Abstract
Background
This study aimed to compare perioperative, long-term oncological, and anorectal functional outcomes of robotic total mesorectal excision (R-TME) and laparoscopic total mesorectal excision (L-TME) sphincter-saving total mesorectal excision in female patients with rectal cancer.
Methods
Retrospective analysis of prospectively maintained database was performed. Sixty-eight cases (L-TME, n = 34; R-TME, n = 34) were performed by a single surgeon (January 2014–January 2019). Patient characteristics, perioperative recovery, postoperative complications, pathology results, and oncological outcomes were compared between the two groups.
Results
Clinical characteristics did not differ between the groups. Mean operating time was longer in R-TME (165.50 ± 95.50 vs. 124.50 ± 82.60 min, p < 0.001). There was no conversion to open surgery in both groups. Mesorectal integrity was complete in both groups (100%). Length of distal and circumferential resection margins (CRM) did not differ between groups. CRM involvement was observed in 1 (2.8%) and 1 (2.8%) in L-TME and R-TME patients, respectively. Incidence of anastomotic leakage was 5.8% (n = 2) in L-TME and 8.8% (n = 3) in R-TME, respectively. Mean length of follow-up was 62.5 (36–102) months for R-TME and 63 (36–103) months for L-TME. Five-year overall survival rates were 92.8% in L-TME and 89.6% in R-TME. Disease-free survival rates were 87.5% in L-TME and 89.6% in R-TME. Local recurrence rates were 3.0% for both groups. Mean Wexner score for L-TME and R-TME patients was: 9.42 ± 8.23 and 9.22 ± 3.64 (p = 0.685), respectively. Daily stool frequency was similar between groups.
Conclusion
Robotic total mesorectal excision (R-TME) and laparoscopic TME (L-TME) have similar perioperative, oncological, and anorectal functional results in female patients with rectal cancer. The robotic approach for rectal cancers in female patients could be not as critical as for male patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimally invasive approaches to rectal cancer surgery has been rapidly adopted in the last decade since large randomized controlled trials (RCTs) have showed faster postoperative recovery with acceptable long-term oncological outcomes, in terms of local recurrence rates and overall survival, with laparoscopic surgery [1,2,3,4,5]. Additionally, laparoscopic approach was reported advantageous compared to open regarding to the preservation of sexual function [6].
Laparoscopic approach is technically challenging for the successful treatment of low rectal cancers especially in male or overweight patients, after neoadjuvant chemoradiotherapy, and in case of bulky tumors because of the narrow space, complex anatomy, and restricted visual field of the pelvic cavity. Straight laparoscopic instruments are not optimized to treat such patients and for this reason robotic platforms were specifically developed to overturn these limitations through improved dexterity, lack of tremor, and introduction of surgeon-controlled 3-D cameras, improvements ideal for complex excisions in restricted spaces (i.e., pelvic cavity) [8]. Robotic total mesorectal excision (R-TME) show comparable short and long-term outcomes with laparoscopic TME (L-TME) [7, 8]. Considering the specific advantage in pelvic surgery, robotic platforms could benefit the patient in the meaning of improved oncological and functional outcomes in expert hands [9,10,11]. However, R-TME is more expensive than L-TME and requires a surgical platform which is not rapidly available to every colorectal team [12,13,14]. Therefore, the adoption of the robotic platform should be tailored in a resource-limited center. Our team previously demonstrated the superiority of R-TME to L-TME in male patients with rectal cancer regarding mesorectal specimen quality and local recurrences [15]. In a recent study, similar perioperative and long-term outcomes were observed for both robotic and laparoscopic TME procedures in male and female patients with rectal cancer, respectively [16]. However, no study has specifically evaluated female patients with rectal cancers for which surgeons could obtain less advantages from the robotic platform since the more ideal pelvic anatomy.
This study aims to evaluate any differences in perioperative, oncological, and anorectal functional outcomes in female patients with rectal cancers treated with either R-TME or L-TME.
Materials and methods
Study population
This retrospective study evaluated a consecutive series of female patients with rectal cancer undergoing L-TME and R-TME performed in parallel between January 2014 and January 2019. Data were extracted from a prospectively maintained colorectal database. Informed consent was obtained.
Primary aim was to report short perioperative and long-term oncological and anorectal functional outcomes of L-TME and R-TME surgeries in female patients with rectal cancer.
Inclusion criteria (Fig. 1): (1) female sex; (2) rectal adenocarcinoma (within 15 cm from anal verge); (3) cT1-4; (4) N any; (5) no evidence of synchronous tumors and distant metastases; (6) elective surgery; (7) minimally invasive approach; 8) sphincter-saving TME: low anterior (LAR) and intersphincteric resection (ISR).
Exclusion criteria: (1) Stage IV; (2) abdominoperineal resection; (3) TAMIS (transanal minimally invasive surgery); (4) open approach; (5) palliative surgery.
AV was defined as the junction between perineal skin and anal mucosa. Distance between caudal edge of the tumor and AV was assessed via digital rectal examination, flexible sigmoidoscopy, and rectal magnetic resonance imaging (MRI) [10].
Preoperative protocol
Preoperative staging included chest X-ray, carcinoembryonic antigen (CEA) levels, total colonic examination with flexible or virtual colonoscopy, thoraco-abdominal computed tomography (CT), pelvic-phased array MRI, and/or endorectal ultrasound.
Patients with clinical stage II and III were submitted to neoadjuvant long-course chemoradiotherapy (50.4 Gy pelvic irradiation and oral capecitabine), or short-course radiotherapy (25 Gy pelvic irradiation). Short-course radiotherapy was preferred in patients without any risk of lateral margin positivity. Waiting period was 4–8 weeks for long-course chemoradiotherapy and 1–4 weeks for short-course radiotherapy.
All patients underwent oral mechanical bowel preparation without oral antibiotics. A single dose of intravenous ciprofloxacin (400 mg) was given 1 h before surgical incision.
Surgical technique
All surgeries (L-TME and R-TME) were performed by a single surgeon (O.A.), with more than 20 years of experience in advanced minimally invasive colorectal surgery at two different centers (Liv Hospital and Maslak Acibadem Hospital, Istanbul, Turkey). Surgical approach was discussed and decided according to surgeon’s indication, patient’s choice, and surgical cost. All patients received extensive explanations of the advantages and disadvantages of both approaches.
Both laparoscopic and robotic approach to LAR and ISR techniques were described in our previous studies [17, 18].
ISR was performed through the correct identification of the intersphincteric plane [19] and following the principles described by Piozzi et al. [20,21,22].
R-TME performed with the Da Vinci Si® or Xi® (Intuitive Surgical, Inc., Sunnyvale, CA, USA) platform were 23 and 11, respectively.
Perfusion of the colonic stump wall was checked before anastomosis using indocyanine green (ICG) in 5 cases in L-TME and 12 cases in R-TME group. For R-TME, the Firefly™ (Fluorescence Imaging Scope; Intuitive Surgical, Inc., Sunnyvale, CA, USA) integrated mode was used.
Conversion was defined as any unplanned laparotomy at any time during surgery, regardless of incision length.
Pathological report
Pathological staging was modified according to American Joint Committee on Cancer (AJCC) 8th edition staging system during data review [23]. All pathology specimens were examined to determine tumor size, number of lymph nodes harvested, distal resection margin (DRM), circumferential resection margin (CRM), and mesorectum integrity. Quality of mesorectum was assessed according to Quirke et al. [24]. Positive CRM was defined as direct tumor extension within 1 mm of the radial, non-peritonealized surface of the resected specimen [25].
Postoperative outcomes and follow-up
Postoperative complications, defined as adverse events occurring within 30 days from surgery, were assessed through Clavien–Dindo’s classification [26]. Anastomotic leak diagnosis and treatment followed the International Study Group of Rectal Cancer [27]. Adjuvant chemotherapy (5-FU, capecitabine, and oxaliplatin) was given to patients with pathological stage III cancers.
Follow-up included control of oncological markers (CEA, carbohydrate antigen 19-9) every 3 months, evaluation with thoraco-abdominal CT annually, and colonoscopy on the first, third, fifth, and tenth year from surgery.
Overall survival (OS) was measured from date of surgery to that of death/last follow-up, disease-free survival (DFS) to that of tumor recurrence. Recurrence was diagnosed through radiological detection of enlarging lesions or by histological confirmation.
This study followed the STROBE statement for cohort studies [28].
Anorectal functional outcomes
Functional outcomes were evaluated only for patients undergoing ISR, for research purpose. Patients undergoing ISR, without recurrence and after a follow-up of 12 months after ileostomy closure were evaluated through an institutional questionnaire. This questionnaire evaluated: feces and flatus discrimination sensation, urgency, fragmentation (two evacuations within 1 h), daily stool frequency, and soiling during day and night. Continence was assessed through Wexner’s score [20] and Kirwan’s classification [21], where 0 represents perfect continence and 20 indicates major incontinence. Functional outcomes were considered “poor” for Wexner scores > 16 and “good” for scores < 10.
Statistical analysis
Patient characteristics were summarized using basic descriptive statistics. Continuous variables were presented as mean ± standard deviation and compared using the two independent sample t test. Categorical variables were expressed as proportions and analyzed using Chi-squared or Fisher’s exact test. Statistical analysis was performed using STATA software package version 9.0 (StataCorp, College Station, TX, USA). Survival and recurrence rates were estimated through Kaplan–Meier model and compared by log-rank test. p values < 0.05 were considered statistically significant.
Results
Clinical outcomes
A total of 68 consecutive female cases of minimally invasive TME were enrolled: L-TME (n = 34) and R-TME (n = 34) (Fig. 1). Patients’ clinico-demographic characteristics are listed in Table 1.
The groups were comparable in demographics, body mass index (BMI), American Association of Anesthesiologists (ASA) score, tumor location, clinical TNM stage, clinical CRM status, and neoadjuvant treatment. Perioperative outcomes are summarized in Table 2. LAR and ISR procedures were performed in both groups with no statistical difference (p = 0.466). There was no conversion to open surgery in both groups. Protective ileostomy rate was significantly lower in the L-TME group (76 vs 91%, p < 0.001) because of the lower rate of neoadjuvant chemoradiotherapy. Median operation time was significantly longer in R-TME (165.5 vs 124.5 min, p < 0.001). There was no difference regarding to blood loss, days to first flatus, or hospital stay. There was no operative, in-hospital, or 30-day mortality.
Histopathological outcomes
Histopathological findings are listed in Table 2. Mean number of retrieved lymph nodes were similar between groups. Mean DRM length, CRM length, and CRM involvement were similar between groups. L-TME group had more advanced stage rectal cancers but no significant difference was reported (p = 0.084). All TME specimens were graded complete. Other histopathological outcomes, including tumor size, grade of tumor differentiation, and pathological T and N stage, were not significantly different between the two groups.
Postoperative complications
The overall complication rate was 17.6% (n = 6) for the L-TME group and 20.5% (n = 7) for the R-TME group (Table 2). No difference was reported regarding Clavien–Dindo’s complication grade. Incidence of anastomotic leakage was 5.8% (n = 2) in L-TME and 8.8% (n = 3) in R-TME, respectively (p = 0.173). All patients with leakage were treated conservatively by maintaining pelvic drainage, until the existing infection was resolved clinically, and postponing the ileostomy closure time. Rectovaginal fistula developed in one patient from both groups, which underwent Martius labial flap interposition for a fistula repair.
Oncological outcomes
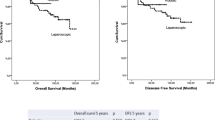
Mean follow-up for L-TME and R-TME was 62.5 (range 36–102) months and 63 (range 36–103) months, respectively. The 5-year OS rate for L-TME and R-TME was 92.8% and 89.6%, respectively (p = 0.235, Fig. 2a). The 5-year DFS rate was 87.5% for L-TME and 89.6% for R-TME (p = 0.347, Fig. 2b). Local recurrence occurred in 1 (3%) patient in L-TME and 1 (3%) patient in R-TME group. Both patients underwent abdominoperineal resection. In L-TME and R-TME groups, distant metastasis developed in 4 (12%) and 3 (9%) patients (p = 0.130), respectively. Six of them died during the follow-up period.
Anorectal functional outcomes
Anorectal functional outcomes are reported in Table 3. Between surviving patients without a permanent colostomy, a total of 31 (45.6%) patients (L-TME n = 14, R-TME n = 17) from low-located tumor were available for the functional outcome questionnaire. Mean Wexner score for L-TME and R-TME patients was: 9.42 ± 8.23 and 9.22 ± 3.64 (p = 0.685), respectively. Daily stool frequency was similar between groups. One patient from the robotic group underwent permanent colostomy due to total fecal incontinence.
Discussion
To authors’ knowledge, this is the first study specifically showing that L-TME provides comparable perioperative, oncological, and anorectal functional results to R-TME in female patients with rectal cancers.
In a resource-limited surgical facility, it is worth to strategically deploy surgical resources according to the outcomes. Sex differences in pelvic anatomy is a critical crossroad for surgical approach indication especially for rectal cancer. Therefore, perioperative, oncological, and anorectal functional results need to be discussed.
This study showed longer operation time in R-TME which is often reported in literature for both sexes [8,9,10, 12]. Longer operating time is associated to reduced surgical daily cases and indirectly to higher costs. This has to be taken into account during surgical planning and justified by better outcomes through the robotic approach.
No conversion was reported in both groups even if the L-TME group had more advanced stage rectal cancers but with no statistical difference (p = 0.084). ROLARR trial demonstrated no significant difference between robotic and laparoscopic TME in conversion rates [29, 30]. However, we recently reported differences in conversion rates between R-TME and L-TME (0 vs. 3.5%, p < 0.001) in male patients with mid-low rectal cancers after neoadjuvant chemoradiotherapy [9]. The present study, despite the limited series, could reinforce the concept that laparoscopic approach is not associated to higher conversion rates in female patients, therefore, not resulting in worse oncological outcomes [3]. The limited maneuverability of laparoscopic instruments could not be critical in female pelvises. More extended studies are needed to demonstrate the role of sex on conversion rates between different approaches.
Obtaining acceptable CRM, DRM, and complete mesorectum quality requires a precise dissection during TME [3, 9, 10, 12]. Those histopathologic metrics have an impact on OS and local recurrence [23]. ROLARR trial demonstrated no significant differences regarding histopathologic parameters [29, 30]. However, the REAL multicenter RCT, between robotic and laparoscopic approach to middle-low rectal cancer, showed lower rates of positive CRM in the robotic group (4.0 vs 7.2%, p = 0.023) [31]. This was confirmed by a recent study of ours showing significantly lower positive CRMs (3.0 vs 4.8%, p = 0.01) and higher rate of complete mesorectum (93 vs 44%, p = 0.03) between R-TME and L-TME in male patients with mid-low rectal cancers after neoadjuvant chemoradiotherapy [9]. Moreover, median CRM length was significantly longer in R-TME group than in L-TME group (p = 0.01). The present study showed no differences in CRM involvement (2.9% for both groups), CRM length (10.5 vs 11.3 mm), and TME specimen quality (all cases were reported complete). This results points out the efficient role of laparoscopy in obtaining oncologically clear dissection in female pelvises. The optimized dissection from advanced articulated robotic instruments could not be as critical for female patients as it is for males to obtain high quality dissection.
Adopting the robotic platform for female rectal cancers did not affect the complication rates, Clavien–Dindo grading, and anastomotic leak rate between the two approaches. Recent meta-analysis that compared robotic (n = 9178) and laparoscopic (n = 13,566) rectal cancer surgeries found similar postoperative complications rates between the two groups [32]. However, the REAL RCT showed lower postoperative complication rate in the robotic group (16.2 vs 23.1%, p = 003) [31]. This difference was confirmed in a recent study considering rectal cancer male patients only, where the robotic technique showed lower postoperative complication rates than laparoscopy (13.6 vs 21.4%, p < 0.05) [24]. Sex dependent differences in pelvic anatomy are critical for complication rates, as Akiyoshi et al. reported, confirming that pelvic outlet is an independent predictor of anastomotic leak (p = 0.0305) [33]. Moreover, Baek et al. demonstrated that robotic approach may overcome difficulties associated with pelvic anatomy and decrease the complication rates [34].
This study reports long-term oncological outcomes with a mean follow-up of 62.5 and 63 months for L-TME and R-TME, respectively. 5-year OS and DFS were excellent for both groups with no significant difference. Long-term local recurrence rate was 3% for both groups. This highlights the fact that laparoscopy can be oncologically safe as the robotic approach in female rectal tumors treated by expert hands. However, Piozzi et al. [11] recently evaluated the oncological impact of the surgical approach (laparoscopic versus robotic) to mid-low rectal cancer after neoadjuvant chemotherapy showing that laparoscopy could be associated to a higher rate of systemic recurrences (27.8 vs 12.1%, p = 0.023). Interestingly, the impact was specifically on lung recurrences (19.4 vs 6.5%, p = 0.019). Lung specific DFS was different between robotic and laparoscopic approach (p = 0.0020) with laparoscopy being a risk factor for lung specific DFS on multivariate analysis (p = 0.030; HR 3.074, 95% CI 1.112–8.496). The authors theorized that the different manipulation of the tumor bearing rectum, especially in hostile pelvises (post neoadjuvant chemoradiotherapy), could lead to an increased release of metastatic cells into the middle/inferior rectal and the internal iliac vein bloodstream with a higher risk of a lung pattern of hematogenous metastases. Further studies are needed to confirm these results and to further evaluate if sex dependent anatomical differences have any impact.
Regarding anorectal function, no statistical differences was found between the two groups. Median Wexner score was 9.42 ± 8.23 and 9.22 ± 3.64 (p = 0.685) for L-TME and R-TME, respectively. However, in the current study the anorectal functional questionnaire was applied only to ISR patients for both groups. Recently, we reported similar anorectal functional results in patients undergoing laparoscopic and robotic ISR [35]. Literature is limited regarding anorectal functional results specifically for rectal cancer surgeries in female patients. Since anus-preserving surgeries are becoming standard with increasingly better oncological outcomes, further multicenter studies with a standardized anorectal functional assessment protocol are needed to explore the functional impact.
This study has several limitations. First, it’s a retrospective study of prospectively collected data potentially suffering from patient selection bias. Second, these results derive from a single surgeon experience, therefore the results may not be valid for other centers. Third, the series is relatively small. A large RCT is required to confirm the non-inferiority of the laparoscopic approach for rectal cancers in female patients. Fourth, this is a series of all rectal cancers (within 15 cm from AV) in female patients. A further study specifically on low rectal cancers is required to confirm these results and evidence any benefit from the robotic approach in female patients. Fifth, cost-effectiveness was not investigated in this study.
Conclusions
This study showed that L-TME and R-TME have similar perioperative, oncological, and anorectal functional results in female patients with rectal cancer. The robotic approach for rectal cancers in female patients could be not as critical as for male patients.
Data availability statement
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.
References
Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ et al (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT Randomized Clinical Trial. JAMA 314:1356–1363
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M et al (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 314:1346–1355
Bademler S, Koza KB, Ucuncu MZ, Tokmak H, Bakir B, Oral EN et al (2019) Standardized laparoscopic sphincter-preserving total mesorectal excision for rectal cancer: median of 10 years’ long-term oncologic outcome in 217 unselected consecutive patients. Surg Laparosc Endosc Percutan Tech 29:354–361
Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr et al (2007) Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg 246:655–662 (discussion 62–4)
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97:1638–1645
Asoglu O, Matlim T, Karanlik H, Atar M, Muslumanoglu M, Kapran Y et al (2009) Impact of laparoscopic surgery on bladder and sexual function after total mesorectal excision for rectal cancer. Surg Endosc 23:296–303
Park EJ, Cho MS, Baek SJ, Hur H, Min BS, Baik SH et al (2015) Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg 261:129–137
Cho MS, Baek SJ, Hur H, Min BS, Baik SH, Lee KY et al (2015) Short and long-term outcomes of robotic versus laparoscopic total mesorectal excision for rectal cancer: a case-matched retrospective study. Medicine (Baltimore) 94:e522
Aliyev V, Tokmak H, Goksel S, Guven K, Bakir B, Kay H et al (2020) Robotic sphincter-saving total mesorectal excision for rectal cancer treatment: a single-surgeon experience in 103 consecutive male patients. Surg Technol Int 37:93–98
Aliyev V, Tokmak H, Goksel S, Meric S, Acar S, Kaya H et al (2020) The long-term oncological outcomes of the 140 robotic sphincter-saving total mesorectal excision for rectal cancer: a single surgeon experience. J Robot Surg 14:655–661
Piozzi GN, Rusli SM, Lee TH, Baek SJ, Kwak JM, Kim J et al (2022) Robotic approach may be associated with a lower risk of lung metastases compared to laparoscopic approach for mid-low rectal cancer after neoadjuvant chemoradiotherapy: a multivariate analysis on long-term recurrence patterns. Int J Colorectal Dis 37:2085–2098
Baek SJ, Kim SH, Cho JS, Shin JW, Kim J (2012) Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg 36:2722–2729
Ielpo B, Duran H, Diaz E, Fabra I, Caruso R, Malave L et al (2017) Robotic versus laparoscopic surgery for rectal cancer: a comparative study of clinical outcomes and costs. Int J Colorectal Dis 32:1423–1429
Quijano Y, Nunez-Alfonsel J, Ielpo B, Ferri V, Caruso R, Duran H et al (2020) Robotic versus laparoscopic surgery for rectal cancer: a comparative cost-effectiveness study. Tech Coloproctol 24:247–254
Aliyev V, Goksel S, Bakir B, Guven K, Asoglu O (2021) Sphincter-saving robotic total mesorectal excision provides better mesorectal specimen and good oncological local control compared with laparoscopic total mesorectal excision in male patients with mid-low rectal cancer. Surg Technol Int 38:160–166
Aliyev V, Piozzi GN, Huseynov E, Mustafayev TZ, Kayku V, Goksel S, Asoglu O (2023) Robotic male and laparoscopic female sphincter-preserving total mesorectal excision of mid-low rectal cancer share similar specimen quality, complication rates and long-term oncological outcomes. J Robot Surg 17(4):1637–1644. https://doi.org/10.1007/s11701-023-01558-2
Serin KR, Gultekin FA, Batman B, Ay S, Kapran Y, Saglam S et al (2015) Robotic versus laparoscopic surgery for mid or low rectal cancer in male patients after neoadjuvant chemoradiation therapy: comparison of short-term outcomes. J Robot Surg 9:187–194
Aliyev V, Goksoy B, Goksel S, Guven K, Bakir B, Saglam S et al (2021) Intersphincteric resection for low rectal cancer: parameters affecting functional outcomes and survival rates. Surg Technol Int 39:166–172
Piozzi GN, Park H, Kim JS, Choi HB, Lee TH, Baek SJ et al (2021) Anatomical landmarks for transabdominal robotic-assisted intersphincteric dissection for ultralow anterior resection. Dis Colon Rectum 64:e87–e88
Piozzi GN, Kim SH (2021) Robotic intersphincteric resection for low rectal cancer: technical controversies and a systematic review on the perioperative, oncological, and functional outcomes. Ann Coloproctol 37:351–367
Piozzi GN, Baek SJ, Kwak JM, Kim J, Kim SH (2021) Anus-preserving surgery in advanced low-lying rectal cancer: a perspective on oncological safety of intersphincteric resection. Cancers 13:4793
Piozzi GN, Park H, Choi TS, Kim SH (2020) Intersphincteric resection for low rectal cancer: a review on anatomy and surgical technique, oncologic and functional outcomes and the role of robotics. Turk J Colorectal Dis 30:76–85
Weiser MR (2018) AJCC 8th edition: colorectal cancer. Ann Surg Oncol 25:1454–1455
Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J et al (2009) Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 373:821–828
Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH, Pathology Review C et al (2002) Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 26:350–357
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A et al (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 147:339–351
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP et al (2008) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J et al (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 318:1569–1580
Corrigan N, Marshall H, Croft J, Copeland J, Jayne D, Brown J (2018) Exploring and adjusting for potential learning effects in ROLARR: a randomised controlled trial comparing robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection. Trials 19:339
Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y et al (2022) Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 7:991–1004
Liu C, Li X, Wang Q (2021) Postoperative complications observed with robotic versus laparoscopic surgery for the treatment of rectal cancer: an updated meta-analysis of recently published studies. Medicine (Baltimore) 100:e27158
Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y et al (2009) Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery 146:483–489
Baek SJ, Kim CH, Cho MS, Bae SU, Hur H, Min BS et al (2015) Robotic surgery for rectal cancer can overcome difficulties associated with pelvic anatomy. Surg Endosc 29:1419–1424
Aliyev V, Piozzi GN, Bulut A, Guven K, Bakir B, Saglam S et al (2022) Robotic vs. laparoscopic intersphincteric resection for low rectal cancer: a case matched study reporting a median of 7-year long-term oncological and functional outcomes. Updates Surg 74:1851–1860
Acknowledgements
We would like to express our gratitude to Aslan Aliyev (Eberhard Karls University, Germany) for his contribution to the statistical analysis.
Author information
Authors and Affiliations
Contributions
Conceptualization: VA, OA; methodology: OA, VA; formal analysis and investigation: VA, GNP; NS; writing—original draft preparation: VA, GNP; writing—review and editing: VA, GNP, NS, KG, BB, SG, and supervision: OA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest to disclose. Vusal Aliyev, Guglielmo Niccolò Piozzi, Niyaz Shadmanov, Koray Guven, Baris Bakir, Suha Goksel, and Oktar Asoglu have no conflict of interest or financial ties to disclose.
Ethical approval
Institutional Review Board (IRB) approval was waived following the retrospective nature of the study.
Informed consent
Informed consent was obtained from each patient.
Compliance with Ethical Standards, Research involving human participants and/or animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aliyev, V., Piozzi, G.N., Shadmanov, N. et al. Robotic and laparoscopic sphincter-saving resections have similar peri-operative, oncological and functional outcomes in female patients with rectal cancer. Updates Surg 75, 2201–2209 (2023). https://doi.org/10.1007/s13304-023-01686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-023-01686-2