Abstract
Mycosphaerella musicola produces various toxins known to play a key role in pathogenicity and disease development. In our study secondary metabolites produced by M .musicola was extracted and investigated using different parameters. The investigation was aimed to understand the phytotoxic resistance of locally growing different Musa genotypes (AB-Elakki (ELA), AAA-G9 (G9) and AAB-Nanjangud Rasa Baley (NRB)) against the crude extract and partially purified lipophilic TLC fractions extracted from the culture filtrates of M. musicola. Susceptible and resistant banana genotypes against the crude extract and partially purified fractions were analyzed by electrolyte leakage assay and leaf puncher assay. The results revealed that the crude extract and TLC fractions showed varied phytotoxicity among the banana leaves belonging to different age groups and different genotypes. Phytotoxicity studies allowed us to observe that ELA and G9 exhibited resistance to crude extract and TLC fractions, indicating that these genotypes are resistant to initial M. musicola infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Musa species was originated in tropical Asia, geographical area ranging from south west India to the island of New Guinea. Growing of Musa species on domestic scale may be started by cultivation of selected wild subspecies of Musa acuminata (AA genome) (Kennedy 2009). Different edible banana genotypes like AA, AAA, AB, AAB, ABB was developed from vegetative propagation and natural crossing over to different genotypes of Musa balbisiana (BB genome) (Perrier et al. 2009). Now banana and plantain are one of the 4th important crop playing a major role in exportation and for food purpose globally (Arias et al. 2003). Approximately 20 different species of Mycosphaerella have been successfully isolated and reported from Musa (Arzanlou et al. 2008). Among them Mycosphaerella musicola and Mycosphaerella fijiensis are known to cause considerable damage (Blomme et al. 2013). Mycosphaerella musicola causes yellow sigatoka or yellow leaf streak disease (YLSD) in banana, which is considered as one of the devastating diseases of banana plantation globally. First symptom of the disease is appearance of yellow pale streaks on adaxial surface measuring around 1-2mm which enlarge to form necrotic lesions, coalesce and destroy large photosynthetic surface area of leaf results in reduced overall yield and premature ripening of the fruit (Surridge et al. 2003). The disease is more severe in regions with high altitudes and cool temperatures (Mouliom et al. 1996). Economic loss due to yellow sigatoka is more than 50% globally (Burt et al. 1997). In India the disease severity ranges between 20-50% as investigated by National Research Centre on Banana (NRCB) (Selvarajan et al. 2001).
Fungal pathogens are known to produce phytotoxins which are toxic at very low concentration to the host plants (Puch et al. 2005). Phytotoxins are defined as low molecular weight, secondary metabolites produced by phytopathogens that may cause necrosis, chlorosis, wilting or a combination of these symptoms in susceptible plant hosts (Stergiopoulos et al. 2013; Ellen and Barbara 2008). Phytotoxins are classified as host-specific (primary determinants of the disease) and non-host specific (secondary determinants of the disease) (Amusa 2006). The phytotoxicity of toxins is due to inhibition of specific enzymes or interference with membrane functions and defense responses (Strange 2007). Mycosphaerella fijiensis produces six major toxins, of this 2,4,8- trihydroxytetralone is a host-specific toxin and has been reported to induce disease symptoms that are similar to those produced by the fungus on both banana and plantain leaves (Stierle et al. 1991). Harelimana et al. (1997) showed that toxins produced by Mycosphaerella fijiensis serves as secondary determinant of the pathogenicity, contributing to the lesion expansion in cultivars exhibiting partial resistance to black leaf streak disease (BLSD). Phytotoxins employed to study or to select the resistant varieties frequently encountered with certain limitations, one among them is the lack of characterized toxins that play a crucial role in disease development and leaf necrosis. Therefore, in our study potentiality of toxin from M. musicola on different verities of banana was evaluated.
Materials and methods
Collection of samples
Infected leaf sample belonging to Nanjangud rasa baley (NRB) variety of AAB genome hybrid was collected from Karnataka state horticulture department, Mysore, Karnataka, India, in the month of June, 2012. Healthy leaves of Elakki (ELA) belonging to genome hybrid AB and G9 (G9) belonging to the AAA genome were collected from local banana growing area in Mysore, Karnataka, India.
Isolation of Mycosphaerella musicola
M. musicola was isolated according to method as described by Alvindia (2012). Infected banana leaves were surface sterilized by washing with running tap water for 1 min, air dried and wiped with 95% ethanol. Sections were cut at 2-3 mm from the margins of young actively growing lesions. Cut leaf segment was fixed with a double sided adhesive tape, attached to the underside of 90 mm Petri dishes, and inverted over 2% water agar. Plates were incubated at 25 °C for 48 h. Under a compound microscope, the germinating ascospores were carefully picked up and inoculated into a slant containing potato dextrose agar (PDA) supplemented with 5% malt-extract (ME). The pure cultures were stored at 28 °C conditions for the further usage.
Molecular identification of M. musicola
Fungal mycelium was grown in 100 ml of PDB and incubated at 25 °C under normal laboratory conditions with intermittent shaking. Yielded mycelium was filtered, freeze dried and used for DNA extraction. Isolation of DNA was done by using DNA isolation kit (HIpurA, Himedia, Mumbai, India) according to the manufacturer’s instructions. Amplification of ribosomal DNA (r DNA) and internally transcribed spacer (ITS) region was done by using ITS1 (5' TCCGTAGGTGAACCTGCGG-3') and ITS 4(5'- TCCTCCGCTTATTGATATGC-3') (Rakshith et al. 2013). The PCR reaction volume was 25 μL, consisting 12.5 μL of 2× PCR master mix (Genei, Bangalore, India), 1 μL each of the forward (ITS1) and reverse (ITS4) primers (10 pmol/μL), template DNA (12–15 ng/μL) and 9.5 μL sterile water. The PCR amplification conditions were initial denaturation step at 95 °C for 10mins, preceded by 35 cycles, 94 °C for 1 min, 55 °C for 1 min, 72 °C for 2 min and final step was at 72 °C for 8 min for extension. The amplicon was sequenced using ITS1 primer and the sequence was submitted to the GenBank database (Samaga et al. 2014).
Preparation of crude cytotoxic extract (CTE)
M. musicola isolate was grown in 1 L Erlenmeyer flasks consisting of PDB supplemented with 5% malt extract (ME) and incubated at 28 °C for 30 days. The culture broth was filtered by using muslin cloth, and extracted with ethyl acetate (400 mlX 5) , vaccum evaporated by using Rota evaporator (Buchi R-3 rotavapor, USA) at 45 °C. The final residue was dissolved in 10% of methanol prior to use for bio assays (Harelimana et al. 1997)
Estimation of electrolyte leakage
Fungal plant pathogens produce various phytotoxins during disease development within a plant, these phytotoxins acts as co determinants of pathogenicity (Buiatti and Ingram 1991). Phytotoxins may damage or harm plants at very low concentration (Graniti 1991).Toxicity effect of CTE on different banana varieties and at different stages of leaf growth was studied on three different locally growing banana varieties ELA, G9 and NRB belonging to 3 different genotypes. 5mm leaf discs from each variety were treated with 100 μl of CTE, incubated for 48 h with intermittent shaking at room temperature. After incubation, solutions were separated and their volume was adjusted to 5 mL with distilled water and their conductivity (C) was measured at 200 μS using digital conductometer (Cistronics, Bangalore, India), as an evaluation of the electrolyte leakage from CTE-treated leaf disks. Heat killed leaf disks under 15 psi pressure, 121°C for 20 min was considered as positive control (Ct). Leaf discs in deionized water served as negative control (Cn). The percentage of tissue integrity (I) and percentage of cell mortality (Mt) was deduced as described previously (Harelimana et al. 1997). Similar protocol was followed for the banana leaves of different age groups. Age of banana leaves was determined by Brun’s scale of cigar leaf development and leaves were selected from Brun’s leaves stage 6 (Ganry et al. 2008). Basal leaf (OL), intermediate leaf (IL) and Apical leaf (YL) were used in assay.
Thin layer chromatography (TLC) analysis
CTE was fractionated by thin layer chromatography (TLC) using pre coated silica gel plates (Merk, India). The TLC was developed using mobile phase containing hexane: ethyl acetate (8.5: 1.5). The separated spots were visualized at 280 nm and 320 nm UV light. The individual separated bands were scraped off, resuspended in ethyl acetate, centrifuged at 8000 rpm. Supernatant was dried using Rota evaporator at 45°C and named depending on Rf values.
Toxicity of TLC fractions on banana leaf discs.
100 μg of each TLC fraction was dissolved in 10% of 100 μl methanol. 5mm of banana leaf discs were treated with separated fractions and incubated at 28°C for 48 h. The volume (100 μl) was adjusted to 5 ml with sterile distilled water. Negative control was taken by infiltrating 5 mm leaf disc with 100 μl of 10% methanol and positive control was taken by sterilizing 5 mm leaf disc in 5 ml of distilled water. Conductivity was measured at 200 μS using digital conductometer. I and Mt were deduced as described by Harelimana et al. (1997).
Leaf puncture assay
Cytotoxic effect of separated TLC fractions on different banana genotypes was assessed by previously reported method (Cruz-Cruz et al. 2009). Briefly, the activity was evaluated on healthy leaves of different banana genotypes (NRB, G9 and ELA). Second youngest leaf (15X10 Cm2) was excised from the 4 month old plant, disinfected with sodium hypochlorite 2% (v/v) for 60 s, rinsed with sterile distilled water, blot dried between paper towels and placed in plastic containers previously disinfected with 70% ethyl alcohol, lined with sterile moist paper towels. 100 μg of dried TLC fractions were dissolved in 100 μl of acetone and water mixture 50:50 (v/v), 10 μl of each TLC fraction was loaded on a wound made with scalpel on the upper surface of leaf and incubated for 6 h at 28°C. Acetone and water mixture 50:50 (v/v) was taken as negative control.
Statistical analysis
Phytotoxicity caused by the CTE and TLC fractions extracted from M. musicola on banana varieties and different age group of leaves were analyzed using by one way ANOVA followed by Tukeys test (p<0.05). Statistics were performed using Graphpad Prism 5.03 software (Graph Pad Software Inc., La Jolla, CA, USA).
Results
Isolation and molecular identification of M. musicola
The pathogen was identified as M. musicola based on growth characteristics and morphological key features (Arzanlou et al. 2008). Growth of fungi was slow on synthetic media, after one month colony grew upto 0.7 cm in diameter. The colonies had raised centre, with both light grey, pink or light green color and black to brown coloration back side Fungal isolate exhibited homology with M. musicola on BLAST analysis of ITS sequence. Morphological and genetic similarities of with M. musicola confirmed the isolate as M. musicola. The ITS sequence was deposited in the GenBank data base under the accession number KM369832.
Electrolyte leakage assay
Cytotoxicity of CTE on leaf discs of different banana varieties can be visualized by observing the discoloration of treated leaf discs (Fig 1). Among three different genotypes, ELA (AB) and G9 (AAA) exhibited resistance to the CTE, where as NRB (AAB) showed no resistance to CTE (Fig 2). Among the different age group of banana leaf OL and IL didn’t show any resistance to CTE where as YL was resistant to the CTE (Fig 3). For further assay intermediate leaves of susceptible variety (NRB) were selected.
TLC analysis and activity of fractions
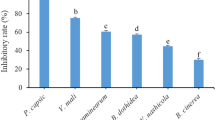
Separation of cytotoxic fractions from CTE was done by thin layer chromatography (TLC) and yielded TLC fractions were coded according to their Rf values (Table 1). Phytotoxic damage of TLC fractions was studied on NRB variety belonging to AAB genotype as this variety exhibited no resistance to the CTE. F2 and F3 showed considerable phytotoxicity compared to control, among this F2 showed more phytotoxicity compared to F3 all 4 TLC fractions showed phytotoxic activity among this F2 and F3 were significant (Fig 4).
Leaf puncture assay
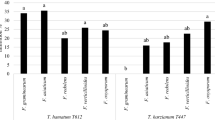
CTE induced varied phytotoxicity on all the 3 different banana genotypes (Fig 5). Phytotoxic activity of TLC fractions on different banana verities (ELA, G9 and NRB) was evaluated by leaf puncture assay F1 was more phytotoxic compared to other 3 fractions on all varieties studied in current work, among 3 different varieties F1 caused more phytotoxic damage on the leaf of NRB followed by ELA, where as G9 variety exhibited certain range of resistance to F1. Remaining fractions also exhibited phytotoxic damage on the leaves of banana varieties, but the phytotoixic damage was less when compared to F1, and varied contrastingly among the different varieties (Fig 6).
Discussion
M. musicola belongs to the class Dothideomycetes. Fungal pathogens belonging to class Dothideomycetes produce host specific toxins (HSTs) during infection (Stergiopoulos et al. 2013). Studies on phyto-pathogen interactions involving the fungi known to produce HSTs always correlated to the susceptibility or resistant to the toxin. Hence this approach is employed in screening of disease resistant or susceptible varieties (Knogge 1996). Studies on electrolyte leakage estimation by treating leaf discs belonging to different banana varieties with lipophilic crude extract indicated that NRB was highly susceptible compared to G9 and ELA.
All separated TLC fractions from lipophilic crude extract showed phytotoxic activity on the leaves of different banana varieties. However, phytotoxic damage induced by F1 was high compared to other TLC fractions. HSTs mimic the pathogen in terms of host range, when tested against different host species and varieties (Graniti 1991; Kohmoto and Otani 1991; Stierle et al. 1991). Non HSTs targets active defense processes including detoxification or stimulation of membrane localized H+ ATPase activity irrespective of host (Knogge 1996; Svabova and Lebeda 2005). Non HST character of lipophilic TLC fraction F1,F3 and F4 was confirmed after the metabolite exhibited similar phytotoxic activity on leaves of non host species (Carica papaya and Ravanella madagascarensis), Phytotoxicity of F2 compared to F1was less, but on leaves of non host species, no phytotoxic damage was observed indicating the HST character of the compound (Data not shown). Further susceptibility and resistant studies by different banana varieties in field conditions and green house conditions indicated the lack of correlation by F1, F3 and F4 confirming their non HST nature. However, lipophilic TLC fraction F2 showed higher phytotoxicity in NRB and lower phytotoxicity in ELA exhibiting its HST character. MIC of phytotoxic lipophilic TLC fractions on leaves of different varieties indicated that MIC value of each fraction varied among all varieties. Non HSTs (F1, F3 and F4) had lower MIC value were as HST F2 showed higher MIC value in all varieties. Further structural analysis of lipophilic crude fraction is in progress and will be published soon.
No attempts have been made till the date to understand the phytotoxicity of the secondary metabolites produced by M. musicola on the different banana genotypes.The potential applications of phytotoxins include their use as probes to study the molecular basis of disease resistance and susceptibility in plants, as tools for the in vitro selection of disease resistant plant lines, plant breeding program and as possible agents for weed control (Buiatti and Ingram 1991).
Conclusion
Our results suggested that toxins produced by M. musicola are non host specific since it caused phytotoxic damage and cell disruption on different locally growing banana varieties, which is one of the first attempt to understand the pathogenicity and phytotoxicity of the toxins produced by M. musicola, outcome of our studies showed that banana variety ELA belonging to AB genome hybrid was more resistant to the toxins, were as G9 belonging to AAA genotype was partially susceptible and NRB belonging to AAB genotype was highly susceptible.
References
Alvindia, D. G. (2012). Inhibitory influence of biocontrol agents, plant oils and an inorganic salt on Mycosphaerella fijiensis and Cordana musae, the causal pathogen of black sigatoka and leaf spot of banana. Afr. J. Microbiol. Res, 19, 4179–4184.
Amusa, N. A. (2006). Microbially produced phytotoxins and plant disease management. Afr. J. Biotechnol., 5, 405–414.
Arias, P., Dankers, C., Liu, P., & Pilkauskas, P. (2003). The world banana economy 1985-2002.F.A.O. Rome: Food and Agriculture Organization of the United Nations.
Arzanlou, P. M., Groenewald, J. Z., Fullerton, R. A., Abeln, E. C. A., Carlier, J., Zapater, M. F., Buddenhagen, I. W., Viljoen, A., & Crous, P. W. (2008). Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia., 20, 19–37.
Blomme, G., Ploetz, R., Jones, D., De Langhe, E., Price, N., Gold, C., Geering, A., Viljoen, A., Karamura, D., Pillay, M., Tinzaara, W., Teycheney, P. Y., Lepoint, P., Karamura, E., & Buddenhagen, I. (2013). A historical overview of the appearance and spread of Musa pests and pathogens on the African continent: highlighting the importance of clean Musa planting materials and quarantine Measures. Ann. Appl. Biol., 162, 4–26.
Buiatti, M., & Ingram, D. S. (1991). Phytotoxins as tools in breeding and selection of disease-resistant plants. Experientia., 47, 811–819.
Burt, J. A., Rutter, J., & Gonzalez, H. (1997). Short distance wind dispersal of the fungal pathogens causing Sigatoka diseases in banana and plantain. Plant. Pathol., 40, 451–458.
Cruz-Cruz, C. A., Garcia-Sosa, K., Escalante-Erosa, F., & Pena-Rodriguez, L. M. (2009). Production of hydrophilic phytotoxins by Mycosphaerella fijiensis. J. Gen. Plant. Pathol., 75, 191–195.
Ellen, M. F., & Barbara, J. H. (2008). Secondary metabolism: regulation and role in fungal biology. Current Opinion in Microbiology., 11, 481–487.
Ganry, J., de Lapeyre de Bellaire, L., & Mourichon, X. (2008). A biological forecasting system to control Sigatoka disease of bananas and plantains. Fruits., 63, 381–387.
Graniti, A. (1991). Phytotoxins and their involvement in plant diseases an introduction. Experientia., 47, 751–755.
Harelimana, G., Lepoivre, P., Jijakli, H., & Mourichon, X. (1997). Use of Mycosphaerella fijiensis toxins for the selection of banana cultivars resistant to black leaf streak. Euphytica., 96, 125–128.
Kennedy, J. (2009). Bananas and people in the homeland of genus Musa: Not just pretty fruit. Ethnobotany Research & Applications., 7, 179–197.
Knogge, W. (1996). Molecular basis of specificity in host/fungus interactions. Eur J Plant Pathol., 102, 807–816.
Kohmoto, K., & Otani, H. (1991). Host recognition by toxigenic plant pathogens. Experientia., 47, 755–764.
Mouliom, P. A., Lassoudiere, A., Foko, J., & Fontem, D. A. (1996). Comparison of development of Mycosphaerella fijiensis and Mycosphaerella musicola on banana and plantain in the various ecological zones in Cameroon. Plant. Dis., 80, 950–954.
Perrier, X., Bakry, F., Carreel, F., Jenny, C., Horry, J. P., Lebot, V., & Hippolyte, I. (2009). Combining biological approaches to shed light on the evolution of edible bananas. Ethnobotany Research & Application., 7, 199–216.
Puch, C. M., Garcia, S. K., & Pena-Rodriguez, L. M. (2005). Optimizing the culture conditions of Mycosphaerella fijiensis Morelet. InfoMusa, 14, 21–23.
Rakshith, D., Santosh, P., & Satish, S. (2013). Isolation and characterization of antimicrobial metabolite producing endophytic Phomopsis sp. from Ficus pumila Linn. (Moraceae). Int. J. Chem. Anal sci, 4, 156–160.
Samaga, P. V., Rai, V. R., & Rai, L. K. M. (2014). Production of an antimicrobial cytochalasan by an endophytic Chaetomium globosum HYML55 from Hypericum mysorense and its RNA secondary structure analysis. Chem. Ecol., 30, 566–578.
Selvarajan, R., Uma, S., & Sathiamoorthy, S. (2001). Etiology and survey of banana leaf spot diseases in India. Advancing banana and plantain R & D in Asia and the Pacific., 10, 94–102.
Stergiopoulos, I., Collemare, J., Mehrabi, R., & De Wit, P. J. (2013). Phytotoxic secondary metabolites and peptides produced by plant pathogenic Dothideomycete fungi. FEMS Microbiol Rev., 37, 67–93.
Stierle, A. A., Upadhyay, R., Hershenhorn, J., Strobel, G. A., & Molina, G. (1991). The phytotoxins of Mycosphaerella fjiensis, the causative agent of black Sigatoka disease of bananas and plantains. Cell. Mol. Life Sci., 47, 853–859.
Strange, R. N. (2007). Phytotoxins produced by microbial plant pathogens. Nat. Prod. Rep., 24, 127–144.
Surridge, A. K. J., Viljoen, A., Crous, P. W., & Wehner, F. C. (2003). Identification of the pathogen associated with Sigatoka disease of bananain South Africa. Australas. Plant Pathol., 32, 27–31.
Svabova, L., & Lebeda, A. (2005). In vitro selection for improved plant resistance to toxin-producing pathogens. J Phytopathol, 153, 52–64.
Acknowledgement
The authors are thankful to University of Mysore and University Grant Commission, New Delhi for providing UGC-NONNET fellowship for the first author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aman, M., Rai, V. Potent toxigenic effect of Mycosphaerella musicola on locally growing banana varieties. Phytoparasitica 43, 295–301 (2015). https://doi.org/10.1007/s12600-015-0456-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-015-0456-3