Abstract
The process of angiogenesis is quite well-known nowadays. Some medicines and extracts affecting this process are already used routinely in supporting the conventional treatment of many diseases that are considered angiogenic such as cancer. However, we must be aware that the area of currently used drugs of this type is much narrower than the theoretical possibilities existing in therapeutic angiogenesis. Plant substances are a large and diverse group of compounds that are found naturally in fruits, vegetables, spices, and medicinal plants. They also have different anticancer properties. The aim of this literature review article is to present the current state of knowledge concerning the molecular targets of tumor angiogenesis and the active substances (polyphenols, alkaloids, phytohormones, carbohydrates, and terpenes) derived from natural sources, whose activity against cancer angiogenesis has been confirmed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global burden of cancer is projected to have more than doubled over the next two decades, raising the prospect of a significant investment in health systems, thus posing a real medical problem [1]. Changes in risk factor distributions and an aging population are both contributing to the growing prevalence and incidence of cancer around the world despite global trends towards lower cancer incidence and mortality rates for a number of cancer types in developed countries [2, 3]. Based on GLOBOCAN data [2], the International Agency for Research on Cancer (IARC) estimated that 14.1 million new cancer cases were diagnosed and that 8.2 million patients died from this disease worldwide in 2012 alone. The increasing number of people with cancer highlights the need for more cancer prevention efforts.
Complementary and alternative medicines, which cover a large spectrum of old to new strategies that purport to extend options to prevent and to treat diseases such as cancer, are gaining more attention in oncology management [4–6]. The emergence of natural bioactive compounds represents a natural experiment, as millions of Americans have begun self-medicating with these products [7]. Natural products have been valuable sources of new therapeutic agents [8]; the number of chemotherapeutic agents and their sources indicates that 80 % of approved drugs are derived from natural compounds [9]. With the new high-throughput screening technologies, natural products are likely to provide many of the lead structures for the modelization of novel substances with enhanced anticancer activities that have high selectivity [5]. One of the most involved factors in cancer invasion and metastasis is angiogenesis; this term was introduced and used for the first time by John Hunter in 1787 [10]. It was described and considered a therapeutic target in cancer therapy by Judah Folkman [11]. It is defined as being the process of creating new thin-walled capillaries based on the existing blood vessel network in the life of an adult organism which should be distinguished from another process of creating vessels—which runs only during the fetal period [12–14]. Furthermore, the process of angiogenesis should be distinguished from arteriogenesis, which is a remodeling of small vessels by the activity of various factors, including the physical ones. Thus, angiogenesis is a complex, multistage process during which many changes must occur [15, 16]. Until now, usually five basic stages of angiogenesis have been distinguished. The factors that stimulate angiogenesis are usually hypoxia, ischemia, or inflammation that acts on cytokines, as well as the basic pro-angiogenic factors of vascular endothelial growth factor (VEGF) or fibroblast growth factor (FGF) type [17, 18].
The initial signal is the flaccidity of the existing vessels caused by nitric oxide (NO). NO provokes the launch of proteolytic enzymes that activate the production of the new vascular structures by the degradation of the basement membrane. Then, the integrins facilitate and regulate the adhesion and migration of endothelial cells (ECs). The stimulation of ECs (especially their mobility and proliferation) is caused by the molecules released from the same cell matrix such us the fibroblast growth factors, commonly known as FGF. The proliferation of ECs determines the genesis of the new tubular structures, which will mature and be stabilized under the effect of angiopoietins and their corresponding receptors [19]. Neovascularization is also important during the process of natural wound healing, initiating the repair processes and contributing to the development of granulation tissue and the limitation of the necrosis area [12, 20]. This process is also the base of the proper maturation of bones and hair growth [21].
The physiological angiogenesis is part of the pathological picture of chronic inflammatory changes associated with asthma, rheumatoid arthritis, and gastrointestinal diseases, such as Crohn’s disease or ulcerative colitis. It can cause blindness as a result of chronic changes in the retina or cornea (diabetic retinopathy, macular degeneration), it plays a role in the development of obesity (adipose tissue development) or endometriosis, and it is added to the production of alveoli, typically at an early postnatal stage. Hypoxia is a powerful stimulus of vessel formation in the cardiovascular system, occurring in ischemic heart disease, atherosclerosis, stroke, and ischemia of the lower limbs (peripheral vascular impairment) [22–24]. In the pathological conditions, the angiogenesis process gets out of control which results in the development and growth of primary tumors and also increases the risk of metastasis [13, 16, 25].
Tumor angiogenesis—the study of new medicines
Intensive research to develop drugs similar to angiostatin (e.g., bevacizumab (Avastin®)) has proved to be disappointing when applied to humans. These medicines might slow the progression of certain types of cancer and might be known for the effective regression of tumors in some cases, but the results achieved tend to be and their action must be supported by other means. There are two methods of inhibiting tumor angiogenesis:
-
1.
Classical angiogenesis inhibitors (targeted therapy), which inhibit the proliferation and formation of new blood vessels
-
2.
Substances which destroy the existing vessels, leading to necrosis within the tumor, and drugs that inhibit angiogenesis in the tumor, leading to its death by cutting off the life-giving blood.
In practice, the antiangiogenic drugs are effective but can only be used in combination with other cytostatics. It is not enough to impair the development of the network of blood vessels to cure the tumor. It is important to remove cancer cells that survive malnutrition, a lack of oxygen, and attacks of immune cells. Tumor cells with time can immunize to the action of lock mechanisms—creating substitute mechanisms. Antiangiogenic molecules are used as monotherapy in situations when it comes to the longest restraint of the development of the tumor classified as incurable. Their relatively low toxicity allows for the long-term use, but such treatment is very expensive. Cancer is a multidimensional disease, which unfortunately very rarely surrenders under the influence of a single agent. However, it is a fact that the control of angiogenesis is essential for the treatment of cancer [26, 27].
There are natural methods, which strongly affect angiogenesis without causing side effects and which can be combined with conventional therapy. These are as follows:
-
A specific diet rich in food products that inhibit angiogenesis, e.g., some types of green tea, roots, and herbs
-
Anything that contributes to the elimination of inflammations which are the direct cause of the development of new blood vessels
In this work, we will focus on recent advances in understanding the pathogenesis of tumor angiogenesis through potential therapeutic targets and the use of natural products as potential prospects in the chemoprevention of cancer.
Angiogenesis: focus on mechanisms and molecular targets
The formation of the vascular system is a complex process, which requires the interaction of some factors and molecular signals. In the developing embryo, elementary blood vessels appear through a procedure known as vasculogenesis, in which blood vessels form de novo by differentiation and adhesion of individual progenitor cells [28]. These progenitors can generate angioblasts in response to growth factor VEGF [26]. These angioblasts produce blood islands which can fuse and remodel to generate the first primitive plexus of vessels. After the formation of the immature plexus, pericytes (mural cells) that interact with the outer surface of the vessel are recruited. During adult life, neovascularization happens through angiogenesis; the growth of vessels from already present capillaries passes by the multistep process and can be represented as a process in two stages. First, the tube formation is observed, in which ECs react to a growth factor which leads to migrating, proliferating, and producing the new sprout. Second, vascular maturation occurs, during which budding vessels are settled by enlisting pericytes or vascular smooth muscle cells and by generating an extracellular matrix [29].
Hellstrom et al. (2001) [30] reported that a lack of pericytes causes endothelial cell hyperplasia associated with an abnormal shape and morphological signs of increased permeability. The regulation function of pericytes on ECs takes place through the cell–cell contact and the secreted factors. Recent studies identified several signaling pathways controlling arterial and venous identities, such as VEGF, complex Eph-Ephrin system, Notch, angiopoietins (ANGPT1 and ANGPT2), placental growth factor (PLGF), platelet-derived growth factor (PDGF), TGF, FGF, interleukin-8 (IL-8), and HGF [31, 32]. The most important pro-angiogenic factors are listed in Table 1.
Angiogenesis is a complex process, directed by the balance between pro- and antiangiogenic molecules expressed by different cell types. It obviously includes more than a simple upregulation of angiogenic activity, and it is postulated to be the result of a balance of positive and negative regulators. When pro-angiogenic factors copy the effect of angiostatic molecules, the tumor gets an angiogenic phenotype that leads to the formation of new blood vessels [33].VEGF is one of the most angiogenic players in the process of the vascular system. It is involved in mitogenesis, in endothelial cell migration, as well as in sprouting and lumen formation. It is known as tumor angiogenic factor or as vascular permeability factor. It also regulates molecules that are involved in endothelial proliferation. And its upregulation is important in physiological processes [31].
FGF
FGFs (pro-angiogenic growth factors) are a family of heparin-binding proteins which are stored in the vascular basal lamina and act both as a reservoir supply and as an upregulator during active angiogenesis. The common forms are FGF-1 or acidic FGF (aFGF) and FGF-2 or basic FGF (bFGF). FGF-1 and FGF-2 induce EC proliferation and differentiation of epiblast cells into ECs. Moreover, FGF-2 induces the production of collagenases and urinary plasminogen activator which works as a chemoattractant for these cells [34, 35].
Angiopoietins
The angiopoietins are a family (angiogenin (Ang)-1–Ang-4) of extracellular ligands that are bound to the ECs’ specific Tie receptors. They are a group of receptor tyrosine kinases which act during vessel remodeling and angiogenesis. Among the four famed angiopoietins, Ang-1 and Ang-2 are the best-characterized cytokines. Their expressions are limited to the tumor cells and in the microvasculature, respectively [36]. Ang-1 boosts endothelial cell survival and sprouting and stabilizes vascular networks by enrolling pericytes to immature vessel segments. On the other hand, Ang-2, expressed at places of vascular remodeling, results in the loss of pericytes and exposes ECs to angiogenic factors [37–39].
TGF-β
The transforming growth factor-β (TGF-β) superfamily is involved in more than 30 structurally identical growth factors and includes the three TGF-β isotypes 1–3 [40]. They are released in a latent structure, and under acidic conditions, they require a cleavage of the associated peptide domain. TGF-β promotes extracellular matrix deposition and integrin receptor upregulation [41]. It is one of the most important interleukins for modulation of the wound EC proliferation, the migration, and the lumen formation [39].
TNF-α
Tumor necrosis factor-α (TNF-α) is an inflammatory cytokine, secreted mostly by activated macrophages during inflammation and immune response. It has many functions including the motivation of GM-CSF and IL-1 and has been argued to work on ECs both directly, by inducing cell differentiation, and indirectly by producing other angiogenic factors from other cells [42, 43].
Notch
The Notch signaling, a cell membrane receptor; is the pathway that plays multiple roles in physiological processes both in the development and in adult life [44]. There are four receptors (Notch 1–4) and five ligands of Notch family, and it has a mechanism of signal transduction that requires cell–cell contact. It initiates when a ligand, expressed on the surface of a cell (signal-sending cell), physically interacts with a receptor, expressed on the surface of another cell (signal-receiving cell). Upon cell–cell contact and the ligand binding, the receptor undergoes two proteolytic cleavages operated by proteases of the disintegrin and metalloproteinase family [(ADAM)/tumor necrosis factor-α (TACE) converting enzyme] and γ-secretase enzyme, respectively. The first split results in a conformational convert, while the other is responsible for the production and the release of the Notch intracellular domain (NICD) [45–47].
Semaphorins
One of the latest regulators of angiogenesis is class 3 semaphorins, a family of secreted proteins. It has a role in pathophysiological angiogenesis [48–50]. Both the antiangiogenic semaphorin-3 (Sema3)A and Sema3F are expressed by ECs, suggesting an autocrine function, while Sema3C is argued to be a pro-angiogenic factor. A loss of Sema3A expression in favor of VEGF may result in the angiogenic switch in some cancers [51].
RhoJ
RhoJ is another signaling protein, an endothelial-specific member of the Cdc42 that controls endothelial motility, tubulogenesis, and lumen formation in vitro depicts [52]. RhoJ plays an important role in endothelial biology and in angiogenesis. Manipulation of RhoJ expression exhibits that it amends the actin cytoskeleton and focal adhesions, crucial elements of endothelial migration during angiogenesis.
CD151
Cluster of differentiation 151 (CD151) is one of the 33 human tetraspanins. They constitute a family of membrane proteins expressed in the endothelium. They function as organizers of the cell surface by allowing the regulation of processes such as cell adhesion, signaling, and intracellular trafficking. CD151 is essential for pathological angiogenesis which is indispensable for endothelial tubulogenesis, perhaps by aiding endothelial cell–cell adhesion [53].
Mechanisms of vessel formation
Egginton [54] reported that the local physical environment may play a distinctive role in response to altered tissue request. Previous studies had reported two different forms of capillary formation: splitting and sprouting angiogenesis. Cellular components involve the glycocalyx (disruption of a vasculoprotective layer), nitric oxide synthase [endothelial nitric oxide synthase (eNOS) is important for vasodilatation], and CD31 (as part of a mechanosensory complex that existed in intercellular junctions).
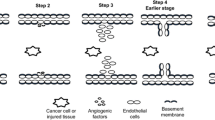
There are many genes (∼600 genes) whose expression is controlled by their environment identified by Serial analysis of gene expression libraries that appear to be organized by shear stress, a lot of which are involved in EC migration, adhesion, and angiogenesis, while low shear stress is found at sites of vascular obstruction (e.g., thrombosis or embolism) [55]. New blood vessel formation happens either by angiogenesis or by vasculogenesis. Vasculogenesis is the formation of new blood vessels de novo from progenitor cells, such as angioblasts, which differentiate into ECs, form tubes, and create primitive vessels. In contrast, angiogenesis is the formation of new vessels from the preexisting blood vessels. It consists of a complex multistep process involving extracellular matrix components. It starts with an enlargement of the original vessel, which then sprouts by intussusception and then splits into individual capillaries. This process is divided into four phases: proteolytic degradation of the basement membrane and enclosing extracellular matrix, EC proliferation and migration, lumen formation, and reorganization [22].
Endothelial cell sprouting
In a normal blood vessel, a basement membrane (BM) rests deep to the EC monolayer in the arterial intima, the BM must be degraded before EC invasion into the surrounding extracellular matrix [56]. ECs, in response to specific pro-angiogenesis signals, change from a quiet to a synthetically active phenotype distinguished by a high mitotic index and raised capacity for migration and matrix proteolysis, such as VEGF. The first event occurring is the detachment of pericytes from the vessel wall and the loosening of endothelial cell junctions. Meanwhile, matrix metalloproteases (MMPs) mediate the proteolytic degradation of the basement membrane and some ECs acquire a motile and invasive phenotype, necessary for the initiation of vessel sprouting. These activated ECs are able to disrupt the tight junctions, adherens junctions, and gap junctions which exist between neighboring intimal ECs and perivascular cells and invade into the basal lamina and surrounding extracellular matrix [57, 58]. Hence, loosed from the capillary intima and in the extravascular area, ECs proliferate and migrate towards chemotactic and angiogenic initiation in a 3-D extracellular environment and sorting of new angiogenic sprouts [59].
This process in which the capillaries expand within itself (way of vessel growth) is called intussusceptive angiogenesis (and subsequent tube formation); it is the capacity of ECs to establish luminal compartments within multicellular chains which allow for the flow of blood from the preexisting vasculature to the neovasculature. Without those new capillary networks, the ability to execute their function of oxygen and nutrient transport to normal or pathologic areas would be disabled [60, 61].
Inoculation is the anastomosis of two luminal parts to form one persistent lumen which resembles a later step of angiogenesis, in which a quiescent vessel senses an angiogenic signal, such as VEGF, VEGF-C, ANG-2, FGFs, or chemokines, released by hypoxia, inflammatory tumor stroma, or a cancer cell. Pericytes first separate from the vessel wall (in response to ANG-2) and release themselves from the basement membrane by proteolytic decadence, which is interposed by metalloproteinases. ECs disconnect their junctions and the new vessel. VEGF increases the permeability of the EC strata, leads plasma proteins to extravasate, and lays down a temporary extracellular matrix (ECM) scaffold [62].
In the case of integrin signaling, ECs migrate onto this ECM surface. Proteases liberate angiogenic signals stored in the ECM like VEGF and FGF and remodel the ECM into angio-competent surroundings. To construct a perfused tube and prevent ECs from moving all together across the angiogenic signal, one endothelial cell, the tip cell, is chosen to lead the tip in the existence of factors such as VEGF receptors, NRPs, and the NOTCH ligands DLL4 and JAGGED1. The neighbors of the tip cell suppose subsidiary postures as stalk cells, which split to extend the stalk (stimulated by Notch signaling pathway, PLGF, and FGFs) and set the lumen. Tip cells are equipped with filopodia to sense environmental instruction cues such as ephrins and semaphorins, while stalk cells liberate molecules such as EGFL7 into the ECM to convey locative information about the position of their neighbors so that the stalk elongates [63]. A hypoxia-inducible program, driven by hypoxia-inducible factor (HIF-1α), renders ECs’ interaction to angiogenic signs. Myeloid bridge cells help in fusion with another vessel branch and let the initiation of blood flow. For a vessel to become efficient, it must become mature and stable. ECs continue their quiet state, and signals such as PDGF-B, ANG-1, TGF-β, ephrin-B2, and NOTCH lead the cells to be wrapped by pericytes. Protease inhibitors known as tissue inhibitors of metalloproteinases and plasminogen activator inhibitor-1 (PAI-1) lead to the precipitation of a basement membrane, and junctions are reestablished to ensure flux distribution [64].
Inhibition of angiogenesis by bioactive compounds
Plant angiogenesis inhibitors
During the past decade, intensive studies in plant material have led to the confirmation of antiangiogenic properties and antitumor activity of a number of commonly used medicinal plants [65–71]. Plant-derived substances are characterized by expected properties in terms of antitumor activity, gaining considerable interest among many synthetic chemotherapeutic agents used in oncology [72–75]. Both untreated plant extracts and purified individual substances are used, which through a number of specific arteries in tumor cells can and lead to their death (through either apoptotic or necrotic activity) as well as by induction of aging [76–80].
Induction of programmed cell death, particularly apoptosis in cancer cells, plays an important role in chemoprevention. Apoptosis, which is an organized process, may be induced by external factors, including the natural substances of plant origin. Until now, various substances that can initiate cell death by apoptosis in multiple tumor types have been identified [26, 81, 82]. These substances have a diverse chemical structure, and their presence was confirmed in vegetables, fruits, spices, and herbs [83–89]. Compounds such as curcumin in turmeric [90–92], naringenin in citrus [93], capsaicin in pepper [94], diallyl trisulphide and allin in garlic [95], carnosic acid in rosemary [96, 97], betulinic acid in almond hull [98], humulone in beer hop [99], and resveratrol in grapes [100, 101] were reported to inhibit angiogenesis by targeting the cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) pathways. Inhibition of the COX-2 pathway, for example, was shown to reduce prostaglandin E2(PGE2) production [102], which may suppress vascular endothelial growth factor (VEGF) expression [103, 104], a key mediator of in vitro angiogenesis [105, 106].
Large-scale world research is focused on the search for new properties of already known natural compounds of plant origin, in order to improve health and the quality and length of life. As a result, they provide knowledge about the most effective substances. The most attractive are those that exhibit pleiotropic activity and those that can also be used against cancer cells [107–109]. The resistance of tumor cells to factors that could be completely eliminated from the organism led to the use of two strategies to fight them. The first is to reduce cell division. The second one, which may be carried out in conjunction with the first, is the induction of cell death. Until now in both cases, the most commonly used were synthetic drugs, so-called antiinfective drugs, and radiotherapy treatment. However, the effectiveness of these treatments is variable and depends on many factors, primarily on the type of cancer and its stage of development. Therefore, substances of plant origin are very important, because they support treatment and stop the development of cancer, protecting healthy cells against malignant transformation [110, 111].
Plants as a source of antiangiogenic drugs: experimental studies
Some plant metabolites are compounds that inhibit angiogenesis. Substances showing anticancer properties can be found in the plants used in herbal medicine as well as in those that are included in our diet. In the search for antiangiogenic drugs, a number of bioactive plant substances and dietary products have been tested for their antiangiogenic potential [77, 112–114]. The evaluation of the performance of natural anticancer compounds and other substances potentially having antiangiogenic properties is done with the use of the chorioallantoic membrane (CAM) test on the chorioallantoic membrane of chicken embryos [115, 116].
In the antiangiogenic therapy, many compounds also found in natural products such as fruits, vegetables, spices, and green tea were used. Many compounds present in natural products such as fruits, vegetables, spices, and green tea are also being used in the antiangiogenic therapy. Medicinal substances are present in the whole plants or in their particular organs (stems, leaves, roots, seeds). The most common plants in discussed area are Selaginella tamariscina Beauv [117], Gleditsia sinensis [118], Acer tegmentosum [119], Viscum album [110], Strychnos nux-vomica [68], Apium graveolens [120–122], Rosmarinus officinalis [123, 124], Brucea javanica [66], and Hypericum perforatum [125]. These and many other plants contain substances of a therapeutic use, including polyphenols and their derivatives (which are the most important group), anthracycline antibiotics and their quinone analogs, alkaloids, and other metabolites of the integrated nitrogen atom in the molecule, terpenoids, polysaccharides, polyamines, and cytokinins [78, 87, 110, 126–129]. Table 2 shows a list of medicinal plants with the bioactive substances extracted from them that directly inhibit angiogenesis. The advantages of antiangiogenic substances found in natural products include the following (see Fig. 1):
-
General availability, as many of these substances are present in our daily diet
-
The low price of products compared to other drugs
-
The fact that they are effectively absorbed from the gastrointestinal tract
-
They long-lasting effect, which can be used in long-term prevention and treatment
-
They rarely have side effects
Studies have shown that antiangiogenic therapy was more effective in combining the inhibitors of angiogenesis with radiotherapy or chemotherapy [79, 130]. Due to a large number of substances used in the treatment and prevention of cancers, the article describes only selected substances of the above-described groups of compounds.
Polyphenolic compounds
Polyphenolic compounds are a group of plant secondary metabolites synthesized mainly from phenylalanine or tyrosine. In chemical terms, polyphenols are compounds having at least one aromatic ring substituted with one or more hydroxyl groups. Depending on the number of hydroxy aromatic rings and the type of functional groups of the phenolic compounds of the phenolic origin, we distinguish acids, polyphenols, and monophenols. Large numbers of derivatives of phenolic compounds are the simple polyphenols, phenylpropanoids, benzoic acid derivatives, flavonoids, stilbenes, and polymers of phenolic compounds such as tanning agents (proanthocyanidins) or lignans [131, 132]. The human diet consists of a mixture of plant polyphenols. It is estimated that the daily intake of plant polyphenols is approximately 1 g [110].
In terms of biology, polyphenolic compounds are strong antioxidants. They prevent dysfunctions of the organism resulting from an inefficient operation of the antioxidant mechanism or excessive production of oxidants, preventing the development of diseases known as civilization diseases (cancer, coronary heart disease, stroke, diabetes). Recent research confirms that the polyphenols show cytotoxic activity and inhibit tumor growth, metastasis, and angiogenesis [131].
Curcumin (Fig. 2a) is a polyphenol built of ferulic acid residues. It is one of the best-known compounds exhibiting angiogenesis inhibitory activity. It is the yellow pigment extracted from the rhizome of Curcuma longa L., characterized by a potent pleiotropic activity [133]. Curcumin is involved in the regulation of many cellular processes, such as keeping the redox balance in nerve cells. In these cells for example, curcumin limits the synthesis of β-amyloid, a key factor for the development of Alzheimer’s disease, which is synthesized during oxidative stress. In addition, curcumin contributes to the reduction of the activity of transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and thus other proteins, including COX-2 and IL-8, and growth factor kinases, including kinases, type MAP, and JAK [134], are involved in the inflammatory reactions [135]. The pleiotropic effect of curcumin is also implicated in the inhibition of hepatic metastasis of tumor cells (CBO140C12) and rat hepatoma (AH109A).
In breast cancer cells (MDA-MB 231), it affects the functioning of estrogen receptors, among others, by reducing the activity of NF-κB/AP-1 factors dependent on metalloproteinases of the type MMP-1 and MMP-2 [131]. Moreover, analogs of curcumin, which is BDMC-A (dimethoxy curcumin) [136], stopping the cell cycle at the G2/M results in inhibition of proliferation of breast cancer cells (MCF-7), with simultaneous initiation of the internal and external apoptotic pathway by inhibiting the activity of B cell lymphoma 2 (Bcl-2) and activation of proteins: p53, Bcl-2-associated X protein (Bax), cytochrome c, apoptotic protease-activating factor (Apaf-1), FasL, and caspases 8, 9, and 3 (Fig. 3) [137]. Additionally, the metabolism products of curcumin, which are ferulic acid and vanillin, also exhibit cytotoxic properties towards ECs [78]. Ferulic acid, by removing free radicals, exhibits chemopreventive activity towards the healthy cells [138], while vanillin is involved in the repair of mutated deoxyribonucleic acid (DNA) of the cancer cells, including colon cancer cells (HT-29) [139], and also reducing the metastasis through angiogenesis of these cells [140].
Epigallocatechin (Fig.2b) is the main polyphenol found in tea leaves along with (−)-epigallocatechin gallate (the main component of green tea), (−)-gallate, (−)-epicatechin gallate, and (−)-epicatechin [141]. These polyphenols have the ability to inhibit the growth of lung cancer cell line PC-9 with high efficiency, and the most effective is the (−)-epicatechin gallate. In addition, the polyphenols activate processes leading to apoptosis in several types of cancer, such as prostate cancer, colon cancer, and lung cancer and inhibit angiogenesis. Epicatechin gallate induces apoptosis in tumor cells by inhibiting the activity of TNF-α [110]. It also contributes to stopping the cell cycle in G0–G1 phase of the cells of Burkitt’s lymphoma, initiating subsequently the classic signs of apoptosis such as DNA fragmentation, visible as a ladder, activation of caspases 9 and 3, inhibition of the activity of antiapoptotic proteins Bcl-2 and Mcl-1, mitochondrial membrane permeability, and the release of pro-apoptotic proteins (including cytochrome C, Smac/DIABLO, and AIF) from the mitochondria into the cytosol and the generation of reactive oxygen species [141].
Carnosic acid (CA) and carnosol (CS) are phenolic compounds existing in fresh and dried leaves of Rosmarinus officinalis L. It is believed that both compounds are responsible for the antioxidant, antiinflammatory, and cytotoxic properties of the raw material and extract [142–144]. CA (Fig.2c) is the major phenolic diterpene showing antioxidant, antibacterial, and antiobesity activity, inhibiting platelet aggregation and having antiangiogenic properties as well as showing the antitumor effect of P-glycoprotein [96, 145–147]. Figure 4 shows anticancer molecular mechanisms of CA.
CS inhibits induced expression of cytokines and adhesion molecules and adhesion of monocytes to ECs by a mechanism which involves NF-κB. The latter mechanism can be associated with antiinflammatory properties of the compound [148]. CA can be subjected to oxidative degradation and rearrangement of the cascade, giving other antioxidant compounds of the rosemary like CS (Fig.2d) (namely, rosmanol, galdosol, and rosmariquinone).
The biological activity of CS is similar to the activity of CA [149]. CA is able to inhibit certain functions of ECs (differentiation, proliferation, and migration and has proteolytic possibilities). Literature data indicates that the growth inhibitory effect exerted on the endothelial cell and tumor proliferation may be due to, at least in part, induction of apoptosis. Inhibition of the mentioned production steps of the in vitro angiogenesis was confirmed by the observed inhibition of angiogenesis in vivo confirmed by a chicken chorioallantoic membrane test. Antiangiogenic activity of CS and CA may help to prevent cancer, and the antimetastatic effects of rosemary extract suggest their potential in the treatment of other cancers related to angiogenesis [150].
Hyperforin and hyperforin derivatives of phloroglucin of acyl phloroglucinol
Numerous studies have confirmed that these substances inhibit the growth of cancer cells. Both hyperforin and adhyperforin are extracted from the herb Hypericum perforatum L. Hyperforin has been proposed as an innovative anticancer drug that induces apoptosis in cancer cells and inhibits angiogenesis in vivo [151, 152]. The inhibitory effect of hyperforin in neovascularization of experimental mouse tumor model was demonstrated by Dona et al. [153]. Hyperforin inhibits angiogenesis in vivo and is capable of inhibiting several key stages of angiogenesis in vitro, including endothelial cell proliferation, differentiation, and invasion, as well as the extracellular matrix degradation by MMP-2 and urokinase [154]. Therefore, the hyperforin seems to be a promising antiangiogenic compound. Determination of the molecular mechanisms related to its antiangiogenic effect is the next step in the evaluation of preclinical and clinical trials (Fig. 2e).
Resveratrol (3,5,4′-trihydroxystilbene) is a derivative of stilbene phytoalexin. First, it is isolated from the roots of veratrum (Veratrum grandilorum) [155]. Currently, it is known that resveratrol (Fig.2f) is present in fruit, grape leaves, berries, and peanuts [156]. It is also the active substance of wine. Resveratrol has proven to have antitumor and chemopreventive properties. It affects each phase of carcinogenesis (i.e., on the initiation, promotion, and progression of the process) by modulating the signal transduction pathways in cells that control cell growth and division, apoptosis, inflammation, angiogenesis, and metastasis. It is believed that this is one of the best factors that can be used in tumor therapy, in many types of cancer (breast, prostate, stomach, colon, pancreas) [100]. It has been shown that resveratrol in breast cancer cells (MCF-7, MDA-MB-231) and murine skin inhibits the cell cycle and induces apoptosis through the mitochondrial pathway releasing cytochrome c, Apaf-1, and activating the caspase and PARP [157, 158]. It also maintains the expression of p53 protein, which reduced activity, is one of the causes of carcinogenesis [159]. Antiangiogenic activity of resveratrol stems from its ability to inhibit the division of human umbilical EC (HUVEC) and reduction of the lytic activity of MMP-2 [160]. Resveratrol inhibits VEGF-induced angiogenesis by disruption of reactive oxygen species-dependent src kinase activation and subsequent VE-cadherin tyrosine phosphorylation [161, 162]. Resveratrol given to rats inhibited the growth of glioma. By inhibiting angiogenesis [163, 164], RSV inhibited HIF-1α accumulation and VEGF secretion induced by cobalt chloride (CoCl2) through SIRT1 in human retinal pigment epithelial (hRPE) cells. Furthermore, resveratrol downregulated VEGFR2 phosphorylation and activation induced by VEGF in ECs via SIRT1. Thus, the inhibitory effect of RSV on the HIF-1α/VEGF/VEGFR2 signaling axis is mediated, at least in part, through SIRT1. The results suggest that targeting SIRT1 could have therapeutic potential for the treatment of CNV (Fig.5) [165]. However, long-term intake of resveratrol-enriched wine products can produce side effects due to the alcohol content. Therefore, there must be a precaution not to encourage people to consume a large quantity of wine rather than a reasonable amount of red wine (two to three glasses per day). Other food products and non-alcoholic drinks could be considered to be alternative and optional resveratrol sources.
Isoflavones are the chemical compounds structurally similar to steroid hormones though they are not derived from cholesterol [166]. The most known isoflavones are quercetin, luteolin, apigenin, kaempferol, and genistein. A large group of these compounds exerts different effects on the human body, showing antioxidant, cardioprotective, and anticancer activity. By blocking proteasome activity, they induce apoptosis in Jurkat T-leukemia cells. Yang et al. [167] have shown that the activity of isoflavones in relation to proteasome is as follows: apigenin ≥ luteolin > quercetin > kaempferol (apigenin acts the strongest). The differential activities of these compounds are due to the chemical structure of molecules in which hydrogen fourth (C4) in the ring C has the highest activity against substrates. In addition, computer modeling has shown that the removal of the hydroxyl group at C3 also extends inhibition of the activity of the proteasome, while inducing apoptosis and inhibiting angiogenesis [168]. This is done by the interaction of specific processes responsible for regulation of apoptosis, such as the release of cytochrome c from mitochondria, activation of caspases 9 and 3, the stimulation of the synthesis of caspase-8 and tBid protein, and the reduction of the expression of antiapoptotic proteins of the Bcl-2 family, such as Bcl-X(L), stimulation of the expression of proteins Bax and Bak as well as interaction with a nuclear factor NF-κB (Fig.6) [169].
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a flavone found in onions, raspberries, red grapes, apples, cherries, broccoli, and leafy greens. Specifically, flavonoids and chalcones regulate expression of VEGF, MMPs, and EGFR and inhibit NF-κB, PI3-K/Akt, and ERK1/2 signaling pathways, thereby causing strong antiangiogenic effects. It has been reported that quercetin (Fig. 2g) showed significant antiangiogenic potential by inhibiting angiogenesis through multiple mechanisms. These include interaction with the COX-2 and LOX-5 enzymes, the EGF receptor, the HER-2 intracellular signaling pathway, and the NF-κB nuclear transcription protein [170–173]. Quercetin may enhance the anticancer effects of tamoxifen through antiangiogenesis [174, 175].
Genistein is an isoflavone isolated from soybean seeds (Glycine maxima L). When combined with cisplatin, it significantly decreases the proliferation of three BxPC cells of pancreatic cancer and their induced apoptosis. The reduction of tumor growth was also observed in vivo in a mouse xenograft model of the BxPC-3 cell [176]. Furthermore, tumor regression was observed in C57B16 mice with Lewis lung carcinoma cells [177]. It is worth mentioning that the pharmacotherapy with the use of genistein can be carried out in conjunction with radiation therapy. Depending on the endogenous estrogen concentrations and the target tissue, genistein and daidzein may interfere with ER signaling. Furthermore, several other ER-independent mechanisms of action, such as the inhibition of steroidogenic enzyme activities, modulation of growth factor action, downregulation of tyrosine and other protein kinases, and the inhibition of angiogenesis, have been suggested for isoflavones on the basis of experiments with animal and cellular models [178]. However, the physiologic relevance of these effects in humans is unclear. Delphinidin is a specific flavonoid present in the skin of Solanum melongena showing cytotoxic activity against human tumor cells. It has been proved that the compound has inhibitory activity against the MMPs, degradation of extracellular matrix during the invasion of tumor cells [179].
Secondary metabolites with integrated nitrogen
Plant secondary metabolites, the nitrogen atom in the molecule, include alkaloids, betalains, cyanogenic glycosides, and glucosinolates (a glucosinolate) [180]. Over 12,000 different alkaloids are known, which are mainly in the seeds, roots, leaves, and bark of plants. Most of the plant species containing alkaloids belong to the families Ranunculaceae, Solanaceae, and poppy. Examples of plants that contain alkaloids include barberry, coltsfoot, henbane, periwinkle pink, pomegranate bark, seeds, coffee, tea leaves, and cocoa [89].
Vinblastine and vincristine (Fig. 2h, i) obtained from Catharanthus roseus show properties valuable in anticancer therapy. Such compounds block the formation of microtubule and karyokinetic spindles, resulting in the inhibition of mitosis at metaphase, cell death, and inhibiting angiogenesis. In addition, they affect the microtubule involved in chemotaxis and migration of organelles and vesicles of the secretory cells and outside. They also affect the structural integrity of some cells [181]. Therefore, vinblastine and vincristine are used to treat leukemia, lymphoma, breast cancer, testicular cancer, lung cancer, and Kaposi’s sarcoma [182]. Different vinca alkaloids have their own unique properties. Vinblastine inhibits angiogenesis or the process by which new blood vessels grow from preexisting ones. Vinblastine is most often applied to treat Hodgkin’s disease, non-Hodgkin’s lymphoma, breast cancer, and germ cell tumors. Side effects of vinblastine include toxicity to white blood cells, nausea, vomiting, constipation, dyspnea, chest or tumor pain, wheezing, and fever. Vinblastine is also occasionally associated with antidiuretic hormone secretion and angina. An antiangiogenic effect of vinblastine was then plainly shown by Vacca et al. [183], who studied multiple events in HUVECs related to angiogenesis in vitro. They also reported the relation dose–response antiangiogenic activity by vinblastine in the in vivo embryonic chick CAM assay. Subsequently, numerous reports have demonstrated marked tumor regression in various mouse models following low doses of vinblastine in combination with VEGF-A-targeting drugs [184–186]. The concept of the antiangiogenic scheduling of chemotherapeutics undoubtedly holds great promise, and numerous studies are presently underway using the metronomic concept [187].
Vincristine’s inhibition of microtubule formation is especially powerful. The reason besides this is that the tubulin protein is dynamic. Its long chain of building blocks is always growing in some places and breaking in others. The less contiguous parts of a tubulin molecule have pieces only two building blocks long, called dimers. Vincristine has a high affinity for tubulin dimers, and the reaction between vincristine and the dimers is rapidly reversible [188]. That means a vincristine molecule will attach to a dimer at one site, break off, and then reattach at another site. This keeps two sites per dimer “poisoned” and unable to reassemble into the protein. So, vincristine’s ability to destabilize tubulin is especially good [189, 190]. Angiogenesis is critical for cancer growth and progression. In the study by Pasquieret et al. [191], the investigators found that treatment with vincristine or in combination with β-blockers significantly inhibited vascular structures by established EC lines (BMH29L cells). However, one major point on vincristine and β-blockers’ mechanism of action is their specificity to cancer-derived ECs. It is well established that cancer-derived ECs show increased proliferation, motility, pro-angiogenesis properties, and resistance to drug treatment compared with “normal” ECs (e.g., HUVECs). For example, human breast cancer-derived ECs and human hepatocellular carcinoma tumor-derived ECs presented increased resistance to vincristine and angiogenesis inhibitors [192, 193].
Cyanogenic glycoside’s induction is a form of protection of plants against herbivores. Furthermore, many compounds such as amygdalin, prunasin, or linamarin show antineoplastic activities [194]. The aim of the study was to evaluate the anticancer properties by inhibiting angiogenesis of amygdalin (medicine under the name “laetrile” or vitamin B17), which occurs, e.g., in the seeds of peaches, apricots, and almonds. Pro-apoptotic effect of amygdalin has been found in prostate cell lines DU145 and LNCaP [195]. Although these compounds are not very effective, they are still used mainly as an alternative or adjunct to the antiangiogenic therapy. Furthermore, the metabolism of cyanogenic glycosides proceeds with a production of poisonous cyanide (HCN), of which accumulation in the body may lead to severe poisoning; therefore, dosages of these compounds should be determined individually for each patient [178, 196].
Glucosinolates are found in plants of the cabbage family, such as cabbage, broccoli, and cauliflower (Brassicaceae Burnett., Cruciferae Juss.). The best-studied glucosinolates occurring in cruciferous plants are synigryna, gluconapin, glucoiberin, and glucoraphanin [180]. Glucosinolate molecule consists of three parts: the glucose in the form of the β-d-tioglucan, sulfuric acid oxime group, and side chain made up of amino acid residues [197]. Glucosinolates and their metabolic products, isothiocyanates (sulforaphane), and indoles (indole-3-carbinol) have antiangiogenic properties [198]. In the anticancer therapy, their function is to activate the enzymes in xenobiotic detox, which are mentioned as potential carcinogens. It has been observed that they activate glutathione transferase in colon cancer cells (HT-29) and liver cancer cells (HepG2) [199, 200]. By reducing the activity of cell cycle proteins, such as cyclin B1 or kinase Cdc25C, they lead to stopping of the cell cycle in G2/M [197].
Polyamines are a group of compounds with a simple chemical structure that includes structure amine groups. Due to the fact that they present typical properties of the hormones, they are included in the group of plant phytohormones. Among these compounds, there is putrescine (1,4-diaminobutane), which is a precursor to other polyamines such as spermine and spermidine [201, 202]. The immediate precursor of polyamines is the amino acid, arginine. Among the polyamines, putrescine arouses the greatest attention. In normal growing cells, it contributes to the regulation of cell cycle proliferation by controlling the transitions between different phases of the cycle and in the correct course of programmed cell death and angiogenesis. Utrescine also affects the limitation of the migration and the spreading of tumor cells. The factor inverting this situation is the compound difluoro-methyl ornithine/eflornithine (α-DFMO) which lowers the level of putrescine and stops the cell cycle of tumor cells in the G1 phase [202, 203]. Moreover, by limiting the availability of nutrients, α-DFMO inhibits angiogenesis in human gastric cancer cells and melanoma cells, leading to the death of these cells [204].
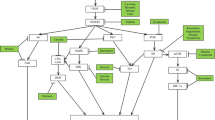
Cytokinins (CKs) are plant hormones that control the process of apoptosis of cancer [205]. In this regard, the strongest activity shows cytokinin ribosides and benzyl aminopurine (BAP). BAP has the ability to inhibit certain human protein kinases including CDK, which may limit cell proliferation, angiogenesis, and activation of apoptosis, which is one of the main goals of tumor therapy. Other cytokines have similar properties but must be used at higher concentrations [126]. Kinetin and isopentenyl adenine (IPA) and benzyl adenine (BA) effectively inhibit cell growth and induce the reduction of MTT and morphological changes in the mature granulocytes of myeloid leukemia [206], whereas cytokinin ribosides effectively inhibit growth and induce apoptosis, limiting the content of cellular ATP [207]. CKs play a crucial role in many physiological processes in plants. One of the interesting functions of CKs is the control of programmed cell death (PCD). It seems that all CK-dependent phenomena including PCD are accompanied by a special multistep phosphorelay signaling pathway. This pathway consists of three elements: histidine kinase receptors, histidine phosphotransfer proteins, and response regulators. A recent review by Kunikowska et al. (2013) shows the highlights of the latest knowledge about CK signaling pathways in many physiological processes in plants, with special attention paid to PCD process [208]. Other natural products that possess antiangiogenic activities recently described in the literature are summarized in Table 3. Besides, a general diagram binding therapeutic targets in angiogenesis and natural products is presented in Fig.7.
Plants as a source of antiangiogenic drugs: clinical trials
Several clinical trials were conducted to evaluate the effect of natural products against tumor angiogenesis. Most of these studies were directed by the pharmaceutical companies OXiGEN and Novartis on combretastatin and vadimezan (ASA 404), respectively.
Combretastastin (CA4-P)
CA4-P acts as antiangiogenic by destabilizing microtubules. Encouraging results in vivo models lead to preliminary clinical trials on CA4-P and its derivatives. CA4-P has been tested alone or in combination with targeted therapy or chemotherapy in patients with advanced cancer in a phase I trial. Many clinical trials studied the combination of CA4-P with other antiangiogenic drugs such as bevacizumab and endostar. It has been reported that when bevacizumab was administered 4 h after CA4-P, agio-permeability and tumor perfusion were statistically and significantly reduced, but these parameters were reversed after administration of CA4-P alone. These results demonstrated that bevacizumab sustained significant vascular modifications induced by CA4-P [222].
Recently, OXiGEN announced the results of the clinical trial Gynecologic Oncology Group protocol 186I (GOG 186I). A randomized phase II study investigated the combination of Avastin® (bevacizumab) with ZYBRESTAT® (CA4P) in recurrent ovarian cancer. GOG 186I showed a statistically significant increase in progression-free survival compared to bevacizumab alone [223]. Higher response rates were observed in relapsed patients with platinum-resistant ovarian cancer treated with standard chemotherapy (carboplatin and paclitaxel) and CA4-P as compared to patients treated with chemotherapy alone [224]. Interestingly, CA-4P revealed therapeutic potential in patients with metastatic anaplastic thyroid cancer [225]. Clinical trials of CA-4P in patients with neovascular age-related macular degeneration showed potential interest and efficacy. However, future clinical use is unlikely due to related adverse side effects observed during this investigation [226]. Other CA4-P clinical trials are listed in Table 4 and Fig. 8 [227]. Recruitment of patients for combretastatin-based clinical trials has evolved from patients in advanced stages of chemotherapy-resistant carcinomas, to phase III clinical trials, thus exhibiting clinical trust in combretastatin-based protocols.
Vadimezan (ASA 404, DMXAA)
ASA 400 is a flavonoid derivative discovered at the Auckland Cancer Society Research Centre at the University of Auckland in New Zealand targeting stimulator of interferon genes (STING) pathway. In 2007, Novartis obtained the worldwide rights for it [228]. ASA 404 had been evaluated in combination with chemotherapy in two phase II trials for advanced non-small cell lung cancer (NSCLC) and showed survival extensions of around 5 months when compared to chemotherapy alone [229]. A phase III trial started in 2008 and showed in March 2010 poor results as a first-line therapy for NSCLC [230]. Temporary poor results in another randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without ASA 404 as the second-line therapy for NSCLC were obtained in 2011 [231]. Two parallel phase I clinical trials were conducted in the UK and New Zealand by Cancer Research UK, finding that ASA404 has anticancer effect at well-tolerated doses. A third phase I trial was designed for patients with recurrent tumors establishing the optimal dose for a phase II trial [232].
The largest two randomized double-blind placebo-controlled phase III trials, ATTRACT-1 and ATTRACT-2, were conducted by Novartis in advanced NSCLC using antiangiogenics. ATTRACT-2 assessed ASA404 in association with docetaxel and carboplatin as first-line therapy in patients with stage IIIB/IV NSCLC, whereas ATTRACT-1 evaluated the same drug associations as the second-line treatment for patients with advanced NSCLC. Both trials were unsuccessful to show survival advantages or significant amelioration [233]. Other clinical trials on ASA 404 are listed in Table 5.
Despite the interesting results at the preclinical stage, ASA 404 failed in human clinical trials. Recent studies have investigated the reason for the inefficacy. ASA 404 was shown to target the STING pathway. However, this activity is mouse specific; it has no effect on human STING target [234].
Other drugs
The toxicity and the anticancer effect of MPC-6827 (verubulin, Azixa®) as a single drug were investigated in patients with advanced cancer. It has also recently been assessed in phase II bevacizumab recurrent glioblastomas [235]. The clinical trial with BCN105P has recently been published [236]. The main objective of this trial was to evaluate the safety, toxicity, pharmacokinetic, and pharmacodynamic effects of this drug. The results from this clinical trial show a favorable toxicity profile of this drug at the advisable dose level and has guided to more evaluation in phase II trials. Importantly, when using dynamic MRI at a higher concentration, significant modifications in vascular ultrastructure were observed in some patients.
CYT997 is the latest inhibitor of the microtubule assembly and antiangiogenic drug [237]. A phase I accelerated dose-escalation study examined the safety, tolerability, pharmacokinetics, and vascular-disrupting effects of orally administered CYT997. Preliminary evidence of significant efficacy was established [238]. Finally, it is required to start other clinical trials on natural products targeting angiogenesis alone (or in combination with chemoradiotherapy) and to carefully investigate the toxicity and the mechanisms of action of these combinations.
Conclusion
Currently in clinical trials, there are many substances that inhibit angiogenesis, acting at the different stages of the process. Mechanisms of these actions are not yet fully known. However, antiangiogenic therapy carries some risks. It can cause systemic, generalized angio-suppression leading to dysfunction of female reproduction, wound healing, and merging fractures, and the therapy targeted on receptors for VEGF may lead to the development of diabetes. Therefore, we must remember the so-called therapeutic angiogenesis, which involves not only the inhibition of the formation of new blood vessels but also stimulating the formation of vessels in diseases characterized by their deficiency (pro-angiogenic therapy). However, nowadays, antiangiogenic therapy is more often used in the treatment.
Thus, results from plant-derived antiangiogenesis agents suggest that, unlike conventional chemotherapeutic agents generally employed at high doses for relatively short time periods, these angiogenic inhibitors may be best employed using less cytotoxic doses over months to years as a potential means to prevent dormant micrometastases from entering a rapid growth phase. The additional clinical testing of newly identified angiogenic inhibitors using a variety of delivery strategies is eagerly awaited. For millennia, the natural products have been used by man as a remedy in traditional medicine. Recent research has shown that such natural substances are able to prevent the development of many cancers. The discovery and characterization of new molecules will expand the drug library of therapeutic molecules, which must grow due to the emergence of new diseases and the emergence of resistance against conventional treatments.
Abbreviations
- AFGF:
-
Acidic fibroblast growth factor
- AP-1:
-
Activator protein 1
- AIF:
-
Apoptosis-inducing factor
- Akt:
-
Ak strain thymoma
- α-DFMO:
-
α-Difluoromethylornithine
- Ang:
-
Angiogenin
- ANGPT:
-
Angiopoietin
- APAF:
-
Apoptotic protease-activating factor
- ATF-4:
-
Activating transcription factor 4
- BAP:
-
6-Benzylaminopurine
- Bax:
-
Bcl-2-associated X protein
- Bcl-2:
-
B cell lymphoma 2
- bFGF:
-
Basic fibroblast growth factor
- CAM:
-
Chorioallantoic membrane
- CD151:
-
Cluster of differentiation 151
- Cdc42:
-
Cell division control protein 42 homolog
- CDK:
-
Cyclin-dependent kinase
- clAP:
-
Calf-intestinal alkaline phosphatase
- COX-2:
-
Cyclooxygenase-2
- CYP4F3:
-
Leukotriene-B(4) omega-hydroxylase 2
- Diablo:
-
Direct IAP-binding protein with low pI
- DLL4:
-
Delta-like ligand 4
- DMXAA:
-
Dimethyl xanthenyl acetic acid
- DNA:
-
Deoxyribonucleic acid
- E2(PGE2):
-
Prostaglandin E2
- ECs:
-
Endothelial cells
- EGF:
-
Epidermal growth factor
- EGFL7:
-
EGF-like domain-containing protein 7
- ERK:
-
Extracellular signal-regulated kinases
- FasL:
-
Fas ligand
- FGF:
-
Fibroblast growth factor
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- GSH:
-
Glutathione
- ROS:
-
Reactive oxygen species
- HER2:
-
Human epidermal receptor 2
- HGF:
-
Hepatocyte growth factor
- HIF:
-
Hypoxia-inducible factor
- IL-8:
-
Interleukin-8
- JAK:
-
Janus kinase
- JNK:
-
c-Jun N-terminal kinases
- LOX:
-
Lipoxygenase
- MAP:
-
Mitogen-activated protein
- MCP-1:
-
Monocyte chemoattractant protein 1
- MRI:
-
Magnetic resonance imaging
- mTOR:
-
Mammalian target of rapamycin
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NRPs:
-
Neuropilins
- pAK:
-
p21 activated kinase
- PARP:
-
Poly ADP ribose polymerase
- PDGF:
-
Platelet-derived growth factor
- PEDF:
-
Pigment epithelium-derived factor
- PERK:
-
PKR-like endoplasmic reticulum kinase
- PI3K:
-
Phosphatidylinositol-3-kinase
- PIGF:
-
Phosphatidylinositol-glycan biosynthesis class F protein
- PLGF:
-
Placental growth factor
- PTN:
-
Pleiotrophin
- Sema3:
-
Semaphorin-3
- SLC7A11:
-
Sodium-independent glutamate transporter
- GCLC:
-
Glutamate-cysteine ligase catalytic subunit
- NRF-2:
-
Nuclear factor (erythroid-derived 2)-like 2
- Smac:
-
Second mitochondria-derived activator of caspases
- STING:
-
Stimulator of interferon genes
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor-α
- TSP-1:
-
Thrombospondin-1
- uPA:
-
Urokinase-type plasminogen activator
- VEGF:
-
Vascular endothelial growth factor
References
Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet. 2014;383:549–57.
Ferlay J et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014;136:359–86.
Siegel RL, Miller KD, Jemal A. Cancer statistics. 2015. CA Cancer J Clin. 2015;65:5–29.
Davis EL. Oh B, Butow PN, Mullan BA, Clarke S. Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: a systematic review. Oncologist. 2012;17:1475–81.
Harvey AL, Edrada-Ebel RA, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nature Rev. Drug Discov. 2015;14(2):111–29.
Wang CZ, He H, Wang X, Yuan CS. Trends in scientific publications of Chinese medicine. Am J Chin Med. 2012;40:1099–108.
Bell RMA. Review of complementary and alternative medicine practices among cancer survivors. Clin J Oncol Nurs. 2010;14:365–70.
Hait WN, Hambley TW. Targeted cancer therapeutics. Cancer Res. 2009;69:1263–7.
Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–43.
Lenzi P, Bocci G, Natale G. John Hunter and the origin of the term “angiogenesis”. Angiogenesis. 2016; 1–2.
Ribatti D. Judah Folkman, a pioneer in the study of angiogenesis. Angiogenesis. 2008;11(1):3–10.
Distler JW, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47:149–61.
Kinja K, Rohit S, Mandloi A, Sharma I, Savita S. Anti-angiogenic therapy—past, present and future. Rec Res Sci Tech. 2001;3:8–15.
Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett. 2012;320:130–7.
Zielonka TM. Angiogeneza – Część I. Mechanizm powstawania nowych naczyń krwionośnych. Alerg Astma Immun. 2003;8:169–74.
King A, Balaji S, Keswani SG, Crombleholme TM. The role of stem cells in wound angiogenesis. Adv Wound Care (New Rochelle). 2014;3:614–25.
Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Kąt N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–13.
Kurzyk A. Angiogenesis—the possibilities, problems and prospects. Progress Biochemistry. 2015;61(1):25–34 [in Polish].
Watt SM, Athanassopoulos A, Harris AL. Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J R Soc Interface. 2010;7(6):731–51.
Wiśniewski T, Makarewicz R, Ziółkowska E, Rystok D, Zekannowska E. Angiogeneza nowotworowa – mechanizmy, czynniki regulujące, leki. Onkologia Info. 2009;6(5):172–8.
Namiecińska M, Marciniak K, Nowak JZ. VEGF jako czynnik angiogenny, neurotroficzny i neuroprotekcyjny. Postepy Hig Med Dośw. 2005;59:573–83.
Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Rev Drug Discov. 2007;6:273–86.
Siemerink MJ, Augustin AJ, Schlingemann RO. Mechanisms of ocular angiogenesis and its molecular mediators. Dev Ophthalmol. 2010;46:4–20.
Xu WH. Large artery: an important target for cerebral small vessel diseases. Ann Transl Med. 2014;2:78.
Shahneh FZ, Baradaran B, Zamani F, Aghebati-Maleki L. Tumor angiogenesis and anti-angiogenic therapies. Hum Antibodies. 2013;22:15–9.
Dorrell MI, Aguilar E, Scheppke L, Barnett FH, Friedlander M. Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc Natl Acad Sci. 2007;104:967–72.
Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–94.
Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–88.
Herbert SP, Stainier DY. Molecular control of endothelial cell behavior during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–64.
Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–53.
Ferrara N, Gerber HP. The biology of VEGF and its receptors. J Nat Med. 2003;9:669–76.
Kloc M, Kubiak JZ, Li XC, Ghobrial RM. Pericytes, microvasular dysfunction, and chronic rejection. Transplantation. 2015;99(4):658–67.
Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10.
Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29(5):196–202.
Zhang J, Li Y. Therapeutic uses of FGFs. Semin Cell Dev Biol. 2015:S1084–9521 (15)00166-4.
Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12(2):125–37.
Kappou D, Sifakis S, Konstantinidou A, Papantoniou N, Spandidos DA. Role of the angiopoietin/Tie system in pregnancy (review). Exp Ther Med. 2015;9(4):1091–6.
Khan KA, Bicknell R. Anti-angiogenic alternatives to VEGF blockade. Clin Exp Metastasis. 2016;33:197–210.
Holderfield MT, Hughes CCW. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102:637–52.
Roedersheimer M et al. A bone-derived mixture of TGFβ-superfamily members forms a more mature vascular network than bFGF or TGF-β2 in vivo. Angiogenesis. 2006;8(4):327–38.
Ucuzian AA, Gassman AA, East AT, Greisler HP. Molecular mediators of angiogenesis. J Burn Care Res. 2010;31(1):158.
Umikawa M, Umikawa A, Asato T, Takei K, Matsuzaki G, Kariya K, Zhang CC. Angiopoietin-like protein 2 induces proinflammatory responses in peritoneal cells. Biochem Biophys Res Commun. 2015;467(2):235–41.
Wagner M et al. Inflamed tumor-associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis. Angiogenesis. 2012;15(3):481–95.
Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16(2):196–208.
Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21(20):2511–24.
Sainson RCA, Harris AL. Regulation of angiogenesis by homotypic and heterotypic notch signalling in endothelial cells and pericytes: from basic research to potential therapies. Angiogenesis. 2008;11(1):41–51.
Gorantla B et al. Notch signaling regulates tumor-induced angiogenesis in SPARC-overexpressed neuroblastoma. Angiogenesis. 2013;16(1):85–100.
Serini G, Maione F, Bussolino F. Semaphorins and tumor angiogenesis. Angiogenesis. 2009;12(2):187–93.
Zhou H et al. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis. 2012;15(3):391–407.
Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11(1):31–9.
Staton CA. Class 3 semaphorins and their receptors in physiological and pathological angiogenesis. Biochem Soc Trans. 2011;39:1565–70.
Leszczynska K, Kaur S, Wilson E, Bicknell R, Heath VL. The role of RhoJ in endothelial cell biology and angiogenesis. Biochem Soc Trans. 2011;39:1606–11.
Bailey RL, Herbert JM, Khan K, Heath VL, Bicknell R, Tomlinson MG. The emerging role of tetraspanin microdomains. Biochem Soc Trans. 2011;39(6):1667–73. doi:10.1042/BST20110745.
Egginton S. In vivo shear stress response. Biochem Soc Trans. 2011;39:1633–8.
Zhuang X, Cross D, Heath VL, Bicknell R. Shear stress, tip cells and regulators of endothelial migration. Biochem Soc Trans. 2011;39:1571–5.
Francis ME, Uriel S, Brey EM. Endothelial cell-matrix interactions in neovascularization. Tissue Eng Part B Rev. 2008;14(1):19–32.
Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–23.
Laurenzana A, Fibbi G, Margheri F, Biagioni A, Luciani C, Del Rosso M, Chillà A. Endothelial progenitor cells in sprouting angiogenesis: proteases pave the way. Curr Mol Med. 2015;15(7):606–20.
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–77.
Makanya AN, Hlushchuk R, Djonov VG. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis. 2009;12:113–23.
Gianni-Barrera R et al. VEGF over-expression in skeletal muscle induces angiogenesis by intussusception rather than sprouting. Angiogenesis. 2013;16(1):123–36.
VanHinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78(2):203–12.
Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, Miura N, Yla-Herttuala S, Fruttiger M, Makinen T, Eichmann A, Pollard JW, Gerhardt H, Alitalo K. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing notch signalling. Nat Cell Biol. 2011;13:1202–13.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307.
Fresco P, Borges F, Diniz C, Marques MPM. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26(6):747–66.
Lau ST, Lin ZX, Zhao M, Leung PS. Brucea javanica fruit induces cytotoxicity and apoptosis in pancreatic adenocarcinoma cell lines. Phytother Res. 2008;22:477–86.
Lee RT, Hlubocky F, Hu JJ, Stafford R, Daugherty C. An international pilot study of oncology physicians? Opinions and practices on complementary and alternative medicine (CAM). Integr Cancer Ther. 2008;7(2):70–5.
Lee SM, Kwon JI, Choi YH, Eom HS, Chi GY. Induction of G2/M arrest and apoptosis by water extract of Strychni semen in human gastric carcinoma AGS cells. Phytother Res. 2008;22(6):752–8.
Yeh JC, Cindrova-Davies T, Belleri M, Morbidelli L, Miller N, Cho CW, Chan K, Wang YT, Luo GA, Ziche M, Presta M, Charnock-Jones DS, Fan TP. The natural compound n-butylidenephthalide derived from the volatile oil of Radix Angelica sinensis inhibits angiogenesis in vitro and in vivo. Angiogenesis. 2011;14(2):187–97.
Rasul A, Yu B, Khan M, Zhang K, Iqbal F, Ma T, Yang H. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int J Oncol. 2012;40(4):1153–61.
Lumlerdkij N et al. Cytotoxicity of medicinal plants used in cancer prevention in Thailand. Planta Med. 2015;81(16) PW_31.
Mehta HJ, Patel V, Sadikot RT. Curcumin and lung cancer—a review. Target Oncol. 2014;9(4):295–310.
de Vogel S, Dindore V, van Engeland M, Goldbohm RA, van den Brandt PA, Weijenberg MP. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372–8.
Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670–95.
Danciu C, Avram S, Gaje P, Pop G, Şoica C, Craina M, Dumitru C, Dehelean C, Peev C. An evaluation of three nutraceutical species in the Apiaceae family from the western part of Romania: antiproliferative and antiangiogenic potential. J Agroalimentary Processes Technol. 2013;19(2):173–9.
Sauer, Heinrich, et al. Herbal ingredients for the inhibition of tumour-induced angiogenesis. Botanical Medicine in Clinical Practice. 2008. p. 335.
Carvalho AA, Costa PMD, Vieira GC, Jamacaru FVF, Moraes MO, Cavalcanti BC, Pessoa C. Natural products used as candidates for angiogenesis inhibitors in cancer therapy. Trends Org Chem. 2011;15:79–93.
Dudkowska M, Kucharewicz K. Natural compounds—modulators of senescence and cell death. Postępy Biochemii. 2014;60(2):207–20.
Lin W, Zhao J, Cao Z, Zhuang Q, Zheng L, Zeng J, Hong Z, Peng J. Livistona chinensis seeds inhibit hepatocellular carcinoma angiogenesis in vivo via suppression of the Notch pathway. Oncol Rep. 2014;31:1723–8.
Rasul A, Khan M, Ali M, Li J, Li X. Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. Sci World J. 2013;2013:9. doi:10.1155/2013/248532.
Jennifer AD, Jennifer EH, Gerald EH. Role of apoptosis in anti-angiogenic cancer therapies. In: Gewirtz DA, Holt SE, Grant S, editors. Apoptosis, senescence and cancer. New York: Humana Press Inc.; 2007. p. 537–56.
Millimouno FM, Dong J, Yang L, Li J, Li X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Am Assoc Cancer Res. 2014;8(26):1–27.
Ludwiczuk A, Najda A, Wolski T, Baj T. Chromatographic determination of the content and the composition of extracts and essential oils from the fruits of three varieties of stalk celery (Apium graveolens L. var dulce Mill. Pers.). JPC-J Planar Chromat. 2001;14(6):400–4.
Buczkowska H, Dyduch J, Najda A. Capsaicinoids in hot pepper depending on fruit maturity stage and harvest date. Acta Sci Pol-Hortorum Cultus. 2013;6:183–96.
Kapłan M, Najda A. Antioxidant activity of vine fruits depending on their colouring. Chemija. 2014;25(1):51–5.
Chinembiri TN, du Plessis LH, Gerber M, Hamman JH, du Plessis J. Review of natural compounds for potential skin cancer treatment. Molecules. 2014;19:11679–721.
Najda A, Dyduch-Siemińska M, Dyduch J, Gantner M. Comparative analysis of secondary metabolites contents in Fragaria vesca L. fruits. Ann Agric Envir Med. 2014;21(2):339–43.
Najda A, Dyduch J, Świca K, Kapłan M, Papliński R, Sachadyn-Król M, Klimek K. Identification and profile of furanocoumarins from the ribbed celery (Apium graveolens L. Var. dulce mill. / Pers.). Food Sci Tech Res. 2015;21(1).
Najda A. Toxic substances in plants with built structure with nitrogen. Episteme. 2014;25(1):65–76.
Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283–99.
Shehzad A, Le J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56–68.
Qiu Y, Yu T, Wang W, Pan K, Shi D, Sun H. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. BiochemBiophys Res Commun. 2014;448:15–21.
Martinez RM, Pinho-Ribeiro FA, Steffen VS, Silva TC, Caviglione CV, Bottura C, Fonseca MJ, Vicentini FT, Vignoli JA, Baracat MM, Georgetti SR, Verri Jr WA, Casagrande R. Topical formulation containing naringenin: efficacy against ultraviolet b irradiation-induced skin inflammation and oxidative stress in mice. PLoS One. 2016;11(1). doi:10.1371/journal.pone.0146296.
Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40(8):1091–7.
Najda A, Błaszczyk L, Winiarczyk K, Dyduch J, Tchórzewska D. Comparative studies of nutritional and health-enhancing properties in the “garlic-like” plant Allium ampeloprasum var. ampeloprasum (GHG-L) and A. sativum. Sci Horticulturae. 2016;201:247–55.
Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods HumNutr. 2010;65:158–63.
Petiwala SM, Berhe S, Li G, Puthenveetil AG, Rahman O, Nonn L, Johnson JJ. Rosemary (Rosmarinus officinalis) extract modulates CHOP/GADD153 to promote androgen receptor degradation and decreases xenograft tumor growth. PLoS One. 2014;9:e89772.
Kwon H.J., Shim J.S., Kim J.H., Cho H.Y., Yum Y.N., Kim S.H., Yu J. Betulinic acid inhibits growth factor-induced angiogenesis via the modulation of mitochondrial function in endothelial cells. Jpn J Cancer Res 2002; 93(4): 417–425.
Shimamura M, Hazato T, Ashino H, Yamamoto Y, Iwasaki E, Tobe H, Yamamoto K, Yamamoto S. Inhibition of angiogenesis by humulone; a bitter acid from beer hop. Biochem.Biophys. Res. Commun. 2001;289(1):220–4.
Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1–8.
Shen T, Xie CF, Wang XN, Luo HX. Stilbenoids. In: Ramawat KG, Merillon JM, editors. Natural products. Berlin: Springer; 2013. p. 1901–49.
Foitzik T, Hotz HG, Hotz B, Wittig F, Buhr HJ. Selective inhibition of cyclooxygenase-2 (COX-2) reduces prostaglandin E2 production and attenuates systemic disease sequelae in experimental pancreatitis. Hepato-Gastroenterology. 2003;50(52):1159–62.
Höper MM, Voelkel NF, Bates TQ, Allard JD, Horan M, Shepherd D, Tudier R. Prostaglandin induce VEGF growth factor in human monocytic cell lines and rat lungs via cAMP. Am J Respir Cell Mol Biol. 1997;17(6):748–56.
Li T, Hu J, Du S, Chen Y, Wang S, Wu Q. ERK1/2/COX-2/PGE2 signaling pathway mediates GPR91-dependent VEGF release in streptozotocin-induced diabetes. Mol Vis. 2014;20:1109–21.
Romano M, Claria J. Cyclooxygenase-2 and 5-lipoxygenase converging function on cell proliferation and angiogenesis: implication for cancer therapy. FASEB J. 2003;17(14):1986–95.
Tuncer S, Banerjee S. Eicosanoid pathway in colorectal cancer: recent updates. World J Gastroenterol. 2015;21(41):11748–66.
Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. Am Soc Clin Oncol. 2006;24(34):5457–64.
Castillo-Pichardo L, Dharmawardhane SF. Grape polyphenols inhibit Akt/mammalian target of rapamycin signaling and potentiate the effects of gefitinib in breast cancer. Nutr Cancer. 2012;64:1058–69.
Huang S et al. Grape seed proanthocyanidins inhibit angiogenesis via the downregulation of both vascular endothelial growth factor and angiopoietin signaling. Nutr Res. 2012;32(7):530–6.
Taraphdar AK, Roy M, Bhattacharya RK. Natural products as inducers of apoptosis: implication for cancer therapy and prevention. Current Sci. 2001;80:1387–96.
Molassiotis A, Fernandez-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, Margulies A, Browall M, Magri M, Selvekerova S. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16:655–63.
Loboda A, Cisowaski J, Zarebski A, Jazwa A, Rivera Nunez D, Kyprotakis Z, Heinrich M, Dulak J. Effect of plant extracts on angiogenic activities of endothelial cells and keratinomycetes. J Physiol Pharmacol. 2005;1:125–37.
Lamy S, Akla N, Ouanouki A, Lord-Dufour S, Béliveau R. Diet-derived polyphenols inhibit angiogenesis by modulating the interleukin-6/STAT3 pathway. Exp Cell Res. 2012;318(13):1586–96.
Kubota Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J Med. 2012;61(2):47–56.
Ribatti D, Nico B, Vacca A, Roncali L, Djonov V. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and antiangiogenesis in vivo. Ant Rec. 2001;264:317–24.
Durupt F, Koppers-Lalic D, Balme B, Budel L, Terrier O, Lina B, Thomas L, Hoeben RC, Rosa-Calatrava M. The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses. Cancer Gene Ther. 2012;19:58–68.
Hsin CH, Wu BC, Chuang CY, Yang SF, Hsieh YH, Ho HY, Lin HP, Chen MK, Lin CW. Selaginella tamariscina extract suppresses TPA-induced invasion and metastasis through inhibition of MMP-9 in human nasopharyngeal carcinoma HONE-1 cells. BMC Complement Altern Med. 2013;13:234–45.
Yi JM, Park JS, Oh SM, Lee J, Kim J, Oh DS, Bang OS, Kim NS. Ethanol extract of Gleditsia sinensis thorn suppresses angiogenesis in vitro and in vivo. BMC ComplementAltern Med. 2012;12:243–51.
Kim EC, Kim SH, Piao SJ, Kim TJ, Bae k, Kim HS, Hong SS, Lee BI, Nam M. Antiangiogenic activity of Acer tegmentosum Maxim water extract in vitro and in vivo. J Korean Med Sci. 2015;30:979–87.
Najda A. Planning and evaluation of phytochemical plants in various growth phases of two varieties of celery (Apium graveolens L. var dulce Mil./Pers.). 2004; PhD Dissertation, University of Life Sciences in Lublin.
Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–78.
Zhang T, Jia W, Sun X. 3-n-Butylphthalide (NBP) reduces apoptosis and enhances vascular endothelial growth factor (VEGF) up-regulation in diabetic rats. Neurol Res. 2010;32:390–6.
Tai J, Cheung S, Wu M, Hasman D. Antiproliferation effect of rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomed. 2012;19:436–43.
Petiwala SM, Johnson JJ. Diterpenes from rosemary (Rosmarinus officinalis): defining their potential for anti-cancer activity. Cancer Lett. 2015;367:93–102.
Agostinis P, Vantieghem A, Merlevede W, de Witte PAM. Hypericin in cancer 85 treatment: more light on the way. Int J Biochem Cell Biol. 2002;34:221–41.
Carimi F, Zottini M, Formentin E, Terzi M, Lo Schiavo F. Cytokinins: new apoptotic inducers in plants. Planta. 2003;216:413–21.
Kuttan G., Pratheeshkumar P., Manu K.A,, Kuttan R. Inhibition of tumor progression by naturally occurring terpenoids. Pharm Biol 2011; 49: 995–1007.
Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. Biotech. 2013;3:439–59.
Parikh NR, Mandal A, Bhatia D, Siveen KS, Sethi G, Bishayee A. Oleanane triterpenoids in the prevention and therapy of breast cancer: current evidence and future perspectives. Phytochem Rev. 2014;13:793–810.
Albini A et al. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012;9(9):498–509.
Weng CJ, Yen GC. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treatment Rev. 2012;38:76–87.
Beghlal D, El Bairi K, Marmouzi I, Haddar L, Mohamed B. Phytochemical, organoleptic and ferric reducing properties of essential oil and ethanolic extract from Pistacia lentiscus (L.). Asian Pac J Trop Dis. 2016;6(4):305–10.
Koohpar ZK, Entezari M, Movafagh A, Hashem M. Anticancer activity of curcumin on human breast adenocarcinoma: role of mcl-1 gene. Iran J Cancer Prev. 2015;8(3):e2331.
Wolanin K, Piwocka K. Curcumin - from natural medicine clinic. Kosmos. 2008;57:53–65.
Kumaravel M, Sankar P, Rukkuma-Ni R. Antiproliferative efect of an analog of curcumin bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione in human breast cancer cells. Eur Rev Med Pharmacol Sci. 2012;16:1900–7.
Devasena T, Rajasekarana KN, Menon VP. Bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione (a curcumin analog) ameliorates DMH-induced hepatic oxidative stressduring colon carcinogenesis. Pharmacol Res. 2002;46:39–45.
Mohankumar K, Pajaniradje S, Sridharan S, Kumar Singh V, Ronsard L, Banerjea AC, Selvanesan Benson C, Selvaraj Coumar M, Rajagopalan R. Mechanism of apoptotic induction in human breast cancer cell, MCF-7, by an analog of curcumin in comparison with curcumin—an in vitro and in silico approach. Chem Biol Interact. 2014;210:51–63.
Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: therapeutic potential through its antioxidant property. J Clinic Biochem Nutr. 2007;40:92–100.
Ho K, Yazan LS, Ismail N, Ismail M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidem. 2009;33:155–60.
Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozukay Y, Singhirunnusorn P, Ruchirawat S, Svasti J, Saiki I. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005;25:57–65.
Nakazato T, Ito K, Ikeda Y, Kizaki M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clinic Cancer Res. 2005;11:6040–9.
Cheung S, Tai J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol Rep. 2007;17:1525–31.
Bai N, He K, Roller M, Lai CS, Shao X, Pan MH, Ho CT. Flavonoids and phenolic compounds from Rosmarinus officinalis. J Agric Food Chem. 2010;58:5363–7.
Mulinacci N, Innocenti M, Bellumori M, Giaccherini C, Martini V, Michelozzi M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: an HPLC/DAD/MS study. Talanta. 2011;85:167–76.
Bernardes WA, Lucarini R, Tozatti MG, Souza MG, Silva ML, Filho AA, et al. Antimicrobial activity of Rosmarinus officinalis against oral pathogens: relevance of carnosic acid and carnosol. Chem Biodivers. 2010;7:1835–40.
Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15:7792–814.
Nabekura T, Yamaki T, Hiroi T, Ueno K, Kitagawa S. Inhibition of anticancer drug efflux transporter P-glycoprotein by rosemary phytochemicals. Pharmacol Res. 2010;61:259–63.
Yu YM, Lin CH, Chan HC, Tsai HD. Carnosic acid reduces cytokine-induced adhesion molecules expression and monocyte adhesion to endothelial cells. Eur J Nutr. 2009;48:101–6.
Johnson JJ. Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011;305:1–7.
López-Jiménez A, García-Caballero M, Medina MA, Quesada AR. Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur J Nutri. 2013;51(1):85–95.
Schempp CM, Kirkin V, Simon-Haarhaus B, Kersten A, Kiss J, Termeer CC, Gilb B, Kaufmann T, Borner C, Sleeman JP, Simon JC. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John’s wort that acts by induction of apoptosis. Oncogene. 2002;21:1242–50.
Hostanska K, Reichling J, Bommer S, Weber M, Saller R. Hyperforin a constituent of St. John’s wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur J Pharm Biopharm. 2003;56:121–32.
Dona M, Dell’Aica I, Pezzato E, Sartor L, Calabrese F, Della Barbera M, Donella-Deana A, Appendino G, Borsarini A, Caniato R, Garbisa S. Hyperforin inhibits cancer invasion and metastasis. Cancer Res. 2004;64:6225–32.
Martínez-Poveda B, Quesada AR, Medina MA. Hyperforin, a bio-active compound of St. John’s wort, is a new inhibitor of angiogenesis targeting several key steps of the process. Inter J Cancer. 2005;17(5):775–80.
Aggarwal BB, Bhardwaj A, Aggarwal RS, Steram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–840.
Roy S, Khanna S, Alessio HM, Vider J, Bagchi D, Bagchi M, Sen CK. Anti-angiogenic property of edible berries. Free Radic Res. 2002;36:1023–31.
Pozo-Guisado E, Merino JM, Mulero-Navarro S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A, Salguero PMF. Resveratrol-induced apoptosis in Mcf-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-κB. Int J Cancer. 2005;115:74–84.
Kiliańska ZM, Żołnierczyk J, Węgierska-Gądek J. Biological activity of poly (ADP-ribose) -1. Postep. Hig Med Dosw. 2010;64:344–63.
Roy P, Kalra N, Prasad S, George J, Skuhla Y. Chemopreventive potential of resveratrol in mouse skin tumors through regulation of mitochondrial and PI3K/AKT signaling pathways. Pharmaceut Res. 2009;26:221–17.
Cao Y, Fu ZD, Wang F, Liu HY, Han R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J Asian Nat Prod Res. 2005;7:205–13.
Igura K, Ohta T, Kuroda Y, Kaji K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001;171:11–6.
Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of src dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003;64:1029–36.
Kanavi MR, Darjatmoko S, Wang S, Azari AA, Farnoodian M, Kenealey JD, van Ginkel PR, Albert DM, Sheibani N, Polans AS. The sustained delivery of resveratrol or a defined grape powder inhibits new blood vessel formation in a mouse model of choroidal neovascularization. Molecules. 2014;19(11):17578–603.
Zhang H, He S, Spee C, Ishikawa K, Hinton DR. SIRT1 mediated inhibition of VEGF/VEGFR2 signaling by resveratrol and its relevance to choroidal neovascularization. Cytokine. 2001;76(2):549–52.
Zhang X, Song Y, Wu Y, et al. Indirubin inhibits tumor growth by antitumor angiogenesis via blocking VEGFR2-mediated JAK/STAT3 signaling in endothelial cell. Int J Cancer. 2011;129:2502–11.
Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013;38(1):15–25.
Yang H et al. Natural compounds with proteasome inhibitory activity for cancer prevention and treatment. Curr Protein Pept Sci. 2008;9(3):227.
Chen DI, Ping Dou Q. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int J Mol Sci. 2008;9(7):1196–206.
Giovannini C, Scazzocchio B, Varì R, Santangelo C, D’archivio M, Masella R. Apoptosis in cancer and atherosclerosis: polyphenol activities. Sanità Ann Ist Super Sanità. 2007;43:406–16.
Huynh H, Nguyen TT, Chan E, Tran E. Inhibition of ErbB-2 and ErbB-3 expression by quercetin prevents transforming growth factor alpha (TGF-α)- and epidermal growth factor (EGF) induced human PC-3 prostate cancer cell proliferation. Int J Oncol. 2003;23:821–9.
Tan WF, Lin LP, Li MH, Zhang YX, Dong JG, Xiao D, Din J. Quercetin, a dietary-derived flavonoid, possesses antiangiogenic potential. Eur J Pharmacol. 2003;459:255–62.
O’Leary KA, de Pascual–Tereasa S, Needs PW, Bao YP, O’Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat Res. 2004;551:245–54.
Dash R, Uddin MM, Hosen SM, Rahim ZB, Dinar AM, Kabir MS, Sultan RA, Islam A, Hossain MK. Molecular docking analysis of known flavonoids as duel COX-2 inhibitors in the context of cancer. Bioinformat. 2015;11(12):543–9.
Ma ZS, Huynh TH, Ng CP, Do PT, Nguyen TH, Huynh H. Reduction of CWR22 prostate tumor xenograft growth by combined tamoxifen-quercetin treatment is associated with inhibition of angiogenesis and cellular proliferation. Int J Oncol. 2004;24:1297–304.
Mojzis J, Varinska L, Mojzisova G, Kostova I, Mirossay L. Anti-angiogenic effects of flavonoids and chalcones. Pharmacol Res. 2008;57(4):259–65.
Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. PNAS. 2008;105:11105–9.
Rastogi T, Devesa S, Mangtani P, Mathew A, Cooper N, Kao R, Sinha R. Cancer incidence rates among south Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol. 2007;37:147–60.
Mense SM, Hei TK, Ganju RK, Bhat HK. Phytoestrogens and breast cancer prevention: possible mechanisms of action. Environ Health Perspect. 2008;116:426–33.
Gutierrez RMP. Flavonoids. In: Handbook of compounds with cytotoxic activity isolated from plants. Commack, NY: Nova Science Publishers Inc.; 2007. p. 158–65.
Sharma P, Kapoor S. Biopharmaceutical aspects of Brassica vegetables. J Pharm Phytochem. 2015;4(1):140–7.
Teicher BA. Newer cytotoxic agents: attacking cancer broadly. Clin Cancer Res. 2008;14:1610–7.
Katz L, Baltz RH. Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol. 2016;43(2–3):155–76.
Vacca A, Iurlaro M, Ribatti D, Minischetti M, Nico B, Ria R, Pellegrino A, Dammacco F. Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood. 1997;94:4143–55.
Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24.
Klement G, Huang P, Mayer B, Green SK, Man S, Bohlen P, Hicklin D, Kerbel RS. Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res. 2002;8:221–32.
Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36.
Stempac D, Seely D, Baruchel S. Metronomic dosing of chemotherapy: applications in pediatric oncology. Cancer Investig. 2006;24:432–43.
Verreault M, Strutt D, Masin D, Anantha M, Yung A, Kozlowski P, Waterhouse D, Bally MB, Yapp DT. Vascular normalization in orthotopic glioblastoma following intravenous treatment with lipid-based nanoparticulate formulations of irinotecan (Irinophore C™), doxorubicin (Caelyx®) or vincristine. BMC Cancer. 2011;11:124–42.
Avramis IA, Kwock R, Avramis VI. Taxotere and vincristine inhibit the secretion of the angiogenesis inducing vascular endothelial growth factor (VEGF) by wild-type and drug-resistant human leukemia T-cell lines. Anticancer Res. 2001;21(4):2281–6.
Mabeta P, Pepper MSA. Comparative study on the anti-angiogenic effects of DNA-damaging and cytoskeletal-disrupting agents. Angiogenesis. 2009;12:81–90.
Pasquier E, Street J, Pouchy C, Carre M, Gifford AJ, Murray J, Norris MD, Trahair T, Andre N, Kavallaris M. Beta-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer. 2013;108:2485–94.
Grange C, Bussolati B, Bruno S, Fonsato V, Sapino A, Camussi G. Isolation and characterization of human breast tumor-derived endothelial cells. Oncol Rep. 2006;15:381–6.
Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang PY, Zhang JB, Wang L, Wu WZ, Qin LX, Tang ZY. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin Cancer Res. 2009;15:4838–46.
Polat U. The effects on metabolism of glucosinolates and their hydrolysis products. J Biol Environ Sci. 2010;4(10):39–42.
Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, Kim J, Kim KH, Kim CJ. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bul. 2006;29:1597–602.
Milazzo S, Lejeune S, Ernst E. Laetrile for cancer: a systematic review of the clinical evidence. Suppor Care Cancer. 2007;15:583–95.
Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Clin Nutr. 2008;47:73–88.
Zalega J, Szostak-Węgierek D. Nutrition in cancer prevention. Part I. Plant polyphenols, carotenoids, dietary fiber. ProblHig Epidemiol. 2013;94:41–9.
Kusznierewicz B., Piasek A., Lewandowska J., Śmiechowska A., Bartoszek A., Anti-carcinogenic properties of white cabbage. Żywność Nauka Technologia Jakość 2007; 6: 20–23.
Traka M, Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem Rev. 2009;8:269–82.
Casero RA, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6(5):373–90.
Zdrojewicz Z, Lachowski M. The importance of putrescine in the human body. Postepy Hig Med Dosw. 2014;68:393–403.
Kucharzewska P, Welch JE, Svensson KJ, Belting M. The polyamines regulate endothelial cell survival during hypoxic stress through PI3K/AKT and MCL-1. Biochem Bioph Res. 2009;380(2):413–8.
Koomoa DLT, Geerts D, Lange I, Koster J, Pegg AE, Feith DJ, Bachmann AS. DFMO/eflornithine inhibits migration and invasion downstream of MYCN and involves p27Kip1 activity in neuroblastoma. Int J Oncol. 2013;42:1219–28.
Jabłońska-Trypuć A, Czerpak R. The role of connective tissue growth factor (CTGF) in fibroproliferative processes and tissues fibrosis. Postepy Biol Komorki. 2009;36:135–54.
Minorsky PV. The hot and the classic. Plant Physiol. 2000;132:1135–6.
Ishii Y, Hori Y, Sakai S, Honma Y. Control of diferentiation and apoptosis of human myeloid leukemia cells by cytokinins and cytokinin nucleosides, plant rediferentiation-inducing hormones. Cell Growth Differ. 2002;13:19–26.
Kunikowska A, Byczkowska A, Doniak M, Kaźmierczak A. Cytokinins resume: their signaling and role in programmed cell death in plants. Plant Cell Rep. 2013;32:771–80.
Coltella N, Valsecchi R, Ponente M, Ponzoni M, Bernardi R. Synergistic leukemia eradication by combined treatment with retinoic acid and HIF inhibition by EZN-2208 (PEG-SN38) in preclinical models of PML-RARα and PLZF-RARα-driven leukemia. Clin Cancer Res. 2015;21(16):3685–94.
Liang C, Guo S, Yang L. Effects of all-trans retinoic acid on VEGF and HIF-1α expression in glioma cells under normoxia and hypoxia and its anti-angiogenic effect in an intracerebral glioma model. Mol Med Rep. 2014;10(5):2713–9.
Aditya NP et al. Antiangiogenic effect of combined treatment with curcumin and genistein on human prostate cancer cell line. J Funct Foods. 2014;8:204–13.
Yu X et al. Anti-angiogenic genistein inhibits VEGF-induced endothelial cell activation by decreasing PTK activity and MAPK activation. Med Oncol. 2012;29(1):349–57.
Mirzoeva S, Franzen CA, Pelling JC. Apigenin inhibits TGF-β-induced VEGF expression in human prostate carcinoma cells via a Smad2/3-and Src-dependent mechanism. Mol Carcinog. 2014;53(8):598–609.
Silvan S, Manoharan S. Apigenin prevents deregulation in the expression pattern of cell-proliferative, apoptotic, inflammatory and angiogenic markers during 7, 12-dimethylbenz [a] anthracene-induced hamster buccal pouch carcinogenesis. Arch Oral Biol. 2013;58(1):94–101.
Kumar S, Raina K, Agarwal R. Chemopreventive and anticancer efficacy of silibinin against colorectal cancer, Multi-Targeted Approach to Treatment of Cancer. Berlin Heidelberg New York: Springer; 2015. p. 339–50.
Yang SH, Lin JK, Chen WS, Chiu JH. Yang. Anti-angiogenic effects of silymarin on colon cancer LoVo cell line. J Surg Res. 2003;113(1):133–4l.
Kim KK et al. Anti-angiogenic activity of cranberry proanthocyanidins and cytotoxic properties in ovarian cancer cells. Int J Oncol. 2012;40(1):227–35.
Wang M-L et al. Antiangiogenic activity of indole-3-carbinol in endothelial cells stimulated with activated macrophages. Food Chem. 2012;134(2):811–20.
Kunimasa K et al. Antiangiogenic effects of indole-3-carbinol and 3,3′-diindolylmethane are associated with their differential regulation of ERK1/2 and Akt in tube-forming HUVEC. J Nutr. 2010;140(1):1–6.
Mohan R et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7(2):115–22.
Hussain S et al. Anti-angiogenic activity of sesterterpenes; natural product inhibitors of FGF-2-induced angiogenesis. Angiogenesis. 2008;11(3):245–56.
Nathan P, Zweifel M, Padhani AR, et al. Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer. Clin Cancer Res. 2012;18(12):3428–39.
Update—OXiGENE announces positive topline results from randomized phase 2 study GOG186I of ZYBRESTAT(R) in combination with Avastin(R) for recurrent ovarian cancer. www.investor.oxigene.com/releasedetail.cfm?releaseid=832026
Zweifel M, Jayson GC, Reed NS, Osborne R, Hassan B, Ledermann J, Shreeves G, Poupard L, Lu SP, Balkissoon J, Chaplin DJ, Rustin GJ. Phase II trial of combretastatin A4 phosphate, carboplatin, and paclitaxel in patients with platinum-resistant ovarian cancer. Ann Oncol. 2011;22:2036–41.
Granata R, Locati L, Licitra L. Therapeutic strategies in the management of patients with metastatic anaplastic thyroid cancer: review of the current literature. Curr Opin Oncol. 2013;25:224–8.
Ibrahim MA, Do DV, Sepah YJ, Shah SM, Van Anden E, Hafiz G, Donahue JK, Rivers R, Balkissoon J, Handa JT, Campochiaro PA, Nguyen QD. Vascular disrupting agent for neovascular age related macular degeneration: a pilot study of the safety and efficacy of intravenous combretastatin A-4 phosphate. BMC Pharmacol Toxicol. 2013;14:7. doi:10.1186/2050-6511-14-7.
Clinical Trials. U.S. National Institutes of Health. 2016. https://clinicaltrials.gov/. Accessed 10 June 2016.
LoRusso PM, Boerner SA, Hunsberger S. Clinical development of vascular disrupting agents: what lessons can we learn from ASA404? J Clin Oncol. 2011;29(22):2952–5.
McKeage MJ, Von Pawel J, Reck M, et al. Randomised phase II study of ASA404 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Br J Cancer. 2008;99(12):2006–12. doi:10.1038/sj.bjc.6604808.
Head M, Jameson MB. The development of the tumor vascular-disrupting agent ASA404 (vadimezan, DMXAA): current status and future opportunities. Expert Opin Investig Drugs. 2010;19(2):295–304.
Lara, P. N., Douillard, J. Y., Nakagawa, K., Von Pawel, J., McKeage, M. J., et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol. 2011;JCO-2011.
Baguley BC, Siemann DW. Temporal aspects of the action of ASA404 (vadimezan; DMXAA). Expert Opin Investig Drugs. 2010;19(11):1413–25.
Lorusso PM, Boerner SA, Hunsberger S. Clinical development of vascular disrupting agents: what lessons can we learn from ASA404? J Clin Oncol. 2011;29(22):2951–2.
Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent DMXAA. J Immunol. 2013;190(10):5216–25. doi:10.4049/jimmunol.1300097.
Tsimberidou AA-MA, Akerley W, Schabel MC, et al. Phase I clinical trial of MPC-6827 (Azixa), a microtubule destabilizing agent, in patients with advanced cancer. Mol Cancer Ther. 2010;6827(12):3410–9.
Patterson DM, Zweifel M, Middleton MR, et al. Phase I clinical and pharmacokinetic evaluation of the vascular disrupting agent OXi4503 in patients with advanced solid tumors. Clin Cancer Res. 2012;18(5):1415–25.
Burns CJ, Fantino E, Powell AK, et al. The microtubule depolymerizing agent CYT997 causes extensive ablation of tumor vasculature in vivo. J Pharmacol Exp Ther. 2011;339(3):799–806.
Burge M, Francesconi AB, Kotasek D, et al. Phase I, pharmacokinetic and pharmacodynamic evaluation of CYT997, an orally-bioavailable cytotoxic and vascular disrupting agent. Investig New Drugs. 2013;31(1):126–35.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalid, E.B., Ayman, EM.EK., Rahman, H. et al. Natural products against cancer angiogenesis. Tumor Biol. 37, 14513–14536 (2016). https://doi.org/10.1007/s13277-016-5364-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5364-8