Abstract
Cisplatin is a common chemotherapeutic agent used in many solid and hematologic malignancies. The main unwanted effect of cisplatin is ototoxicity, for which no standard treatment has been reported. The present study examined the protective efficacy of resveratrol on cisplatin-dependent ototoxicity through an experimental model. Fifteen rats were randomized into three groups. Group 1 (control group) (n = 5) received intraperitoneal (i.p.) 15 mg/kg cisplatin; group 2 (resveratrol group) (n = 5) received i.p. 100 mg/kg resveratrol, followed by i.p. 15 mg/kg cisplatin; group 3 (n = 5) served as a vehicle group and received i.p. 1 ml dimethyl sulfoxide. All rats underwent the auditory brainstem response (ABR) test before and 72 h after the treatment. Pretreatment ABR values of the groups were not significantly different. The pretreatment hearing threshold values of the groups were 30 ± 6.60 and 28.5 ± 5.29 dB in groups 1 and 2, respectively (p > 0.05). The post-ABR-I and post-ABR-IV values were, respectively, 1.41 ± 0.18 and 5.83 ± 0.16 ms in the control subjects and 1.19 ± 0.22 and 4.58 ± 0.27 ms in the study group. The ABR-I and ABR-IV durations in rats treated with resveratrol were significantly shorter (p < 0.01). A comparison of threshold values shows that the resveratrol-treated rats had significantly lower values than the control rats. After cisplatin injection, ABR I–IV intervals were compared among the groups. The ABR I–IV interval duration was 4.42 ± 0.16 ms in the control group, while the resveratrol-treated rats showed a significantly shorter ABR I–IV interval duration of 3.49 ± 0.27 ms (p < 0.001). Resveratrol attenuated cisplatin-dependent inner-ear damage, as shown by the ABR-I, ABR-IV, ABR I–IV interval, and hearing threshold values. Our results suggest that this natural antioxidant may be effectively used in reducing the unwanted effects of cisplatin on the ear physiology of patients, particularly those undergoing chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin is a common antineoplastic agent used for the treatment of several malignant neoplasms [1]. Animal and human research studies show that cisplatin significantly affects stria vascularis and the organ of Corti. The first affected structures are the outer hair cells on the basal turn of the cochlea and other cells on the apex of the cochlea. The inner hair cells (IHCs) are affected subsequently [2]. Therefore, the initial sign of cisplatin ototoxicity is hearing loss at high frequencies on a pure-tone audiogram. The loss of low frequencies, including the speech sounds, occurs later. The production of reactive oxygen species (ROS) in the cochlea is a contributing factor in the development of cisplatin-dependent ototoxicity. The generation of ROS is a consequence of decreased intracellular glutathione levels and a weakened antioxidant defence mechanism [3]. A weak antioxidant defence system causes an increase in lipid peroxidation, which is the main cause of apoptosis of hair cells, support cells, stria vascularis, and auditory nerves [4].
Although various chemoprotective agents have been used to prevent cisplatin-dependent ototoxicity, there is currently no standard treatment for it. Resveratrol (trans-3,4,5-trihydroxystilbene), a natural polyphenolic, nonflavonoid antioxidant, is a phytoalexin found in various food products, with particularly high levels present in grape skins (50–100 μg/g) and red wine (1.52 mg/l) [5]. Resveratrol has been shown to have significant anti-inflammatory, antioxidant, and immunomodulatory properties [6]. Its antioxidant properties mainly depend on the upregulation of endogenous cellular antioxidant systems, but the compound also shows direct ROS-scavenging properties [6]. The protective effects of resveratrol on the cochlea have been previously reported in treating aminoglycoside toxicity and in ototoxicity due to acoustic trauma. However, its role in the prevention of cisplatin-dependent ototoxicity is largely ignored [7, 8]. On the basis of this background, the present study aimed to investigate the effects of intraperitoneal (i.p.) injection of resveratrol on cisplatin-dependent ototoxicity in rats using the auditory brainstem response (ABR) test.
Materials and methods
Animal care and treatment
This study was carried out in the Experimental Research Laboratory. It had the approval of the ethics committee and complied with the guidelines for the care and use of experimental animals. Fifteen adult male Wistar rats, each weighing between 240 and 330 g, were purchased from an animal laboratory. All the rats were examined by a veterinarian and were determined to be in good health. The rats were housed in plastic cages and maintained under standard conditions: 12-h light and 12-h dark periods, 20 °C constant temperature, and a humidity range of 40–60 %. The rats had free access to standard dry pellets and tap water ad libitum until the end of the study. The external ears and the tympanic membranes of all rats were examined. Cerumen in the external ear canal was removed. Rats that showed any sign of otitis, opacification, and/or tympanic membrane perforation were excluded from the study.
Drug preparation
The 15 rats were randomized (using random number tables) into three groups: [1] control group (n = 5), [2] resveratrol group (n = 5), and [3] vehicle group (n = 5). In group 1 (control group), a single dose of 15 mg/kg of cisplatin (Cisplatin DBL, Australia) was administered intraperitoneally to the rats. The rats in group 2 (study group) were treated with a single dose of i.p. 100 mg/kg resveratrol (Sigma Aldrich, Saint-Quentin-Fallavier, France), followed by i.p. 15 mg/kg cisplatin 30 min later. In group 3, a single dose of i.p. 1 ml dimethyl sulfoxide (DMSO, Sigma Aldrich, Saint-Quentin-Fallavier, France), the vehicle of resveratrol, was injected. All injections were done under general anesthesia.
ABR measurements
The rats in all experimental groups were anesthetized with 10 mg/kg xylazine (Alfazyne®, Alfasan International B.V., Woerden, The Netherlands) and 60 mg/kg ketamine (Ketalar®, Eczacıbaşı Parke-Davis, Istanbul,Turkey) in recording the baseline ABR data at the beginning of the study and the final ABR measurements carried out 72 h after the treatment. The animals were examined under anesthesia and were confirmed to have normal external auditory canals and tympanic membranes before the baseline and final audiometric measurements. During the tests, the room temperature was maintained at 21 °C. The rats under general anesthesia were warmed up using an electrical heater to stabilize their normal body functions.
The procedure for recording ABRs in Sprague–Dawley rats has been described elsewhere [9, 10]. Briefly, a stainless-steel reference needle electrode was affixed to the vertex, and an active recording needle electrode was attached to the ipsilateral mastoid. A disk ground electrode was placed on the dorsum. Medelec Audiostar Portable Evoked Response Audiometers usually measure fast, middle, and late latencies of ABR in patients. Because the measurement of late latency responses (300 ms) requires alertness, only the fast latency responses (1–10 ms), which are normally used for electrophysiological evaluations, were recorded in the anesthetized rats. A special probe (Medelec ear tips, Neonatal) was introduced into the external auditory canal. Then, sound stimuli were delivered to the animals via a Medelec intra-auricular headset. An alternating click stimulus with a 10-s repetition rate was used to elicit action potentials in the auditory system. Each waveform was obtained from 1,024 sweeps. A Brüel and Kjaer microphone placed above the animal’s head was used to calibrate the click stimuli (duration of 100 ms, stimulation rate of 10/s, and frequencies of 0–10, 150 Hz). ABR measurements were elicited as waves I, IV, and I–IV interpeak intervals bilaterally. A complete ABR testing of each animal lasted approximately 20–30 min.
Statistical analysis
For the statistical analyses, the values were expressed as mean ± standard deviation whenever appropriate. The normalities of the distributions were tested using the Kolmogorov–Smirnov test. We also conducted a one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc and Chi-square tests to compare the groups. The homogeneity of the groups was confirmed by Levene’s test. Data analyses were performed using a statistical software package (SPSS version 15.0, SPSS, Chicago, IL, USA). For all comparisons, the statistical significance was determined as p < 0.05.
Results
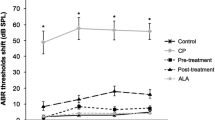
A total of 30 records from 15 animals were taken. In the vehicle group, the pre- and posttreatment ABR-I, ABR-IV, and hearing threshold levels were similar. Therefore, this group was excluded from the statistical analysis. The results presented include those from the control and treatment groups. The pretreatment ABR-I and ABR-IV values in control subjects were 1.02 ± 0.11 (0.86–1.29) ms and 4.26 ± 0.26 (4.04–4.90) ms, respectively. In resveratrol-treated rats, the pretreatment ABR-I and ABR-IV values were 1.08 ± 0.20 (0.70–1.34) ms and 4.08 ± 0.18 (3.80–4.42) ms, respectively. The groups revealed no difference in the pretreatment ABR-I and ABR-IV values (Table 1). Similarly, the pretreatment hearing threshold values of the groups were not significantly different (p = 0.58). After cisplatin injection, the ABR-I, ABR-IV, and hearing threshold values of the groups are presented in Table 2. The resveratrol treatment was associated with a significantly decreased duration of ABR-1, ABR-IV, and hearing threshold values (p < 0.05). The ABR I–IV interval duration was shorter in the treatment group. However, the difference was not statistically significant (Table 3). After the treatment, the ABR I-IV interval duration was significantly shorter in the resveratrol-treated rats than in the control subjects (p < 0.01). The change in the ABR I–IV interval is presented in Fig. 1. When the ABR I–IV interval durations were compared within the same group, the results showed no significant difference (p = 0.654 and p = 0.374).
Discussion
The results of the current study revealed that resveratrol, a potent natural antioxidant, has protective effects on cisplatin-dependent ototoxicity, which is one of the most common dose-limiting side effects of cisplatin [1]. Compared with the drug’s other side effects, including nephrotoxicity, ototoxicity has unique properties—being irreversible and cumulative. In the relevant literature, several chemoprotective agents, such as sodium thiosulfate, amifostine, d-methionine, glutathione, n-acetylcysteine, tocopherol, and vitamin C, have been used to prevent cisplatin-dependent ototoxicity; however, none of these agents has marked effects upon the occurrence or progress of the inner-ear damage [11–15].
The protective effects of resveratrol in different tissues have long been studied. Rubiolo et al. [16] showed that resveratrol increases the activity of antioxidant enzymes in primary rat hepatocyte cultures, thus protecting these cells from oxidative damage. Furthermore, Lee et al. [17] reported that resveratrol directly induces the production of antioxidative enzymes in vascular tissues. Recently, Di Franco et al. [18] pointed out that resveratrol could be effective in reducing skin toxicity that results from external beam radiotherapy for patients with breast cancer.
One of the main mechanisms involving cisplatin-dependent ototoxicity is the overproduction of ROS in the cochlea [19, 20]. ROS overproduction results in increased lipid peroxidation and apoptosis of IHC connective tissue, stria vascularis, and auditory nerves [4]. Therefore, preventing ROS overproduction and combating the increased oxidative stress might be important steps to avoid cisplatin-dependent ototoxicity. In the present study, we found that ABR-I, ABR-IV, and hearing threshold values were significantly shorter after resveratrol treatment in rats exposed to cisplatin. Eventually, one can conclude that resveratrol provides a significant protective effect on the cochlea, which is shown by shorter ABR I–IV interval values and lower hearing threshold measurements in the experimental model. Recently, Yumusakoglu et al. [21] reported that a systemic administration of resveratrol to guinea pigs significantly protected the cochlea from cisplatin ototoxicity. They also showed that the cochlear level of ROS was considerably decreased in resveratrol-treated animals. In a similar experimental model, Erdem et al. [22] investigated the effects of resveratrol on otoacoustic emission measurements in rats treated with cisplatin. They reported that resveratrol pretreatment is associated with a significant prevention of ototoxicity, as shown by stable values from their otoacoustic emission measurements at 1,418, 2,003, 3,363, 5,560, 8,003, and 9,515 Hz frequencies. These results collectively imply that cisplatin-dependent ototoxicity mainly depends on the ROS increase in the cochlea and that the antioxidant activity of resveratrol could prevent this toxicity.
The present experimental study has clear limitations that warrant a consideration. For example, because of the relatively small number of rats in each group, we were unable to identify the minor differences in the physiological studies. Another limitation was the lack of measurements of oxidative and antioxidative parameters in either cochlear tissue or plasma. The measurement and comparison of oxidative parameters or antioxidant enzyme levels between the groups could be more valuable in future studies on the topic.
We conclude that pretreating rats with i.p. resveratrol can significantly protect the cochlear function due to the compound’s antioxidant properties. As resveratrol is a common product that is available as a dietary supplement, it can be easily given to patients undergoing cisplatin chemotherapy to avoid the irreversible loss of hearing, which is the most significant dose-limiting side effect of the anti-cancer agent.
References
Boulikas T, Vougiouka M (2004) Recent clinical trail using sisplatin, karboplatin and their combination chemotherapy drugs. Oncol Rep 11:559–595
Meech RP, Campbell KC, Hughes LP et al (1998) A semiquantitative analysis of the effects of sisplatin on the rat stria vascularis. Hear Res 124:44–59
Ravi R, Somani SM, Rybak LP (1995) Mechanism of sisplatin ototoxicity: antioxidant system. Pharmacol Toxicol 76:386–394
Rybak LP, Whitworth C, Somani S (1999) Satu: application of antioxidants and other agents to prevent sisplatin ototoxicity. Laryngoscope 109:1740–1744
Vidavalur R, Otani H, Singal PK, Maulik N (2006) Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp Clin Cardiol 11(3):217–225
Rodrigo R, Bosco C (2006) Oxidative stress and protective effects of polyphenols: comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol 142(3–4):317–327
Bonabi S, Caelers A, Monge A, Huber A, Bodmer D (2008) Resveratrol protects auditory hair cells from gentamicin toxicity. Ear Nose Throat J 87(10):570–573
Seidman M, Babu S, Tang W, Naem E, Quirk WS (2003) Effects of resveratrol on acoustic trauma. Otolaryngol Head Neck Surg 129(5):463–470
Derin A, Agirdir B, Derin N, Dinc O, Guney K, Ozcaglar H et al (2004) The effects of l-carnitine on presbyacusis in the rat model. Clin Otolaryngol Allied Sci 29:238–241
Ozcaglar HU, Agirdir B, Dinc O, Turhan M, Kilinçarslan S, Oner G (2001) Effects of cadmium on the hearing system. Acta Otolaryngol 121:393–397
Muldoon LL, Pagel MA, Kroll RA, Brummett RE, Doolittle ND, Zuhowski EG, Egorin MJ, Neuwelt EA (2000) Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res 6(1):309–315
Campbell KCM, Meech RP, Rybak LP, Hughes LF (1999) d-Methionine protects against sisplatin damage to the stria vascularis. Hear Res 138:13–28
Feghali JG, Liu W, Van De Water TR et al (2001) l-n-Acetyl-cysteine protection against sisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope 111:1147–1155
Riggs LC, Matz GJ, Ryback RP (1998) Ototoxicity. In: Bailey BJ, Calhoun KH (eds) Head and neck surgery: otolaryngology, 2nd edn. Lippincott-Raven, Philadelphia, pp 2165–2170
Rybak LP, Husain K, Morris C et al (2000) Effect of protective agents against sisplatin ototoxicity. Am J Otol 21:513–520
Rubiolo JA, Mithieux G, Vega FV (2008) Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol 591:66–72
Li Y, Cao Z, Zhu H (2006) Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res 53:6–15
Di Franco R, Calvanese M, Murino P et al (2012) Skin toxicity from external beam radiation therapy in breast cancer patients: protective effects of Resveratrol, Lycopene, Vitamin C and anthocianin (Ixor®). Radiat Oncol 30(7):12
Peters RC, Mommersteeg PMC, Heijmen PS (1999) The electroreceptor organ of the catfish, Ictalurus melas, as a model for sisplatin-induced ototoxicity. Neuroscience 91:745–751
Dehne N, Lautermann J, Petrat F et al (2001) Sisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol 174:27–34
Yumusakhuylu AC, Yazici M, Sari M, Binnetoglu A, Kosemihal E, Akdas F, Sirvanci S, Yuksel M, Uneri C, Tutkun A (2012) Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol 76(3):404–408
Erdem T, Bayindir T, Filiz A, Iraz M, Selimoglu E (2011) The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur Arch Otorhinolaryngol 269(10):2185–2188 (Epub ahead of print)
Acknowledgments
The authors in this manuscript indicate that they do not have any financial relationship with the organization that sponsored the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Şimşek, G., Tokgoz, S.A., Vuralkan, E. et al. Protective effects of resveratrol on cisplatin-dependent inner-ear damage in rats. Eur Arch Otorhinolaryngol 270, 1789–1793 (2013). https://doi.org/10.1007/s00405-012-2183-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-012-2183-4