Abstract
Salvage mastectomy is regarded as the treatment of first choice for ipsilateral breast cancer recurrence (IBCR), even if a second breast conserving surgery (BCS) is feasible. The purpose of this study was to compare the long-term oncological outcomes of IBCR patients who had undergone either mastectomy or second BCS, performing a propensity score matching (PSM) analysis to reduce the selection bias. All the consecutive patients with IBCR were retrospectively reviewed and divided into two different groups of treatment: repeat BCS versus salvage mastectomy. The propensity score predicting the probability of surgical treatment was determined for each patient and a 1:1 matching was performed. Disease-free survival (DFS), distant disease-free survival (DDFS), overall survival (OS), and breast cancer-specific survival (BCSS) were analyzed and compared between the two groups. A total of 309 patients underwent surgical treatment for IBCR. After PSM, 108 patients treated with repeat BCS and 108 patients treated with salvage mastectomy were included in the analysis. There was no significant difference in terms of DFS between patients with IBCR receiving repeat BCS or salvage mastectomy (p = 0.167). However, patients with IBCR undergoing second BCS had significantly better DDFS, OS, and BCSS compared to salvage mastectomy (p < 0.001). Salvage mastectomy should not be considered the optimal treatment for IBCR and it does not seem to improve prognosis compared to repeat conserving surgery. Second BCS for IBCR is a safe option with encouraging long-term oncological outcomes and should be proposed to all patients, when technically feasible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast conserving surgery (BCS) is considered the standard treatment for early-stage breast cancer [1, 2]. Over the past decades, breast preservation has been progressively used due to the improvement in patient quality of life, decrease in post-operative risks, and availability of neo-adjuvant chemotherapy [3,4,5,6]. However, within 10 years, approximately 5–10% of patients treated with BCS and subsequent radiotherapy will develop ipsilateral breast cancer recurrence (IBCR) [1, 2, 7,8,9]. Various factors have been associated with an increased risk of developing IBCR, including tumor characteristics and type of treatment (higher tumor grade, positive excision margins, and omission of adjuvant chemo-radiotherapy) [10,11,12,13]. Determining the most appropriate treatment option for IBCR represents a complex surgical decision. Salvage mastectomy has been regarded as the treatment of first choice; however, it still does not completely remove the possibility of a second loco-regional recurrence, metastatic disease, or cancer-related death [14,15,16]. In clinical practice, many patients with IBCR desire a second conservative surgical approach [17, 18]; therefore, it is of utmost importance to determine whether these patients have the same prognosis as those who undergo mastectomy. Up to now, there have been no prospective randomized trials to demonstrate the superiority of mastectomy compared to a second BCS in terms of oncological safety for patients with IBCR. However, several studies have retrospectively evaluated the prognostic difference between repeat conservative treatment and mastectomy for IBCR [19,20,21,22,23,24,25,26]. Recently, a retrospective analysis performed at our institution [27] suggested that there is no significant difference in terms of recurrence between IBCR patients receiving BCS or salvage mastectomy; although patients undergoing repeat conserving surgery might present a better survival compared to patients undergoing mastectomy. However, it should be noted that numerous selection bias may have affected the conclusions of the previous analyses, including ours. The purpose of this study was to compare the long-term oncological outcomes of patients with IBCR who had undergone either salvage mastectomy or second BCS, performing a propensity score matching (PSM) analysis to reduce the selection bias and to consolidate the results of our previous analysis.
Methods
Study design and patient management
As it was previously performed [27], data about patients with histologically confirmed IBCR were collected, retrospectively reviewed and analyzed. All IBCR patients were consecutively treated at the Breast Unit of Humanitas Clinical and Research Center (Milan, Italy), between January 2008 and December 2018. Patients were divided into two different groups of treatment: repeat BCS versus salvage mastectomy. The following exclusion criteria were used: primary breast cancer treated with mastectomy, contralateral recurrence, new ipsilateral primary tumor, recurrent benign breast tumors, breast sarcomas, synchronous metastatic disease, isolated axillary lymph node recurrence, inoperable IBCR, previous non-breast malignancies, disease-free interval (DFI) ≤ 6 months, and follow-up < 24 months. Indication for repeat BCS were as follows: unifocality of the recurrence and a breast-to-tumor ratio which was favourable for cosmetic results. A multidisciplinary tumour board composed of breast surgeons, oncologists, radiotherapists, radiologists, plastic surgeons, and pathologists discussed the management of every patient. Patients with IBCR did not receive routine adjuvant radiotherapy; the indication for re-irradiation was given based on specific clinical and pathological risk factors. Follow-up was performed every 6 months. All patients gave the informed consent for operation and clinical data acquisition.
Definitions
IBCR can be classified into two different entities: true recurrence represents the regrowth of malignant cells, whereas new ipsilateral primary tumor is a de novo malignancy [28]. Classification guidelines are not standardized yet; however, we defined IBCR as either true recurrence or new primary on the basis of the histologic subtype and tumour location. An IBCR was designated true recurrence if its histologic subtype was in accordance with the primary breast cancer and if it was located within 3 cm of the primary tumor bed or in the surgical scar (by breast imaging or physical examination). If the IBCR had a change in histology, or a change from infiltrating carcinoma to carcinoma in situ, or was more than 3 cm from primary breast cancer site, it was considered a new primary. All the analyzed patients with IBCR were affected by true recurrence, based on the cited criteria.
DFI was defined as the period from the date of first BCS for primary breast cancer to the date of appearance of IBCR. Disease-free survival (DFS) was defined as the period from the date of surgical treatment for IBCR (either repeat BCS or salvage mastectomy) to the date of any tumor progression including loco-regional recurrence or distant metastasis. Distant disease-free survival (DDFS) was defined as the period from the date of surgery for IBCR and the date of detection of distant metastasis. Overall survival (OS) was defined as the time interval from IBCR treatment to death from any cause or to the date of last contact. Breast cancer-specific survival (BCSS) was determined by selecting breast cancer as the cause of death and recording the follow-up duration after censoring deaths from other causes.
Propensity score matching method
After comparing the two different groups of treatment (repeat BCS versus salvage mastectomy) and considering that most variables were not equally distributed between the groups, PSM analysis was applied to control factors that may confound the association between the type of surgical treatment and long-term oncological results. The propensity score predicting the probability to receive second BCS compared with mastectomy was determined for each individual patient with IBCR, using multivariable logistic regression including the following covariates: tumor grading, tumor stage (T and N), pathological tumor dimension, complete resection. These covariates were chosen because they are strongly associated with the selection of surgical treatment or with the prognosis, even though some of them did not show statistical significance in the crude model. Given the propensity scores for all patients with IBCR, pairs of them were identified (one patient who underwent repeat BCS, and one patients who underwent mastectomy), and a 1:1 matching (without replacement) was performed using the nearest neighbor matching within a caliper width equal to 0.2 standard deviations [29]. Additionally, the differences in propensity scores in each pair of patients were no more than 0.01.
Statistical analysis
Patients were selected from the same prospectively maintained institutional dataset used in the previous study [27], with the same observation period (last follow-up was updated up to July 1, 2020). No patient was lost to follow-up. Differences in clinical and tumor characteristics of the two different groups of treatment (repeat BCS versus salvage mastectomy) were compared using the Chi-square test or Fisher’s exact test, both before and after adjustment by the PSM. After PSM, the Kaplan–Meier method was used to generate the recurrence and survival curves and to estimate the DFS, DDFS, OS, and BCSS rates. The log-rank test was used to evaluate the difference in long-term oncological outcomes considering demographic, tumor, and treatment characteristics. The multivariate analyses were performed using the Cox proportional hazards model, to identify independent risk and protective factors of DFS, DDFS, OS, and BCSS. Hazard ratios and 95% confidence intervals were calculated. Statistical significance was set at p < 0.05; all statistical tests were two-tailed. Data analyses and figures were performed with IBM SPSS 25.0 software.
Results
Characteristics of patients before propensity score matching
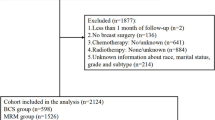
A total of 309 patients underwent surgical treatment for IBCR, 166 patients (53.7%) underwent salvage mastectomy and 143 patients (46.3%) underwent repeat BCS. Table 1 details and compares patient, tumor, and adjuvant treatment characteristics before PSM, according to the surgical method used (second BCS versus salvage mastectomy). Several factors were significantly different between the two groups. Patients in the repeat BCS group were more likely to be older (p < 0.001) with a longer DFI (p = 0.008). Recurrent tumors treated with second BCS were smaller (p < 0.001), with lower T and N stage (p < 0.001, p = 0.015, respectively). The repeat BCS group had a higher proportion of luminal-like tumors (p < 0.001), and a lower proportion of HER2-enriched, triple negative tumors (p = 0.003, p = 0.024, respectively). Patients who underwent second BCS received adjuvant radiotherapy and hormone therapy more frequently (p < 0.001, p = 0.012, respectively), and post-operative chemotherapy less frequently (p = 0.015) compared with patients who underwent salvage mastectomy.
Characteristics of patients after propensity score matching
After PSM, 216 patients with IBCR were included in the analysis: 108 patients treated with repeat BCS and 108 patients treated with salvage mastectomy. Overall, the median age was 65 years (range 32–90), and the median DFI was 82 months (range 8–365). The two treatment groups were more balanced compared to the unmatched cohort (Table 2); however, patients in the mastectomy group were still younger and with shorter DFI (p = 0.018, p = 0.027, respectively). Moreover, recurrent tumors of patients treated with mastectomy had higher Ki67 and vascular invasion (p = 0.006, p < 0.001, respectively). Patients who underwent second BCS received adjuvant radiotherapy more frequently and post-operative chemotherapy less frequently (p < 0.001, p = 0.032, respectively) compared with patients who underwent salvage mastectomy.
Long-term oncological outcomes
The median follow-up of the matched cohort was 69 months (range 24–224). At the time of the last follow-up, 57 patients (/216, 26.4%) had re-recurrence. In the repeat BCS group, 20 (/108, 18.5%) and 8 patients (/108, 7.4%) had loco-regional recurrence and distant metastases, respectively. In the mastectomy group, 6 (/108, 5.6%) and 23 patients (/108, 21.3%) had loco-regional recurrence and distant metastases, respectively. Overall, 35 patients (/216, 16.2%) died: 8 (/108, 7.4%) and 27 patients (/108, 25.0%) in the second BCS and mastectomy group, respectively. The DFS rate at 3-, 5-, and 10-years was 85.8%, 68.6%, 35.6%, and 71.5%, 60.7%, 36.4%, in patients receiving repeat BCS or mastectomy, respectively. The DDFS rate at 3-, 5-, and 10-years was 94.1%, 90.3%, 82.1%, and 75.7%, 65.3%, 41.1%, in patients receiving repeat BCS or mastectomy, respectively. The OS rate at 3-, 5-, and 10-years was 96.9%, 92.8%, 84.1%, and 84.0%, 68.3%, 42.9%, in patients receiving repeat BCS or mastectomy, respectively. The BCSS rate at 3-, 5-, and 10-years was 98.8%, 94.6%, 85.7%, and 86.7%, 70.5%, 43.7%, in patients receiving repeat BCS or mastectomy, respectively. There was no significant difference in terms of DFS between patients with IBCR receiving repeat BCS or salvage mastectomy (p = 0.167). However, patients with IBCR undergoing second BCS had significantly better DDFS, OS, and BCSS compared to salvage mastectomy (p < 0.001). Comparison of long-term oncological outcomes is summarized in Table 3. Figure 1 and Fig. 2 show the Kaplan–Meier recurrence and survival curves of the matched cohort.
Disease-free survival (a) and distant disease-free survival (b) of matched ipsilateral breast cancer recurrence patients according to treatment. This figure depicts the recurrence curves [disease-free (a) and distant disease-free (b)] of the matched cohort of ipsilateral breast cancer recurrence patients according to different surgical treatment (either breast conserving surgery or salvage mastectomy). This figure was created with IBM SPSS 25.0 software. BCS Breast conserving surgery
Overall survival (a) and breast cancer-specific survival (b) of matched ipsilateral breast cancer recurrence patients according to treatment. This figure depicts the survival curves [overall (a) and breast cancer-specific (b)] of the matched cohort of ipsilateral breast cancer recurrence patients according to different surgical treatment (either breast conserving surgery or salvage mastectomy). This figure was created with IBM SPSS 25.0 software. BCS Breast conserving surgery
Risk and protective factors
Table 4 details the results of the multivariate analyses performed in the matched cohort, in which the Cox proportional hazards model was used to identify the risk and protective factors associated with patient recurrence and survival. Regarding risk factors, dimension of the recurrent tumor > 18 mm decreased DDFS, and presence of vascular invasion decreased DFS, OS, and BCSS. Additionally, age > 65 years decreased OS. Conversely, adjuvant radiotherapy increased DFS, hormone therapy increased DFS and DDFS, and post-operative chemotherapy increased OS.
Discussion
In patients with IBCR after BCS and adjuvant radiotherapy, the choice of treatment between two different therapeutic options, either salvage mastectomy or repeat BCS, is currently based only on retrospective studies with small patient cohorts. There is no significant evidence for considering second BCS as well as considering mastectomy as the standard of care in case of IBCR. In our retrospective analysis with PSM we aimed to provide additional evidence in the decision-making process for the treatment of patients with IBCR.
To begin with, the surgical treatment of IBCR may be influenced by its biological and pathological features. Houvenaeghel et al. [30] evaluated the tumor features associated with ipsilateral local recurrence after BCS and found that estrogen-receptor negative tumors, with high tumor grade presented shorter DFI. Moreover, HER2-enriched sub-type and patients’ age ≤ 40 years may negatively influence DFI and OS. The same topic was further analyzed by Corso et al. [31], which similarly found that metastatic axillary lymph nodes (p = 0.0004), high tumor grade G3 (p = 0.04), HER-enriched and triple negative tumors (p = 0.008, p = 0.02, respectively) were significantly associated with an increased risk for IBCR. Additionally, adjuvant hormone therapy, chemotherapy, and radiotherapy (p = 0.0003, p = 0.001, p = 0.0005, respectively) emerged as protective factors for IBCR. More recently, the same authors [32] constructed and validated novel nomograms predicting the risk of IBCR in patients treated either with BCS or mastectomy. The authors were able to identify the following features: young age at onset (age < 35 years), T2-T4, metastatic lymph nodes (≥ 4 positive nodes), G2-G3 tumor grade, vascular invasion, HER2-enriched, luminal sub-types, and lobular histology to be significantly associated with IBCR.
The previously cited biological and pathological features associated with the nomograms may help the breast surgeon and guide the multidisciplinary team in quantifying and stratifying the IBCR risk; however, the studies referring to the prognostic impact of the surgical procedure (either mastectomy or repeat BCS) for IBCR report contrasting results. Chen et al. [19] discouraged the use of second BCS for IBCR, reporting the results of 568 and 179 patients who underwent salvage mastectomy or repeat BCS, respectively. The BCS group had significantly lower 5-year OS compared to the salvage mastectomy group (67% versus 78%, respectively, p = 0.03). Su et al. [21] performed a large retrospective analysis on 5089 IBCR patients; 4,048 (79.4%) and 1050 patients (20.1%) underwent mastectomy or second BCS, respectively. At multivariate analysis, second BCS was associated with increased overall mortality (p < 0.001) and cancer-specific mortality (p < 0.001). However, some studies reported the results of patients with IBCR who were treated with repeat BCS with no significantly inferior outcomes compared to salvage mastectomy. Kurtz et al. [22], analyzed the results of 118 patients with IBCR; among them, 52 received second BCS. The authors reported that repeat BCS was feasible and safe with no significantly inferior 10-year OS compared to mastectomy (64% versus 54%, respectively). Salvadori et al. [23] selected a sub-group of patients with small ipsilateral recurrence undergoing repeat BCS. The 5-year OS was significantly worse in the group treated with salvage mastectomy compared with repeat conserving surgery (70% versus 85%). Alpert et al. [24] reported the results of 30 patients who underwent repeat BCS and 116 patients who received salvage mastectomy for IBCR. With a follow-up of 13.8 years, there was no significant difference in the 10-year OS between the second BCS and salvage mastectomy group (58% versus 65%, respectively). Additionally, there was no significant difference in the second recurrence rate between the mastectomy and BCS cohort (32% versus 24%, respectively). Gentilini et al. [25] found a sub-group of patients with ipsilateral recurrence < 2 cm occurring after 48 months from primary breast cancer surgery representing the best candidates for second BCS with 5-year OS of 84%.
The reason why the previous retrospective analyses showed no difference in long-term oncological outcomes between repeat BCS and mastectomy might be reflected by the lack of balancing of the confounding factors between the two different treatment groups. For instance, patients who underwent repeat BCS for IBCR tended to have smaller, solitary tumors than those receiving salvage mastectomy [23, 25]; therefore a better prognosis. However, the PSM method can be used to reduce and eliminate the potential bias caused by the observational nature of these retrospective studies that may have caused differences of clinical characteristics between the two groups of treatment. Yoshida et al. [33] were the first authors using the PSM method for the analysis of 271 patients with IBCR, showing no difference in terms of recurrence and survival between patients who received repeat BCS and those who received salvage mastectomy (p = 0.6, p = 0.09, respectively). Recently, Wu et al. [34] analyzed data of IBCR patients from the Surveillance, Epidemiology, and End Results (SEER) database using the PSM method, observing no significant difference in terms of BCSS and OS between the two groups of treatment. Additionally, Baek et al. [35] analyzed the oncological outcomes of 180 patients with IBCR using the PSM, founding no significant difference in terms of BCSS and OS between the two groups of treatment. These studies seem to indicate that second BCS is a safe and feasible alternative for patients with IBCR. Our retrospective analysis is the first study showing the superiority of DDFS, OS, and BCSS in IBCR patients treated with repeat BCS compared to salvage mastectomy; therefore, corroborating the results of the previous studies and providing additional evidence in support of second conserving surgery.
On multivariate analysis after PSM, we found that adjuvant re-irradiation after second BCS for IBCR represents an independent protective factor for DFS. However, there is no consensus about the optimal post-operative treatment for patients with IBCR who have been previously treated with radiotherapy. Previous studies reported that re-irradiation is required in order to achieve satisfactory results in terms of DFS [36,37,38,39]. Often, the necessity of second radiotherapy represents the reason for not offering repeat BCS to patients with IBCR. It is commonly thought that a repeat course of adjuvant radiotherapy is not well tolerated by the tissues, leading to unacceptable toxicity. Nevertheless, many authors reported that re-irradiation represents a safe and feasible option with encouraging results in terms of long-term oncological outcomes. Deutsch [40] reported the outcomes of 39 patients with IBCR treated with second BCS and a repeat course of external beam radiation with a 5-year OS and DFS of 77.9% and 68.5%, respectively. Additionally, new techniques that target only the tumor bed have been proposed. Vavassori et al. [41] reported the outcomes of 31 patients with IBCR treated with repeat BCS and post-operative interstitial high-dose-rate brachytherapy. The 5-year OS and DFS was 87.1% and 83.9%, respectively. In our matched cohort, only 44 patients (40.7%) treated with second BCS underwent post-operative radiotherapy. Re-irradiation was not mandatory and its indication was discussed individually for each patient in the multidisciplinary tumor board. In our investigation on long-term oncological outcomes of patients with IBCR, we found the protective role of adjuvant chemotherapy on OS. Previously, the effectiveness of chemotherapy after surgical excision of isolated loco-regional recurrences was examined by the prospective randomized Chemotherapy as Adjuvant for LOcally Recurrent breast cancer (CALOR) trial [42]. The final analysis of the CALOR trial demonstrated the benefit of adjuvant chemotherapy on patients with resected estrogen receptor-negative isolated loco-regional breast cancer recurrence [43].
It is necessary to underline that our study has some limitations. First, this is a single-center study, subject to limitations due to its retrospective design using observational data collected at a specific moment. Second, although PSM was performed, we could not replicate the randomized assignment of the prospective clinical trial. However, no prospective trials have been performed to demonstrate the superiority of mastectomy compared to conserving surgery in terms of long-term outcomes, because it would be unethical to randomize patients between the two different treatment options. Additionally, of the original 309 patients with IBCR, only 216 patients were analyzed after the PSM. Despite these limitations, this study also presents several strong points. First, the PSM method was used to eliminate the potential bias owing to the observational nature of the study. Furthermore, the classification method and inclusion criteria were clearly stated and used for the selection of a homogeneous group of IBCR patients. Moreover, all patients had a long follow-up duration and none was lost to follow-up.
Conclusions
In conclusion, our study suggests that salvage mastectomy should not be considered the optimal treatment for IBCR and it does not seem to improve prognosis compared to repeat conserving surgery. Second BCS for IBCR is a safe option with encouraging long-term oncological outcomes and should be proposed to all patients, when technically feasible.
Change history
28 July 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s13304-022-01342-1
References
Fisher B, Anderson S, Bryant J et al (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241
Veronesi U, Cascinelli N, Mariani L et al (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232
Al-Hilli Z, Thomsen KM, Habermann EB et al (2015) Reoperation for complications after lumpectomy and mastectomy for breast cancer from the 2012 National surgical quality improvement program (ACS-NSQIP). Ann Surg Oncol 22:459–469
Curran D, Van Dongen JP, Aaronson NK et al (1998) Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC trial 10801. Eur J Cancer 34:307–314
Clough KB, Acosta-Marín V, Nos C et al (2015) Rates of neoadjuvant chemotherapy and oncoplastic surgery for breast cancer surgery: a French national survey. Ann Surg Oncol 22:3504–3511
Golshan M, Cirrincione CT, Sikov WM et al (2015) Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg 262:434–438
Bosma SCJ, Van Der Leij F, Van Werkhoven E et al (2016) Very low local recurrence rates after breast-conserving therapy: analysis of 8485 patients treated over a 28-year period. Breast Cancer Res Treat 156:391–400
Wapnir IL, Anderson SJ, Mamounas EP et al (2006) Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five national surgical adjuvant breast and bowel project node-positive adjuvant breast cancer trials. J Clin Oncol 24:2028–2037
McBain CA, Young EA, Swindell R et al (2003) Local recurrence of breast cancer following surgery and radiotherapy: incidence and outcome. Clin Oncol 15:25–31
Il YuJ, Choi DH, Huh SJ et al (2015) Proportion and clinical outcomes of postoperative radiotherapy omission after breast-conserving surgery in women with breast cancer. J Breast Cancer 18:50–56
Bodilsen A, Bjerre K, Offersen BV et al (2015) The influence of repeat surgery and residual disease on recurrence after breast-conserving surgery: a Danish breast cancer cooperative group study. Ann Surg Oncol 22:476–485
Yoshida T, Takei H, Kurosumi M et al (2009) Ipsilateral breast tumor relapse after breast conserving surgery in women with breast cancer. Breast 18:238–243
Mechera R, Viehl CT, Oertli D (2009) Factors predicting in-breast tumor recurrence after breast-conserving surgery. Breast Cancer Res Treat 116:171–177
Walstra CJEF, Schipper RJ, Poodt IGM et al (2019) Repeat breast-conserving therapy for ipsilateral breast cancer recurrence: a systematic review. Eur J Surg Oncol 45:1317–1327
Doyle T, Schultz DJ, Peters C et al (2001) Long-term results of local recurrence after breast conservation treatment for invasive breast cancer. Int J Radiat Oncol Biol Phys 51:74–80
Fodor J, Major T, Polgár C et al (2008) Prognosis of patients with local recurrence after mastectomy or conservative surgery for early-stage invasive breast cancer. Breast 17:302–308
Al-Ghazal SK, Fallowfield L, Blamey RW (2000) Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer 36:1938–1943
Schain WS, d’Angelo TM, Dunn ME et al (1994) Mastectomy versus conservative surgery and radiation therapy. Psychosocial consequences. Cancer 73:1221–1228
Chen SL, Martinez SR (2008) The survival impact of the choice of surgical procedure after ipsilateral breast cancer recurrence. Am J Surg 196:495–499
Galper S, Blood E, Gelman R et al (2001) Prognosis following local recurrence after conservative surgery and radiation therapy for early-stage breast cancer. Breast Cancer Res Treat 69:212
Su Y, Guo R, Xue J et al (2019) Increased mortality with repeat lumpectomy alone after ipsilateral breast tumor recurrence. Oncologist 24:818–827
Kurtz JM, Amalric R, Brandone H et al (1988) Results of wide excision for mammary recurrence after breast-conserving therapy. Cancer 61:1969–1972
Salvadori B, Marubini E, Miceli R et al (1999) Reoperation for locally recurrent breast cancer in patients previously treated with conservative surgery. Br J Surg 86:84–87
Alpert TE, Kuerer HM, Arthur DW et al (2005) Ipsilateral breast tumor recurrence after breast conservation therapy: outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int J Radiat Oncol Biol Phys 63:845–851
Gentilini O, Botteri E, Veronesi P et al (2012) Repeating conservative surgery after ipsilateral breast tumor reappearance: criteria for selecting the best candidates. Ann Surg Oncol 19:3771–3776
Ishitobi M, Fukui R, Hashimoto Y et al (2017) Safety for repeat lumpectomy without radiotherapy for ipsilateral breast tumor recurrence. Anticancer Res 37:5293–5299
Sagona A, Gentile D, Anghelone CAP et al (2021) Ipsilateral breast cancer recurrence: characteristics, treatment, and long-term oncologic results at a high-volume center. Clin Breast Cancer. https://doi.org/10.1016/j.clbc.2020.12.006
Veronesi U, Marubini E, Del Vecchio M et al (1995) Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst 87:19–27
Austin PC (2011) Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 10:150–161
Houvenaeghel G, de Nonneville A, Cohen M et al (2019) Isolated ipsilateral local recurrence of breast cancer: predictive factors and prognostic impact. Breast Cancer Res Treat 173:111–122
Corso G, Maisonneuve P, Santomauro GI et al (2018) Ipsilateral breast tumor reappearance and contralateral breast cancer after primary breast cancer treatment: a comprehensive retrospective study of 15,168 patients. Oncol 95:147–155
Corso G, Maisonneuve P, Massari G et al (2020) Validation of a novel nomogram for prediction of local relapse after surgery for invasive breast carcinoma. Ann Surg Oncol 27:1864–1874
Yoshida A, Takahashi O, Okumura Y et al (2016) Prognosis after mastectomy versus repeat lumpectomy in patients with ipsilateral breast cancer recurrence: a propensity score analysis. Eur J Surg Oncol 42:474–480
Wu Y, Shi X, Li J et al (2021) Prognosis of surgical treatment after ipsilateral breast tumor recurrence. J Surg Res 258:23–37
Baek SY, Kim J, Chung IY et al (2021) Long-term survival outcomes of repeat lumpectomy for ipsilateral breast tumor recurrence: a propensity score-matched analysis. Breast Cancer Res Treat 185:155–164
Kauer-Dorner D, Pötter R, Resch A et al (2012) Partial breast irradiation for locally recurrent breast cancer within a second breast conserving treatment: alternative to mastectomy? Results from a prospective trial. Radiother Oncol 102:96–101
Shah C, Wilkinson JB, Jawad M et al (2012) Outcome after ipsilateral breast tumor recurrence in patients with early-stage breast cancer treated with accelerated partial breast irradiation. Clin Breast Cancer 12:392–397
Hannoun-Levi JM, Castelli J, Plesu A et al (2011) Second conservative treatment for ipsilateral breast cancer recurrence using high-dose rate interstitial brachytherapy: preliminary clinical results and evaluation of patient satisfaction. Brachytherapy 10:171–177
Hannoun-Levi JM, Resch A, Gal J et al (2013) Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: multicentric study of the GEC-ESTRO Breast Cancer Working Group. Radiother Oncol 108:226–231
Deutsch M (2002) Repeat high-dose external beam irradiation for in-breast tumor recurrence after previous lumpectomy and whole breast irradiation. Int J Radiat Oncol Biol Phys 53:687–691
Vavassori A, Riva G, Cavallo I et al (2020) High-dose-rate brachytherapy as adjuvant local reirradiation for salvage treatment of recurrent breast cancer (balestra): a retrospective mono-institutional study. J Contemp Brachytherapy 12:207–215
Aebi S, Gelber S, Anderson SJ et al (2014) Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol 15:156–163
Wapnir IL, Price KN, Anderson SJ et al (2018) Efficacy of chemotherapy for ER-negative and ER-positive isolated locoregional recurrence of breast cancer: final analysis of the CALOR trial. J Clin Oncol 36:1073–1079
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Author information
Authors and Affiliations
Contributions
Study conception and design: GD, SA, BE, AL, LA, TC. Material preparation and data collection: GD, SA, BE, AL, LA, TC. Analysis and interpretation of data: GD, SA, BE, FD, FB, TC. Drafting of manuscript: GD, SA, BE, FD, FB, TC. Critical revision and final approval: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
The present study complied with the guidelines for human studies. The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The Institutional Review Board of our hospital approved this retrospective study.
Informed consent
Each patient provided informed consent for operation and clinical data acquisition.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s13304-022-01342-1
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gentile, D., Sagona, A., Barbieri, E. et al. RETRACTED ARTICLE: Breast conserving surgery versus salvage mastectomy for ipsilateral breast cancer recurrence: a propensity score matching analysis. Updates Surg 74, 479–489 (2022). https://doi.org/10.1007/s13304-021-01122-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-021-01122-3