Abstract
Cancer is the second death causing disease all over the world and until today 100 different types of cancer have been identified whose treatment methods consist of serious side effects on human body. To reduce the frequency of adverse effects of cancer treatment, nowadays plant derived natural components are getting priority. The plant Morus latifolia is widely available in northern part of Bangladesh. The earlier researches suggested that popular varieties of some Morus sp. like Morus alba, Morus indica etc. have good anti-proliferative activity. Hence, this study was designed to evaluate the anti-proliferative activity of leaf and bark extracts of M. latifolia against Ehrlich’s ascites carcinoma (EAC) in vivo. The leaf and bark extracts of M. latifolia were used in several bioassays including Brine shrimp lethality test, hemagglutination activity test, antioxidant activity test, and cell growth inhibition test. Besides, fluorescence microscopy was performed to study apoptotic features in EAC cells, and molecular analysis like real-time PCR were also conducted. The results of Brine shrimp lethality test, hemagglutination activity test, and antioxidant activity assay supported the cell growth inhibition capability of leaf and bark extracts which was confirmed by in vivo cell growth inhibition bioassay. Moreover, the experimental extracts were able to induce cell apoptotis through altering the expression pattern of Bcl-2 and Bax genes. All of the results of this study suggest that several noble compounds are present in M. latifolia plant extracts which are capable for healing cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morus latifolia is a shrub type plant growing as wild or under cultivation in many temperate regions of the world that is known as “Mulberry” in English and “Tut gach” in Bengali. The green leaves of this plant are broadly used in silk industries specially in feeding the silkworms in several countries [1]. It is belonging to the Moraceae family with Morus genus which comprises another 10–16 species including M. laevigata, M. alba, M. indica, M. rubra, M. nigra etc. It is fast growing plant with 15–20 feet long normally and 35–40 feet long in rare cases [2].
The Morus sp. plants are rich in a number of ayurvedic properties and medicinal components including vitamin-C, phenolic acid, iron, tannin etc. [3]. In Indian subcontinent and China, the ayurvedic properties of plants are thoroughly used against different kinds of disorders and diseases from the ancient time to today. Moreover, it has a tremendous contribution in modern medical sciences to diminish the human difficulties [4,5,6]. The M. latifolia plant is abundant with secondary metabolites called anthocyanin which is one of most excellent materials to many scientists for research on different human abnormalities or diseases especially on cancer [7].
Cancer is a class of diseases with unusual cell growth due to fail in controlling the cell cycle. It also involve in unorganized and unidentified cell division and destruction of massive human life [8]. A statistics reported that in the year of 2012, about 8.2 millions of people were died worldwide and about 14.1 millions of new people were diagnosed with cancer. But these frequencies are gradually increasing day by day [9]. Though cancer is a devastating and leading life threatening disease, its treatment outcomes are still now so poor with various adverse side effects. Due to severe drawbacks of cancer treatment, scientists are still working to look for plant derived natural compounds to treat cancer with comparatively lower side effects [10, 11].
Plants are strongly considered as an important natural resources of phytochemicals with different efficient bio-activities that offer as drugs against several types of disorders and diseases like cardiovascular disease, neurodegenerative disorders, inflammation, cancer etc. [12, 13]. About 70–80% of world population is directly or indirectly depends on plants materials as their major treatment source and over 60% of antitumor supplements are generated from a number of medicinal plants or plant parts. For these pharmacological activities, plant samples become of a great interest on bio-medical research for human benefit [14].
Anything that may cause to develop unusual potentiality in a normal body cell is responsible for cancer. Generally, different kinds of physical and chemical agents or activities including radiation, smoking, some pathogens, obesity etc. play a vital role for cancer development by inducing DNA damages as well as altering the normal sequence of human genetics [15,16,17]. The alteration of genome sequence is known as mutation and it can change the normal protein formation associated with regular cell division which subsequently create a mass of cells (tumor) as well as cancer [18]. In our body, this mutation can be done by the action of free radicals which are being produced during normal physiological processes and due to oxidative stress (OS). The antioxidant property of plant materials can remove excessive free radicals which lead to perform natural cell division and growth [19, 20].
Lectins are an important carbohydrate binding protein with several bioactivities like agglutination activity, cytotoxic activity, antimicrobial activity, anti-proliferative activity and so on. This protein is found in plant and animal as well as some kinds of microorganism including fungi, bacteria, viruses etc. It is proved by many researchers that plant lectin inhibits the cancer cell growth up to 30–60% and in rare case this proportion reached near about 80% [21, 22]. Moreover, these proteins are responsible for cancer cell agglutination as well as cancer cell aggregation. They can also deflect the normal cell cycle by altering the non-apoptotic G1 phase, arresting the G2/M phase, and ensuing cell apoptosis [23].
Apoptosis is a normal physiological process that allows removing abnormal or unwanted cells from the body through a series of programmed cell death. In normal body, it is very essential to keep the body fit and healthy by protecting from pathogen invaded cells, discarding inflammatory cells, and knocking out invasive cells [24]. The apoptosis process is regulated by the balanced function of different types of pro-apoptotic (Bax, p 53, Bak) and anti-apoptotic (Bcl-2, Bcl-X) genes. But the abnormal functions of these genes alter the natural cell apoptosis which triggers the cell division and cell proliferation in an irregular way leading to development of tumor as well as cancer [25]. However, induced apoptosis by plant based natural remedies are now being used frequently in cancer treatment [26].
Mulberry is a well known medicinal plant in Bangladesh and its several species have wonderful biological activities including antioxidant, cytotoxicity, anti-inflammatory, anti-diabetic, hepato-protective, anti-microbial, and anti-proliferation activity [27, 28]. But there is evident that no research work has been done earlier on leaf and bark extracts of M. latifolia in Bangladesh and that’s why these plant materials have been chosen for the current investigation. In this research work, the effects of selected extracts on EAC cells growth through apoptosis process have been concentrated by estimating the expression of apoptosis related genes like Bax and Bcl-2.
Materials and methods
Chemical and reagents
1,1-Diphenyl-2-picrylhydrazyl (DPPH), Sodium Chloride (NaCl), Sodium Citrate, Butylated hydroxyl toluene (BHT), Trypan blue, and 4, 6-diamidino-2-phenylindole (DAPI) were bought from Sigma Aldrich (USA). Hemagglutination buffer (20 mM Tris–HCl buffer, pH 7.8 containing 1% NaCl and 10 mM CaCl2) and Phosphate buffer saline (PBS) were prepared in our laboratory. RNAsimple Total RNA kit and M-MLV (Moloney Murine Leukemia Virus) reverse-transcriptase were purchased from Tiangen (Beijing, China). Primers were custom synthesized from IDT (Integrated DNA Technologies), Malaysia. GoTaq® qPCR Master Mix (2x) was purchased from Promega, USA. All other chemicals and reagents were of analytical grade.
Collection of plant materials and preparation of extracts
The experimental plant materials (fresh leaf and bark of M. latifolia) were collected from Bangladesh Sericulture Research and Training Institute (BSRTI), Rajshahi, Bangladesh. The plant samples were identified by Dr. Saidur Rahman, Chief Scientific Officer, BSRTI, Rajshahi, Bangladesh. The collected materials were then washed with distilled water for several times and kept for air drying. Later, the dry leaf and bark were cut into small size and allowed for drying in drier at 40 °C for 3 days and 10 days respectively. By using an electrical grinder machine (Jaipan, India), the experimental specimens were grinded into fine powder and preserved at room temperature. The resultant powders were then dissolved into methanol followed by keeping on a shaking (160 rpm) incubator at 37 °C for 24 h. Later, the samples were sonicated by using sonicator (Soniprep 150, China) at 10 kHz for 20 min and filtered with vacuum pump filtration system. The filtered materials were lyophilized and stored at 4 °C for further used.
Hemagglutination assay
Hemagglutination assay for detecting the presence of lectin and lectin like protein in leaf and bark extracts of M. latifolia was conducted in U-bottomed 96-well micro titer plate as stated by Kabir et al. [29] and Hasan et al. [30]. In brief, firstly the mouse blood was collected into a beaker with anti-coagulant reagent (sodium citrate), washed for several times by using PBS, and prepared as 2% red blood cells (RBCs) (v/v) in PBS. After that, the wells of micro titer plate were filled by 50 µl of leaf and bark extracts of M. latifolia which were serially diluted with same volume of hemagglutination buffer. Lastly, the 50 µl of 2% RBC suspension was added into every well of the titer plate and kept at room temperature for shaking. After 30 min, the titer plate was observed carefully and agglutination of maximum dilution was recorded which indicated the positive agglutination activity of the experimental extracts.
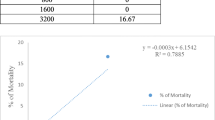
Brine shrimp lethality test
Brine shrimp lethality assay of leaf and bark extract of M. latifolia was performed by using shrimp nauplii (Artemia salina L) as described previously [29]. According to the method, initially the fresh brine shrimp eggs were taken into brine solution (pH = 7.0) prepared by dissolving 38 g of NaCl in 1 L distilled water for hatching. Then, 10 test tubes were taken with 10 different concentrations (25, 50, 75, 100, 125, 150, 175, 200, 225, and 250 µg/ml) of leaf and bark extracts separately and made the final volume up to 10 ml by adding brine solution. Subsequently, 20 immature brine shrimp nauplii were transferred into each test tube and the test tubes were kept under observation at room temperature. After 24 h, the proportion of mortality of the nauplii was evaluated for every concentration and the LC50 values were calculated by the Probit Analysis [31].
Antioxidant activity assay
Antioxidant activity of leaf and bark extracts were estimated by using DPPH free radical scavenging bioassay that is described as Brand-Williams et al. with a little changes [32]. Based on this protocol, stock solution of experimental extracts (1 mg/ml concentration) and methanolic solution of DPPH (1 mg/25 ml concentration) were prepared. Then, ten different concentrations (25, 50, 75, 100, 125, 150, 175, 200, 225 and 250 µg/ml) of stock solution were taken into ten different test tubes and made volume up to 1 ml by adding absolute methanol. After that, 1.5 ml of DPPH solution was added in each test tube and kept them in a dark place at room temperature to complete the reaction. After 30 min, the optical density of each solution was measured by using a spectrophotometer (GENESYS 10S UV–Vis, Thermo Scientific, USA) at 517 nm. The BHT, methanol, and DPPH solution were used as standard, blank, and control respectively. The scavenging percentage of DPPH was evaluated by using the following formula:
where A0 = absorbance of the control; A1 = absorbance of the leaf and bark extracts.
The 50% inhibition concentration (IC50) values was calculated by using regression line of gained scavenging percentages and corresponding concentrations.
Experimental design and ethical clearence
Throughout the study, about 5–6 weeks old, fresh and healthy Swiss Albino mice weighting 25 ± 2 g were used which were collected from the animal house of Department of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh. This experiment was authenticated by the members of IAMEBBC (Institutional Animal, Medical Ethics, Bio-safety and Bio-security Committee), Institute of Biological Sciences (IBSc), University of Rajshahi, Bangladesh for Experimentations on Animal, Human, Microbes and Living Natural Sources, Memo no. 31/320-IAMEBBC/IBSc.
The experimental animals were nurtured in an animal house with proper environmental condition (12 h dark and 12 h light cycle, 25 ± 2 °C temperature). The animals were provided with food and water ad libitum. The Ehrlich’s ascites carcinoma (EAC) cells used in this investigation were initially collected from Protein and Enzyme laboratory, Department of Biochemistry and Molecular Biology, University of Rajshahi, Bangladesh and there after these cells were propagated in intra-peritoneal cavity of fresh Swiss albino mouse. Before starting treatment, the mice were divided into mainly three (03) groups and they are expressed as control group, leaf treated group and bark treated group. The leaf and bark treated groups were again divided into three sub-groups namely group-1, group-2, and group-3 which were received 50, 100, and 200 mg/kg of experimental extracts respectively. Another group was used as standard (Bleomycin treated) that was used only for determination of cell growth inhibition. All of the groups contained 6 animals. The EAC cells were injected into all mice of each group at 1.72 × 106 cells/ml concentration by successive needle aspiration with maintaining suitable sterilized condition. After 24 h of cell inoculation, the treatments were begun for 5 consecutive days. Extracts were administered to mice by dissolving in distilled water.
Determination of EAC cell growth inhibition ratio
The in vivo growth inhibition of EAC cells was estimated using a standard method that narrated by Sur et al. [33]. For this study, all the extract treated mice were sacrificed after 24 h of last treatment and EAC cell was harvested from the intra-peritoneal cavity by using narrow needle. The collected EAC cells were diluted initially with normal saline water (1% NaCl) and then with trypan blue. The total number of viable cells was counted on the hemocytometer using trypan blue dye (0.5%) exclusion assay. The total viable cells in every mouse of all groups were calculated by following the equation:
The percentage of growth inhibition was measured by using following equation:
where Tw = average number of EAC cells of the treated mice group and Cw = average of number of EAC cells of the control mice group.
The group without any treatment and the group treated with Bleomycin (anti cancer drug) were used as control and standard.
Morphological observation of EAC cells
DAPI staining technique is one of the most promising methods to observe cellular morphology and this process was used in the present investigation to examine the alteration of EAC cells as well as cell apoptosis with slight modification [9]. In this method, the EAC cells were collected from peritoneal cavity of control and treated mice and kept into small glass tubes with 1% saline water. Then, 1 ml of isolated cells was taken into 1.5 ml ependrop tubes and washed with PBS for several times and every time the cells were centrifuged at 1200 rpm for 2 min. After washing well, 5 µl of DAPI staining solution was added into every microcentrifuge tubes and kept them in dark for 30 min. Later, 200 µl of PBS was taken into each test tube and again centrifuged at 1200 rpm for 2–3 min. Finally, the supernatant was removed and 200 µl of PBS was added for another time to prepare a number of slides for observing the morphological changes in EAC cells under fluorescence microscope (Olympus iX71, Korea).
Gene expression analysis
The gene expression analysis was carried out through real-time polymerase chain reaction (real-time PCR) technique. The total RNA was extracted from both control and treated mice EAC cells using RNAsimple Total RNA kit (Tiangen, Beijing, China) following the manufacturer’s instructions. The quantity of isolated RNA was measured by using a nano-drop spectrophotometer (NanoDrop 2000, Thermo Scientific, USA). The isolated RNA was reverse transcribed into cDNA in a final volume of 20 µl reaction mixture containing 3 µl total RNA, 2 µl of 10 mM oligo(dT), 2 µl of 10 mM dNTPs, 1 µl of TIANscript MMLV reverse transcriptase (Beijing, China), buffer 4 µl and 8 µl nuclease free water according to the company’s guidelines. The amplification of two genes Bcl-2 and Bax associated with tumor development was performed using the synthesized cDNA as template in real-time PCR technique where GAPDH gene was used as reference. The primer sequences for GAPDH, Bcl-2, and Bax given in Table 1. PCR reactions in all cases were performed in triplicate in 10 µl volume containing 5 μl GoTaq® qPCR Master Mix (2x) (Promega, USA), 0.5 μl (10 mM) of each primer, 3 μl nuclease free water and 1 μl template. Thermal cycling was carried out for specific genes on corresponding cDNA samples in 48-well reaction plates by using the Eco™ Real-Time PCR System (Illumine®, USA). PCR was conducted with the following cycling parameters: 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 52 °C for 30 s and 72 °C for 25 s. Reaction specificity was confirmed by melt curve analysis at 95 °C for 15 s, 60 °C for 15 s, 95 °C for 15 s. The ∆∆Cq method was used for the quantitation of gene expression using GAPDH as an endogenous control.
Statistical analysis
All of experiments were done for thrice (n = 3) except the cell growth inhibition study where n = 6. All the experimental results are represented as mean ± SD (Standard Deviation). The significance test was carried out by using SPSS-16 software following one way ANOVA by Dunnett Post hoc test by comparing with control. The levels of significant were set up at 5%, 1% and 0.1% level where *P < 0.05, **P < 0.01 and ***P < 0.001 respectively.
Results
Hemagglutination assay
The hemagglutination activity of leaf extract and bark extract of M. latifolia was determined to check the presence of lectin or lectin like protein that is related to inhibit cancer development. The minimum concentration of leaf and bark extract to agglutinate the 2% RBC of mouse was found as 1.56 µg/ml and 6.25 µg/ml respectively (Fig. 1). This result indicated that leaf extract contains more lectin protein and required little amount to agglutinate the RBC than bark extract.
Brine shrimp lethality test
In vitro cytotoxic activity of leaf and bark extract of M. latifolia at different concentrations was evaluated by using the brine shrimp lethality bioassay. The less toxic effects were appeared at lower concentration of experimental extract and it was increasing gradually at comparatively higher concentration (Fig. 2). The LC50 value for leaf and bark extract were 146 ± 12 µg/ml and 205 ± 18 µg/ml respectively. The higher LC50 values of leaf and bark extract expounded that they had less toxic effect.
Antioxidant activity assay
Antioxidant activity of leaf and bark extract was in dose-depended fashion (Fig. 3). The IC50 value of leaf and bark extracts were 98.58 ± 7.54 µg/ml and 233.08 ± 12.35 µg/ml respectively, whereas the IC50 value of standard (BHT) was 53.12 ± 5.24 µg/ml. Based on the result, leaf extract showed better activity than bark extract.
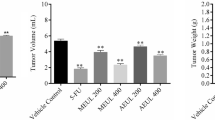
Determination of EAC cell growth inhibition ratio
The growth of EAC cell was effectively inhibited by the leaf and bark extract of M. latifolia in a dose-dependent manner. The total number of EAC cells/ml for control, Bleomycin, leaf, and bark treated mice are mentioned in Table 2. The viability of EAC cells considerably went down in all treated mice when compared with control group. After five consecutive days of treatment, the percentages of EAC cell growth inhibition were 42.3, 51.1, and 56 at 50, 100, and 200 mg/kg leaf extract and 36.6, 46.9, and 52.2 at 50, 100, and 200 mg/kg bark extract respectively. Whereas, treatment with Bleomycin demonstrated 79% growth inhibition. However, the leaf extract has relatively more anti-proliferative capacity than the bark extract.
Morphological observation of EAC cells
According to the fluorescence microscopic observation, the cells of control group mice were regular and round shaped whereas the apoptotic cells or on-going apoptotic cells were appeared with condensed chromatin, cell shrinkage, membrane bleebing, and nuclear degradation as a result of treatment with extracts. (Figure 4). The mean number of apoptotic cells/slide (Fig. 5) illustrates that the leaf extract exhibit comparatively more activity than bark extract.
Gene expression analysis
The Bcl-2 and Bax genes are related to cell apoptosis and in the current experiment, down regulation in Bcl-2 and up regulation in Bax were observed (Fig. 6). In case of bark treatment, expression of Bcl-2 was decreased by 0.69, 0.51, and 0.21 fold at 50, 100, and 200 mg/kg dose respectively. On the other hand, expression of Bax was increased by 1.61, 2.99, and 5.17 fold at 50, 100, and 200 mg/kg dose respectively. The expression of Bcl-2 was decreased by 0.77, 0.54, and 0.27 fold at 50, 100, and 200 mg/kg leaf extract respectively. Whereas expression of Bax was increased by 1.54, 2.75, and 4.81 fold due to treatment with leaf extract at 50, 100, and 200 mg/kg dose respectively.
Effect of bark and leaf extract of M. latifolia on expression pattern of Bcl-2 and Bax gene in EAC cells in vivo. In case of bark, A = Bcl-2 and C = Bax. On the other hand, in case of leaf, B = Bcl-2 and D = Bax. The results are represented as mean ± SD (n = 3). Significance levels were determined and set at *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control
Discussion
Cancer is one of the deadly diseases that characterized by abnormal and unorganized cell proliferation. There are over 100 different types of cancer including lungs, prostate, breast, liver, blood, brain, bone cancer etc. found in both developed and developing countries with massive economical losses [34, 35]. This life threatening disease is caused by both internal and external factors as well as alteration of DNA repair genes, oncogenes and tumor suppressor genes. These abnormalities of genes take place by free radicals that are generated by excessive OS through normal body’s functions [36]. The antioxidant property of phytochemicals plays a central role to balance the OS that remove the free radicals [37]. In this study, the leaf and bark extracts showed pleasant antioxidant activity compared to BHT (standard). But according to the results of IC50 value, the leaf extract showed better antioxidant activity than bark extract.
The brine shrimp lethality assay was performed to detect the cytotoxicity of experimented plant materials. The considerable LC50 values of plant specimens reported the suitability of further research and helped in considering the extracts as biologically potent [38, 39]. However, the results regarding brine shrimp lethality demonstrated considerable LC50 values indicating that they contain different kinds of biologically active compounds which can inhibit cellular growth.
Lectins are one kind of carbohydrates binding protein that normally found in whole plant or plant parts [40]. It is well known that the plant lectins are able to bind with cell membrane of different types of cancer cells leading to stun their multiplication [41, 42]. A few researches evident that the lectin has anti-proliferative activity against EAC cell line [43, 44]. However, the hemagglutination assay reported the minimal concentration of leaf and bark extracts to agglutinate RBC of mouse at 1.56 µg/ml and 6.25 µg/ml respectively. The result indicates that, the leaf extract contained more lectin protein than bark extract which support the higher EAC growth inhibitory effect of leaf extract.
The result of hemocytometric cell counting represented that the leaf and bark extract treatment suppressed EAC cell growth significantly. The leaf extract showed better EAC growth inhibitory effect than bark when they were compared with the inhibition frequency. These results proved that the anti-proliferation activity of leaf and bark extracts of M. latifolia is similar to other species of Morus genus as well as other natural resources like tea, honey etc. [19, 45, 46].
According to the results regarding the morphological study, several structural changes such as irregular shape, shrinked cell membrane, chromatin condensation etc. were appeared in EAC cells of extract treated mice, meanwhile regular round shaped cells were found in control mice. A significant number of abnormal cells were counted in treated mice compared to control mice. Therefore, it can be said that the experimental extracts deflect the normal structure of EAC cells as well as prohibit their uncontrolled growth through apoptosis.
Apoptosis is a natural path to eliminate the unhealthy and unorganized cells from body without any harm of neighboring cells. It is characterized by membrane blebbing, nucleus condensation, contraction of cell membrane, nuclear fragmentation, cell shrinkage etc. [47, 48]. It occurs by arresting the cells at G1, S and G2/M phase of cell cycle to inhibit the formation of tumor [49]. The abnormalities of apoptosis process play a vital role in formation of mass of cells called tumor, subsequently allow the cells to divide and organize in an unusual way which lead to cancerous development [50, 51]. This defect of apoptosis is largely depend on the imbalanced interactions between anti-apoptotic proteins (Bcl-2, Bcl-X, Bcl-W, Mcl-1, etc.) and pro-apoptotic proteins (Bax, Bak, Bok, Mtd, etc.). The down regulation of Bcl-2 family genes and up-regulation of Bax family genes trigger the formation of pore on membrane of mitochondria and eventually take place cell apoptosis through mitochondria mediated intrinsic pathway [9, 52, 53].
In this present investigation, expression of Bcl-2 and Bax gene was analyzed. We found that leaf and bark extract reduced the expression of Bcl-2 and increased the expression of Bax in dose-depended fashion. So, the leaf and bark samples of M. latifolia inhibit the EAC cell growth through intrinsic apoptotic process by creating a number of cell abnormalities and can become a good source for further research on cancer.
References
Todaro M, Bonanno A, Tornambè G, Di Grigoli A, Luisa Scatassa M, Giaccone P (2009) Utilization of mulberry leaves (Morus latifolia cv. Kokusou 21) in diets for dairy ewes. Ital J Anim Sci 8:438–440
Bernal J, Mendiola JA, Ibez E, Cifuentes A (2011) Advanced analysis of nutraceuticals. J Pharm Bbiomed Anal 55:758–775
Iqbal S, Younas U, Chan KW, Sarfraz RA, Uddin MK (2012) Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): a comparative study. Int J Mol Sci 13:6651–6664
Islam MS, Rahi MS, Koli HK, Jerin I, Sajib SA, Hoque KMF, Reza MA (2018) Evaluation of phytochemical, antioxidant, cytotoxicity and in vitro antibacterial activity of aqueous extract of Ganoderma lucidum cultivated in Bangladeshi habitat. Malaya J Biosci 5:1–13
Niemi M, Sthle G (2016) The use of ayurvedic medicine in the context of health promotiona mixed methods case study of an ayurvedic centre in Sweden. BMC Complement Altern Med 16:62–75
Narayanaswamy V (1981) Origin and development of ayurveda:(a brief history). Anc Sci Life 1:1–7
Hou DX (2003) Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med 3:149–159
Islam MS, Rahi MS, Jerin I, Hasan KE, Sajib SA, Ferdaus KMKB, Hoque KMF, Reza MA (2017) Antiproliferative, antioxidant and antibacterial activity of seed extract of Thuja occidentalis. Int J Biosci 11:372–386
Al-Mamun MA, Husna J, Khatun M, Hasan R, Kamruzzaman M, Hoque KMF, Reza MA, Ferdousi Z (2016) Assessment of antioxidant, anticancer and antimicrobial activity of two vegetable species of Amaranthus in Bangladesh. BMC Complement Altern Med 16:157–167
Tagne RS, Telefo BP, Nyemb JN, Yemele DM, Njina SN, Goka SMC, Lienou LL, Kamdje AHN, Moundipa PF, Farooq AD (2014) Anticancer and antioxidant activities of methanol extracts and fractions of some Cameroonian medicinal plants. Asian Pac J Trop Med 7:S442–S447
Krishnamoorthy M, Ashwini P (2011) Anticancer activity of Cynodon dactylon L. extract on Ehrlich ascites carcinoma. J Environ Res Dev 5:551–557
Fadeyi SA, Fadeyi OO, Adejumo AA, Okoro C, Myles EL (2013) In vitro anticancer screening of 24 locally used Nigerian medicinal plants. BMC Complement Altern Med 13:79–87
Cragg G, Newman D (2003) Plants as a source of anticancer and anti HIV agents. Ann Appl Biol 143:127–133
Gao PF, Watanabe K (2011) Introduction of the World Health Organization project of the international classification of traditional medicine. Zhong Xi Yi Jie He Xue Bao 9:1161–1164
Ferguson LR, Chen H, Collins AR, Connell M, Damia G, Dasgupta S, Malhotra M, Meeker AK, Amedei A, Amin A (2015) Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol 35:5–24
Bernstein C, Bernstein H (2015) Epigenetic reduction of DNA repairs in progression to gastrointestinal cancer. World J Gastrointest Oncol 7:30–46
Du L, Kim JJ, Chen B, Zhu S, Dai N (2017) Marital status is associated with superior survival in patients with esophageal cancer: a Surveillance, Epidemiology, and End Results study. Oncotarget 8:95965–95972
Roukos DH (2009) Genome wide association studies: how predictable is a persons cancer risk? Expert Rev Anticancer Ther 9:389–392
Alam AK, Hossain AS, Khan MA, Kabir SR, Reza MA, Rahman MM, Islam MS, Rahaman MAA, Rashid M, Sadik MG (2016) The antioxidative fraction of white mulberry induces apoptosis through regulation of p53 and NFKB in EAC cells. PLoS ONE 11:e0167536
Lü JM, Lin PH, Yao Q, Chen C (2010) Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med 14:840–860
Brudner M, Karpel M, Lear C, Chen L, Yantosca LM, Scully C, Sarraju A, Sokolovska A, Zariffard MR, Eisen DP (2013) Lectin dependent enhancement of Ebola virus infection via soluble and transmembrane C type lectin receptors. PLoS ONE 8:e60838
Kabir SR, Nabi MM, Nurujjaman M, Reza MA, Alam AK, Zaman RU, Khalid BFK, Ruhul MA, Hasan MMK, Hossain MA (2015) Momordica charantia seed lectin: toxicity, bacterial agglutination and antitumor properties. Appl Biochem Biotechnol 175:2616–2628
Rutishauser U, Sachs L (1975) Cell-to-cell binding induced by different lectins. J Cell Biol 65:247–257
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Reed JC (2000) Mechanisms of apoptosis. Am J Pathol 157:1415–1430
Searle J, Lawson T, Abbott P, Harmon B, Kerr J (1975) An electron microscope study of the mode of cell death induced by cancer chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol 116:129–138
Balasubramanian A, Ramalingam K, Krishnan S, Ajm C (2005) Anti-inflammatory activity of Morus indica Linn. Iran J Pharmacol Ther 4:13–15
Khalid N, Fawad SA, Ahmed I (2011) Antimicrobial activity, phytochemical profile and trace minerals of black mulberry (Morus nigra L.) fresh juice. Pak J Bot 43:91–96
Kabir S, Islam F, Jahangir Alom M, Abu Zubair M, Absar N (2012) Purification, characterizations of a snake guard seeds lectin with antitumor activity against Ehrlich ascites carcinoma cells in vivo in mice. Protein Pept Lett 19:360–368
Hasan MM, Islam MS, Hoque KMF, Haque A, Reza MA (2019) Effect of Citrus macroptera fruit pulp juice on alteration of caspase pathway rendering anti-proliferative activity against Ehrlich’s ascites carcinoma in mice. Toxicol Res 35:271–277
Le VQA, Ahn JY, Heo MY, Cho SJ, Yoon H, Park J, Ko JH, Lee L, Han J, Kim SY (2017) Proteomic profiles of Daphnia magna exposed to lead (II) acetate trihydrate and atrazine. Genes Genomics 39:887–895
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28:25–30
Sur P, Ganguly DK (1994) Tea plant root extract (TRE) as an antineoplastic agent. Planta Med 60:106–109
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics, 2005. CA Cancer J Clin 55:10–30
Aruoma OI (1998) Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 75:199–212
Mukherjee PK, Kumar V, Houghton PJ (2007) Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother Res 21:1142–1145
Lpez-Lzaro M (2010) A new view of carcinogenesis and an alternative approach to cancer therapy. Mol Med 16:144–153
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DJ, McLaughlin JL (1982) Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 45:31–34
Rieser MJ, Gu Z-M, Fang X-P, Zeng L, Wood KV, McLaughlin JL (1996) Five novel mono-tetrahydrofuran ring acetogenins from the seeds of Annona muricata. J Nat Prod 59:100–108
Jeyaprakash AA, Jayashree G, Mahanta S, Swaminathan C, Sekar K, Surolia A, Vijayan M (2005) Structural basis for the energetics of jacalinsugar interactions: promiscuity versus specificity. J Mol Biol 347:181–188
De Meja EG, Prisecaru VI (2005) Lectins as bioactive plant proteins: a potential in cancer treatment. Crit Rev Food Sci Nutr 45:425–445
Liu B, Bian HJ, Bao JK (2010) Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Lett 287:1–12
Kabir SR, Zubair MA, Nurujjaman M, Haque MA, Hasan I, Islam MF, Hossain MT, Hossain MA, Alam MT (2011) Purifica- tion and characterization of a Ca2+ dependent novel lectin from Nymphaea nouchali tuber with antiproliferative activities. Biosci Rep 31:465–475
Ahmed H, Chatterjee B, Debnath A (1988) Interaction and in vivo growth inhibition of Ehrlich ascites tumor cells by jacalin. J Biosci 13:419–424
Jaganathan SK, Mondhe D, Wani Z, Pal HC, Mandal M (2010) Effect of honey and eugenol on Ehrlich ascites and solid carcinoma. Biomed Res Int 2010:989163
Bhattacharyya A, Choudhuri T, Pal S, Chattopadhyay S, Datta GK, Sa G, Das T (2003) Apoptogenic effects of black tea on Ehrlichs ascites carcinoma cell. Carcinogenesis 24:75–80
Islam M, Rahi M, Jahangir CA, Rahman MH, Jerin I, Amin R, Hoque KMF, Reza MA (2018) In vivo anticancer activity of Basella alba leaf and seed extracts against Ehrlich’s ascites carcinoma (EAC) cell line. Evid Based Complement Altern Med 2018:1537896
Hyun JH, Kang JI, Kim SC, Kim E, Kang JH, Kwon JM, Park DB, Lee YJ, Yoo ES, Kang HK (2008) The effects of Crinum asiaticum on the apoptosis induction and the reversal of multidrug resistance in HL-60/MX2. Toxicol Res 24:29–36
Gomes A, Giri B, Alam A, Mukherjee S, Bhattacharjee P, Gomes A (2011) Anticancer activity of a low immunogenic protein toxin (BMP1) from Indian toad (Bufo melanostictus, Schneider) skin extract. Toxicon 58:85–92
Brown JM, Attardi LD (2005) The role of apoptosis in cancer development and treatment response. Nat Rev Cancer 5:231–237
Sa DJ, Lee EJ, Yoo BS (2009) Apoptosis induction by menadione in human promyelocytic leukemia HL-60 cells. Toxicol Res 25:113–118
Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH (2001) Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene 20:2927–2936
Giannakakou P, Robey R, Fojo T, Blagosklonny MV (2001) Low concentrations of paclitaxel induce cell type dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel- induced cytotoxicity. Oncogene 20:3806–3813
Acknowledgements
All the authors are grateful to Dr. Saidur Rahman (Chief Scientific Officer, Bangladesh Sericulture Research and Training Institute, Rajshahi, Bangladesh) for providing the experimental plant materials.
Funding
There was no significant financial support for this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that there are no conflicts of interest about this paper for publication.
Additional information
Md. Shihabul Islam and Chowdhury Arif Jahangir have eqaully contributed and considered as first authors.
Rights and permissions
About this article
Cite this article
Islam, M.S., Jahangir, C.A., Rahi, M.S. et al. In-vivo antiproliferative activity of Morus latifolia leaf and bark extracts against Ehrlich’s ascites carcinoma. Toxicol Res. 36, 79–88 (2020). https://doi.org/10.1007/s43188-019-00011-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-019-00011-7