097/MAO.0000000000002283
Dai J, Long W, Liang Z et al (2018) A novel vehicle for local protein delivery to the inner ear: injectable and biodegradable thermosensitive hydrogel loaded with PLGA nanoparticles. Drug Dev Ind Pharm 44:89–98. https://doi.org/10.1080/03639045.2017.1373803
Davis AC, Hoffman HJ (2019) Hearing loss: rising prevalence and impact. Bull World Health Organ 97:646-646A. https://doi.org/10.2471/BLT.19.224683
De Araujo JG, Serra LSM, Lauand L et al (2019) Protective effect of melatonin on cisplatin-induced ototoxicity in rats. Anticancer Res 39:2453–2458. https://doi.org/10.21873/anticanres.13364
Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA (2005) Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther 314:1052–1058. https://doi.org/10.1124/jpet.105.087601
Ding S, Xie S, Chen W et al (2019) Is oval window transport a royal gate for nanoparticle delivery to vestibule in the inner ear? Eur J Pharm Sci 126:11–22. https://doi.org/10.1016/j.ejps.2018.02.031
Doolittle ND, Muldoon LL, Brummett RE et al (2001) Delayed sodium thiosulfate as an otoprotectant against carboplatin-induced hearing loss in patients with malignant brain tumors. Clin Cancer Res 7:493–500
Dormer K, Mamedova N, Kopke R, et al (2005) Feasibility of Superparamagnetic nanoparticles for drug delivery to the inner ear. In: NSTI-Nanotech, pp 132–135
Dormer NH, Nelson-Brantley J, Staecker H, Berkland CJ (2019) Evaluation of a transtympanic delivery system in Mus musculus for extended release steroids. Eur J Pharm Sci 126:3–10. https://doi.org/10.1016/j.ejps.2018.01.020
Du X, Chen K, Kuriyavar S et al (2013a) Magnetic targeted delivery of dexamethasone acetate across the round window membrane in guinea pigs. Otol Neurotol 34:41–47. https://doi.org/10.1097/MAO.0b013e318277a40e
Du X, Li W, Gao X et al (2013b) Regeneration of mammalian cochlear and vestibular hair cells through Hes1/Hes5 modulation with siRNA. Hear Res 304:91–110. https://doi.org/10.1016/j.heares.2013.06.011
Edmunds AL (2017) Otiprio: an FDA-approved ciprofloxacin suspension gel for pediatric otitis media with effusion. P T 42:307–311
Ekdale EG (2016) Form and function of the mammalian inner ear. J Anat 228:324–337. https://doi.org/10.1111/joa.12308
El Kechai N, Agnely F, Mamelle E et al (2015) Recent advances in local drug delivery to the inner ear. Int J Pharm 494:83–101. https://doi.org/10.1016/j.ijpharm.2015.08.015
El Kechai N, Mamelle E, Nguyen Y et al (2016) Hyaluronic acid liposomal gel sustains delivery of a corticoid to the inner ear. J Control Release 226:248–257. https://doi.org/10.1016/j.jconrel.2016.02.013
Endo T, Nakagawa T, Kita T et al (2005) Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope 115:2016–2020. https://doi.org/10.1097/01.mlg.0000183020.32435.59
Engleder E, Honeder C, Klobasa J et al (2014) Preclinical evaluation of thermoreversible triamcinolone acetonide hydrogels for drug delivery to the inner ear. Int J Pharm 471:297–302. https://doi.org/10.1016/j.ijpharm.2014.05.057
Erdem T, Bayindir T, Filiz A et al (2012) The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur Arch Oto-Rhino-Laryngol 269:2185–2188. https://doi.org/10.1007/s00405-011-1883-5
Eshraghi AA, He J, Mou CH et al (2006) D-JNKI-1 treatment prevents the progression of hearing loss in a model of cochlear implantation trauma. Otol Neurotol 27:504–511. https://doi.org/10.1097/01.mao.0000217354.88710.13
Fakhari A, Corcoran M, Schwarz A (2017) Thermogelling properties of purified poloxamer 407. Heliyon 3:e00390. https://doi.org/10.1016/j.heliyon.2017.e00390
Feng L, Ward J, Li S et al (2014) Assessment of PLGA-PEG-PLGA copolymer hydrogel for sustained drug delivery in the ear. Curr Drug Deliv 11:279–286. https://doi.org/10.2174/1567201811666140118224616
Ferreira Soares DC, Domingues SC, Viana DB, Tebaldi ML (2020) Polymer-hybrid nanoparticles: current advances in biomedical applications. Biomed Pharmacother 131:110695. https://doi.org/10.1016/j.biopha.2020.110695
Fetoni AR, Eramo SLM, Di Pino A et al (2018) The antioxidant effect of rosmarinic acid by different delivery routes in the animal model of noise-induced hearing loss. Otol Neurotol 39:378–386. https://doi.org/10.1097/MAO.0000000000001700
Fettiplace R (2017) Hair cell transduction, tuning, and synaptic transmission in the mammalian cochlea. Comprehensive physiology. Wiley, New York, pp 1197–1227
Freitas LM, Antunes FTT, Obach ES et al (2021) Anti-inflammatory effects of a topical emulsion containing Helianthus annuus oil, glycerin, and vitamin B3 in mice. J Pharm Investig 51:223–232. https://doi.org/10.1007/s40005-020-00508-6
Fritzsch B (1997) The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci 20:159–164. https://doi.org/10.1016/S0166-2236(96)01007-7
Fritzsch B, Beisel KW, Hansen LA (2006) The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? BioEssays 28:1181–1193. https://doi.org/10.1002/bies.20502
Fujita K, Hakuba N, Hata R et al (2007) Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci Lett 415:113–117. https://doi.org/10.1016/j.neulet.2007.01.005
Fujiwara T, Hato N, Nakagawa T et al (2008) Insulin-like growth factor 1 treatment via hydrogels rescues cochlear hair cells from ischemic injury. NeuroReport 19:1585–1588. https://doi.org/10.1097/WNR.0b013e328311ca4b
Gacek RR (2021) On the nature of hearing loss in Méniere’s disease. ORL 83:144–150. https://doi.org/10.1159/000511113
Gao G, Liu Y, Zhou C-H et al (2015) Solid lipid nanoparticles loaded with edaravone for inner ear protection after noise exposure. Chin Med J (engl) 128:203–209. https://doi.org/10.4103/0366-6999.149202
Gao W-Q (1998) Therapeutic potential of neurotrophins for treatment of hearing loss. Mol Neurobiol 17:17–31. https://doi.org/10.1007/BF02802022
Gao Y, Sun Y, Ren F, Gao S (2010) PLGA–PEG–PLGA hydrogel for ocular drug delivery of dexamethasone acetate. Drug Dev Ind Pharm 36:1131–1138. https://doi.org/10.3109/03639041003680826
Gausterer JC, Saidov N, Ahmadi N et al (2020) Intratympanic application of poloxamer 407 hydrogels results in sustained N-acetylcysteine delivery to the inner ear. Eur J Pharm Biopharm 150:143–155. https://doi.org/10.1016/j.ejpb.2020.03.005
Ge X, Jackson RL, Liu J et al (2007) Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol Neck Surg 137:619–623. https://doi.org/10.1016/j.otohns.2007.04.013
Ghiasi B, Sefidbakht Y, Rezaei M (2019) Hydroxyapatite for biomedicine and drug delivery. Springer, Cham, pp 85–120
Gong C, Qi T, Wei X et al (2012) Thermosensitive polymeric hydrogels as drug delivery systems. Curr Med Chem 20:79–94. https://doi.org/10.2174/0929867311302010079
Goycoolea MV, Lundman L (1997) Round window membrane. Structure function and permeability: a review. Microsc Res Tech 36:201–211. https://doi.org/10.1002/(SICI)1097-0029(19970201)36:3%3c201::AID-JEMT8%3e3.0.CO;2-R
Graziola F, Candido TM, de Oliveira CA et al (2016) Gelatin-based microspheres crosslinked with glutaraldehyde and rutin oriented to cosmetics. Braz J Pharm Sci 52:603–612. https://doi.org/10.1590/s1984-82502016000400004
Guo G, Li B, Wang Y et al (2010) Effects of salvianolic acid B on proliferation, neurite outgrowth and differentiation of neural stem cells derived from the cerebral cortex of embryonic mice. Sci China Life Sci 53:653–662. https://doi.org/10.1007/s11427-010-3106-5
Gusmão de Araujo J, Sampaio ALL, Ramos Venosa A, de Oliveira CACP (2014) The potential use of melatonin for preventing cisplatin ototoxicity: an insight for a clinical approach. Adv Otolaryngol 2014:1–8. https://doi.org/10.1155/2014/185617
Hadinoto K, Sundaresan A, Cheow WS (2013) Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm 85:427–443. https://doi.org/10.1016/j.ejpb.2013.07.002
Haile LM, Kamenov K, Briant PS et al (2021) Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet 397:996–1009. https://doi.org/10.1016/S0140-6736(21)00516-X
Honeder C, Engleder E, Schöpper H et al (2014) Sustained release of triamcinolone acetonide from an intratympanically applied hydrogel designed for the delivery of high glucocorticoid doses. Audiol Neurotol 19:193–202. https://doi.org/10.1159/000358165
Inaoka T, Nakagawa T, Kikkawa YS et al (2009) Local application of hepatocyte growth factor using gelatin hydrogels attenuates noise-induced hearing loss in guinea pigs. Acta Otolaryngol 129:453–457. https://doi.org/10.1080/00016480902725197
Iwai K, Nakagawa T, Endo T et al (2006) Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope 116:529–533. https://doi.org/10.1097/01.mlg.0000200791.77819.eb
Jero J, Tseng CJ, Mhatre AN, Lalwani AK (2001) A surgical approach appropriate for targeted cochlear gene therapy in the mouse. Hear Res 151:106–114. https://doi.org/10.1016/S0378-5955(00)00216-1
Jiang M, Zhang Y-Q, He G-X, Sun H (2007) Protective effect of NT-3 gene mediated by hydroxyapatite nanoparticle on the cochlea of guinea pigs injured by excitotoxicity. Zhong Nan Da Xue Xue Bao Yi Xue Ban 32:563–567
Kamali H, Khodaverdi E, Kaffash E et al (2020) Optimization and in vitro evaluation of injectable sustained-release of levothyroxine using PLGA-PEG-PLGA. J Pharm Innov. https://doi.org/10.1007/s12247-020-09480-y
Kikkawa YS, Nakagawa T, Tsubouchi H et al (2009) Hepatocyte growth factor protects auditory hair cells from aminoglycosides. Laryngoscope 119:2027–2031. https://doi.org/10.1002/lary.20602
Kim D-H, Nguyen TN, Han Y-M et al (2021) Local drug delivery using poly(lactic-co-glycolic acid) nanoparticles in thermosensitive gels for inner ear disease treatment. Drug Deliv 28:2268–2277. https://doi.org/10.1080/10717544.2021.1992041
Kim D-K, Park S-N, Park K-H et al (2015) Development of a drug delivery system for the inner ear using poly(amino acid)-based nanoparticles. Drug Deliv 22:367–374. https://doi.org/10.3109/10717544.2013.879354
Kopke RD, Wassel RA, Mondalek F et al (2006) Magnetic nanoparticles: inner ear targeted molecule delivery and middle ear implant. Audiol Neurotol 11:123–133. https://doi.org/10.1159/000090685
Kurabi A, Keithley EM, Housley GD et al (2017) Cellular mechanisms of noise-induced hearing loss. Hear Res 349:129–137. https://doi.org/10.1016/j.heares.2016.11.013
Lajud SA, Nagda DA, Qiao P et al (2015) A novel chitosan-hydrogel-based nanoparticle delivery system for local inner ear application. Otol Neurotol 36:341–347. https://doi.org/10.1097/MAO.0000000000000445
Lambert PR, Nguyen S, Maxwell KS et al (2012) A randomized, double-blind, placebo-controlled clinical study to assess safety and clinical activity of OTO-104 given as a single intratympanic injection in patients with unilateral Ménière’s disease. Otol Neurotol 33:1257–1265. https://doi.org/10.1097/MAO.0b013e318263d35d
Lechner M, Sutton L, Ferguson M et al (2019) Intratympanic steroid use for sudden sensorineural hearing loss: current otolaryngology practice. Ann Otol Rhinol Laryngol 128:490–502. https://doi.org/10.1177/0003489419828759
Lee JS, Feijen J (2012) Polymersomes for drug delivery: design, formation and characterization. J Control Release 161:473–483. https://doi.org/10.1016/j.jconrel.2011.10.005
Lee KY, Nakagawa T, Okano T et al (2007) Novel therapy for hearing loss. Otol Neurotol 28:976–981. https://doi.org/10.1097/MAO.0b013e31811f40db
Liberman MC, Gao J, He DZZ et al (2002) Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419:300–304. https://doi.org/10.1038/nature01059
Liu B, Zhang S, Leng Y et al (2015a) Intratympanic injection in delayed endolymphatic hydrops. Acta Otolaryngol 135:1016–1021. https://doi.org/10.3109/00016489.2015.1052984
Liu H, Bu M, Tang J et al (2015b) Enhanced bioavailability of nerve growth factor with phytantriol lipid-based crystalline nanoparticles in cochlea. Int J Nanomed. https://doi.org/10.2147/IJN.S82944
Liu H, Chen S, Zhou Y et al (2013a) The effect of surface charge of glycerol monooleate-based nanoparticles on the round window membrane permeability and cochlear distribution. J Drug Target 21:846–854. https://doi.org/10.3109/1061186X.2013.829075
Liu H, Feng L, Tolia G et al (2014) Evaluation of intratympanic formulations for inner ear delivery: methodology and sustained release formulation testing. Drug Dev Ind Pharm 40:896–903. https://doi.org/10.3109/03639045.2013.789054
Liu H, Wang Y, Wang Q et al (2013b) Protein-bearing cubosomes prepared by liquid precursor dilution: inner ear delivery and pharmacokinetic study following intratympanic administration. J Biomed Nanotechnol 9:1784–1793. https://doi.org/10.1166/jbn.2013.1685
Liu L, Gao Q, Lu X, Zhou H (2016) In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J Pharm Sci 11:673–683. https://doi.org/10.1016/j.ajps.2016.07.001
Luo J, Xu L (2012) Distribution of gentamicin in inner ear after local administration via a chitosan glycerophosphate hydrogel delivery system. Ann Otol Rhinol Laryngol 121:208–216. https://doi.org/10.1177/000348941212100311
Maeda Y, Fukushima K, Kawasaki A et al (2007) Cochlear expression of a dominant-negative GJB2R75W construct delivered through the round window membrane in mice. Neurosci Res 58:250–254. https://doi.org/10.1016/j.neures.2007.03.006
Mair EA, Park AH, Don D et al (2016) Safety and efficacy of intratympanic ciprofloxacin otic suspension in children with middle ear effusion undergoing tympanostomy tube placement. JAMA Otolaryngol Neck Surg 142:444. https://doi.org/10.1001/jamaoto.2016.0001
Maxwell KS, Robinson JM, Hoffmann I et al (2021) Intratympanic administration of OTO-313 reduces tinnitus in patients with moderate to severe, persistent tinnitus: a phase 1/2 study. Otol Neurotol 42:e1625–e1633. https://doi.org/10.1097/MAO.0000000000003369
Mungan Durankaya S, Olgun Y, Aktaş S et al (2021) Effect of Korean red ginseng on noise-induced hearing loss. Turkish Arch Otorhinolaryngol 59:111–117. https://doi.org/10.4274/tao.2021.2021-1-5
Murata T, Kutsuna T, Kurohara K et al (2018) Evaluation of a new hydroxyapatite nanoparticle as a drug delivery system to oral squamous cell carcinoma cells. Anticancer Res 38:6715–6720. https://doi.org/10.21873/anticanres.13040
Naveentaj S, Muzib YI (2020) A review on liquid crystalline nanoparticles (cubosomes): emerging nanoparticulate drug carrier. Int J Curr Pharm Res. https://doi.org/10.22159/ijcpr.2020v12i1.36820
Neuwelt EA, Brummett RE, Remsen LG et al (1996) In vitro and animal studies of sodium thiosulfate as a potential chemoprotectant against carboplatin-induced ototoxicity. Cancer Res 56:706–709
Nordang L, Linder B, Anniko M (2003) Morphologic changes in round window membrane after topical hydrocortisone and dexamethasone treatment. Otol Neurotol 24:339–343. https://doi.org/10.1097/00129492-200303000-00034
Nyberg S, Abbott NJ, Shi X et al (2019) Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci Transl Med 11:eaao0935. https://doi.org/10.1126/scitranslmed.aao0935
Omotehara Y, Hakuba N, Hato N et al (2011) Protection against ischemic cochlear damage by intratympanic administration of AM-111. Otol Neurotol 32:1422–1427. https://doi.org/10.1097/MAO.0b013e3182355658
Özel HE, Özdoğan F, Gürgen SG et al (2016) Comparison of the protective effects of intratympanic dexamethasone and methylprednisolone against cisplatin-induced ototoxicity. J Laryngol Otol 130:225–234. https://doi.org/10.1017/S0022215115003473
Paliwal R, Paliwal SR, Kenwat R et al (2020) Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin Ther Pat 30:179–194. https://doi.org/10.1080/13543776.2020.1720649
Pararas EEL, Borkholder DA, Borenstein JT (2012) Microsystems technologies for drug delivery to the inner ear. Adv Drug Deliv Rev 64:1650–1660. https://doi.org/10.1016/j.addr.2012.02.004
Paulson DP, Abuzeid W, Jiang H et al (2008) A novel controlled local drug delivery system for inner ear disease. Laryngoscope 118:706–711. https://doi.org/10.1097/MLG.0b013e31815f8e41
Peng YY, Glattauer V, Ramshaw JAM (2017) Stabilisation of collagen sponges by glutaraldehyde vapour crosslinking. Int J Biomater 2017:1–6. https://doi.org/10.1155/2017/8947823
Piu F, Wang X, Fernandez R et al (2011) OTO-104: a sustained-release dexamethasone hydrogel for the treatment of otic disorders. Otol Neurotol 32:171–179. https://doi.org/10.1097/MAO.0b013e3182009d29
Plontke SK, Biegner T, Kammerer B et al (2008a) Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol 29:401–406. https://doi.org/10.1097/MAO.0b013e318161aaae
Plontke SK, Mikulec AA, Salt AN (2008b) Rapid clearance of methylprednisolone after intratympanic application in humans versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol 29:732–733. https://doi.org/10.1097/MAO.0b013e318173fcea
Plontke SK, Mynatt R, Gill RM et al (2007) Concentration gradient along the scala tympani after local application of gentamicin to the round window membrane. Laryngoscope 117:1191–1198. https://doi.org/10.1097/MLG.0b013e318058a06b
Postema RJ, Kingma CM, Wit HP et al (2008) Intratympanic gentamicin therapy for control of vertigo in unilateral Menière’s disease: a prospective, double-blind, randomized, placebo-controlled trial. Acta Otolaryngol 128:876–880. https://doi.org/10.1080/00016480701762458
Praetorius M, Brunner C, Lehnert B et al (2007) Transsynaptic delivery of nanoparticles to the central auditory nervous system. Acta Otolaryngol 127:486–490. https://doi.org/10.1080/00016480600895102
Pyykkö I, Zou J, Zhang W, Zhang Y (2011) Nanoparticle-based delivery for the treatment of inner ear disorders. Curr Opin Otolaryngol Head Neck Surg 19:388–396. https://doi.org/10.1097/MOO.0b013e32834aa3a8
Rahmanian-Devin P, Baradaran Rahimi V, Askari VR (2021) Thermosensitive chitosan-β-glycerophosphate hydrogels as targeted drug delivery systems: an overview on preparation and their applications. Adv Pharmacol Pharm Sci 2021:1–17. https://doi.org/10.1155/2021/6640893
Rathnam C, Chueng S-TD, Ying Y-LM et al (2019) Developments in bio-inspired nanomaterials for therapeutic delivery to treat hearing loss. Front Cell Neurosci. https://doi.org/10.3389/fncel.2019.00493
Rauch SD (2011) Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss. JAMA 305:2071. https://doi.org/10.1001/jama.2011.679
Rizk HG, Lee JA, Liu YF et al (2020) Drug-induced ototoxicity: a comprehensive review and reference guide. Pharmacother J Hum Pharmacol Drug Ther 40:1265–1275. https://doi.org/10.1002/phar.2478
Roßberg W, Goetz F, Timm ME et al (2020) Intratympanic application of triamcinolone in sudden hearing loss—radiologic anatomy in cone beam CT and its’ correlation to clinical outcome. Eur Arch Oto-Rhino-Laryngol 277:1931–1937. https://doi.org/10.1007/s00405-020-05920-0
Roy S, Glueckert R, Johnston AH et al (2012) Strategies for drug delivery to the human inner ear by multifunctional nanoparticles. Nanomedicine 7:55–63. https://doi.org/10.2217/nnm.11.84
Roy S, Johnston AH, Newman TA et al (2010) Cell-specific targeting in the mouse inner ear using nanoparticles conjugated with a neurotrophin-derived peptide ligand: potential tool for drug delivery. Int J Pharm 390:214–224. https://doi.org/10.1016/j.ijpharm.2010.02.003
Russo E, Villa C (2019) Poloxamer hydrogels for biomedical applications. Pharmaceutics 11:671. https://doi.org/10.3390/pharmaceutics11120671
Saber A, Laurell G, Bramer T et al (2009) Middle ear application of a sodium hyaluronate gel loaded with neomycin in a Guinea pig model. Ear Hear 30:81–89. https://doi.org/10.1097/AUD.0b013e31818ff98e
Saber A, Strand SP, Ulfendahl M (2010) Use of the biodegradable polymer chitosan as a vehicle for applying drugs to the inner ear. Eur J Pharm Sci 39:110–115. https://doi.org/10.1016/j.ejps.2009.11.003
Salt AN, Hartsock JJ, Hou J, Piu F (2019) Comparison of the pharmacokinetic properties of triamcinolone and dexamethasone for local therapy of the inner ear. Front Cell Neurosci. https://doi.org/10.3389/fncel.2019.00347
Salt AN, Plontke SK (2009) Principles of local drug delivery to the inner ear. Audiol Neurotol 14:350–360. https://doi.org/10.1159/000241892
Salt AN, Plontke SK (2018) Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications. Hear Res 368:28–40. https://doi.org/10.1016/j.heares.2018.03.002
Santi PA, Mancini P, Barnes C (1994) Identification and localization of the GM1 ganglioside in the cochlea using thin-layer chromatography and cholera toxin. J Histochem Cytochem 42:705–716. https://doi.org/10.1177/42.6.8189033
Schuknecht HF (1956) Ablation therapy for the relief of Meniere’s disease. Laryngoscope 66:859. https://doi.org/10.1288/00005537-195607000-00005
Seidman M (2003) Effects of resveratrol on acoustic trauma. Otolaryngol Head Neck Surg 129:463–470. https://doi.org/10.1016/S0194-5998(03)01586-9
Selivanova O, Maurer J, Ecke U, Mann WJ (2003) The effects of Streptolysin-O and sodium hyaluronate on the permeability of the round window membrane in guinea pigs—an electrophysiologic study. Laryngorhinootologie 82:235–239. https://doi.org/10.1055/s-2003-38937
Şen Karaman D, Kettiger H (2018) Silica-based nanoparticles as drug delivery systems. In: Inorganic frameworks as smart nanomedicines. Elsevier, pp 1–40
Silverstein H, Choo D, Rosenberg SI, et al (1996) Intratympanic steroid treatment of inner ear disease and tinnitus (preliminary report). Ear Nose Throat J 75:468–471, 474, 476 passim
Şimşek G, Tokgoz SA, Vuralkan E et al (2013) Protective effects of resveratrol on cisplatin-dependent inner-ear damage in rats. Eur Arch Oto-Rhino-Laryngol 270:1789–1793. https://doi.org/10.1007/s00405-012-2183-4
Smith ME, Scoffings DJ, Tysome JR (2016) Imaging of the Eustachian tube and its function: a systematic review. Neuroradiology 58:543–556. https://doi.org/10.1007/s00234-016-1663-4
Sohmer H (1997) Pathophysiological mechanisms of hearing loss. J Basic Clin Physiol Pharmacol. https://doi.org/10.1515/JBCPP.1997.8.3.113
Somdaş MA, Güntürk İ, Balcıoğlu E et al (2020) Protective effect of N-acetylcysteine against cisplatin ototoxicity in rats: a study with hearing tests and scanning electron microscopy. Braz J Otorhinolaryngol 86:30–37. https://doi.org/10.1016/j.bjorl.2018.08.002
Hellstrom S, Spandow O, Anniko M (1990) Structural changes in the round window membrane following exposure to Escherichia coli lipopolysaccharide and hydrocortisone. Laryngoscope 100(9):995–1000
Sreenivas S, Pai K (2008) Thiolated chitosans: novel polymers for mucoadhesive drug delivery—a review. Trop J Pharm Res. https://doi.org/10.4314/tjpr.v7i3.14694
Staecker H, Jokovic G, Karpishchenko S et al (2019) Efficacy and safety of AM-111 in the treatment of acute unilateral sudden deafness—a double-blind, randomized, placebo-controlled phase 3 study. Otol Neurotol 40:584–594. https://doi.org/10.1097/MAO.0000000000002229
Sun C, Wang X, Chen D et al (2016) Dexamethasone loaded nanoparticles exert protective effects against Cisplatin-induced hearing loss by systemic administration. Neurosci Lett 619:142–148. https://doi.org/10.1016/j.neulet.2016.03.012
Sun H, Jiang M, Zhu S-H (2008) In vitro and in vivo studies on hydroxyapatite nanoparticles as a novel vector for inner ear gene therapy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 43:51–57
Surovtseva EV, Johnston AH, Zhang W et al (2012) Prestin binding peptides as ligands for targeted polymersome mediated drug delivery to outer hair cells in the inner ear. Int J Pharm 424:121–127. https://doi.org/10.1016/j.ijpharm.2011.12.042
Suzuki J, Corfas G, Liberman MC (2016) Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep 6:24907. https://doi.org/10.1038/srep24907
Syamchand SS, Sony G (2015) Multifunctional hydroxyapatite nanoparticles for drug delivery and multimodal molecular imaging. Microchim Acta 182:1567–1589. https://doi.org/10.1007/s00604-015-1504-x
Szeto B, Chiang H, Valentini C et al (2020) Inner ear delivery: challenges and opportunities. Laryngoscope Invest Otolaryngol 5:122–131. https://doi.org/10.1002/lio2.336
Tabatabaei Mirakabad FS, Nejati-Koshki K, Akbarzadeh A et al (2014) PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev 15:517–535. https://doi.org/10.7314/APJCP.2014.15.2.517
Tahir N, Tahir Haseeb M, Madni A, et al (2020) Lipid polymer hybrid nanoparticles: a novel approach for drug delivery. In: role of novel drug delivery vehicles in nanobiomedicine. IntechOpen
Tamura T, Kita T, Nakagawa T et al (2005) Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope 115:2000–2005. https://doi.org/10.1097/01.mlg.0000180174.81036.5a
Thaler M, Roy S, Fornara A et al (2011) Visualization and analysis of superparamagnetic iron oxide nanoparticles in the inner ear by light microscopy and energy filtered TEM. Nanomed Nanotechnol Biol Med 7:360–369. https://doi.org/10.1016/j.nano.2010.11.005
van de Heyning P, Muehlmeier G, Cox T et al (2014) Efficacy and safety of AM-101 in the treatment of acute inner ear tinnitus—a double-blind, randomized, placebo-controlled phase II study. Otol Neurotol 35:589–597. https://doi.org/10.1097/MAO.0000000000000268
Vona B, Haaf T (eds) (2016) Genetics of Deafness. S. Karger AG
Wakabayashi K, Fujioka M, Kanzaki S et al (2010) Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci Res 66:345–352. https://doi.org/10.1016/j.neures.2009.12.008
Wang N, Gao X, Li M et al (2020) Use of solid lipid nanoparticles for the treatment of acute acoustic stress-induced cochlea damage. J Nanosci Nanotechnol 20:7412–7418. https://doi.org/10.1166/jnn.2020.18522
Wang P, Chu W, Zhuo X et al (2017) Modified PLGA–PEG–PLGA thermosensitive hydrogels with suitable thermosensitivity and properties for use in a drug delivery system. J Mater Chem B 5:1551–1565. https://doi.org/10.1039/C6TB02158A
Wang X, Dellamary L, Fernandez R et al (2009) Dose-dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol Neurotol 14:393–401. https://doi.org/10.1159/000241896
Wang X, Dellamary L, Fernandez R et al (2011) Principles of inner ear sustained release following intratympanic administration. Laryngoscope 121:385–391. https://doi.org/10.1002/lary.21370
Wang X, Partlow B, Liu J et al (2015) Injectable silk-polyethylene glycol hydrogels. Acta Biomater 12:51–61. https://doi.org/10.1016/j.actbio.2014.10.027
Wang X, Truong T, Billings PB et al (2003) Blockage of immune-mediated inner ear damage by etanercept. Otol Neurotol 24:52–57. https://doi.org/10.1097/00129492-200301000-00012
Wang Y, Qu Y, Chen X et al (2019) Effects of D-methionine in mice with noise-induced hearing loss mice. J Int Med Res 47:3874–3885. https://doi.org/10.1177/0300060519860679
Wareing M, Mhatre AN, Pettis R et al (1999) Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear Res 128:61–69. https://doi.org/10.1016/S0378-5955(98)00196-8
Wen X, Ding S, Cai H et al (2016) Nanomedicine strategy for optimizing delivery to outer hair cells by surface-modified poly(lactic/glycolic acid) nanoparticles with hydrophilic molecules. Int J Nanomed 11:5959–5969. https://doi.org/10.2147/IJN.S116867
WHO (2021a) Deafness and hearing loss. In: World Heal. Organ. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss
WHO (2021b) WHO: 1 in 4 people projected to have hearing problems by 2050. https://www.who.int/news/item/02-03-2021b-who-1-in-4-people-projected-to-have-hearing-problems-by-2050
Wise AK, Tan J, Wang Y et al (2016) Improved auditory nerve survival with nanoengineered supraparticles for neurotrophin delivery into the deafened cochlea. PLoS ONE 11:e0164867. https://doi.org/10.1371/journal.pone.0164867
Wu T-H, Liu C-P, Chien C-T, Lin S-Y (2013) Fluorescent hydroxylamine derived from the fragmentation of PAMAM dendrimers for intracellular hypochlorite recognition. Chemistry 19:11672–11675. https://doi.org/10.1002/chem.201300494
Wu X, Ding D, Jiang H et al (2012) Transfection using hydroxyapatite nanoparticles in the inner ear via an intact round window membrane in chinchilla. J Nanoparticle Res 14:708. https://doi.org/10.1007/s11051-011-0708-1
Xu X, Chen H, Wu X et al (2018) Hollow mesoporous Silica@zeolitic imidazolate framework capsules and their applications for gentamicin delivery. Neural Plast 2018:1–9. https://doi.org/10.1155/2018/2160854
Yamahara K, Yamamoto N, Nakagawa T, Ito J (2015) Insulin-like growth factor 1: a novel treatment for the protection or regeneration of cochlear hair cells. Hear Res 330:2–9. https://doi.org/10.1016/j.heares.2015.04.009
Yang CJ, Chung JW (2016) Pathophysiology of noise induced hearing loss. Audiol Speech Res 12:S14–S16. https://doi.org/10.21848/asr.2016.12.S1.S14
Yang K-J, Son J, Jung SY et al (2018) Optimized phospholipid-based nanoparticles for inner ear drug delivery and therapy. Biomaterials 171:133–143. https://doi.org/10.1016/j.biomaterials.2018.04.038
Yoon JY, Yang K-J, Kim DE et al (2015) Intratympanic delivery of oligoarginine-conjugated nanoparticles as a gene (or drug) carrier to the inner ear. Biomaterials 73:243–253. https://doi.org/10.1016/j.biomaterials.2015.09.025
Yoon JY, Yang K-J, Park S-N et al (2016) The effect of dexamethasone/cell-penetrating peptide nanoparticles on gene delivery for inner ear therapy. Int J Nanomed 11:6123–6134. https://doi.org/10.2147/IJN.S114241
Yu D, Sun C, Zheng Z et al (2016) Inner ear delivery of dexamethasone using injectable silk-polyethylene glycol (PEG) hydrogel. Int J Pharm 503:229–237. https://doi.org/10.1016/j.ijpharm.2016.02.048
Yu D, Wu H, Sun C et al (2015) A single dose of dexamethasone encapsulated in polyethylene glycol-coated polylactic acid nanoparticles attenuates cisplatin-induced hearing loss following round window membrane administration. Int J Nanomed. https://doi.org/10.2147/IJN.S77912
Yu Z, Yu M, Zhang Z et al (2014) Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Res Lett 9:343. https://doi.org/10.1186/1556-276X-9-343
Zhang W, Pyykko I, Zou J et al (2011a) Nuclear entry of hyperbranched polylysine nanoparticles into cochlear cells. Int J Nanomedicine. https://doi.org/10.2147/IJN.S16973
Zhang Y, Zhang W, Löbler M et al (2011b) Inner ear biocompatibility of lipid nanocapsules after round window membrane application. Int J Pharm 404:211–219. https://doi.org/10.1016/j.ijpharm.2010.11.006
Zhao J, Li G, Zhao X et al (2020) Down-regulation of AMPK signaling pathway rescues hearing loss in TFB1 transgenic mice and delays age-related hearing loss. Aging 12:5590–5611. https://doi.org/10.18632/aging.102977
Zheng J, Shen W, He DZZ et al (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405:149–155. https://doi.org/10.1038/35012009
Zheng Z, Wang Y, Yu H et al (2020) Salvianolic acid B inhibits ototoxic drug–induced ototoxicity by suppression of the mitochondrial apoptosis pathway. J Cell Mol Med 24:6883–6897. https://doi.org/10.1111/jcmm.15345
Zou J, Saulnier P, Perrier T et al (2008) Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J Biomed Mater Res Part B 87B:10–18. https://doi.org/10.1002/jbm.b.31058
Zou J, Sood R, Ranjan S et al (2010a) Manufacturing and in vivo inner ear visualization of MRI traceable liposome nanoparticles encapsulating gadolinium. J Nanobiotechnology 8:32. https://doi.org/10.1186/1477-3155-8-32
Zou J, Sood R, Ranjan S et al (2012) Size-dependent passage of liposome nanocarriers with preserved posttransport integrity across the middle-inner ear barriers in rats. Otol Neurotol 33:666–673. https://doi.org/10.1097/MAO.0b013e318254590e
Zou J, Zhang W, Poe D et al (2010b) MRI manifestation of novel superparamagnetic iron oxide nanoparticles in the rat inner ear. Nanomedicine 5:739–754. https://doi.org/10.2217/nnm.10.45
Zou J, Zhang Y, Zhang W et al (2009) Preclinical Nanomedicine: Internalization of liposome nanoparticles functionalized with TrkB ligand in rat cochlear cell populations. Eur J Nanomed. https://doi.org/10.1515/EJNM.2009.2.2.7
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1A2C1011176).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (T.N. Nguyen and J.S. Park) declare that they have no conflicts of interest.
Research involving human and animal rights
This article does not contain any studies with human and animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, T.N., Park, JS. Intratympanic drug delivery systems to treat inner ear impairments. J. Pharm. Investig. 53, 93–118 (2023). https://doi.org/10.1007/s40005-022-00586-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-022-00586-8