Abstract
The objective of this work was to assess the incidence of Yellow Sigatoka in banana plants cultivated with deficiencies of nitrogen, phosphorus, potassium, calcium, magnesium, sulfur or boron. The experimental design was a randomized complete block with 8 treatments, 4 repetitions and 1 plant per repetition. The treatments were supplied in solution culture and consisted of all the nutrients (control) or nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulphur (S) or boron (B) deficiency. Leaves 1 and 2 were inoculated on the abaxial surface with a suspension of conidia and assessed every 5 days to with a total of 5 assessments. The average number of lesions were integrated for the area under the disease progress curve (AUDPC). The greatest AUDPC occurred in plants deficient in K, N, P, S, or Mg. Plants deficient in N, P, K, Ca, Mg, S or B had lower leaf contents of these nutrients and showed morphological changes expressed in visual deficiency symptoms. Thus, banana plants deficient in K, N, P, S or Mg had a greater incidence of Yellow Sigatoka, compared with plants with full nutrients and plants deficient Ca or B.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The banana plant (Musa spp.) is among the most cultivated fruit species in tropical and subtropical countries because the fruit is appreciated by many consumers. World banana production in 2013 was 106.71 million tons; with the largest producers being India, China, Philippines, Brazil and Ecuador (Faostat 2015). There are a large number of diseases that can decrease banana productivity (Furtado et al. 2009); however, Yellow Sigatoka (Mycosphaerella musicola Leach (Stat. Conid. Pseudocercospora musae Zimm.)) has become increasngly important in recent years (Gomes et al. 2013). This disease is wide-spread in several producing regions where it causes early leaf death and a consequent reduction in plant growth and development that severely limits production (Surridge et al. 2003). Loses caused by Yellow Sigatoka are estimated by 50 to 100 % depending on the microclimate (Aman and Rai 2015; Cordeiro and Matos 2005; Rocha et al. 2012). According to Cordeiro et al. (2005), Yellow Sigatoka is difficult to control. The best way to manage this disease is to use several control measures, among which is to keep the plant well nourished. It is well known that fast leaf emergence, in shorter breaks, provids not only gene expression for horizontal resistance, but also the improvement of physical and chemical barriers to pathogen infection (Marschner 2012; Taiz and Zeiger 2013).
The banana plant grows and develops rapidly, necessitating an appropriate concentration of nutrients in the soil with a subsequent balanced nutrition if the plant is to reach high productivity (Santos et al. 2009; Silva et al. 2011). Due to this fact, many researchers describe the effects of mineral nutrition on the growth and productivity of the banana plant; however, few of them show the relationship of nutrition with the intensity of disease (Freitas et al. 2015).
The relationship between plant nutrition and disease is hard to study because it is difficult to isolate external factors or the effect of individual nutrients in the infectious process. Studies in nutrient solution permit the isolation of each nutrient to enable studying the relationship between the nutritional effects and the intensity of disease (Lima et al. 2010).
Considering the shortage of information related to nutrient effects on the intensity of Yellow Sigatoka of banana and the necessity of adopting management strategies that can reduce the use and impact of agricultural pesticides on the environment, our objective was to assess the affect of individual macronutrients and Boron on the incidence of Yellow Sigatoka on banana plants cultivated in nutrient solution.
Materials and methods
This experiment, with the same treatments, was repeated in order to verify repeatability of the results. In both experiments, banana seedlings (Musa spp.‘AAA Cavendish’) micropropagated from Grande Naine were selected for uniformity after the 4th leaf appearance. The seedlings were initialy gorwn for 15 days in 16 L trays containing the basic nutrient solution of Hoagland and Arnon (1950) at 50 % ionic strength with continuous aeration. After this initial adaptation, the plants were transferred to 6 L vessels painted on the external surface with reflective paint. These vessels contained the 100 % ionic strength nutrient solution of Hoagland and Arnon (1950) under continuous aeration modified for the 8 treatments consisting of a complete nutrient solution with all the nutrients (control) or with nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulphur (S) or boron (B) deficiency (Table 1). The experimental design was a completely randomized block design with four repetitions of one plant per repetition.
The pH of the nutrient solution was monitored weekly, and kept between 5.5 and 6.0 with the addition of HCl 0.1 mol L−1 or NaOH 0.1 mol L−1. Whenever necessary, the volume in the vessels was replenished with deionized water. The nutrient solution was changed in the different treatments when there was a 30 % depletion of K+ in the control treatment as determined by a Compaction Meter® (Horiba-CARDY).
Mycosphaerella musicola was isolated from banana leaves, cultivar Prata Anã (Musa spp.), following the methodology described by Cordeiro et al. (2011). Six 5 mm diameter plugs of sporulating mycelium removed from 23 day old malt agar colonies were transferred to 50 ml Erlenmeyer flasks containing 30 ml of V8 liquid medium (100 ml of V8 juice, 1 g of CaCO3 and 900 ml of distilled water). After inoculation, the Erlenmeyer flasks were agitated (120 rpm) for 6 days. After this period, the content was distributed into two Petri dishes containing V8 solid medium (100 ml of juice V8, 20 g agar, 1 g of CaCO3 and 900 ml of distilled water). To facilitate drying, the dishes were kept open in the BOD with four 20 W fluorescent bulbs at 25 °C and 24 h photoperiod. After the culture medium dried (approximately 2 days of incubation), 10 ml of sterile distilled water was added to each dish and toothbrushes were used to facilitate the release of conidia. The suspension obtained was filtered through a double gauze layer and the spore concentration was adjusted using an optical microscope fitted with a Neubauer chamber based on an average of 4 readings.
Plants were inoculated 28 days after transferring them to the individual treatments by atomizing a suspension of 2 × 104 conidia ml−1 to the abaxial surface of leaves 1 and 2 for full coverage without draining (Lhomme and Jimenez 1992). After inoculation, the plants were kept in a moist chamber maintained at 95 % relative humidity with an ultrasonic humidifier for 48 h.
Seventeen days after inoculation, the number of lesions were recorded using a template with a rectangular area of 50 cm2 to evaluate the four quadrants of the leaf blade (Cordeiro 1997). Five assessments were made at 5 day intervals. The average number of lesions in the four quadrants were used to calculate the area under the disease progress curve (AUDPC) according to the equation proposed by Shaner and Finney (1977):
In which:
- yi:
-
proportion of disease in i-th observation
- ti:
-
time, in days, in i-th observation
- n:
-
total number of observations.
After completing the disease assessments, leaf 3 was dissected discarding the central vein, washed in distilled water, separately packaged in paper bags and kiln dried at 60 °C. After drying to a constant mass, the samples were ground and analyzed (Malavolta et al. 1997), to determine their nitrogen, phosphorus, potassium, calcium, magnesium, sulphur, boron, copper, iron, manganese and zinc content.
The Shapiro-Wilk test (Shapiro and Wilk 1965) was applied to the data to assess the normal distribution using software R (R Core Team 2014). In the absence of normality, the data were transformed through the algorithm of Box and Cox (1964). After these procedures, the variation between the two experiments of each variable was submitted to joint analysis over time, using the PROC GLM procedure of SAS (v. 9.2; SAS Institute Inc.). The variables were then submitted to an analysis of variance analysis (ANOVA). Significant variables (F test) were compared through the Scott-Knott test (P < 0.05) of software R included with the Agricolae (Mendiburu 2014) and Scott-Knott (Jelihovschi et al. 2014) packages.
Results and discussion
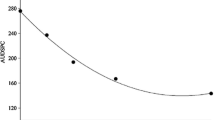
Since there was no difference between the two experiments, the data presented are the average of the two experiments. The area under the disease progress curve (AUDPC) showed significant differences (P < 0.05) between the treatments. The highest AUDPC occurred in plants that were deficient in K, N, P, S or Mg. The plants with all the nutrients (control) had the least AUDPC, but did not differ statistically from the Ca or B deficient treatments (Fig. 1).
Area under the disease progress curve (AUDPC) of Yellow Sigatoka in banana plants cultivated in full nutrient solution (control) and deficient in nitrogen (−N), phosphorus (−P), potassium (−K), calcium (−Ca), magnesium (−Mg), sulphur (−S) and boron (−B). *Means followed by the same letter do not differ statistically through the test of Scott-Knott, at P = 0.05
The highest values of AUDPC with a deficiency of N or K can be due to these nutrients being required in higher quantities than the others in banana culture (Diniz et al. 1999; Santos et al. 2009) (Fig. 1). The role of these two nutrients in plant diseases is specific in each pathosystem and dependant on the host species and its interaction with the pathogen and the environment. It can occur with higher or lower disease intensity depending on the supply of the element and its balance with other nutrients (Pozza and Pozza 2012).
Belan et al. (2014) reported low K in leaves of coffee plants close to lesions of necrotrophic and biotrophic pathogens and high K in tissues more distant from them. These authors related their results to the effects of K in the plant’s defense to pathogens and to the breaking of the vacuole. The beneficial effects of K in suppressing disease have been verified for necrotrophic and biotrophic pathogens (Dordas 2009). Although K deficient plants are generally more susceptible to infection than plants properly nourished (Huber and Graham 1999), the susceptibility of K deficient plants is related to several metabolic functions infuenced by this nutrient in plant metabolism (Amtmann et al. 2008; Marschner 2012). According to Marschner (2012), K is involved with cellular permeability that maintains turgor and keeps water available for essential enzymatic activity and several other metabolic processes. According to Huber and Arny (1985), K increases the accumulation of phytoalexines and phenols around infected areas to reduce the colonization and reproduction of pathogens, and decrease the production of initial inoculum. Moreover, K reinforces the cell wall and enhances the formation of inter and intracelular material to make the penetration of pathogenic agents difficult by stimulating the cicatrization of wounds (Li et al. 2010; Pozza and pozza 2012).
In banana culture, several studies report that K is important in reducing the intensity of diseases. Through the use of geostatistics, reduced severity of Black Sigatoka was evident (Mycosphaerella fijiensis) along areas with higher K (Uchôa et al. 2011). The number of plants with symptoms of Panama Disease (Fusarium oxysporum f. sp. cubense) was also higher in areas where the plants showed lower leaf K (Furtado et al. 2009). Silva and Rodrigues (2013) also reported that fertilization with increasing rates of K2O (up to 800 kg ha−1 year) reduced the percentage of banana plants infected by Panama Disease. There are also accounts of K affecting other pathosystems. Doreto et al. (2012) reported a 36 % reduction in the incidence of soybean rust (Phakopsora pachyrhizi) by applying 80 kg ha−1 of K2O. Increasing concentrations of K (up to 7 mmol L−1) in liquid culture reduced the intensity of Phoma leaf spot (Phoma tarda) (Lima et al. 2010) and Brown Eye Spot (Cercospora coffeicola Berk and Cooke (1881) (Garcia Júnior et al. 2003) of coffee trees, although this concentration resulted in an imbalance with other nutrients, and the over-all intensity of disease increased. In wheat, the severity of foliar blight (Cochliobolus sativus and/or Pyrenophora tritici-repentis) was reduced 51 % by applying 60 kg ha−1 of K2O (Sharma et al. 2005).
The influence of N on resistance or susceptibility of plants to disease varies depending on other factors such as the pathogen, genotype, concentration and nutrient source used and interaction of N with other nutrients. Appropriate concentrations of N contributed to the synthesis of lignin, phytoalexines and tannins, but in excess, N reduces the production of these compounds due to the demand for carbon in photosynthesis via the Krebs cycle, risking the synthesis of secondary metabolites through the shikimic acid cycle that is important for disease resistance. Excess N also contributes to the release of polysaccharides on the leaf surface which can be used by several pathogens as an energy source (Huber and Thompson 2007). Therefore, the effect of N on plant diseases can not be generalized because specific diseases can be favorable or not with the increase of N (Pozza and Pozza 2012). Similar to the effect of K, Furtado et al. (2009) confirmed a higher number of banana plants with symptoms of Panama Disease in areas where the plants contained lower leaf N. Brown Eye Spot of coffee trees, (Pozza et al. 2001) was reduced 20.7 % with increased N in the nutrient solution. Cao et al. (2011) reported a 16 % increase in leaf lesions caused by Pseudomonas syringae pv. syringae on N deficient peach plants. The application of 67.2 kg ha−1 of N to soil reduced leaf diseases up to 14 %, in wheat following pasture (Krupinsky and Tanaka 2001). In other pathosystems, the application in high rates of N and an imbalance with other nutrients has been reported to increase the intensity of several diseases.
After K and N, plants deficient in P, S and Mg had the next highest AUDPCs (Fig. 1). Phosphorus is an essential mineral for the production of energy in cellular metabolism (ATP synthesis) and is also a constituent of phospholipids in cell membranes, proteins, nucleic acids, and is involved with the accumulation of lignin and tissues maturation besides other activities. Thus, it is possible for P to influence plant resistance to several diseases (Taiz and Zeiger 2013). Increasing applications of P2O5, from 0 to 170.0 kg ha−1 reduced the rate of Soybean Rust (Phakopsora pachyrhizi) development from 0.42 to 0.29 (Balardin et al. 2006). In the same way, potato plants fertilized with P (240 kg ha−1) and N (300 kg ha−1) had a lower incidence of Verticillium wilt (Verticillium dahliae) compared to non-fertilized plants (Davis et al. 1994). The incidence and severity of Brown Spot in cowpea were also significantly reduced with applications of 90 and 120 kg ha−1 of P2O5. Furthermore, fertilization also increased the number of petioles, pods, nodules, seed/pod, leaf area and crop yield. These results were attributed to increased growth and development of the roots that improved the absorption of nutrients for vigorous vegetative growth to decrease the intensity of disease in the crown of the plant by escaping from this disease (Owolade et al. 2006).
Sulfur participates in important processes during plant growth and development (Marschner 2012) to influence resistance to plant diseases. The production of toxic compounds, emission of volatile component (hydrogen sulphide, dimethyl sulphide and dimethyl disulfide), and production of gluatathione, phytoalexines and glucosinolates are important functions to avoid fungal infection (Haneklaus et al. 2007). A few studies developed to monitor the reduction of diseases by using S have reported some promising results.
Similar to reports for K and N, Furtado et al. (2009) confirmed the higher number of plants with symptoms of Panama Disease in areas where the plants showed lower levels of S. Reduced intensity of diseases of other crops with S has also been reported. Salac et al. (2005) reported a lower incidence and severity of leaf blotch (Pyrenopeziza brassicae) in rape plants nourished with S. These authors attributed these results to the increase in gluatathione, cysteine and glucosinolates. Similar results were found by Klikocka et al. (2005) who reported a significant decrease of thine infection by Rhizoctonia solani, and a noticeable increase in the production of potato tubers when plants were nourished with S. Klikocka (2009) also observed a decrease in the incidence and severity of Streptomyces scabies and Rhizoctonia solani on potato tubers in areas where S was applied to the soil.
Magnesium is a component of structural tissues and participates in several physiological and biochemical functions; moreover, it is a component of the chlorophyll molecule and necessary for protein synthesis (Marschner 2012). Due to Mg participation in these processes, deficient or extremely under nourished plants with this nutrient tend to be more susceptible to diseases (Huber and Jones 2013). Relatively few studies have shown the effects of Mg on plant diseases. Among those reported, most of them confirm the reduction of disease with a sufficient supply of Mg (Jones and Huber 2007). In coffee, Alves et al. (2009) reported a higher intensity of rust (Hemileia vastatrix) and brown eye spot in the leaves and fruits of plants deficient in Mg, S, N and Cu. Likewise, leaf sheath blight (Rhizoctonia solani) in rice was reduced when the concentration of Mg was increased from 0.062 to 0.5 mM (Schurt et al. 2014).
Plants deficient in Ca and B had higher AUDPCs than the full nutrient treated plants (control), although the AUDPC was lower than plants deficient in the other nutrients (Fig. 1). It is important to highlight the importance of these nutrients in the reduction of plant diseases. The role of Ca in the management of plant diseases is well described in the literature (Pozza and Pozza 2012). This nutrient aids recognition of pathogen infection in the plasma membrane, in biomembrane stability and cell wall structure (Huber et al. 2012). Thus, there are many reports of reduced disease with the application of Ca to soil (Pozza and Pozza 2012). In banana plant, Freitas et al. (2015) and Gerald et al. (2003), reported less Yellow Sigatoka in areas with higher contents of Ca. The same effect was confirmed in other pathosystems, including coffee (Coffea arabica/Cercospora coffeicola) (Garcia Júnior et al. 2003), citrus (Citrus paradisi/Mycosphaerella citri) (Mondal and Timmer 2003) and oak (Quercus ilex/Phytophthora cinnamomi) (Serrano et al. 2013). In contrast to Ca, only a few studies report the effects of B on plant diseases. Some mechanisms involved with a sufficiency of B include detoxification of microbial metabolites, strength and integrity of the cell wall, and synthesis of phenolic compounds and lignin (Stangoulis and Graham 2007).
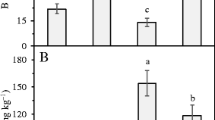
Nutritional deficiency of the plants with the various treatments was confirmed through leaf analyses and the expression of visual symptoms (Tables 2 and 4). Leaf content of N, P, K, Ca, Mg, S or B were significantly lower with all treatments compared with the full nutrient control, (Table 2). Nutrients in the full treatment (control) were close to the range considered sufficient (Borges and Souza 2004) for the culture of banana (Tables 2 and 3). This result could explain why the lowest AUDPC was observed in the nutrient sufficient plants (Fig. 1) since nutrients are responsible for the expression of horizontal resistance dependent on physical and chemical barriers (Marschner 2012; Taiz and Zeiger 2013).
Furtado et al. (2009), studying leaf content of nutrientes in healthy banana plants compared with those showing symptoms of Panama disease, confirmed the higher content of N, K and S in asymptomatic plants and lower content of these minerals in diseased plants. The highest N content was found in the Ca deficient treatment (Table 2). A deficiency of Ca can favor the accumulation of N in leaf tissue because of a general disintegration in membrane structure and the loss of cellular compartmentation (Marschner 2012). The highest content of P, Ca and Fe were found in the Mg deficient treatment (Table 2). According to Malavolta et al. (1997) Ca, Fe and Mg participate in the process known as competitive inhibition; in other words, these cations compete for the same spot of absorption. Thus, the deficiency of Mg possibly favored the absorption of Ca and Fe because of the reduced competitive inhibition between Mg and these nutrients. The highest content of Mg, S and Zn were found in the K deficient treatment (Table 2). These results also can be explained based on competitive inhibition since K competes with Mg and Zn (Malavolta et al. 1997). In contrast, the highest content of K was found in the treatment with all the nutrients (Table 2). This result reinforces the importance of K in the reduction of Yellow Sigatoka because the AUDPC was lowest in plants with the full nutrient treatment and highest in K deficient plants (Fig. 1). Plants receiving the full treatment had a balanced nutritional program (Tables 2 and 3). The banana plant is sensitive to nutritional imbalance; thus, the balance of nutrients can be as important as the specific level of a nutrient (Silva et al. 2008; Souza et al. 1999). Nutritional imbalance created by a deficiency of individual nutrients was also confirmed in the other treatments. A deficiency of N resulted in a higher content of B and Cu while the deficiency of B increased Mn (Table 2).
No visual expression of deficiency symptoms were seen in the full treatment (control) plants; however, morphological changes and visual symptoms characteristic of individual nutritional deficiencies were observed in plants deficient in specific nutrients with the other treatments (Table 4). Experiments in nutrient solution with missing elements have been used to characterize deficiency symptoms in other plants such as soursop (Annona muricata) (Batista et al. 2003), camu camu (Myrciaria dubia) (Viégas et al. 2004), pineapple ‘Imperial’ (Ramos et al. 2009) and ornamental ginger (Zingiber spectabile) (Coelho et al. 2012).
Conclusions
-
1.
Banana plants deficient in K, N, P, S and Mg had a greater area under the disease progress curve for Yellow Sigatoka than non-deficient and plants deficient in Ca or B.
-
2.
The deficiency of individual nutrients causes an imbalance in the concentration of other nutrientes that is expressed as visual morphological changes and symptoms characteristic of each nutritional deficiency.
References
Alves MC, Silva FM, Pozza EA, Oliveira MS (2009) Modeling spatial variability and pattern of rust and brown eye spot in coffee agroecosystem. J Pest Sci 82:137–148. doi:10.1007/s10340-008-0232-y
Aman M, Rai VR (2015) Potent toxigenic effect of Mycosphaerella musicola on locally growing banana varieties. Phytoparasitica 43:295–301. doi:10.1007/s12600-015-0456-3
Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant 133:682–691. doi:10.1111/j.1399-3054.2008.01075.x
Balardin RS, Dallagnol LJ, Didoné HT, Navarini L (2006) Influence of phosphorus and potassium on severity of soy bean rust. Phakopsora Pachyrhizi Fitopatol Bras 31:462–467. doi:10.1590/S0100-41582006000500005
Batista MMF, Viégas IDJ, Frazão DAC, Thomaz MAA, Silva RD (2003) Effect of macronutrient omission in growth, symptoms of nutritional deficiency and mineral composition in soursop plants (Annona muricata). Rev Bras Frutic 25:315–318. doi:10.1590/S0100-29452003000200033
Belan LL, Pozza EA, Freitas ML, Pozza AAA, Abreu MS, Alves E (2014) Nutrients distribution in diseased coffee leaf tissue. Australas Plant Pathol 44:105–111. doi:10.1007/s13313-014-0329-0
Berk, Cooke (1881) Cercospora coffeicola. Grevillea 9:99
Borges AL, Souza LS (2004) O cultivo da bananeira. Cruz das Almas, Brazil
Box GE, Cox DR (1964) An analysis of transformations. J R Stat Soc Ser B Methodol 26:211–252
Cao T, Kirkpatrick BC, Shackel KA, Dejong TM (2011) Influence of mineral nutrients and freezing-thawing on peach susceptibility to bacterial canker caused by Pseudomonas syringae pv. syringae. Fruits 66:441–452. doi:10.1051/fruits/2011057
Coelho VAT, Rodas CL, Coelho LC, Carvalho JG, Almeida EFA, Figueiredo MA (2012) Macronutrients and boron deficiency visual symptoms characterization in ornamental ginger. Rev Bras Hortic Ornamental 18:47–55
Cordeiro ZJM (1997) Variabilidade patogênica de isolados de Mycosphaerella musicola e resistência induzida e genética em genótipos de bananeira. Dissertation, Escola Superior de Agricultura “Luiz de Queiroz”, Piracicaba
Cordeiro ZJM, Matos AP (2005) Expression of resistance of banana varieties to yellow Sigatoka. Fitopatol Bras 30:534–535. doi:10.1590/S0100-41582005000500013
Cordeiro ZJM, Matos AP, Kimati H (2005) Doenças da bananeira. In: Kimati H, Amorim L, Resende JAM, Bergamin Filho A, Camargo LEA (ed) Manual de fitopatologia: doenças das plantas cultivadas. 4rd edn. São Paulo, São Paulo, pp 99–117
Cordeiro ZJM, Rocha HS, Araújo AG (2011) Metodologia para manuseio de Mycosphaerella musicola em laboratório. EMBRAPA Mandioca e Fruticultura, Cruz das Almas, p 32
Davis JR, Stark JC, Sorensen LH, Schneider AT (1994) Interactive effects of nitrogen and phosphorus on Verticillium wilt of Russet Burbank potato. Am Potato J 71:467–481
Diniz JDN, Gonçalves AN, Hernandez FFF, Torres AC (1999) Macronutrient absorption by banana explants in vitro. Pesq Agrop Brasileira 34:1201–1209. doi:10.1590/S0100-204X1999000700012
Dordas C (2009) Role of nutrients in controlling plant diseases in sustainable agriculture: a review. Sustainable Agriculture 33–46. doi:10.1051/agro:2007051
Doreto RBS, Gavassoni WL, Silva EF, Marchetti ME, Bacchi LMA, Stefanello FF (2012) Asian rust and soybean yield under potassium fertilization and fungicide, in the 2007/08 crop season. Semin: Ciências Agrárias 33:941–952. doi:10.5433/1679-0359.2012v33n3p941
Faostat (2015). Food and Agriculture Organization of the United Nations. http://faostat3.fao.org/home/E. Accessed 27 Jul 2015
Freitas AS, Pozza EA, Alves MC, Coelho G, Rocha HS, Pozza AAA (2015) Spatial distribution of Yellow Sigatoka Leaf Spot correlated with soil fertility and plant nutrition 1–15. Precis Agric. doi:10.1007/s11119-015-9409-6
Furtado EL, Bueno CJ, Oliveira ALD, Menten JOM, Malavolta E (2009) Relationship between occurrence of Panama disease in banana trees of cv. Nanicão and nutrients in soil and leaves. Trop Plant Pathol 34:201–215. doi:10.1590/S1982-56762009000400002
Garcia Júnior D, Pozza EA, Pozza AA, Souza PE, Carvalho JG, Balieiro AC (2003) Incidence and severity of the brown eye spot of coffee according to supply of potassium and calcium in nutrient solution. Fitopatol Bras 28:286–291. doi:10.1590/S0100-41582003000300010
Gerald SF, White SD, Dickinson AA, Goldman B (2003) A survey of Sigatoka leaf disease (Mycosphaerella musicola Leach) of banana and soil calcium levels in North Queensland. Anim Prod Sci 43:1157–1161. doi:10.1071/EA02094
Gomes LIS, Douhan GW, Bibiano LB, Maffia LA, Mizubuti ES (2013) Mycosphaerella musicola identified as the only pathogen of the Sigatoka disease complex present in Minas Gerais State, Brazil. Plant Dis 97:1537–1543. doi:10.1094/PDIS-12-12-1212-RE
Haneklaus S, Bloem E, Schnug E (2007) Sulfur and plant disease. In: Datnoff LE, Elmer WH, Huber DM (eds) Mineral nutrition and plant disease. The American Phytopathological Society, Saint Paul, pp 101–118
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California Agricultural Experiment Station, Berkeley, 32 p
Huber DM, Jones JB (2013) The role of magnesium in plant disease. Plant Soil 368:73–85. doi:10.1007/s11104-012-1476-0
Huber DM, Arny DC (1985) Interactions of potassium with plant disease. In: Munson RD (ed) Potassium in Agriculture. ASA, CSSA, SSA, Madison, pp 467–488
Huber DM, Graham RD (1999) The role of nutrition in crop resistance and tolerance to disease. In: Rengel Z (ed) Mineral nutrition of crops fundamental mechanisms and implications. Food Product Press, New York, pp 205–226
Huber DM, Thompson IA (2007) Nitrogen and plant disease. In: Datnoff LE, Elmer WH, Huber DM (eds) Mineral nutrition and plant disease. The American Phytopathological Society, Saint Paul, pp 31–44
Huber D, Römheld V, Weinmann M (2012) Relationship between nutrition, plant diseases and pests. In: Marschner H (ed) Mineral nutrition of higher plants, 3rd edn. Academic Pres, San Diego, pp 283–298
Jelihovschi EG, Faria JC, Allaman IB (2014) The Scott-Knott clustering algoritm. Ilheus, Brasil
Jones JB, Huber DM (2007) Magnesium and plant disease. In: Datnoff LE, Elmer WH, Huber DM (eds) Mineral nutrition and plant disease. The American Phytopathological Society, Saint Paul, pp 95–100
Klikocka H (2009) Influence of NPK fertilization enriched with S, Mg, and micronutrients contained in liquid fertilizer Insol 7 on potato tubers yield (Solanum tuberosum L.) and infestation of tubers with Streptomyces scabies and Rhizoctonia solani. J Elem 14:271–288
Klikocka H, Haneklaus S, Bloem E, Schnug E (2005) Influence of sulfur fertilization on infection of potato tubers with Rhizoctonia solani and Streptomyces scabies. J Plant Nutr 28:819–833. doi:10.1081/PLN-200055547
Krupinsky JM, Tanaka DL (2001) Leaf spot diseases on winter wheat influenced by nitrogen, tillage, and haying after a grass-alfalfa mixture in the Conservation Reserve Program. Plant Dis 85:785–789. doi:10.1094/PDIS.2001.85.7.785
Lhomme JP, Jimenez FO (1992) Estimating dew duration on banana and plantain leaves from standard meteorological observations. Agric For Meteorol 62:263–274
Li WJ, Ping HE, Jin JY (2010) Effect of potassium on ultrastructure of maize stalk pith and young root and their relation to stalk rot resistance. Agric Sci China 9:1467–1474. doi:10.1016/S1671-2927(09)60239-X
Lima LMD, Pozza EA, Torres HN, Pozza AA, Salgado M, Pfenning LH (2010) Relationship between nitrogen/potassium with Phoma spot and nutrition of coffee seedlings cultivated in nutrient solution. Trop Plant Pathol 35:223–228. doi:10.1590/S1982-56762010000400003
Malavolta E, Vitti GC, Oliveira SA (1997) Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd edn. POTAFOS, Piracicaba, 319 p
Marschner H (2012) Mineral nutrition of higher plants, 3rd edn. Academic, San Diego, 643 p
Mendiburu F (2014) Statistical Procedures for Agricultural Research. R package version 1.2-1. IOP Publishing PhysicsWeb. http://CRAN.R-project.org/package=agricolae. Accessed 03 Nov 2014
Mondal SN, Timmer LW (2003) Effect of urea, CaCO3, and dolomite on pseudothecial development and ascospore production of Mycosphaerella citri. Plant Dis 87:478–483. doi:10.1094/PDIS.2003.87.5.478
Owolade OF, Akande MO, Alabi BS, Adediran JA (2006) Phosphorus level affects Brown Blotch Disease, development and yield of Cowpea. World J Agric Sci 2:105–108
Pozza AAA, Martinez HEP, Caixeta SL, Cardoso AA, Zambolim L, Pozza EA (2001) Influence of the mineral nutrition on intensity of brown-eye spot in young coffee plants. Pesq Agrop Brasileira 36:53–60. doi:10.1590/S0100-204X2001000100007
Pozza EA, Pozza AAA (2012) Relação entre nutrição e as doenças de plantas: implicações práticas. SIMPÓSIO AVANÇOS NA OTIMIZAÇÃO DO USO DE DEFENSIVOS AGRÍCOLAS NO MANEJO FITOSSANITÁRIO, 12., , Lavras. Anais…Lavras: UFLA, 2012. 1 CD-ROM
Ramos MJM, Monnerat PH, Carvalho AJCD, Pinto JLDA, Silva JD (2009) Visual symptons of macronutrients and boron deficiency in ‘imperial’ pineapple. Rev Bras Frutic 31:252–256. doi:10.1590/S0100-29452009000100035
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. Accessed 03 nov. 2014
Rocha HS, Pozza EA, Uchôa CDN, Cordeiro ZJM, Souza PE, Sussel ÂAB, Rezende CA (2012) Temporal progress of yellow Sigatoka and aerobiology of Mycosphaerella musicola Spores. J Phytopathol 160:277–285. doi:10.1111/j.1439-0434.2012.01897.x
Salac I, Haneklaus SH, Bloem E, Booth EJ, Sutherland KG, Walker KC, Schnug E (2005) Sulfur nutrition and its significance for crop resistance: a case study from Scotland. Agric Res 283:111–119
Santos VPD, Fernandes PD, Melo ASD, Sobral LF, Brito MEB, Dantas JDDM, Bonfim LV (2009) Fertigation of banana cv Prata Anã with N and K in an ultisol. Rev Bras Frutic 31:567–573. doi:10.1590/S0100-29452009000200035
Schurt DA, Lopes UP, Duarte HS, Rodrigues FÁ (2014) Effect of magnesium on the development of sheath blight in rice. J Phytopathol 162:617–620. doi:10.1111/jph.12240
Serrano MS, Fernández-Rebollo P, Vita P, Sánchez ME (2013) Calcium mineral nutrition increases the tolerance of Quercus ilex to Phytophthora root disease affecting oak rangeland ecosystems in Spain. Agrofor Syst 87:173–179. doi:10.1007/s10457-012-9533-5
Shaner G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildew resistance in Knox wheat. Phythopatology 67:1051–1056
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Sharma S, Duveiller E, Basnet R, Karki CB, Sharma RC (2005) Effect of potash fertilization on Helminthosporium leaf blight severity in wheat, and associated increases in grain yield and kernel weight. Field Crop Res 93:142–150. doi:10.1016/j.fcr.2004.09.016
Silva JTA, Silva IP, Moura Neto A, Costa ÉL (2008) Potassium, magnesium and limestone application in banana tree seedlings ‘Prata anã’ (AAB). Rev Bras Frutic 30:782–786. doi:10.1590/S0100-29452008000300037
Silva JTA, Rodrigues MGV (2013) Nutricional evaluation, production and incidence panamá disease in banana “prata anã” fertilized with K, on the 4th cycle. Rev Bras Frutic 35:1170–1177. doi:10.1590/S0100-29452013000400028
Silva JTA, Silva IP, Pereira RD (2011) Phosphorus fertilization in banana ‘Prata anã’ (AAB) cultivated in two latosols. Rev Ceres 58:238–242. doi:10.1590/S0034-737X2011000200016
Souza L, Borges LA, Silva JTA (1999) Características físicas e químicas de solos cultivados com bananeira, sob irrigação, na região norte de Minas Gerais. Cruz das Almas, Bahia
Stangoulis JCR, Graham RD (2007) Boron and plant diseases. In: Datnoff LE, Elmer WH, Huber DM (eds) Mineral Nutrition and Plant Disease. The American Phytopathological Society, Saint Paul, pp 207–214
Surridge AKJ, Viljoen A, Crous PW, Wehner FC (2003) Identification of the pathogen associated with Sigatoka disease of banana in South Africa. Australas Plant Pathol 32:27–31
Taiz L, Zeiger E (2013) Fisiologia vegetal, 5th edn. Artmed, Porto Alegre, 918p
Uchôa CN, Pozza EA, Pozza AAA, Silva Moraes W (2011) Geostatistical modeling of black-sigatoka and relation to soil fertility. Biosci J 27:357–362
Viégas IDJM, Thomaz MAA, Silva JD, Conceição HD, Naiff APM (2004) Effect of omission of macronutrient and boron on growth, on symptoms of nutritional deficiency and mineral composition in camucamuzeiro plants (Myrciaria dubia). Rev Bras Frutic 26:315–319. doi:10.1590/S0100-29452004000200032
Acknowledgments
The National Council for Scientific and Technological Development (CNPq) and the Foundation for Supporting Research in the state of Minas Gerais (FAPEMIG) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freitas, A.S., Pozza, E.A., Pozza, A.A.A. et al. Impact of nutritional deficiency on Yellow Sigatoka of banana. Australasian Plant Pathol. 44, 583–590 (2015). https://doi.org/10.1007/s13313-015-0371-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-015-0371-6