Abstract
In last three decades, several studies were carried out on the d-galactose-specific lectin of Momordica charantia seeds (MCL). In the present study, in vitro growth inhibition (8–23 %) at different concentrations (6–24 μg/ml) of MCL was observed against Ehrlich ascites carcinoma (EAC) cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MCL also showed 28, 45, and 75 % growth inhibitions against EAC cells when administered 1.2, 2.0, and 2.8 mg/kg/day (i.p.), respectively for five consequent days in vivo in mice. After lectin treatment, the level of red blood cell and hemoglobin was increased significantly with the decrease of white blood cell and maintained the normal level when compared with EAC-bearing control and normal mice without EAC cells. Although MCL caused cell cycle arrest at G0/G1 phase of EAC cells, any irregular shape or apoptotic morphological alterations in the lectin-treated EAC cells was not observed by an optical and fluorescence microscope. Lectin showed toxicity against brine shrimp nauplii with an LC50 value of 49.7 μg/ml. Four out of seven pathogenic bacteria were agglutinated by MCL in the absence of inhibitory sugar d-lactose/d-galactose. In conclusion, MCL showed strong cytotoxic effect and therefore can be used as a potent anticancer chemotherapeutic agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lectins are the heterogeneous group of proteins of non-immune origins that bind reversibly to mono-and oligosaccharides with high specificity and have no catalytic activity [1]. Plant lectins, a unique group of proteins and glycoproteins with potent biological activity, occur in foods like wheat, corn, tomato, peanut, kidney bean, banana, pea, lentil, soybean, mushroom, rice, and potato. Thus, a dietary intake of lectins by human is necessary [2]. Many lectins resist digestion, survive gut passage, and bind to gastrointestinal cells and/or enter the circulation intact to maintain full biological activity. Several lectins have been found to possess anticancer properties in vitro, in vivo, and in human case studies; they are used as therapeutic agents, preferentially binding to cancer cell membranes or their receptors, causing cytotoxicity, apoptosis, and inhibition of tumor growth [2]. In the recent years, accumulating evidence suggests that plant lectins have become an attractive research field due to their role in cell agglutination, toxicity, and antifungal, antibacterial, antiviral, antiproliferative, and antitumor effects [3–11].
Plant lectins are divided into 12 different families according to their different carbohydrate-binding specificities, such as (1) Agaricus bisporus agglutinin homologs, (2) amaranthins, (3) class V chitinase homologs with lectin activity, (4) cyanovirin family, (5) EEA family, (6) GNA family, (7) proteins with hevein domains, (8) jacalins, (9) proteins with legume lectin domains, (10) LysM domain, (11) Nictaba family, and (12) ricin B family [12]. Proteins with legume lectin domains have been most widely studied because of their presence in large quantities in the seeds. Proteins with legume lectin domain family have been widely reported to have a number of links to many pathological processes including cancer [5].
Momordica charantia lectin (MCL) is a galactose-specific tetrameric glycoprotein with a Mr of 120 kDa and α2β2-type subunit architecture, with the subunit masses being 28 and 30 kDa [13] that was found in the seeds of bitter gourd and has been the subject of active research for the past three decades. Partial N-terminal sequencing of MCL exhibits about 40 and 30 % homology to the lectins from Sambucus nigra and Ricinus communis, respectively [14]. MCL also exhibits strong type 1 and weak type 2 ribosome-inactivating protein activities as well as insulinomimetic activity [15–17]. The macromolecular properties and carbohydrate binding specificities of this lectin towards monosaccharides and disaccharides have been investigated elaborately [14, 18, 19]. Using fluorescence spectroscopy and isothermal titration calorimetry, thermodynamic parameters associated with the binding of different carbohydrates to MCL have been characterized [19–21].The results obtained indicate that carbohydrate binding to MCL is governed primarily by enthalpic forces. MCL contains 36 % β-sheet, 21 % β-turns, 13 % α-helix, and the rest are unordered structure as determined by circular dichroism [22]. The differential scanning calorimetric and spectroscopic studies of MCL suggested that the structure of the protein was stable over a wide pH range [23] and crystallization and preliminary X-ray studies of MCL were also reported [24]. Although several chemical and biological activities have been reported on MCL, the anticancer property against Ehrlich ascites carcinoma (EAC) cells along with its antibacterial and toxicity activities against different organisms or cell lines remains unknown. In this study, for the first time, we are reporting the anticancer activity of MCL against EAC cells in vivo in mice, bacterial agglutination activity, and toxicity against brine shrimp nauplii.
Materials and Methods
Preparation of MCL
M. charantia seeds were sun dried and crushed into powder, which was subsequently washed with n-hexane to remove fat. Fat-free dried powder was then dissolved in 10 mM Tris–HCl buffer and centrifuged at 10,000 g for 30 min. The supernatant was then added to 20 ml of lactose-sepharose (lactose linked to epoxy-activated sepharose-4B) previously equilibrated with 10 mM of Tris–HCl buffer at pH 8.2. NaCl and MnCl2 salt solutions were added to the gel to the final concentration of 150 and 10 mM, respectively, and kept overnight at 4 °C with shaking for effective binding of lectin. A column was prepared with the gel, and the unbound proteins were washed out by 10 mM of Tris–HCl buffer saline (TBS) at pH 8.2 containing 2 mM of MnCl2. Lectin was eluted from the column by TBS containing 0.2 M lactose. The eluted fraction was dialyzed against 10 mM of Tris–HCl buffer containing 1 mM of MnCl2. Purity of the eluted protein was assessed by PAGE where it gave a single band, consistent with earlier reports [24]. Protein concentration was determined by the Lowry method [25], and the lectin activity was assessed by the hemagglutination assay as described previously [9].
MTT Colorimetric Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) colorimetric assay was used to detect EAC cell proliferation. EAC cells (4.5 × 105 in 200 μl RPMI-1640 medium) were plated in the 96-well flat-bottom culture plate in the presence and absence of different concentrations of MCL (6–24 μg/ml) and incubated for at 37 °C in CO2 incubator for 24 h. An aliquot was removed from each well, and 180 μl of phosphate buffer saline (PBS) and 20 μl of MTT (5 mg/ml) were added to each well and incubated at 37 °C. After 8 h, the aliquot was removed again and 200 μl of acidic isopropanol was added into each well. The plate was agitated for 5 min and incubated for 1 h at 37 °C. Absorbance was taken at 570 nm using a titer plate reader, and cell proliferation inhibition ratio was calculated as follows:
where A is optical density (OD)570 nm of the cellular homogenate (control) without MCL and B is OD570 nm of the cellular homogenate with MCL.
Trypan Blue Exclusion Assay
EAC cell proliferation in vitro and in vivo was determined by trypan blue exclusion assay. Exactly 20 μl of cell suspensions was stained with an equal volume of trypan blue (0.4 % in 10 mM phosphate buffer saline) for 2 min. Then the numbers of viable cells were counted by light microscopy. Cells that retained a blue color were considered as dead cells. The proliferation inhibition ratio was calculated by the following equation:
Determination of Ehrlich Ascites Carcinoma Cell Growth Inhibition in Mice
EAC cells were collected from a donor Swiss albino mouse bearing 6–7-day-old ascites tumors, and the viability was observed by trypan exclusion assay. EAC cells (1 × 106) in 0.1 ml normal saline showing about 99 % viability were injected intraperitoneally to each Swiss albino mouse. After 24 h of tumor inoculation, the mice were randomly distributed into four groups (six mice per group). Three groups of mice were treated intraperitoneally with MCL at the doses of 2.8, 2.0, and 1.2 mg/kg/day, respectively, for five consecutive days. The remaining group was used as the control. Mice in each group were sacrificed on the seventh day of EAC cell inoculation, and the total intraperitoneal tumor cells were harvested by normal saline and counted by light microscopy. Then the total numbers of viable cells in every mouse of the treated groups were compared with those of the control group (EAC treated only). Finally, the percent of inhibition was calculated by the formula mentioned in section “Trypan Blue Exclusion Assay.”
Hematological Parameters of Normal, EAC-Inoculated, and MCL-Treated EAC-Bearing Mice
EAC cells were injected in 18 mice as described above, and six were kept as control for hematological parameter. After 24 h, 18 mice were distributed in three groups and two groups were treated with MCL for 10 days at 2.8 and 2.0 mg/kg/day of doses. The rest of the group was used as EAC-bearing control. On the 12th day, blood was drawn from the tail of each mouse. The percentage of hemoglobin was measured by a hematometer. Total white blood cell (WBC) and red blood cell (RBC) were counted by light microscopy.
Cell Morphology Study by Optical and Fluorescence Microscopy
EAC cells were collected from MCL-treated and untreated mice (2.8 mg/kg/day; five consecutive days) and washed thrice with PBS. Cells were then stained with 0.1 μg/ml of Hoechst 33342 at 37 °C for 20 min in the dark and washed again with PBS. Finally, cell morphology was observed under a fluorescence and optical microscope (Olympus iX71, Korea).
Cell Cycle Analysis
EAC cells were collected from MCL-treated (2.0 mg/kg/day; five consecutive days) and untreated mice and washed thrice with cold PBS. The cells were then fixed with 70 % ethanol for about 24 h at 4 °C and washed three times with cold PBS. Before analysis by flow cytometry, the cells in 1 ml PBS were treated with 50 μg of RNase A for 30 min at 37 °C followed by staining with 5 μg of propidium iodide in the dark at 4 °C for 5 min. The fractions of cells in G0/G1, S, and G2/M phases were analyzed by a FACS flow cytometer (Partec CyFlow SL, Germany).
Brine Shrimp Nauplii Lethality Assay
Lethality assay of MCL lectin against brine shrimp nauplii (Artemia salina L.) was carried out according to Kabir et al. [8] at the protein concentration ranging from 12.5 to 200 μg/ml, and the percentage of mortality of the nauplii was calculated for each concentration. The LC50 values were determined using Probit analysis as described by Finney [26].
Bacterial Agglutinating Activity Assay
Escherichia coli, Listeria monocytogenes, Shigella flexneri, Staphylococcus aureus, Salmonella typhi, Shigella boydii, and Salmonella enteritidis were used to check bacterial agglutination activity. At first, bacteria were grown at 37 °C overnight in nutrient broths (liquid nutrient medium), and the bacteria were collected by centrifugation at 1027 g for 5 min. Then the bacteria were washed with 10 mM TBS (pH 7.8) and re-suspended in the same buffer, and the optical density was adjusted around 2.0 at 630 nm. Precisely 50 μl of each bacterial suspension was mixed with a serial dilution of MCL (0.5 mg/ml) to get a final volume of 100 μl in 96-well microtiter plates. The plates were agitated and kept at room temperature for 2 and 60 min, respectively. Finally, bacterial agglutinating activity was monitored using a light microscope. Bacterial agglutination inhibition was studied by the method above in the presence of d-lactose/d-galactose.
Result
MTT Assay
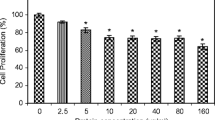
MTT assay was used to investigate the effect of MCL in vitro on EAC cells. Lectin-induced EAC cell death was in a dose-dependent manner as shown in Fig. 1. At a concentration of 24 μg/ml, the inhibitory effect of the protein was 23 %. When protein concentration was decreased gradually, the inhibitory effect was also reduced to 15 and 8 % at 12 and 6 μg/ml, respectively.
MCL Inhibits the Growth of EAC Cells
EAC cell proliferation in mice was effectively inhibited with MCL treatment in a dose-dependent manner. At 1.2 mg/kg/day, growth inhibition of EAC cells was 28 % and the inhibition increased to 45 and 75 % at 2.0 and 2.8 mg/kg/day of protein concentrations, respectively (Fig. 2).
RBC and Hemoglobin Level of Normal, EAC-Inoculated, and MCL-Treated EAC-Bearing Mice
Different hematological parameters were observed among the normal, tumor-bearing, and MCL-treated tumor-bearing mice. The total RBC of EAC-bearing control and normal mice were 1.35 × 109 and 2.5 × 109/ml, respectively. After treatment with 2.0 and 2.8 mg/kg/day lectin, the RBC level of EAC-bearing mice increased towards the normal. The difference in concentration did not affect the result. On the other hand, WBC of EAC-bearing control mice and normal mice was 53 × 106 and 20 × 106/ml, respectively. When the mice were treated with 2.0 mg/kg/day lectin, WBC level decreased significantly and it went towards the normal at 2.8 mg/ml protein concentration. Hemoglobin level of the EAC-bearing mice and normal mice were 5.8 and 10.0 g/dl, respectively. After treatment with lectin (2 mg/kg/day), the percent of hemoglobin of EAC-bearing mice increased significantly (i.e., around 9.6 g/dl) to the normal level as shown in Fig. 3.
Cell Cycle Analysis
The cell cycle was analyzed by FACS, and the healthy EAC cells exhibited three phases, G0/G1, S, and G2/M. The percentages of G0/G1, S, and G2/M phases were 58.5, 21.2, and 20.3 %, respectively, in the untreated EAC cells. But the G0/G1 and S phases increased to 71 and 26 %, respectively, with the decreased of G2/M phase to 3 % after treatment with MCL (Fig. 4). The result demonstrated that MCL inhibited the cellular proliferation of EAC cells via G0/G1 phase arrest.
Cell cycle analysis of MCL-treated EAC cells. a Bar diagram showing the percentages of each cell cycle evaluated by flow cytometry based on mean values obtained from three independent experiments, and the results are expressed as means ± SD. Histogram representing flow cytometry results of b control and showing the effects c of MCL on the cell cycle phase. The x-axis (FL3) is representing the intensity of the PI staining which is directly proportional to the amount of DNA in cells and y-axis representing the cell number
Cell Morphology Study by Optical and Fluorescence Microscopy
MCL-treated (2.8 mg/kg/day) and control EAC cells were stained with Hoechst 33342 in order to observe the morphological changes. In control EAC cells, nuclei were round as observed by optical microscopy (Fig. 5a) and homogeneously stained with Hoechst 33342 (Fig. 5b). Any irregular shape or apoptotic morphological alterations (e.g., manifest fragmented DNA in nuclei, membrane, and nuclear condensation) were not observed by optical (Fig. 5c) and fluorescence microscopy (Fig. 5d) in the MCL-treated EAC cells.
Effects of MCL on the morphological changes of EAC cells. Cells were collected from nontreated EAC-bearing mice and MCL-treated EAC-bearing mice and stained with Hoechst 33342 and observed by optical and fluorescence microscopy (Olympus iX71). Control cells in a optical and b fluorescence; MCL-treated cells in c optical and d fluorescence
Brine Shrimp Nauplii Lethality Assay
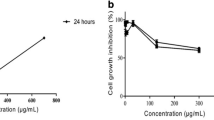
At the MCL concentration of 12.5–50 μg/ml, mortality rate was 30.6–36.5 % that increased to 60 % when the MCL concentration was increased to 100 μg/ml. At 200 μg/ml, the mortality rate increased to 90.6 % and the LC50 value was calculated to be 49.7 μg/ml (Fig. 6).
Bacterial Agglutinating Assay
MCL agglutinated two gram-positive bacteria (L. monocytogenes and S. aureus) and two gram-negative bacteria (E. coli and S. enteritidis), but did not agglutinate S. typhi, S. flexneri, and S. boydii in the absence of inhibitory sugar. In the presence of inhibitory sugars, MCL did not show any agglutination activity against the four bacteria. The minimum concentration of MCL solution needed for the agglutination of each bacterium was summarized in Table 1.
Discussion
Several kinds of plant lectins provide the ability to stop multiplication of cancer cells [5–11, 27], and each lectin exhibits differences in their antiproliferative effect against tumor cell lines due to the differences in their sugar specificity [28]. Although several experiments were carried out to study the anticancer effect of lectins on different cancer cells [5], only a few reported against EAC cells in vivo in mice [6–10, 29, 30]. In the present study, in vitro growth inhibition (8–23 %) against EAC cells was observed in RPMI-1640 medium at different MCL concentrations (6–24 μg/ml) as determined by MTT assay, and the result showed that the inhibition was dose dependent. The antitumor effect of MCL was also evaluated on EAC-bearing mice. The result showed that MCL decreased the EAC cell growth by 28, 45, and 75 % at 1.2, 2.2, and 2.8 mg/kg/day of protein concentrations, respectively. This result is significant when compared with EAC cell growth inhibition results of Kaempferia rotunda lectin (KRL), Nymphaea nouchali tuber lectin (NNTL), snake gourd seed lectin (TCSL), and pea lectin [6–9]. The toxicity of MCL on mice was checked previously by the intraperitoneal route, and the LD50 value was calculated to be 316 ± 1.4 μg/ml [16]. It was also reported that MCL partly inhibited the protein synthesis of Yoshida ascites cells at 100 μg/ml of concentration. When the concentration was decreased to 10 μg/ml, inhibition was not observed [16]. Therefore, the doses applied for this antitumor study would be mildly toxic for mice. In tumor-bearing mice, anemia occurs due to the decrease in RBC or hemoglobin percentage. Administration of MCL at a 2 mg/kg/day dose in EAC-bearing mice restored the hemoglobin content, and a 2.8 mg/kg/day MCL dose reinstates the total RBC and WBC content close to the normal level.
The regulation of cell cycle dynamics of different phases is due to different factors and different mechanisms. Many antitumor agents and DNA-damaging agents induce apoptotic cell death by arresting the cell cycle at the G0/G1, S, or G2/M phase. Lectin can also induce cell cycle arrest either in one or a combination of different phases during apoptosis. In this study, MCL induced cell cycle arrest of EAC cells in G0/G1 phase. It was found that pea lectin induced cell cycle arrest in EAC cells at G2/M phase [9]. On the other hand, K. rotunda lectin induced cell apoptosis by arresting EAC cells at G0/G1 phase [10]. Not only lectins but also other cytotoxic proteins can induce cell cycle arrest, e.g., cytotoxic protein BMP1 effectively arrested cell cycle progression of EAC cells at the G1 phase [31]. Cape aloe [32], Ottelione A [33], and Paullinia cupana Mart. var. sorbilis, Guarana [34] also arrest EAC cells at G1 cell cycle phase. Apoptosis is intrinsic cell suicidal mechanisms that can be regulated by numerous cellular signaling pathways and characterized morphologically by cell shrinkage, apoptotic body formation, and condensation of chromatin. These morphological changes and death of apoptotic cells are caused by a series of proteases termed caspases, such as caspase-3, -6, -7, -8, and -9. In our previous study, it was observed that pea lectin and K. rotunda lectin [9, 10] remarkably changed the EAC cell shape and nuclear morphology. However, in this study, such type of any change was not observed. The said results suggested that MCL-induced cell growth inhibition was not the effect of an apoptosis cell death.
Some lectins are toxic and showed toxicity against brine shrimp nauplii. The mortality rate of brine shrimp nauplii was raised by the increase of the concentration of lectin. Here, 50 % mortality (LC50) of the nauplii occurred at a 49.7 μg/ml concentration of MCL, whereas LC50 of mannose-specific mulberry seed lectin (MSL) and K. rotunda lectin was 21.87 and 18 ± 6 μg/ml [6, 35], respectively. The results revealed that MCL is lower toxic than mulberry seed lectin and K. rotunda lectin but higher than N. nouchali tuber lectin (LC50 value of 120 ± 29 μg/ml [7] and snake gourd seed lectin (LC50 value of 261 ± 29 μg/ml) [8].
Agglutination of several bacterial strains confirmed the interaction between the lectins and the strains. We found that L. monocytogenes and E. coli were the most sensitive to MCL as they were agglutinated at 3 μg/ml of protein concentration. On the other hand, S. enteritidis and S. aureus were agglutinated at 100 μg/ml of protein concentration. Thus, MCL agglutinated both gram-positive and gram-negative bacteria. These results indicated that MCL recognized the surface molecules on both gram-positive and gram-negative bacteria. The bacterial agglutination might be due to the presence of cognate glycan antigen on the cell surface of induced bacteria. Bacterial agglutination was also reported for some other plant lectins. In the absence of sugar, K. rotunda lectin, N. nouchali tuber lectin, snake gourd seed lectin, and Eugenia uniflora L. seed lectin also agglutinated both gram-positive bacteria and gram-negative bacteria [6–8, 36]. In the presence of sugar, MCL did not show any agglutination activity. A similar result was also observed in the case of K. rotunda lectin and snake gourd seed lectin [6, 8]. The inhibition study suggested that bacterial agglutination occurred by the interaction of lectin with bacterial surface carbohydrates [37].
In conclusion, MCL inhibited the growth of EAC cells in vitro and in vivo in mice with the increase of hemoglobin, RBC, and decrease of WBC towards the normal level through G0/G1 phase cell cycle arrest. Lectin agglutinated some pathogenic bacteria and showed toxicity against brine shrimp nauplii. MCL might be a possible candidate of potent anticancer chemotherapeutic agents.
References
Lis, H., & Sharon, N. (1998). Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chemical Reviews, 98, 637–674.
De Mejía, E. G., & Prisecaru, V. I. (2005). Lectins as bioactive plant proteins: a potential in cancer treatment. Critical Reviews in Food Science and Nutrition, 45, 425–445.
Sitohy, M., Doheim, M., & Badr, H. (2007). Isolation and characterization of a lectin with antifungal activity from Egyptian Pisum sativum seeds. Food Chemistry, 104, 971–979.
Tian, Q., Wang, W., Miao, C., Peng, H., Liu, B., Leng, F., Dai, L., Chen, F., & Bao, J. (2008). Purification, characterization and molecular cloning of a novel mannose-binding lectin from rhizomes of Ophiopogon japonicus with antiviral and antifungal activities. Plant Science, 175, 877–884.
Liu, B., Bian, H.-J., & Bao, J.-K. (2010). Plant lectins: potential antineoplastic drugs from bench to clinic. Mini-review. Cancer Letters, 287, 1–12.
Kabir, S. R., Hossen, M. A., Zubair, M. A., Alom, M. J., Islam, M. F., Hossain, M. A., & Kimura, Y. A. (2011). A new lectin from the tuberous rhizome of Kaempferia rotunda: isolation, characterization, antibacterial and antiproliferative activities. Protein and Peptide Letters, 18, 1140–1149.
Kabir, S. R., Zubair, M. A., Nurujjaman, M., Haque, M. A., Hasan, I., Islam, M. F., Hossain, M. T., Hossain, M. A., Rakib, M. A., Alam, M. T., Shaha, R. K., Hossain, M. T., Kimura, Y., & Absar, N. (2011). Purification and characterization of a Ca2+-dependent novel lectin from Nymphaea nouchali tuber with antiproliferative activities. Bioscience Reports, 31, 465–475.
Kabir, S. R., Islam, M. F., Alom, M. J., Zubair, M. A., & Absar, N. (2012). Purification, characterizations of a snake guard seeds lectin with antitumor activity against Ehrlich ascites carcinoma cells in vivo in mice. Protein and Peptide Letters, 19, 360–368.
Kabir, S. R., Nabi, M. M., Haque, A., Zaman, R. U., Mahmud, Z. H., & Reza, M. A. (2013). Pea lectin inhibits growth of Ehrlich ascites carcinoma cells by inducing apoptosis and G2/M cell cycle arrest in vivo in mice. Phytomedicine, 20, 1288–1296.
Kabir, S. R., & Reza, M. A. (2014). Antibacterial activity of Kaempferia rotunda rhizome lectin and its induction of apoptosis in Ehrlich ascites carcinoma cells. Applied Biochemistry and Biotechnology, 172, 2866–2876.
Rafiq, S., Majeed, R., Qazi, A. K., Ganai, B. A., Wani, I., Rakhshanda, S., Qurishi, Y., Sharma, P. R., Hamid, A., Masood, A., & Hamid, R. (2013). Isolation and antiproliferative activity of Lotus corniculatus lectin towards human tumour cell lines. Phytomedicine, 21, 30–38.
Van Damme, E. J. M., Peumans, W. J., Barre, A., & Rougé, P. (1998). Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. C.R.C. Critical Reviews in Plant Sciences, 17, 575–692.
Mazumder, T., Gaur, N., & Surolia, A. (1981). The physicochemical properties of the galactose-specific lectin from Momordica charantia. European Journal of Biochemistry, 113, 463–470.
Wang, H., & Ng, T. B. (1998). Ribosome inactivating protein and lectin from bitter melon (Momordica charantia) seeds: sequence comparison with related proteins. Biochemical and Biophysical Research Communications, 253, 143–146.
Barbieri, L., Lorenzoni, E. F., & Stripe, F. (1979). Inhibition of protein synthesis in vitro by a lectin from Momordica charantia and by other haemagglutinins. Biochemical Journal, 182, 633–635.
Barbieri, L., Zamboni, M., Lorenzoni, E., Montanaro, L., Sperti, S., & Stripe, F. (1980). Inhibition of protein synthesis in vitro by proteins from the seeds of Momordica charantia (bitter pear melon). Biochemical Journal, 186, 443–452.
Ng, T. B., Wong, C. M., Li, W. W., & Yeung, H. W. (1986). Isolation and characterization of a galactose binding lectin with insulinomimetic activities. From the seeds of the bitter gourd Momordica charantia (Family Cucurbitaceae). International Journal of Peptide and Protein Research, 28, 163–172.
Padma, P., Komath, S. S., & Swamy, M. J. (1998). Fluorescence quenching and time-resolved fluorescence studies on Momordica charantia (bitter gourd) seed lectin. Biochemistry and Molecular Biology International, 45, 911–920.
Khan, M. I., Mazumder, T., Pain, D., Gaur, N., & Surolia, A. (1981). Binding of 4-methylumbelliferyl beta-D-galactopyranoside to Momordica charantia lectin: fluorescence-quenching studies. European Journal of Biochemistry, 113, 471–476.
Sultan, N. A. M., & Swamy, M. J. (2003). Thermodynamic analysis of binding of 4-methylumbelliferyl-α- and β-d-galactopyranosides to Momordica charantia lectin. Current Science, 84, 200–203.
Sultan, N. A. M., & Swamy, M. J. (2005). Energetics of carbohydrate binding to Momordica charantia (bitter gourd) lectin: an isothermal titration calorimetric study. Archives of Biochemistry and Biophysics, 437, 115–125.
Sultan, N. A. M., Maiya, B. G., & Swamy, M. J. (2004). Thermodynamic analysis of porphyrin binding to Momordica charantia (bitter gourd) lectin. European Journal of Biochemistry, 271, 3274–3282.
Kavitha, M., Bobbili, K. B., & Swamy, M. J. (2010). Differential scanning calorimetric and spectroscopic studies on the unfolding of Momordica charantia lectin. Similar modes of thermal and chemical denaturation. Biochimie, 92, 58–64.
Chandran, T., & Sharma, A. (2010). M. Acta Vijayan. Crystallization and preliminary X-ray studies of a galactose-specific lectin from the seeds of bitter gourd (Momordica charantia). Acta crystallographica. Section F, Structural biology and crystallization communications, 66, 1037–1040.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Finney, D. J. (1971). Probit analysis (p. 333). London: Cambridge University Press.
Kheeree, N., Sangvanich, P., Puthong, S., & Karnchanatat, A. (2010). Antifungal and antiproliferative activities of lectin from the rhizomes of Curcuma amarissima Roscoe. Applied Biochemistry and Biotechnology, 162, 912–925.
Yan, Q., Zhu, L., Kumar, N., Jiang, Z., & Huang, L. (2010). Characterisation of a novel monomeric lectin (AML) from Astragalus membranaceus with anti-proliferative activity. Food Chemistry, 122, 589–595.
Ahmed, H., Chatterjee, B. P., & Debnath, A. K. (1988). Interaction and in vivo growth inhibition of Ehrlich ascites tumor cells by jacalin. Journal of Biosciences, 13, 419–424.
Akev, N., Turkay, G., Can, A., Gurel, A., Yildiz, F., Yardibi, H., Eki, E. E., & Uzun, H. (2007). Tumour preventive effect of Aloe vera leaf pulp lectin (Aloctin I) on Ehrlich ascites tumours in mice. Phytotherapy Research, 21, 1070–1075.
Bhattacharjee, P., Giri, B., & Gomes, A. (2011). Apoptogenic activity and toxicity studies of a cytotoxic protein (BMP1) from the aqueous extract of common Indian toad (Bufo melanostictus schneider) skin. Toxicon, 57, 225–236.
Kametani, S., Oikawa, T., Kojima-Yuasa, A., Kennedy, D. O., Norikura, T., Honzawa, M., & Matsui-Yuasa, I. (2007). Mechanism of growth inhibitory effect of Cape aloe extract in ehrlich ascites tumor cells. Journal of Nutritional Science and Vitaminology, 53, 540–546.
El-Missiry, M. A., Othman, A. I., Amer, M. A., & Mohamed, E. (2012). Ottelione A inhibited proliferation of Ehrlich ascites carcinoma cells in mice. Chemico-Biological Interactions, 200, 119–127.
Fukumasu, H., Latorre, A. O., & Zaidan-Dagli, M. L. (2011). Paullinia cupana Mart. var. sorbilis, guarana, increases survival of Ehrlich ascites carcinoma (EAC) bearing mice by decreasing cyclin-D1 expression and inducing a G0/G1 cell cycle arrest in EAC cells. Phytotherapy Research, 25, 11–16.
Absar, N., Yeasmin, T., Raza, M. S., Sarkar, S. K., & Arisaka, F. (2005). Single step purification, characterization and N-terminal sequences of a mannose specific lectin from mulberry seeds. Protein Journal, 24, 369–377.
Oliveira, M. D. L., Andrade, C. A. S., Santos-Magalha, N. S., Coelho, L. C. B. B., Teixeira, J. A., Carneiro-da-Cunha, M. G., & Correia, M. T. S. (2008). Purification of a lectin from Eugenia uniflora L. seeds and its potential antibacterial activity. Letters in Applied Microbiology, 46, 371–376.
Ghanekar, A., & Pérombelon, M. C. M. (1980). Interactions between potato lectin and some phytobacteria in relation to potato tuber decay caused by Erwinia carotovora. Journal of Phytopathology, 98, 137–149.
Acknowledgments
This research work was funded by the Ministry of Science and Technology (sanction order no. 39.009.006.01.00.042.2012-2013/BS-67/401), Bangladesh.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kabir, S.R., Nabi, M.M., Nurujjaman, M. et al. Momordica charantia Seed Lectin: Toxicity, Bacterial Agglutination and Antitumor Properties. Appl Biochem Biotechnol 175, 2616–2628 (2015). https://doi.org/10.1007/s12010-014-1449-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1449-2