Abstract
Recently, a lectin was purified from the potato cultivated in Bangladesh locally known as Sheel. In the present study cytotoxicity of the lectin against Ehrlich ascites carcinoma (EAC) cells was studied by MTT assay in vitro in RPMI-1640 medium and 8.0–36.0 % cell growth inhibition was observed at the range of 2.5–160 μg/ml protein concentration when incubated for 24 h. The lectin-induced apoptosis in EAC cells was confirmed by fluorescence and optical microscope. The apoptotic cell death was also confirmed by using caspase inhibitors. Cells growth inhibition caused by the lectin (36 %) was remarkably decreased to 7.6 and 22.3 % respectively in the presence of caspase-3 and -8 inhibitors. RT-PCR was used to evaluate the expression of apoptosis-related genes Bcl-X, p53, and Bax. An intensive expression of Bcl-X gene was observed in untreated control EAC cells with the disappeared of the gene in Sheel-treated EAC cells. At the same time, Bax gene expression appeared only in Sheel-treated EAC cells and the expression level of the p53 gene was increased remarkable after the treatment of EAC cells with the lectin. The lectin showed strong agglutination activity against EAC cells. Flow cytometry was used to study the cell cycle phases of EAC cells and it was observed that the lectin arrested the G2/M phase. In conclusion, Sheel lectin inhibited EAC cells growth by inducing apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lectins are a group of proteins that usually contains two or more binding sites for sugars or carbohydrate units and have the ability to agglutinate erythrocytes with known sugar specificity. Lectins are usually found in plants, animals, virus, bacteria, and fungi. The main role of lectin in animals is to facilitate cell-cell contact and play numerous functions in plant such as: carbohydrate transport, protein storage, physiological regulation, etc. Plant lectins, usually found in bark, leaf, root, leave, seeds, and rhizomes, represent a unique group of proteins with potent biological activities [1–3]. Several lectins were purified from plant sources and the mechanism of actions of most of the lectins against different cancer cell lines are unknown, although some literatures reported elucidation of the anticancer mechanism of lectins against different cancer cell lines [2–12].
A 20,000-kDa chitin-binding lectin was isolated from Solanum tuberosum locally known as Sheel potato and characterized in our previous studies [1, 13]. The lectin inhibited the growth of Listeria monocytogenes, Escherichia coli, Salmonella enteritidis, and Sigella boydii bacteria and also inhibited biofilm formation by Pseudomonus aeruginosa [13]. The lectin exhibited antifungal activity against Rhizopus spp, Penicillium spp., and Aspergillus niger [13]. The lectin was toxic and inhibited 79.84 % EAC cells growth in vivo in mice with the increase of hemoglobin level and red blood cells (RBC) towards the normal and the decreased level of white blood cells [1]. But the antitumor mechanism of this lectin is still unknown. In this study, we are reporting the antitumor activity of potato lectin is due to cell agglutination, G2/M cell cycle arrest and the induction of apoptosis that was confirmed by MTT assay, cell morphological study, using caspase inhibitors and apoptosis-related genes expression.
Materials and methods
Chemicals and reagents
For protein purification, DEAE cellulose and chitin were purchased from Wako (Japan). For cell culture, fetal calf serum and penicillin-streptomycin from Invitrogen (USA) and RPMI-1640 medium from Sigma were purchased. MTT was purchased from Carl Roth (Germany), propidium iodide and Hoechst-33342 from Sigma (USA), and caspase inhibitors z-DEVD-fmk and z-IETD-fmk were purchased from Biovision (USA). All other chemicals/reagents were of analytical grade. The Sheel potato was collected from Rangpur district of Bangladesh.
Purification of Sheel lectin
Sheel lectin was purified according to Hasan et al. [1] and the hemagglutination assay was carried out in a 96-well microtiter U-bottomed plates as described by Kabir et al. [6].
Cell culture
RPMI-1640 medium was supplemented with 10 % fetal calf serum, and 1 % (v/v) penicillin-streptomycin was used for EAC cells culture in a humidified atmosphere of 5 % CO2 at 37 °C.
MTT colorimetric assay
MTT colorimetric assay was used to detect the antiproliferative activity of Sheel lectin against EAC cell as described by Kabir et al. [6]. At first 5 × 105 EAC cells in 100 μl RPMI-1640 media (with 10 % fetal calf serum and 1 % penicillin-streptomycin) were plated in each well of the 96-well flat-bottom culture plate containing serially diluted lectin in 100 μl RPMI-1640 medium and incubated for 24 h at 37 °C in CO2 incubator. Wells of the culture plate containing the medium without lectin was used as control. The aliquot was removed from each well and then EAC cells were washed thrice and stained with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). After 4 h of incubation at 37 °C, an aliquot was removed again from each well and acidic isopropanol was added and kept in dark for 30 min. All experiments were done in triplicate and the absorbance of the treatment and control groups were measured at 570 nm by titer plate reader and the cell proliferation inhibition ratio was determined by using the equation:
where A is the OD570 nm of the cellular homogenate (control) without Sheel lectin and B is the OD570 nm of the cellular homogenate with Sheel lectin.
Cell morphological change and nuclear damage
After treatment of EAC cells with lectin (160 μg/ml) and without lectin, EAC cells were washed thrice with phosphate buffer saline (PBS) and stained with 0.1 μg/ml of Hoechst-33342 at 37 °C for 20 min in dark and then apoptosis in EAC cells was morphologically observed under a fluorescent microscope (Olympus iX71, Korea) as described by Kabir et al. [6].
Effect of caspase inhibitors on Sheel lectin-induced cytotoxicity in EAC cells
Caspase inhibitors were used in order to check the role of caspases in the lectin-induced cell death. EAC cells inoculated in RPMI-1640 medium were incubated for 2 h in the presence and absence of 2 μmol/ml of each caspase-3 inhibitor (z-DEVD-fmk) and caspase-8 inhibitor (z-IETD-fmk). Then the cells were treated with Sheel lectin at the final concentration of 160 μg/ml and incubated for 24 h at 37 °C in 5 % CO2 and the cell growth inhibition was detected by MTT assay.
Reverse transcriptase polymerase chain reaction
In this step, EAC cells were treated with lectin (160 μg/ml) and then total RNA was extracted from lectin-treated and untreated EAC cells using RNA isolation kit according to manufacturer direction (Tiangen, China). A micro-spectrophotometer (K2800 nucleic acid analyzer, Beijing Kaiao Technology Development Co., Ltd, China) was used to determine the amount of RNA and to check the purity. High capacity RNA-to-cDNA Kit was used to reverse transcribe RNA into cDNA according to manufacturer instruction (Applied Biosystems, USA). These cDNAs were used as template for PCR and the expression level of β-actin, p53, Bax, and Bcl-X genes were studied. A GeneAtlas (Japan) thermo cycler was used for the amplification of cDNA and the reaction mixture was prepared with dNTP, Taq polymerase buffer, platinum Taq polymerase (Tiangen, China) and 25 pmol each of forward and reverse primer (IDT Singapore). Expression of β-actin was used to check the quality of RNA. The primers were used for β-actin, Bax, p53, and Bcl-X was described previously [6, 14]. The steps involved were 3 min PCR activation step at 95 °C, followed by 40 cycles of 95 °C/30 s, 55 °C/30 s, 72 °C/50 s and a final extension of 72 °C/10 min for each of Bax, Bcl-X, p53, and β-actin genes. PCR products were analyzed by 1.0 % agarose gel where 100 bp DNA ladder and 1 kb DNA ladder was used as markers.
Agglutination of EAC cells
U-bottomed 96-well microtiter plate was used for EAC cells agglutination assay and hemagglutination buffer was prepared by mixing of 1 M Tris (pH 7.8), 3 M NaCl and 1 M CaCl2 to the final concentration of 20 mM Tris-HCl containing 150 mM NaCl and 10 mM CaCl2. For EAC cells agglutination assay, 50 μl of hemagglutination buffer was added to each well of the microtiter plate and equal amount of lectin (0.4 mg/ml) in same buffer was mixed. Then, 50 μl of 2 % EAC cells suspension previously washed with saline was added to each well and agitated with micro-shaker for 5 min and kept at room temperature for 30 min and the visual agglutination titer of the maximum dilution giving the positive agglutination was recorded.
Cell cycle analysis
Cell cycle analysis was used to assess the effect of Sheel lectin on the EAC cells. In cell cycle analysis, dsDNA is determined by FACS Flow cytometer (Partec CyFlow SL, Germany) after staining with propidium iodide. Shortly after, EAC cells were incubated in RPMI-1640 medium in the presence and absence of Sheel lectin (160 μg/ml) for 24 h at 37 °C in 5 % CO2. Phosphate buffer saline was used to wash EAC cells three times and the cells were fixed with 70 % ethanol. Before flow cytometric analysis, the cells were washed with PBS, then treated with RNase-A and stained with propidium iodide. The amount of fragmented DNA and cell cycle phases were analyzed from the acquired data as describe by Kabir et al. [6].
Statistical analysis
The experimental results are expressed as the mean ± standard deviation (S.D.). One-way ANOVA was used for the calculation of data followed by Dunnett’s t test using SPSS software of 16 version.
Result
MTT assay
The effect of Sheel lectin on EAC cells was investigated by MTT assay. Inhibitory effect was found to be 8 % at 2.5 μg/ml lectin concentration. When the concentration increased to 5 μg/ml, the inhibitory effect reached to 17.2 %. The inhibitory effects were almost same (around 26 %) from 10 to 80 μg/ml lectin concentration. Finally, 36 % inhibitory effect was observed at 160 μg/ml lectin concentration, as shown in Fig. 1.
Observation of EAC cells growth inhibition by lectin. The cell proliferation was measured by the MTT assay (n = 3, mean ± S.D.) after cells were treated with various doses of Sheel lectin and without lectin for 24 h in CO2 incubator at 37 °C. *p < 0.05, as compared lectin-treated EAC cells with untreated control
Cell morphological examination
EAC cells were treated with and without Sheel lectin and then stained with Hoechst-33342 and finally cell morphological changes were examined by fluorescence and optical microscope. In untreated EAC cells, a round and homogeneously stained nucleus was observed (Fig. 2a, b) while apoptotic morphological alteration (e.g., blebbing, cell shrinkage, chromatin condensation, and nuclear fragmentation) for lectin-treated EAC cells was observed (Fig. 2c, d, e) clearly. The above results confirmed Sheel lectin-induced apoptosis in EAC cells.
Apoptotic morphological changes of lectin-treated and untreated EAC cells. Apoptotic cell morphological changes were observed after staining of EAC cells with Hoechst-33342 by optical and fluorescence microscopy (Olympus iX71). Control EAC cells in a (optical) and b (fluorescence) and Sheel lectin-treated EAC cells in c (optical), d and e (fluorescence). Arrows indicate apoptotic cells
Effect of caspase inhibitors on Sheel lectin-induced cytotoxicity in EAC cells
Two caspase inhibitors z-DEVD-fmk (caspase-3 inhibitor) and z-IETD-fmk (caspase-8 inhibitor) were used to check the involvement of caspases in the lectin-induced apoptotic cell death of EAC cells. It was observed that EAC cell growth was inhibited to 36 % in the presence of Sheel lectin and this inhibitory effect reduced to 22.3 and 7.6 % when cells were incubated in the presence of z-IETD-fmk and z-DEVD-fmk respectively, as shown in Fig. 3.
Sheel lectin-induced apoptosis in EAC cells in a caspase-dependent manner. EAC cells were pre-treated with and without 2 μmol/ml of z-DEVD-fmk and z-IETD-fmk at 37 °C for 2 h, then the cells were incubated for 24 h at the same environment and finally cell growth inhibition was determined by MTT assay (n = 3, mean ± SD). *p < 0.05, as compared lectin-treated EAC cells with and without inhibitors
Reverse transcriptase polymerase chain reaction
To check the quality of RNAs, β-actin primers was used for reverse transcriptase polymerase chain reaction (RT-PCR) and clear amplification products were obtained for RNAs isolated from Sheel lectin-treated and untreated EAC cells. Then the expression of apoptosis-related p53, Bax and Bcl-X genes in the Sheel lectin-treated and untreated EAC cells were investigated as shown in Fig. 4. In untreated EAC cells (control), an intensive expression of Bcl-X gene (expressed as the isoform Bcl-XL) was detected while no expression of the gene was observed in Sheel-treated EAC cells. At the same time Bax gene expression was appeared only in Sheel-treated EAC cells with disappear of the gene expression in control EAC cells. Expression of p53 gene was increased remarkably after the treatment of EAC cells with the lectin as shown in Fig 4.
Observation of amplification of tumor related genes Bax, p53, Bcl-X (as Bcl-XL), and control gene β-actin. Total RNA extracted from treated and untreated EAC cells and then reverse transcription was performed using random hexamer. After that PCR reaction was carried out using primers specific for Bax, p53, Bcl-X and β-actin. PCR reaction products separated on 1 % agarose gel stained with ethidium bromide. L representing DNA ladder; T RNA from Sheel lectin-treated EAC cells; C, RNA from lectin untreated EAC cells
EAC cells agglutination by Sheel lectin
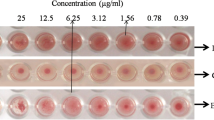
EAC cells without lectin did not show agglutination activity (Fig. 5a, c) but after treatment with Sheel lectin a strong agglutination activity against EAC cells were observed at 25 μg/ml and higher concentrations (Fig. 5b, d) and the minimum protein concentration for agglutination activity was calculated to be 6.25 μg/ml.
EAC cells agglutination by Sheel lectin. EAC cells with and without Sheel lectin in 96 well titer plate was agitated in micro-shaker for 5 min and then kept at room temperature for 30 min finally agglutination was observed by optical microscope. EAC cells without lectin (a and c) and EAC cells with 25 μg/ml lectin (b and d)
Cell cycle analysis by FACS
Cell cycle of each cell has three phases G0/G1, S, and G2/M. The effect of Sheel lectin on the different phases of cell cycle of EAC cells was studied by using FACS flow cytometry. The percentages of G0/G1, S, and G2/M phases were calculated to be 62.5, 7.5, and 30.0 %, respectively after treatment of EAC cells with Sheel lectin. Whereas in untreated EAC cells 66.16, 17.2, and 16.6 % were determined for G0/G1, S, and G2/M phases, respectively. The above results revealed that the lectin arrested the G2/M phase of EAC cells (Fig. 6).
Different phases of EAC cells before and after treatment with lectin. The percentages of each cell cycle were evaluated by flow cytometry based on mean values obtained from three independent experiments. Results are expressed as means ± SD as shown in (a). b and c representing flow cytometry results for control and the effects of Sheel lectin on the cell cycle phases respectively. The amount of DNA directly proportional to the intensity of the PI staining is representing by the X-axis and the Y-axis representing cell number. **p <0.01 as compared with the control
Discussion
EAC cells may be a fine cell line for anticancer drug test due to the lack of H-2 histocompatibility antigens, which may be the reason for their rapid proliferation and for studying them in almost any mouse host [15]. In our previous study, it was observed that Sheel lectin inhibited 79.84 % EAC cells growth in vivo in mice when 1.38 mg/kg/day dose administered intraperitoneally for five consecutive days [1]. In the present study, cytotoxicity was examined against EAC cells in the RPMI-1640 medium by MTT assay and it was observed that Sheel lectin inhibited 36 % growth inhibition at 160 μg/ml protein concentration. The difference between in vitro and in vivo studies may be due to the length of treatment and like wheat germ agglutinin (WGA), Sheel lectin which is also an agglutinin may induce lectin-dependent macrophage-mediated cytotoxicity against EAC cells and can induce tumoricidal activity of blood monocytes in vivo [16, 17]. An opposite result also observed for pea lectin that caused 84 % EAC cell growth inhibition in vitro in RPMI-1640 medium whereas 63 % EAC cells growth inhibition was observed in vivo in mice [6].
Apoptosis is the process of programmed cell death with the change of cell morphology including nuclear fragmentation, blebbing, cell shrinkage, chromatin condensation, and DNA fragmentation. A group of proteases play essential role in the apoptosis process by a series of reaction is known as caspases. At least 12 caspases have been identified in humans which can be categorized as initiator caspases (e.g., Casp-2, −8, −9, and −10) and effector (executioner) caspases (e.g., Casp-3, −6, and −7). Initiator caspases cleaves the pro-forms of effector caspases and activates them. Then, activated effector caspases cleave other protein substance within the cell to trigger the apoptotic process. It was reported that lectin of different families induce apoptosis in various cancer cell lines [5, 7, 18–23]. In our earlier experiments, apoptotic morphological changes in EAC cells were observed for pea lectin and KRL [6, 24]. In the present study, when EAC cells were treated with Sheel lectin, cell shape was changed and the nucleus was fragmented and condensed; these are comparable with the control EAC cells. The initiation of caspase cascade reaction is regulated by different caspase inhibitors. Here, 36 % of cell growth inhibition was observed at 160 μg/ml of Sheel lectin while the inhibitory effects decreased to 7.2 and 22.3 % in the presence of caspase-3 and -8 inhibitors respectively in the medium. The above results suggest apoptotic-induced cell death of EAC cells in the presence of Sheel lectin.
Several genes are involved in apoptosis process and among them Bcl-2 family is predominantly known for the regulation of this process. Bcl-X is a member of the Bcl-2 family that is functionally similar to Bcl-2, which works together with Bax. The gene product of Bcl-X exists as Bcl-XL (long) and Bcl-XS (short) [25]. In the present study, when EAC cells were treated with lectin, Bcl-XL gene expression fully disappeared at the same time intensive genes expression of Bax was observed. Beside these, a significant increase of p53 gene expressions was determined in Sheel lectin-treated EAC cells that indicate p53 pathway is involved in apoptosis. Several lectin has the ability to agglutinate cancer cells like red blood cell and the affinity of lectin towards human cancer cell line is higher than that of healthy cells [23]. It was also reported that the link between membrane glycoproteins and lectins is weak, but a stronger one is formed by multiple binding sites of a lot weak joints and through this mechanism, lectins can induce apoptosis, cytotoxicity, and inhibition of tumor growth [18, 22]. In the present study, a very strong agglutination of EAC cells was observed when agitated with Sheel lectin. This strong agglutination suggests that the binding of lectin to the cell surface receptors of EAC cells. Binding of potato lectin to human hepatoma (H3B), mouse melanoma (B16), rat osteosarcoma (ROS), and human choriocarcinoma (JAr) cell lines were also reported [22].
The regulation of cell cycle dynamics of different phases is due to different factors and different mechanism [26]. Many antitumor agents arrest the cell cycle at the G0/G1, S, or G2/M phases and induce apoptotic cell death. The cytotoxic protein BMP1 effectively arrested cell cycle progression of EAC cells at the G1 phase [27]. Lectins also retain the ability to induce cell cycle arrest either in one or in a combination of different phases. It was reported that Musca domestica larva lectin (MLL) induced apoptosis by arresting at G1 phase in BEL-7402 cells [28]. Here, he observed Sheel lectin arrested G2/M phase of EAC cells cycle. In our earlier study it was observed that KRL and pea lectin inhibited EAC cells growth by induced G0/G1 and G2/M phases cell cycle arrest respectively and induced apoptosis, while Momordica charantia seeds lectin-induced G2/M phase cell cycle arrest without causing any apoptosis [6, 24, 29]. Induction of apoptosis by G2/M cell cycle arrest also reported for M. charantia seeds lectin in hepatocellular carcinoma [8] and Sophora flavescens lectin in MCF-7 cells [11].
In this present study, due to limitation of facilities, it was not possible to show the mitochondrial conditions but we found that the lectin strongly binds with rapidly growing EAC cells and caused apoptosis-related cell death as proved by different experiments. The lectin can be designated as a potent anticancer agent but further studies are necessary before any clinical trial.
References
Hasan I, Islam F, Ozeki Y, Kabir SR. Antiproliferative activity of cytotoxic tuber lectins from Solanum tuberosum against experimentally induced Ehrlich ascites carcinoma in mice. African J of Biotechnol. 2014;13:1679–85.
Ferriz-Martinez RA, Torres-Arteaga IC, Blanco-Labra A, Garcia-Gasca T. New Approaches in the Treatment of Cancer. New York: Nova Science; 2010. p. 71–89.
Kabir SR, Hasan I, Zubair MA. Lectins from Medicinal Plants: Characterizations and Biological Properties. In: Recent Progress in Medicinal Plants-Nutraceuticals & Functional Food (Vol 42). USA: Studium Press LLC; 2015. p. 339–56.
Lin P, Ye X, Ng T. Purification of melibiose-binding lectins from two cultivars of Chinese black soybeans. Acta Biochim Biophys Sin (Shanghai). 2008;40:1029–38.
Liu B, Bian HJ, Bao JK. Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Lett. 2010;287:1–12.
Kabir SR, Nabi MM, Haque A, Zaman RU, Mahmud ZH, Reza MA. Pea lectin inhibits growth of Ehrlich ascites carcinoma cells by inducing apoptosis and G2/M cell cycle arrest in vivo in mice. Phytomedicine. 2013;20:1288–96.
Liu B, Li CY, Bian HJ, Min MW, Chen LF, Bao JK. Antiproliferative activity and apoptosis-inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch Biochem Biophys. 2009;482:1–6.
Zhang CZ, Fang EF, Zhang HT, Liu LL, Yun JP. Momordica charantia lectin exhibits antitumor activity towards hepatocellular carcinoma. Invest New Drugs. 2015;33:1–11.
Panda PK, Mukhopadhyay S, Behera B, Bhol CS, Dey S, Das DN, et al. Antitumor effect of soybean lectin mediated through reactive oxygen species-dependent pathway. Life Sci. 2014;111:27–35.
Silva Fde O, Santos P, Figueirôa Ede O, de Melo CM, de Andrade Lemoine Neves JK, Arruda FV, et al. Antiproliferative effect of Canavalia brasiliensis lectin on B16F10 cells. Res Vet Sci. 2014;96:276–82.
Shi Z, Chen J, Li CY, An N, Wang ZJ, Yang SL, et al. Antitumor effects of concanavalin A and Sophora flavescens lectin in vitro and in vivo. Acta Pharmacol Sin. 2014;35:248–56.
Zhou W, Gao Y, Xu S, Yang Z, Xu T. Purification of a mannose-binding lectin Pinellia ternata agglutinin and its induction of apoptosis in Bel-7404 cells. Protein Expr Purif. 2014;93:11–7.
Hasan I, Ozeki Y, Kabir SR. Purification of a novel chitin-binding lectin with antimicrobial and antibiofilm activities from a Bangladeshi cultivar of potato (Solanum tuberosum). Indian J Biochem Biophys. 2014;51:142–8.
Islam F, Khanam JA, Khatun M, Zuberi N, Khatun L, Kabir SR, et al. P-menth-1-ene-4, 7-diol from Eucalyptus camaldulensis Dhnh. inhibitEhrlich Ascites Carcinoma (EAC) cells growth by apoptosis and G2/M cell cycle arrest. Phytother Res. 2015;29:573–81.
Chen L, Watkins JF. Evidence against the presence of H2 histocompatibility antigens in Ehrlich ascites tumour cells. Nature. 1970;225(5234):734–5.
Ogawara M, Utsugi M, Yamazaki M, Sone S. Induction of human monocyte-mediated tumor cell killing by a plant lectin, wheat germ agglutinin. Jpn J Cancer Res (Gann). 1985;76:1107–14.
Ogawara M, Sone S, Ogura T. Human alveolar macrophages: wheat germ agglutinin-dependent tumor cell killing. Jpn J Cancer Res (Gann). 1987;78:288–95.
Suen YK, Fung KP, Choy YM, Lee CY, Chan CW, Kong SK. Concanavalin A induced apoptosis in murine macrophage PU5-1.8 cells through clustering of mitochondria and release of cytochrome c. Apoptosis. 2000;5:369–77.
Hostanska K, Vuong V, Rocha S, Soengas MS, Glanzmann C, Saller R, et al. Recombinant mistletoe lectin induces p53-independent apoptosis in tumour cells and cooperates with ionising radiation. Br J Cancer. 2003;88:1785–92.
Kim MS, Lee J, Lee KM, Yang SH, Choi S, Chung SY, et al. Involvement of hydrogen peroxide in mistletoe lectin-II-induced apoptosis of myeloleukemic U937 cells. Life Sci. 2003;73:1231–43.
Miyoshi N, Koyama Y, Katsuno Y, Hayakawa S, Mita T, Ohta T, et al. Apoptosis induction associated with cell cycle dysregulation by rice bran agglutinin. J Biochem. 2001;130:799–805.
Wang H, Ng TB, Ooi VE, Liu WK. Effects of lectins with different carbohydrate-binding specificities on hepatoma, choriocarcinoma, melanoma and osteosarcoma cell lines. Int J Biochem Cell Biol. 2000;32:365–72.
Zhang ZT, Peng H, Li CY, Liu JJ, Zhou TT, Yan YF, et al. Polygonatum cyrtonema lectin induces murine fibrosarcoma L929 cell apoptosis via a caspase-dependent pathway as compared to Ophiopogon japonicus lectin. Phytomedicine. 2010;18:25–31.
Kabir SR, Reza MA. Antibacterial activity of Kaempferia rotunda rhizome lectin and its induction of apoptosis in Ehrlich ascites carcinoma cells. App Biochem Biotech. 2014;172:2866–76.
Gradilone A, Gazzaniga P, Ribuffo D, Scarpa S, Cigna E, Vasaturo F, et al. Survivin, bcl-2, bax, and bcl-X gene expression in sentinel lymph nodes from melanoma patients. J Clin Oncol. 2003;21:306–12.
Yan Q, Li Y, Jiang Z, Sun Y, Zhu L, Ding Z. Antiproliferation and apoptosis of human tumor cell lines by a lectin (AMML) of Astragalus mongholicus. Phytomedicine. 2009;16:586–93.
Bhattacharjee P, Giri B, Gomes A. Apoptogenic activity and toxicity studies of a cytotoxic protein (BMP1) from the aqueous extract of common Indian toad (Bufo melanostictus Schneider) skin. Toxicon. 2011;57:225–36.
Zhao Q, Cao X, Zeng B, Wang C, Yan L, Xu C. Musca domestica larva lectin induces apoptosis in BEL-7402 cells through a mitochondria-mediated reactive oxygen species way. Biol Pharm Bull. 2010;33:1274–8.
Kabir SR, Nabi MM, Nurujjaman M, Haque A, Mahmud ZH, Reza MA, et al. Momordica charantia seeds lectin: toxicity, bacterial agglutination and antitumor properties. App Biochem Biotech. 2015;175:2616–28.
Acknowledgement
This research work was funded by the Faculty of Science, Rajshahi University and Ministry of Science and Technology (Grant No. 39.009.002.01.00.053.2014-2015/MEDI’S-165), Bangladesh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interests.
Rights and permissions
About this article
Cite this article
Kabir, S.R., Rahman, M.M., Amin, R. et al. Solanum tuberosum lectin inhibits Ehrlich ascites carcinoma cells growth by inducing apoptosis and G2/M cell cycle arrest. Tumor Biol. 37, 8437–8444 (2016). https://doi.org/10.1007/s13277-015-4735-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4735-x