Abstract
Silicon (Si) is known to reduce the severity of pathogens on many plants. This study aimed to determine the effect of silicon (Si) in reducing the severity of yellow Sigatoka (Mycosphaerella musicola) on banana plants cultivated in nutrient solution. The treatments included five concentrations of silicic acid (H4SiO4): 0, 0.5, 1.0, 1.8 and 3.6 mmol L−1. The area under the disease severity progress curve (AUDSPC) was calculated for each treatment, and the nutrient content of the leaves determined. AUDSPC for plants receiving 3.05 mmol L−1 H4SiO4 was 49.27% lower compared to plants receiving no supplemental H4SiO4. In contrast, plants grown in a solution of 3.6 mmol L−1 H4SiO4 had 23.53% more Si content in the leaves compared to plants cultivated without supplemental H4SiO4. These results suggest Si could be used as a component in the integrated management of yellow Sigatoka.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Banana (Musa spp.) is one of the world’s most valuable agricultural commodities (Ploetz et al. 2015). In addition to being a complementary diet food, it is energy rich and nutritionally dense, and cultivation promotes the financial and social sustainability of populations in areas where it is cultivated (Su et al. 2015). Production losses are mostly caused by various plant diseases, one of which is yellow Sigatoka caused by Mycosphaerella musicola Leach (Stat. Conid. Pseudocercospora musae Zimm). This necrotrophic pathogen reduces the photosynthetic areas of the plant, thus adversely affecting the fruit quality and causing losses that can exceed 50% of production (Burt et al. 1997). Regular application of fungicide is the most common method of controlling the disease (Patel 2009). However, systematic chemical control raises the production costs, can select for fungicide-resistant strains of the pathogen and can have an adverse effect on the environment. Providing various plant nutrients, including mineral elements, to ensure optimal plant health can enhance disease resistance (Marschner 2012; Freitas et al. 2015a). Ensuring that banana plants are not deficient in nutrients thus helps minimize the losses incurred by yellow Sigatoka (Freitas et al. 2015a; Freitas et al. 2015b).
Silicon (Si) is an example of a mineral element that has been found to induce resistance (Resende et al. 2013) and thus reduce development of diseases (Datnoff et al. 2007; Pozza et al. 2015). Although Si is a required mineral for only some species of plants, it is considered beneficial to many, including banana (Marschner 2012). Henriet et al. (2006) demonstrated that banana not only absorbs Si from the soil but also accumulates substantial quantities in its tissues. The capacity to accumulate Si is often associated with reduced severity of diseases (Pereira et al. 2009). Two hypotheses have been proposed for the mode of action of Si. Firstly, it facilitates the formation of a physical barrier, preventing the pathogens from entering and colonizing the host, and secondly, Si has the ability to activate biochemical host defence mechanisms (Pereira et al. 2009; Marschner 2012; Pozza et al. 2015).
Utilizing Si to reduce diseases in banana has been demonstrated. Kablan et al. (2012) reported reduced severity of black Sigatoka (M. fijiensis) from 20.5 to 18.8% when plants were grown in a nutrient-solution, and from 68.9 to 39.5% when plants were grown in soil with 2 mmol L−1 silicic acid (H4SiO4). The authors suggested that the high Si content in the banana leaves reduced the severity of the disease symptoms. Si also effectively decreased diseases caused by soil pathogens of banana. A 2 mmol L−1 soil application of H4SiO4 reduced the severity of disease caused by Cylindrocladium spathiphyllum by almost 50% (Vermeire et al. 2011). Similarly, when 1.75 g of calcium silicate was added kg−1 of soil, Fusarium wilt (Fusarium oxysporum f. sp. cubense) was reduced by 15.98% compared to the non-treated control (Fortunato et al. 2012).
Si thus offers several benefits to various plant species, including banana, by playing a role in the reduction of disease severity caused by pathogens (Botelho et al. 2011; Lima et al. 2010; Moraes et al. 2006). However, there is virtually no information on the ability of Si to reduce the severity of yellow Sigatoka. Therefore, the objective of this study was to determine the effect of Si on the severity of yellow Sigatoka.
Materials and methods
Plant materials and growth conditions
The experiment was performed in a greenhouse with a mean relative humidity of 80% and a mean temperature of 25 °C. Micropropagated banana seedlings (Musa acuminata ‘Grande Naine AAA Cavendish’) were grown in commercial substrate (Bioplant® – Nova Ponte, Brazil) for forty-nine days, when they were placed in trays holding 16 L of non-amended nutrient solution (Hoagland and Arnon 1950) at 50% ionic strength and aerated continuously for 15 days to adapt to the experimental conditions. Subsequently, the plants were transferred to 6 L pots containing nutrient solution (Hoagland and Arnon 1950) at 100% ionic strength with continuous aeration. Macronutrients were added as follows: 1 mL L−1 KH2PO4 (Vetec® química, Belo Horizonte, Brazil), 5 mL L−1 KNO3 (Êxodo® científica, Sumaré, Brazil), 5 mL L−1 Ca(NO3)2·5H2O (Êxodo® científica) and 2 mL L−1 MgSO4·7H2O (Êxodo® científica). Micronutrients were added as follows: 1 mL L−1 of the stock solution, which included 2.86 g L−1 boric acid, 1.81 g L−1 manganese chloride, 0.10 g L−1 of zinc chloride, 0.04 g L−1 copper chloride and 0.02 g L−1 molybdic acid. A 1 mL aliquot of a stock solution containing iron was added (the stock solution contained 33.3 g L−1 Na2-EDTA, 100.4 mL L−1 1 N NaOH, 24.9 g L−1 FeSO4·7H2O and 4 mL L−1 1 N HCl) per liter of nutrient solution (Lima et al. 2010).
The experimental design was a randomized complete block with five treatments and four replicates. The treatments included five concentrations of silicic acid (H4SiO4): 0, 0.5, 1.0, 1.8 and 3.6 mmol L−1. Each replicate consisted of one plant in a pot (experimental unit). The entire experiment was repeated once.
The nutrient solution was monitored weekly to maintain the pH between 5.5 and 6.0 – achieved by adding 0.1 mol L−1 HCl or 0.1 mol L−1 NaOH as needed. Deionized water was added to the vessel, whenever required, to maintain the volume. Ion depletion from the nutrient solution was checked for weekly using a compact potassium meter (Horiba-CARDY®, Irvine, United States of America). The nutrient solution was replaced when depletion was 30% of the initial value of K+ (Braccini et al. 1999).
Inoculation of banana plants
Several isolates of Pseudocercospora musae were obtained from banana leaves (Musa acuminate × Musa balbisiana ‘Prata Anã AAB Prata’) following the methodology of Cordeiro et al. (2011). After isolation, the mycelium was grown on Petri dishes containing malt agar (20 g malt extract [Acumedia®, Indaiatuba, Brazil] and 20 g agar [Dinâmica®, Jaraguá do Sul, Brazil] per L of distilled water). Conidia were obtained from the colonies using the methodology of Freitas et al. (2015b). Ten mycelial areas were removed from 26-day-old colonies with a borer (5-mm diameter) and were macerated. The macerate was diluted in 15 mL of tomato juice (200 mL tomato juice [Superbom®, Redondo, Brazil], 1 g CaCO3, and 900 mL distilled water) and transferred to 9-cm diameter Petri dishes containing culture medium (200 mL tomato juice [Superbom®], 20 g agar [Dinâmica®, Jaraguá do Sul, Brazil], 1 g CaCO3, and 900 mL distilled water). To facilitate drying, the plates were left open inside a sterile incubator under continuous light (four 20-W fluorescent bulbs) at a temperature of 25 °C for approximately 48 h. After drying, sterilized distilled water (10 mL) was added to each Petri dish and a previously sterilized glass rod was used to release the conidia from the dry mycelium. The resulting suspension was filtered through a double layer of gauze, and the concentration of the suspension adjusted to 4 × 104 conidia mL−1 using a Neubauer hemocytometer and taking an average of four readings.
Inoculation was performed three months after transferring plants to the nutrient solutions. The abaxial surfaces of leaves 1, 2, 3 and 4 were inoculated by spraying 0.7 mL of the conidial suspension onto a 36 cm2 area. After inoculation, the plants were individually covered with transparent plastic bags for 60 h, to provide adequate conditions for the fungus to infect (recorded temperature and relative humidity were 23 °C and 92%, respectively using a Model HT-500 data logger (Instrutherm®, Pompeia, Brazil).
Assessment of disease severity
Once the first symptoms were observed, disease severity assessments on the abaxial leaf surface of each inoculated leaf was commenced. A total of six evaluations were performed, one every six days, with the aid of a diagrammatic scale (Stover 1971), with modifications incorporated by Gauhl (1994). The scale has 5 grades: 0 = no symptoms, 1 = up to 10 lesions per leaf, 2 = 1 to 5% leaf area covered by lesions, 3 = 6 to 15% leaf area covered by lesions, 4 = 16 to 33% leaf area covered by lesions, and 5 = 34 to 50% leaf area covered by lesions.
The mean disease severity of the four leaves was integrated to calculate the area under the disease severity progress curve (AUDSPC) (Shaner and Finney 1977):
AUDSPC = \( \sum_{i=1}^{n-1}{\left(\frac{\Big({y}_i+{y}_{i+1}}{2}\right)}^{\ast}\left({t}_{i+1}-{t}_i\right) \).
in which.
- yi:
-
severity of disease at the i-th observation;
- ti:
-
time in days at the i-th observation;
- n:
-
total number of observations.
Determination of nutrients in the leaves
The nutrient content of the leaves was determined at the end of the experiments. The second and third leaves were rinsed thoroughly in distilled water, except the midrib, and packed separately in paper bags. Leaves were oven-dried at 60 °C to constant weight, ground and analyzed (Martinez et al. 1999). The Si contents were determined by the method of alkaline digestion and dosage by a colorimetric method, as modified by Korndörfer et al. (2004). Contents of Ca, Mg, Cu, Zn, Fe and Mn were determined by atomic absorption spectrophotometry; K by flame emission photometry; S by turbidimetry; P by colorimetry; N according to the Kjeldahl method; and B by the Azomethine-H colorimetric method (Malavolta et al. 1997).
Data analysis
Normality of data (variables AUDSPC and Si content) were confirmed using the Shapiro-Wilk test (Shapiro and Wilk 1965). The combined data from both experiments were submitted to analysis of variance (ANOVA) to determine whether there were differences between the two experiments for each variable. A further ANOVA was performed on the data to determine treatment effects, and when significant at P ≤ 0.05, were subject to further analysis using linear or non-linear regression. The significance of the slope and intercept parameters were tested using a t-test, the coefficient of determination (R2), the Mean Square of Deviations (MSD), and inspection of residual distributions. Depending on the variable analyzed, to estimate the minimum and maximum points of the functions, first-order derivative techniques were applied, matching them to zero. The analyses were performed using R (R Core Team 2013). Regression plots were prepared using SigmaPlot® (Systat Software Inc. 2006).
Results
There was no significant difference between the data from the two trials so the experimental data were combined in the analysis.
Severity of yellow Sigatoka as a function of the Si concentrations in the nutrient solution
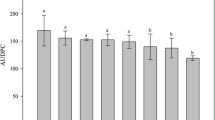
The first symptoms of yellow Sigatoka appeared on the leaves inoculated with P. musae at 28 days after inoculation. The concentration of H4SiO4 had a significant effect (P ≤ 0.05) on the AUDSPC. The greatest AUDSPC value was at a concentration of 0 mmol L−1 H4SiO4 (275.29). Using the first-order derivative of the adjusted regression model, the lowest AUDSPC was on plants grown in 3.05 mmol L−1 H4SiO4 (139.65), which was 49.27% less compared to the value for the control plants that did not receive additional Si (Fig. 1).
Nutritional aspects of banana as a function of the Si concentrations in the nutrient solution
The nutrition of the banana plants was affected by the concentration of Si in the nutrient solution in which they were grown. The greater the concentration of H4SiO4 in the nutrient solution, the greater the foliar content of Si (P ≤ 0.05). With increasing concentration of H4SiO4 from 0 to 3.6 mmol L−1, there was a corresponding increase of 23.53% in the Si content in leaves (Fig. 2). The mean leaf nutrient content of the remaining nutrients tested revealed that in all the treatments the values were mostly within the range considered adequate for banana (Table 1).
Discussion
The results of these experiments demonstrated that a 3.05 mmol L−1 H4SiO4 nutrient solution resulted in a 49.27% reduction in AUDSPC of yellow Sigatoka on banana compared with when plants were grown in the absence of Si. Work with black Sigatoka showed similar but less dramatic results (Kablan et al. 2012), with a decrease in black Sigatoka from 20.5 to 18.8% in plants grown in nutrient solution containing 0 and 2 mmol L−1 H4SiO4, respectively. But when Si was applied to soil, there was a greater reduction in severity of black Sigatoka (a severity of 68.9 and 39.5% in soil-cultivated plants without and with a supply of 1.7 mmol L−1 H4SiO4, respectively). In another study, the application of 2 mmol L−1 H4SiO4 to the soil reduced severity of disease caused by C. spathiphyllum by approximately 50% (Vermeire et al. 2011), while Fortunato et al. (2012) reported a 15.98% decrease of Fusarium wilt in Macã plants cultivated with Si compared to non-treated control plants. The results from our study are further evidence of the disease suppressive effects of Si.
Specifically, our results, and those from other studies, show that Si can potentially decrease disease severity in banana. Pereira et al. (2009) purported that this was due to Si absorbed by the plant roots being transported to the leaves where it consequently occurred at high concentrations. In our study, there was an increase in Si content in leaves from 1.193 to 1.56 g kg−1 with increasing concentrations of H4SiO4 in nutrient solution from 0 to 3.6 mmol−1. These results concur with those of Henriet et al. (2006), confirming that banana plants accumulate Si in their tissues. There was a corresponding drop in the severity of the banana disease. Similarly, Kablan et al. (2012) reported that the mean Si contents rose from 0.42 to 22.07 g kg−1 in plants raised in a nutrient solution at the highest concentration of Si, and from 1.2 to 22.6 g kg−1 in plants cultivated in soil supplemented with different amounts of Si. In a study limited to soil-raised plants (Vermeire et al. 2011) there was a 2.09 to 16.71 g kg−1 increase in the leaf contents of Si in banana. In addition to the foliar Si contents, the presence of calcium silicate in the soil also induced a spike in the Si contents in the roots and rhizomes of the plant (Fortunato et al. 2012).
The mechanism and role of Si in resistance of plants to pathogens is still not completely understood (Kablan et al. 2012; Pereira et al. 2009; Pozza et al. 2015). Si acts as a physical barrier, preventing pathogen penetration (Pozza et al. 2015). Asmar et al. (2013) observed an increase in cellulose content from 1.44 to 1.9% and hemicellulose from 1.05 to 1.83 in banana shoots grown in culture medium with 1 g L−1 sodium silicate (Na2SiO3) than shoots grown without Si (control). Sangster et al. (2001) suggested that Si can produce a double layer just beneath the cuticle. Kim et al. (2002) proposed that this double layer retarded the entry of Pyricularia grisea in leaves of rice. Pozza et al. (2004) reported a thicker cuticle present on the undersurface of coffee plants that received supplementary Si, which was principally due to the more highly developed epicuticular wax layer. The authors attributed the decrease in leaf damage caused by Cercospora coffeicola to this layer.
Si may also activate the natural defense system of the plant (Pozza et al. 2015). When 1 g L−1 sodium silicate was added to banana shoots, the tannin content increased by 57.52% compared to the control (Asmar et al. 2013). Khan et al. (2009) suggested that tannins are secondary metabolites possessing antimicrobial properties. Therefore, Si might also induce banana to boost its defense reaction to pathogens, although this has not been studied. Research has identified the role of Si in both the formation of a physical barrier and the activation of plant defense enzymes (Pozza et al. 2015). In work on the role of Si protecting sorghum plants, against infection by Colletotrichum sublineolum, high Si deposits were found at diseased sites on the leaf, with decreased lesion sizes and fewer acervuli. In addition, there were notable increases in the peroxidase and polyphenoloxidase activity (Resende et al. 2013).
Si is needed for plant nutrition (Pozza et al. 2015) and, therefore, when supplied to allow optimal health, can increase disease resistance. However, in the present study, other nutrients were maintained at values close to those considered sufficient for banana (Martinez et al. 1999; Borges and Souza 2004), and H4SiO4 is a very available source of Si. Thus, it can be concluded that Si nutrition in banana directly affects the severity of yellow Sigatoka. There remains much to understand: Botelho et al. (2011) noted that other sources of Si, including calcium, potassium and/or magnesium silicates may affect the results as a function of the levels of the cation, rather than the silicate molecule (Marschner 2012).
Addition of H4SiO4 to a nutrient solution decreased the severity of yellow Sigatoka and increased the Si content in banana leaves. These observations require further research, but have the potential to be used in an integrated management approach to the control of yellow Sigatoka.
References
Asmar SA, Pasqual M, Araujo AG, Silva RAL, Rodrigues FA, Pio LAS (2013) Morphophysiological characteristics of acclimatized ‘Grande Naine’ banana plants in response to in vitro use of silicon. Semina: Ciências Agrárias 34:73–82. https://doi.org/10.5433/1679-0359.2013v34n1p73
Borges AL, Souza LS (2004) O cultivo da bananeira. Cruz das Almas, Brazil
Botelho DMS, Pozza EA, Alves E, Botelho CE, Pozza AAA, Júnior PR, Souza PE (2011) Effect of silicon on the intensity of brown eye spot and on the mineral nutrition of coffee seedlings. Arq Inst Biol 78:23–29
Braccini MDCL, Lucca A, Martinez HEP (1999) Critérios para renovação ou manutenção de solução nutritiva em cultivo hidropônico. Semina: Ciências Agrárias 20:48–58. https://doi.org/10.5433/1679-0359.1999v20n1p48
Burt JA, Rutter J, Gonzalez H (1997) Short distance wind dispersal of the fungal pathogens causing Sigatoka diseases in banana and plantain. Plant Pathol 40:451–458
Cordeiro ZJM, Rocha HS, Araújo AG (2011) Metodologia para manuseio de Mycosphaerella musicola em laboratório. Documentos/Embrapa Mandioca e Fruticultura, Cruz das Almas, p 32
Datnoff LE, Rodrigues FA, Seebolt KW (2007) Silicon and Plant Disease. In: Datnoff LE, Elmer WH, Huber DM (eds) Mineral Nutrition and Plant disease. American Phytopathological Society, St. Paul, MN, pp 233–246
Fortunato AA, Rodrigues FÁ, Baroni JCP, Soares GCB, Rodriguez MAD, Pereira OL (2012) Silicon suppresses Fusarium wilt development in banana plants. J Phytopathol 160:674–679. https://doi.org/10.1111/jph.12005
Freitas AS, Pozza EA, Alves MC, Coelho G, Rocha HS, Pozza AAA (2015a) Spatial distribution of Yellow Sigatoka Leaf Spot correlated with soil fertility and plant nutrition. Precis Agric 17:93–107. https://doi.org/10.1007/s11119-015-9409-6
Freitas AS, Pozza EA, Pozza AAA, Oliveira MGF, Silva HR, Rocha HS, Galvão LR (2015b) Impact of nutritional deficiency on Yellow Sigatoka of banana. Australas Plant Pathol 44:583–590. https://doi.org/10.1007/s13313-015-0371-6
Gauhl F (1994) Epidemiology and ecology of black Sigatoka (Mycosphaerella fijiensis Morelet) on plantain and banana (Musa spp) in Costa Rica, Central América. INIBAP, Montpellier 120 p
Henriet C, Draye X, Oppitz I, Swennen R, Delvaux B (2006) Effects, distribution and uptake of silicon in banana (Musa spp.) under controlled conditions. Plant Soil 287:359–374. https://doi.org/10.1007/s11104-006-9085-4
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California Agricultural Experiment Station, Berkeley 32 p
Kablan L, Lagauche A, Delvaux B, Legrève A (2012) Silicon reduces black Sigatoka development in banana. Plant Dis 96:273–278. https://doi.org/10.1094/ PDIS-04-11-0274
Khan R, Islam B, Akram M, Shakil S, Ahmad A, Ali SM, Siddiqui M, Khan AU (2009) Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules 14:586–597. https://doi.org/10.3390/molecules14020586
Kim SG, Kim KW, Park EW, Choi D (2002) Silicon-induced cell wall fortification of rice leaves: a possible cellular mechanism of enhanced host resistance to blast. Phytopathology 92:1095–1103. https://doi.org/10.1094/PHYTO.2002.92.10.1095
Korndörfer GH, Pereira HS, Nola A (2004) Análise de silício: Solo, Planta e Fertilizante. Universidade Federal de Uberlândia, Uberlândia MG. GPSi-ICIAG-UFU
Lima LM, Pozza EA, Pozza AAA, Fonseca TAPS, Carvalho JG (2010) Quantification of Asian rust and nutritional aspects of soybean due to the use of silicon supplied via nutrient solution. Summa Phytopathol 36:51–56. https://doi.org/10.1590/S0100-54052010000100009
Malavolta E, Vitti GC, Oliveira SA (1997) Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd edn. POTAFOS, Piracicaba 319 p
Marschner H (2012) Mineral nutrition of higher plants, 3rd edn. Academic, San Diego 643 p
Martinez HEP, Carvalho JG, Souza RB (1999) Diagnose foliar. In: Ribeiro AC, PTG G, Alvarez VVH (eds) Recomendações para uso de corretivos e fertilizantes em Minas Gerais. 5ª aproximação. CFSEMG, Viçosa, pp 143–168
Moraes SR, Pozza EA, Alves E, Pozza AA, Carvalho JG, Lima PH, Botelho AO (2006) Effects of silicon sources on the incidence and severity of the common beans anthracnose. Fitopatol Bras 31:69–75. https://doi.org/10.1590/S0100-41582006000100012
Patel P (2009) Chemical control of sigatoka leaf spot (Mycosphaerella musicola) of banana. International. J Plant Protect 2:98–100
Pereira SC, Rodrigues FA, Carré-Missio V, Oliveira MGA, Zambolim L (2009) Effect of foliar application of silicon on soybean resistance against soybean rust and on the activity of defense enzymes. Trop Plant Pathol 34:164–170. https://doi.org/10.1590/S1982-56762009000300005
Ploetz RC, Kema GH, Ma LJ (2015) Impact of diseases on export and smallholder production of banana. Annu Rev Phytopathol 53:269–288. https://doi.org/10.1146/annurev-phyto-080614-120305
Pozza AAA, Alves E, Pozza EA, Carvalho JG, Montanari M, Guimarães PTG, Santos DM (2004) Effect of silicon on the control of brown eye spot in three coffee cultivars. Fitopatol Bras 29:185–188. https://doi.org/10.1590/S0100-41582004000200010
Pozza EA, Pozza AAA, Botelho DMDS (2015) Silicon in plant disease control. Revista Ceres 62:323–331. https://doi.org/10.1590/0034-737X201562030013
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria URL http://www.R-project.org/
Resende RS, Rodrigues FA, Gomes RJ, Nascimento KJT (2013) Microscopic and biochemical aspects of sorghum resistance to anthracnose mediated by silicon. Ann Appl Biol 163:114–123. https://doi.org/10.1111/aab.12040
Sangster AG, Hodson MJ, Tubb HJ (2001) Silicon deposition in higher plants. In: Datnoff LE, Snyder GH, Korndörfer GH (eds) Silicon in Agriculture. Elsevier, Amsterdam, pp 85–113
Shaner G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildew resistance in Knox wheat. Phythopatology 67:1051–1056
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Stover RH (1971) A proposed international scale for estimating intensity of banana leaf spot (Mycosphaerella musicola). Trop Agric 48:185–196
Su L, Ruan Y, Yang X, Wang K, Li R, Shen Q (2015) Suppression on plant-parasitic nematodes using a soil fumigation strategy based on ammonium bicarbonate and its effects on the nematode community. Sci Rep 5. https://doi.org/10.1038/srep17597
Systat Software Inc. (2006) SigmaPlot User’s Guide. Version 10.0. Systat Software Inc., Chicago.
Vermeire ML, Kablan L, Dorel M, Delvaux B, Risède JM, Legrève A (2011) Protective role of silicon in the banana-Cylindrocladium spathiphylli pathosystem. Eur J Plant Pathol 131:621–630. https://doi.org/10.1007/s10658-011-9835-x
Acknowledgements
The research was funded by the National Council for Scientific and Technological Development (CNPq) and the Foundation for Research Support of the State of Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freitas, A.S., Pozza, E.A., Soares, M.G.O. et al. Severity of yellow Sigatoka in banana cultivated in silicon nutrient solution. Australasian Plant Pathol. 46, 515–520 (2017). https://doi.org/10.1007/s13313-017-0521-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-017-0521-0